Abstract
Introduction
The introduction of immunotherapy (IO) in the treatment of patients with cancer has significantly improved clinical outcomes. Population level information on actual IO utilization is limited.
Methods
We conducted a retrospective cohort study using provincial health administrative data from Ontario, Canada to: (1) assess the extent of IO use from 2011 (pre-IO funding) to 2019; and (2) identify factors associated with IO use in patients with advanced cancers for which IO is reimbursed including melanoma, bladder, lung, head and neck, and kidney tumors. The datasets were linked using a unique encoded identifier. A Fine and Gray regression model with death as a competing risk was used to identify factors associated with IO use.
Results
Among 59 510 patients assessed, 8771 (14.7%) received IO between 2011 and 2019. Use of IO increased annually from 2011 (3.3%) to 2019 (39.2%) and was highest in melanoma (52%) and lowest in head and neck cancer (6.6%). In adjusted analysis, factors associated with lower IO use included older age (hazard ratio (HR) 0.91 (95% CI, 0.89-0.93)), female sex (HR 0.85 (95% CI, 0.81-0.89)), lower-income quintile, hospital admission (HR 0.78 (95% CI, 0.75-0.82)), high Charlson score and de novo stage 4 cancer. IO use was heterogeneous across cancer centers and regions.
Conclusion
IO utilization for advanced cancers rose substantially since initial approval albeit use is associated with patient characteristics and system-level factors even in a universal healthcare setting. To optimize IO utilization in routine practice, survival estimates and potential inequity in access should be further investigated and addressed.
Keywords: immunotherapy, utilization, advanced cancer, population-based study, universal healthcare system
Since the advent of immunotherapy (IO), the treatment paradigm for cancer has dramatically changed for multiple tumor sites. This study used provincial administrative health data to estimate the proportion of patients with advanced cancer for which IO had been approved received the treatment and explored factors associated with IO use.
Implications for Practice.
The utilization of immunotherapy has substantially increased over the years in patients with advanced melanoma, bladder, lung, head and neck, and kidney tumors. Immunotherapy use was associated with patient characteristics and system-level factors such as age, sex, income quintile, and institution type suggesting potential inequity in access to treatment even in a universal healthcare system. Given its rapid adoption in routine practice, understanding patterns of immunotherapy use and reporting on survival estimates is crucial in optimizing the utilization of these novel therapies.
Introduction
The past decade has witnessed notable advances in cancer treatment; the emergence of immune-based therapies, in particular, has sparked the promise to revolutionize cancer therapy.1,2 Since the advent of immunotherapy (IO), the treatment paradigm for cancer has dramatically changed for multiple tumor sites. The survival benefit achieved with the use of IO compared to conventional chemotherapy, in addition to its manageable toxicity profile, has set a new standard of care for many patients with advanced cancers.3-7
To date, most evidence on the benefits of IO has come from randomized trials. Data from randomized trials are based on highly selected patients, which may limit the generalizability of the results. A recently published cross-sectional study from the US reported that the estimated percentage of American patients with cancer who are eligible for IO increased from 1.54% in 2011 to 43.63% in 2018.8 Data on actual IO utilization at the system level, including in a universal healthcare setting such as Canada is limited. Real-world evidence can provide information on the extent of IO use including whether patients with advanced cancer outside clinical trials have equitable access to IO and whether the treatment has been adopted homogeneously over time. This may identify factors associated with IO use that could be addressed in publicly funded healthcare systems to optimize access and equity to treatment and improve patient outcomes. Such information can also assist with health system planning given the substantial cost of IO.
In Ontario, Canada’s most populous province, IO is approved for the treatment of adult patients with select advanced cancers. We used provincial administrative health data from Ontario to estimate what proportion of patients with advanced cancer for which IO has been approved receive it and explored factors associated with IO use.
Methods
Study Design and Objectives
To assess the use of IO and to identify factors associated with IO use, we conducted a retrospective cohort study in Ontario, Canada between 2011 and 2019. Ontario is Canada’s largest province and provides health care through a government-administered single-payer system. As such, our study used health administrative data representing the whole population. Analysis was performed at ICES, an independent, non-profit research institute that facilitates health services research in the province (Supplementary Appendix 1). This study adhered to the RECORD reporting guidelines.9
Data Sources
The databases we used included: Ontario Cancer Registry (OCR), Registered Persons Database (RPDB), Discharge Abstract Database (DAD), Ontario Health Insurance Plan (OHIP) claims database, information about Ontario health care institutions funded by the Ministry of Health and Long-Term Care (INST), Cancer Activity Level Reporting (ALR) database, ICES physician database (IPDB), Drug list (DIN) and New Drug Funding Program (NDFP). These datasets were linked using unique encoded identifiers and analyzed at ICES (Supplementary Table S1).
Participants
The target population consisted of patients 18 years of age or older diagnosed between January 2011 and December 2019 with a new malignancy for which IO has been approved in Ontario up to 2019 (melanoma, lung, bladder, head and neck, and kidney cancers). The index date to create our cohort was the date of diagnosis. IO approvals in the province started in 2012; the timing of individual approvals is summarized in Supplementary Table S2. Eligible patients were identified using the OCR database. Given that all IO approvals during this time frame focused on patients with advanced disease, the eligible population included patients with either stage 4 at diagnosis or those with evidence of having received systemic therapy with palliative intent at any point during the study period. The codes used to identify eligible patients based on their cancer site are summarized in Supplementary Table S3.
