Abstract
Background
The plasma cell disorders (PCDs), multiple myeloma (MM), and light-chain amyloidosis (AL) are disproportionately diseases of older adults, whose care may be complicated by frailty associated with advancing age. We sought to evaluate the prevalence of functional deficits and symptoms in a cohort of persons with PCDs and associations of demographic, disease-related, functional, and psychosocial measures with quality of life (QoL).
Patients and Methods
Adults with PCDs were recruited into an observational registry in 2018-2020. Patients completed a functional assessment and European Organization for Research and Treatment of Cancer QoL questionnaire (QLQ-C30). Associations of covariates of interest with QoL were evaluated via univariate linear regression.
Results
Among 121 adults, the mean age was 68.6. Diagnoses were 74% MM, 14% AL, 7% both MM and AL, and 5% other PCDs. The median time from diagnosis was 34.9 months. Median lines of therapy were 2, with 11% having received ≥4th-line