Baseline Characteristics and Covariates
The following baseline patient and tumor characteristics were identified: age at diagnosis, sex, income quintile, rurality, Local Health Integration Network (LHIN) which are health authorities responsible for regional administration of public healthcare services in Ontario (Supplementary Appendix 2), year of diagnosis, tumor site, whether patients had a subsequent different stage 4 cancer, whether patients had de novo metastatic disease, cancer center facility level at the time of diagnosis with the level of complexity of care delivered and the availability of services differentiating one level from another (4 levels with level 1 being the most complex) (Supplemental Appendix 2),10 and Charlson score with a lookback period of 5 years prior to diagnosis. Some key pathologic information such as PD-L1 status and molecular aberrations were not available. Other variables included were treatment with radiation therapy and hospital admission since diagnosis. The details on how each variable was operationalized are shown in Supplementary Table S4.
Exposure of Interest
Immunotherapy included any treatment with an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and/or anti-programmed death-(ligand) 1 (PD-1/PD-L1) inhibitor administered in any line in the metastatic setting including whether this was received as part of a clinical trial or not. This included: atezolizumab, avelumab, durvalumab, ipilimumab, nivolumab, pembrolizumab, or any combination of IO with/without other systemic therapies. Drug identification numbers are presented in Supplementary Table S3. To identify whether a patient received IO, we followed patients from the time of diagnosis until death or the latest available follow-up date. Patients with more than one stage 4 cancer were censored at the time of the second stage 4 cancer diagnosis as we are not able to identify for which cancer site the IO was given.
Outcomes
The primary outcome was the use of IO in the target population. IO use was reported as the proportion of patients diagnosed with any cancer site of interest between 2011 and 2019 who received at least one dose of IO out of the total number of patients who were diagnosed with any cancer site of interest during the same period. We also reported the IO use by tumor site and drug type per year. For each tumor site, we reported the proportion of patients with a specific cancer site diagnosed in a specific year who received IO at any time during follow-up period out of the total number of patients diagnosed with the same cancer of interest during the same year. For drug type, we reported the proportion of patients who were diagnosed during a specific year with any cancer site of interest and received a specific IO out of the total number of patients who were diagnosed with any cancer of interest during the same year. Our secondary aim was to identify factors associated with IO use in the same target population.
Statistical Analysis
We described and compared the patients and their tumor characteristics and receipt of IO to those without, as well as between those receiving IO through a clinical trial vs. those who received it in routine care. The codes used to identify clinical trials are shown in Supplementary Table S5. All continuous variables were reported as means with SD and medians with interquartile ranges as appropriate. All categorical variables were reported as frequency counts and proportions. To test for significance, a one-way ANOVA or Kruskal-Wallis test for continuous variables or chi square for categorical variables were used. Standardized differences and tests for significant differences between IO use were reported. Missingness categories were included for variables with missing values. In accordance with ICES privacy policies, cell sizes less than or equal to five were not reported. A Fine and Gray regression model with death as a competing risk was used to evaluate factors associated with IO use. We adjusted for the following variables: age, sex, income quintile, rurality, year of cohort entry, region, tumor site, multiple stage 4 cancers, cancer center level, Charlson score, hospital admission, radiation therapy, and de novo stage 4 cancer. Adjusted hazard ratios (HRs) and 95% CIs were reported. Collinearity and model validity were assessed.
We further conducted a sensitivity analysis excluding patients with stage 4 disease who never received systemic therapy (chemotherapy or immunotherapy) after diagnosis. This alternate analysis was undertaken because it may be argued that patients who did not receive any systemic therapy may have been too sick or unfit to be eligible to receive systemic treatment including IO. Throughout, p-values of ≤.05 and standardized differences of >.10 were considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Cohort Characteristics
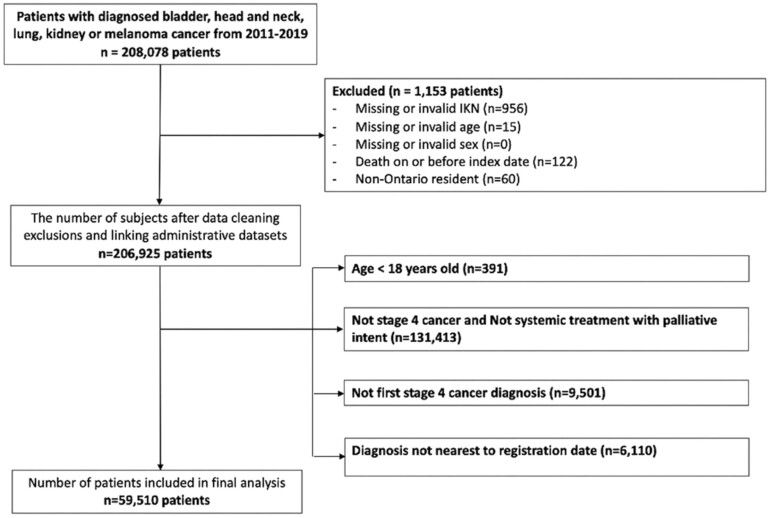
After applying all inclusion and exclusion criteria, the final cohort consisted of 59 510 patients (Fig. 1). As shown in Table 1, the median age of the cohort was 69 years old (IQR 61-77) and 42% of patients were female. Almost 70% of patients had lung cancer and 33% were diagnosed at a level 1 cancer center. More than half of the patients (57%) received radiation therapy and 68% were admitted to the hospital at some point following diagnosis. Almost 73% of patients had de novo metastatic disease. Pembrolizumab (41.6%) and Nivolumab (37.4%) were the two most prescribed IO drugs.
Figure 1.
Cohort creation.
Table 1.
Baseline characteristics.
| Total (N = 59 510) | Unexposed (to immunotherapy) (N = 50 739) | Exposed (to immunotherapy) (8771) | P value | |
|---|---|---|---|---|
| Age at index date | ||||
| Mean (SD) | 68.33 ± 11.34 | 68.84 ± 11.33 | 65.37 ± 10.97 | <.001 |
| Median (IQR) | 69 (61-77) | 69 (61-77) | 66 (59-73) | <.001 |
| Age <65 | 21 417 (36.0%) | 17 505 (34.5%) | 3912 (44.6%) | <.001 |
| Age ≥65 | 38 093 (64.0%) | 33 234 (65.5%) | 4859 (55.4%) | |
| Female, n (%) | 24 756 (41.6%) | 21, 294 (42.0%) | 3462 (39.5%) | <.001 |
| Income quintile, N (%) | ||||
| Quintile 1 | 13 606 (22.9%) | 11 967 (23.6%) | 1639 (18.7%) | <.001 |
| Quintile 2 | 12 964 (21.8%) | 11 126 (21.9%) | 1838 (21.0%) | |
| Quintile 3 | 11 652 (19.6%) | 9874 (19.5%) | 1778 (20.3%) | |
| Quintile 4 | 10 886 (18.3%) | 9146 (18.0%) | 1740 (19.8%) | |
| Quintile 5 | 10 213 (17.2%) | 8459 (16.7%) | 1754 (20.0%) | |
| Rural, yes, n (%) | 8845 (14.9%) | 7556 (14.9%) | 1289 (14.7%) | .704 |
| Year of cohort entry, n (%) | ||||
| 2011 | 7195 (12.1%) | 6956 (13.7%) | 239 (2.7%) | <.001 |
| 2012 | 7363 (12.4%) | 6980 (13.8%) | 383 (4.4%) | |
| 2013 | 7481 (12.6%) | 6966 (13.7%) | 515 (5.9%) | |
| 2014 | 7216 (12.1%) | 6526 (12.9%) | 690 (7.9%) | |
| 2015 | 7059 (11.9%) | 6057 (11.9%) | 1002 (11.4%) | |
| 2016 | 7105 (11.9%) | 5853 (11.5%) | 1252 (14.3%) | |
| 2017 | 6913 (11.6%) | 5355 (10.6%) | 1558 (17.8%) | |
| 2018 | 5681 (9.5%) | 3920 (7.7%) | 1761 (20.1%) | |
| 2019 | 3497 (5.9%) | 2126 (4.2%) | 1371 (15.6%) | |
| Local Health Integration Network for Ontario (LHIN), n (%) | ||||
| A | 3813 (6.4%) | 3272 (6.4%) | 541 (6.2%) | <.001 |
| B | 5238 (8.8%) | 4539 (8.9%) | 699 (8.0%) | |
| C | 3151 (5.3%) | 2736 (5.4%) | 415 (4.7%) | |
| D | 7321 (12.3%) | 6270 (12.4%) | 1051 (12.0%) | |
| E | 2448 (4.1%) | 1998 (3.9%) | 450 (5.1%) | |
| F | 3512 (5.9%) | 2929 (5.8%) | 583 (6.6%) | |
| G | 4226 (7.1%) | 3677 (7.2%) | 549 (6.3%) | |
| H | 5870 (9.9%) | 4951 (9.8%) | 919 (10.5%) | |
| I | 6692 (11.2%) | 5700 (11.2%) | 992 (11.3%) | |
| J | 3401 (5.7%) | 2872 (5.7%) | 529 (6.0%) | |
| K | 6280 (10.6%) | 5343 (10.5%) | 937 (10.7%) | |
| L | 2513 (4.2%) | 2099 (4.1%) | 414 (4.7%) | |
| M | 3814 (6.4%) | 3272 (6.4%) | 542 (6.2%) | |
| N | 1231 (2.1%) | 1081 (2.1%) | 150 (1.7%) | |
| Tumor site, n (%) | ||||
| Head and neck | 7253 (12.2%) | 6775 (13.4%) | 478 (5.4%) | <.001 |
| Melanoma | 3838 (6.4%) | 1845 (3.6%) | 1993 (22.7%) | |
| Kidney | 3387 (5.7%) | 2499 (4.9%) | 888 (10.1%) | |
| Bladder | 3708 (6.2%) | 3137 (6.2%) | 571 (6.5%) | |
| Lung | 41 324 (69.4%) | 36 483 (71.9%) | 4841 (55.2%) | |
| More than one (stage 4) cancer diagnosis, n (%) | 537 (0.9%) | 505 (1.0%) | 32 (0.4%) | <.001 |
| Teaching | 16 685 (28.0%) | 12 823 (25.3%) | 3862 (44.0%) | |
| Other/missing | 28 924 (48.6%) | 27 575 (54.3%) | 1349 (15.4%) | |
| Diagnosis cancer center level, n (%) | ||||
| 1 | 19 769 (33.2%) | 16 917 (33.3%) | 2852 (32.5%) | <.001 |
| 2 | 15 078 (25.3%) | 12 837 (25.3%) | 2241 (25.6%) | |
| 3 | 14 221 (23.9%) | 12 234 (24.1%) | 1987 (22.7%) | |
| 4 | 10 386 (17.5%) | 8715 (17.2%) | 1671 (19.1%) | |
| Other/missing | 56 (0.1%) | 36 (0.1%) | 20 (0.2%) | |
| Radiation therapy, Yes, n (%) | 33 924 (57.0%) | 28 024 (55.2%) | 5900 (67.3%) | <.001 |
| Hospitalizations, Yes (%) | 40 328 (67.8%) | 35 377 (69.7%) | 4951 (56.4%) | <.001 |
| IO regimen part of clinical trial, for patients who had the outcome, n (%) | N/A | N/A | 637 (7.3%) | N/A |
| Charlson Comorbidty Index in the previous 5 years, n (%) | ||||
| 0 | 7857 (13.2%) | 6741 (13.3%) | 1116 (12.7%) | <.001 |
| 1 | 4961 (8.3%) | 4392 (8.7%) | 569 (6.5%) | |
| 2 | 3891 (6.5%) | 3423 (6.7%) | 468 (5.3%) | |
| 3+ | 5309 (8.9%) | 4834 (9.5%) | 475 (5.4%) | |
| No hospitalizations | 37 492 (63.0%) | 31 349 (61.8%) | 6143 (70.0%) | |
| IO drug type, n (%) | ||||
| Atezolizumab | N/A | N/A | 267 (3.0%) | N/A |
| Avelumab | N/A | N/A | 48 (0.5%) | |
| Combination | N/A | N/A | 754 (8.6%) | |
| Durvalumab | N/A | N/A | 238 (2.7%) | |
| Ipilimumab | N/A | N/A | 423 (4.8%) | |
| Nivolumab | N/A | N/A | 3283 (37.4%) | |
| Pembrolizumab | N/A | N/A | 3647 (41.6%) | |
| De novo stage 4 cancer, n (%) | 43 297 (72.8%) | 39 849 (78.5%) | 3448 (39.3%) | <.001 |
*index date: date of cancer diagnosis. Abbreviations: IO, immunotherapy; NA, not applicable.
Compared to patients who did not receive IO, patients treated with IO were younger, a greater proportion was male, and a greater proportion had a high-income quintile and a lower Charlson comorbidity score. Moreover, on a patient level, there was geographic variation across the province (LHINs) and differences in the level of cancer centers where the diagnosis was made. Patients who received IO were more frequently treated with radiation therapy and were less likely to have de novo metastatic disease and be admitted to the hospital.
Among patients treated with IO, 637 (7.3%) received it as part of a clinical trial (Supplementary Table S6). Patients who received IO in a clinical trial were younger and lived in urban areas. They were more frequently diagnosed in level 1 cancer centers, more frequently diagnosed with de novo metastatic disease, and received radiation therapy. In addition, they had a lower Charlson score and a shorter time to treatment. The IO drugs prescribed on trial differed, with the most frequently used IO drug on trial being durvalumab (30%) which was only used in 0.6% of patients outside trials.
Immunotherapy Utilization
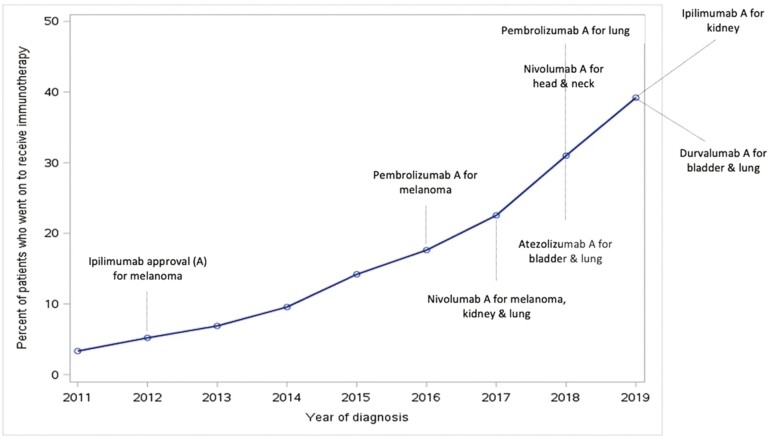
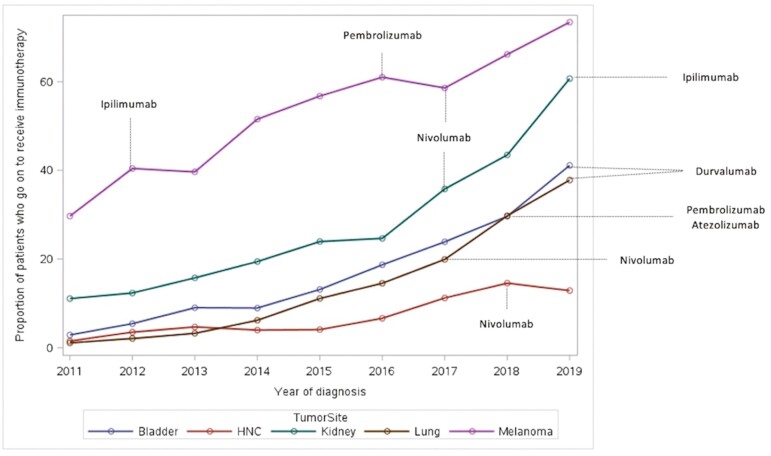
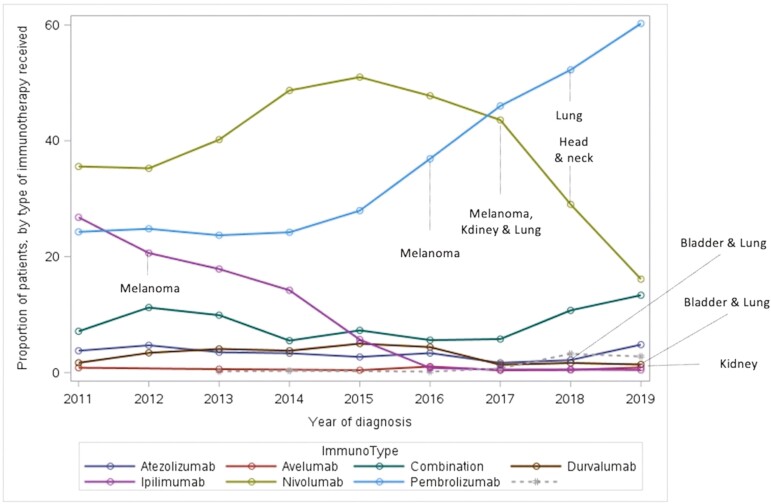
Overall, 8771 patients out of 59 510 diagnosed with any advanced cancer of interest in the province between 2011 and 2019 received IO (14.7%) (Fig. 2). The IO use increased yearly across all tumor sites from 2011 to 2019 as presented in Table 2. Utilization varied by tumor site and was the highest in patients with melanoma (52%) and the lowest in patients with head and neck cancer (6.6%) acknowledging the fact that IO was first approved for melanoma in 2012 and subsequently for other sites from 2017 onward (Fig. 3). The type of IO used also changed over time (Fig. 4). While ipilimumab and nivolumab were frequently used in 2012 for melanoma, nivolumab, and pembrolizumab use increased with time for multiple tumor sites. The use of other IO (durvalumab, atezolizumab, and avelumab) was limited particularly outside clinical trials.
Figure 2.
Immunotherapy use in Ontario. X axis: time/year of diagnosis. Y axis: percentage of patients diagnosed with any of the cancer site of interest at a certain year who received immunotherapy out of the total number of patients diagnosed with any of the cancer site of interest during the same year.
Table 2.
Immunotherapy use by year of diagnosis and tumor site.
| IO use N = 59 510 | Bladder N = 3708 (%) | HN N = 7253 (%) | Kidney N = 3387 (%) | Lung N = 41 324 (%) | Melanoma N = 3838 (%) | Overall use per year (%) |
|---|---|---|---|---|---|---|
| 2011 | 2.9 | 1.5 | 11.1 | 1.1 | 29.7 | 3.3 |
| 2012 | 5.4 | 3.5 | 12.3 | 2.1 | 40.4 | 5.2 |
| 2013 | 9.0 | 4.7 | 15.7 | 3.2 | 39.6 | 6.9 |
| 2014 | 8.9 | 4.0 | 19.4 | 6.2 | 51.5 | 9.6 |
| 2015 | 13.1 | 4.1 | 23.9 | 11.1 | 56.7 | 14.2 |
| 2016 | 18.7 | 6.6 | 24.7 | 14.5 | 61.0 | 17.6 |
| 2017 | 23.9 | 11.2 | 35.8 | 19.9 | 58.6 | 22.5 |
| 2018 | 29.6 | 14.5 | 43.5 | 29.7 | 66.2 | 31.0 |
| 2019 | 41.1 | 12.9 | 60.7 | 37.8 | 73.4 | 39.2 |
| Overall use per site | 15.4 | 6.6 | 26.2 | 11.7 | 51.9 | 14.7 |
Ontario approval of immunotherapy (IO).
Bladder cancer: atezolizumab approved in 2018, durvalumab approved in 2019.
Head and neck cancers: nivolumab approved in 2018.
Kidney cancer: ipilimumab approved in 2019, nivolumab approved in 2017.
Lung cancer: pembrolizumab approved in 2018, nivolumab approved in 2017, atezolizumab approved in 2018, durvalumab approved in 2019.
Melanoma: ipilimumab approved in 2012, pembrolizumab approved in 2016, nivolumab approved in 2017.
Figure 3.
Immunotherapy use by tumor site. X axis: time/year of diagnosis. Y axis: percentage of patients diagnosed with one of the cancer sites of interest at a certain year who received immunotherapy out of the total number of patients diagnosed with the same cancer site of interest during the same year.
Figure 4.
Immunotherapy use by drug type. X axis: time/year of diagnosis. Y axis: percentage of patients diagnosed with any of the cancer sites at a certain year who received a specific IO out of the total number of patients diagnosed with any of the same cancer sites during the same year.
Factors Associated with Utilization
The results of the unadjusted analysis are presented in Supplementary Table S7. Younger patients, those residing in higher-income neighborhood or diagnosed with melanoma were more likely to receive IO. Similarly, patients without significant comorbidities (Charlson score <3) and patients who received radiation therapy had a higher likelihood to be treated with IO. In contrast, patients with de novo metastatic disease, patients with history of a hospital admission, and patients with more than one stage 4 cancer were less likely to receive IO. Furthermore, utilization varied across different geographic regions and types of cancer facilities where the initial diagnosis was made.
The results of the adjusted analysis are presented in Table 3. After adjusting for known possible confounders, all identified covariates in the unadjusted model remained significant except for patients having more than one stage 4 cancer. Additionally, sex became associated with IO use. Older age (every additional 10 years) (HR 0.91 (95% CI, 0.89–0.93)), female sex (HR 0.85 (95% CI, 0.81-0.89)) and lower-income quintile (HR 0.85 (95% CI, 0.8-0.92)) for income quintile 1 and HR 0.91 (95% CI, 0.85-0.98) for income quintile 2 compared to quintile 5) were associated with a lower likelihood of treatment with IO. As well, patients diagnosed later during the study period (HR 1.78 (95% CI, 1.75-1.8)), patients with melanoma (compared to other disease sites), and patients treated with radiation therapy (HR 1.55 (95% CI, 1.48-1.62)) had a higher likelihood of getting IO. IO use was heterogeneous across LHINs and diagnosis cancer center levels. Patients with a higher Charlson score (HR 0.86 (95% CI, 0.77-0.95)) for Charlson score 1 and HR 0.84 (95% CI, 0.76-0.94) for Charlson score 2 compared to Charlson score 0), patients who were admitted to hospital after their diagnosis (HR 0.78 (95% CI, 0.75-0.82)) and patients diagnosed with de novo metastatic disease (HR 0.8 (95% CI, 0.77-0.84)) had a lower likelihood of receiving IO.
Table 3.
Adjusted analysis for factors associated with immunotherapy use.
| Factors associated with immunotherapy use | Adjusted hazard ratio of immunotherapy treatment vs. no IO treatment (95% CI) | |||||
|---|---|---|---|---|---|---|
| Unit | N in category | HR | Lower CL | Upper CL | P value | |
| Age | Every additional 10 years | 0.91 | 0.89 | 0.93 | <.0001 | |
| Sex | Reference = “Male” | 24 756 | 0.85 | 0.81 | 0.89 | <.0001 |
| Income quintile | Reference = “Quintile 5” | |||||
| Quintile 1 | 13 606 | 0.85 | 0.80 | 0.91 | <.0001 | |
| Quintile 2 | 12 964 | 0.91 | 0.85 | 0.98 | .007 | |
| Quintile 3 | 11 652 | 0.97 | 0.91 | 1.04 | .338 | |
| Quintile 4 | 10 886 | 0.96 | 0.89 | 1.02 | .186 | |
| Missing | 189 | 0.92 | 0.46 | 1.84 | .811 | |
| Rural | Reference = “Urban” | |||||
| Rural | 8845 | 1.02 | 0.95 | 1.09 | .620 | |
| Missing | 80 | 0.94 | 0.39 | 2.25 | .890 | |
| Year of cohort entry | per year | 1.78 | 1.75 | 1.80 | <.0001 | |
| LHIN | Ref = G | |||||
| A | 3813 | 1.10 | 0.98 | 1.25 | .110 | |
| B | 5238 | 1.11 | 0.99 | 1.25 | .072 | |
| C | 3151 | 1.07 | 0.94 | 1.22 | .302 | |
| D | 7321 | 1.18 | 1.06 | 1.31 | .002 | |
| E | 2448 | 1.23 | 1.08 | 1.39 | .002 | |
| F | 3512 | 1.10 | 0.90 | 1.14 | .888 | |
| H | 5870 | 1.02 | 0.92 | 1.14 | .677 | |
| I | 6692 | 1.22 | 1.10 | 1.36 | .000 | |
| J | 3401 | 1.31 | 1.16 | 1.48 | <.0001 | |
| K | 6280 | 1.15 | 1.03 | 1.28 | .012 | |
| L | 2513 | 1.30 | 1.14 | 1.49 | .000 | |
| M | 3814 | 1.19 | 1.05 | 1.35 | .007 | |
| N | 1231 | 0.86 | 0.71 | 1.04 | .119 | |
| Tumor site | Per site (reference melanoma) | |||||
| Lung | 41 324 | 0.45 | 0.42 | 0.48 | <.0001 | |
| Kidney | 3387 | 0.54 | 0.50 | 0.59 | <.0001 | |
| HNC | 7253 | 0.08 | 0.08 | 0.09 | <.0001 | |
| Bladder | 3708 | 0.30 | 0.27 | 0.33 | <.0001 | |
| More than one (stage 4) cancer diagnosis | Yes vs. no | 537 | 0.88 | 0.62 | 1.25 | .471 |
| Diagnosing cancer center level | Ref = (level 1) | 19 769 | ||||
| 2 | 15 078 | 1.12 | 1.05 | 1.20 | .001 | |
| 3 | 14 221 | 1.06 | 0.99 | 1.13 | .109 | |
| 4 | 10 386 | 0.95 | 0.89 | 1.02 | .138 | |
| Missing | 56 | 1.21 | 0.78 | 1.90 | .397 | |
| Charlson score | Ref = 0 | |||||
| No hosp | 37 492 | 0.96 | 0.87 | 1.06 | .414 | |
| 1 | 7857 | 0.86 | 0.77 | 0.95 | .005 | |
| 2 | 4961 | 0.84 | 0.76 | 0.94 | .002 | |
| 3+ | 3891 | 1.01 | 0.95 | 1.08 | .692 | |
| Hospitalization | yes vs. no | 40 459 | 0.78 | 0.75 | 0.82 | <.0001 |
| Radiation therapy | yes vs no | 33 892 | 1.55 | 1.48 | 1.62 | <.0001 |
| De novo stage 4 cancer | yes vs no | 43 297 | 0.80 | 0.77 | 0.84 | <.0001 |
Abbreviation: IO, immunotherapy.
Sensitivity Analysis
After excluding patients who never received any systemic therapy, the IO use was almost two-fold higher (26%, 8771 out of 34 043). Most variables associated with IO use identified in the adjusted model remained significant. The results of the sensitivity analysis are presented in Supplementary Table S8. Older patients, female sex, lower-income quintile, and being diagnosed with a cancer of interest other than melanoma remained associated with lower IO use. Similarly, patients with higher Charlson scores and patients with a history of hospital admission after diagnosis were less likely to get IO while patients who received radiation therapy and patients who had de novo metastatic disease were more likely. The IO use remained heterogeneous across the LHINs and between diagnosis cancer center levels.
Discussion
In this population-based retrospective cohort study in Ontario, we found that IO utilization has increased considerably over time across all tumor sites for which IO has been approved. This is in line with previous reports showing rapid adoption of IO in the US after FDA approval.11,12 We also identified several factors associated with IO use including age, sex, income quintile, geography, tumor site, cancer center level, Charlson score, previous radiation therapy, and history of hospital admission. IO use differed across tumor sites, which is likely explained by the timing of approvals and the clinical scenario (line of therapy and biomarker status (ie, PD-L1)). Unsurprisingly, we saw the highest use (52%) among patients with melanoma which is likely due to the fact that public IO funding approval occurred first for melanoma (in 2012), compared with other cancer sites where patients were only eligible for IO after 2016. Furthermore, patients with melanoma were able to receive IO in the first-line setting and independent of any biomarker (ie, PD-L1). Patients with head and neck tumors had the lowest use (6.6%); which is likely explained by a later approval date (2018) and only in the second-line metastatic setting where historically less than 50% of patients receive second-line therapies.13 As for patients with lung cancer, IO approval was granted for several IO agents for second-line treatment, at first independent of PD-L1 status, then in the first-line setting for patients whose tumors had high PD-L1 expression (≥50%). We would expect higher IO use than 11.7% if we were able to capture biomarker information to only include patients who were eligible to receive IO based on PD-L1 status. Patients whose tumors have high PD-L1 expression (≥50%) account for approximately one-third of patients with advanced non–small cell lung cancer.14 Additionally, similar to patients with head and neck cancer, less than 50% of patients with advanced lung cancer receive second-line therapies in practice.15
We saw several differences in patients’ characteristics depending on whether IO was received as part of a clinical trial. Compared to patients treated outside trials, patients receiving IO as part of a trial were younger, more frequently diagnosed at level 1 cancer centers and were more likely to have been treated with radiation therapy. This is not surprising, as patients in clinical trials are typically highly selected and may differ from the general population.
We also identified several patient and tumor characteristics associated with IO use in routine care. Some were expected such as age, tumor site, and year of diagnosis, Charlson score, and history of hospital admission as one would expect that IO is offered for patients who are likely more fit for systemic therapy. For patients diagnosed in earlier years, it is likely that those with higher comorbidity burden or older age, were not as fit by the time of IO approval for their particular cancer but we are unable to ascertain this hypothesis as we do not have information on comorbidity burden or performance status at the time of IO approval. However, other factors need to be further explored to address potential inequity in access to IO. These include sex (female patients) and lower income in a publicly funded system. Disparities in relation to factors such as socioeconomic status are consistent with prior studies predating the IO era16,17 and need to be considered in future studies.
Our study needs to be interpreted in the context of its limitations. Since we did not have a reliable way to identify cancer recurrence, we limited our eligible population to those diagnosed during the immunotherapy era and started the inception date as one year prior to first public IO approval in Ontario. We did not include patients who were diagnosed earlier but were still alive and potentially eligible to receive IO. We did not have information on variables such as PD-L1, EGFR, ALK status, use of oral agents that target some of these aberrations, and line of therapy; some of which could exclude patients from being “eligible” to receive IO. Furthermore, the lag between the cancer diagnosis, IO use, and data availability, did not allow us to accurately assess IO use in the last year of diagnosis (2019). We also did not have detailed information on IO used through compassionate access programs that may have allowed patients to receive IO before public approval. As a result, with additional information on biomarkers, line of therapy, and compassionate access programs, we would have identified “eligible patients” more precisely and we may have underestimated the absolute number of patients who received IO.
This study provides an important contribution to the oncology literature, as to our knowledge, it is the first population-based study of IO use in a publicly funded health care system. In this study, we were able to include patients not only based on their cancer stage (stage 4) but also intent of therapy (palliative intent) albeit we limited the cohort to those first diagnosed during the IO era. Typically, patients are included based on their stage, determined at the time of diagnosis. Therefore, patients who had recurrence or progression afterward would be missed and not included in the analysis. Because of the multiple linked population-based administrative data sources, we were able to avoid selection biases that may be associated with institutional case series.
Lastly, our study provides unique insights into the population-based use of novel therapies in patients with advanced cancer in Ontario and potential disparities in access to treatment that need to be explored further. Building an understanding of population-level outcomes is especially important given the resource constraints inherent to Ontario’s publicly funded health system. The rapid increase in IO use has major financial implications for the healthcare system. In Ontario, where this study was conducted, the total annual cost of cancer treatment drugs increased by nearly 40-fold from 1997 to 2016 (≈$40 million).18 In order for this additional cost to have value, the substantial increase in IO utilization needs to translate into a survival benefit similar to what the randomized trials have shown. However, recent real-world data analyses have shown that survival estimates seem to be shorter than those reported in registration trials.19,20 Therefore, further studies investigating survival outcomes, cost-utility, and barriers to receipt of IO treatment among advanced cancer patients are warranted given the rapid increase in usage and its expansion to more cancer sites.
Conclusion
In summary, IO utilization has increased substantially in Ontario over time across relevant tumor sites which reflects the increasing clinical evidence and funding of new anti-cancer agents. This results in a higher cost on the healthcare system that needs to be considered. Potential inequities to access to therapy were also identified in a publicly funded healthcare system and need to be explored.
Supplementary Material
Contributor Information
Jacques Raphael, Division of Medical Oncology, London Regional Cancer Program, Western University, London, ON, Canada; ICES, London, ON, Canada.
Lucie Richard, ICES, London, ON, Canada.
Melody Lam, ICES, London, ON, Canada.
Phillip S Blanchette, Division of Medical Oncology, London Regional Cancer Program, Western University, London, ON, Canada; ICES, London, ON, Canada.
Natasha B Leighl, Division of Medical Oncology & Hematology, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada.
George Rodrigues, Division of Radiation Oncology, London Health Sciences Centre, London, ON, Canada.
Maureen E Trudeau, Division of Medical Oncology, Odette Cancer Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Monika K Krzyzanowska, Division of Medical Oncology & Hematology, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada; ICES Central, Toronto, ON, Canada.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). The study was completed at the ICES Western site. This study received funding from the Medical Oncology Research Fund Grant (MORF). The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Conflict of Interest
Jacques Raphael: Hoffmann-La Roche (H), Lilly, Merk, Novartis, AstraZeneca (SAB); Maureen E. Trudeau: RNA Diagnostics (OI, RF—inst.); Monika K. Krzyzanowska: Eisai (C/A), Lilly (SAB), Eisai, Exelixis, Ipsen, Lilly (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Author Contributions
Conception/design: J.R., L.R., M.L., P.B., N.L., G.R., M.T., M.K. Provision of study material/patients: J.R., L.R., M.L., M.K. Collection and/or assembly of data: J.R., L.R., M.L., M.K. Data analysis and interpretation: J.R., L.R., M.L., P.B., N.L., G.R., M.T., M.K. Manuscript writing: J.R., L.R., M.L., M.K. Final approval of manuscript: All authors.
Data Availability
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
References
- 1. Zugazagoitia J, Guedes C, Ponce S, et al. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551-1566. 10.1016/j.clinthera.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 2. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335-3337. 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haslam A, Prasad V.. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;3(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. 10.1371/journal.pmed.1001885 PMID: 26440803; PMCID: PMC4595218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandenberg T, Coakley N, Nayler J, et al. A framework for the organization and delivery of systemic treatment. Curr Oncol. 2009;16(1):4-15. 10.3747/co.v16i1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. 10.1001/jamaoncol.2018.0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krimphove MJ, Tully KH, Friedlander DF, et al. Adoption of immunotherapy in the community for patients diagnosed with metastatic melanoma. J Immunotherapy Cancer. 2019;7:289. 10.1186/s40425-019-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byrne K, Hallworth P, Monfared AAT, Moshyk A, Shaw JW.. Real-world systemic therapy treatment patterns for squamous cell carcinoma of the head and neck in Canada. Curr Oncol. 2019;26(2):e167-e174. 10.3747/co.26.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vrankar M, Kern I, Stanic K.. Prognostic value of PD-L1 expression in patients with unresectable stage III non-small cell lung cancer treated with chemoradiotherapy. Radiat Oncol. 2020;15:247. 10.1186/s13014-020-01696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies J, Patel M, Gridelli C, de Marinis F, Waterkamp D, McCusker ME.. Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: A systematic review of recently published studies. PLoS One. 2017;12(4):e0175679. Published 2017 Apr 14. 10.1371/journal.pone.0175679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradley CJ, Yabroff KR, Mariotto AB, et al. Antineoplastic treatment of advanced-stage non-small-cell lung cancer: treatment, survival, and spending (2000 to 2011). J Clin Oncol. 2017;35:529-535. 10.1200/JCO.2016.69.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. David EA, Daly ME, Li C-S, et al. Increasing rates of no treatment in advanced-stage non-small cell lung cancer patients: a propensity-matched analysis. J Thorac Oncol. 2017;12:437-445. 10.1016/j.jtho.2016.11.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng SY, Saxena FE, Seung SJ, Earle CC, Chan K, Mittmann N.. Demographic characteristics and cost of treatment among oncology patients in a publicly funded system, the Ontario Trillium Drug Program: a retrospective cohort study. CMAJ Open. 2019;7(3):E516-E523. 10.9778/cmajo.20190011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parikh RB, Min EJ, Wileyto EP, et al. Uptake and survival outcomes following immune checkpoint inhibitor therapy among trial-ineligible patients with advanced solid cancers. JAMA Oncol. 2021;7(12):1843-1850. doi: 10.1001/jamaoncol.2021.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kehl KL, Greenwald S, Chamoun NG, Manberg PJ, Schrag D.. Association between first-line immune checkpoint inhibition and survival for medicare-insured patients with advanced non–small cell lung cancer. JAMA Netw Open. 2021;4(5):e2111113. doi: 10.1001/jamanetworkopen.2021.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.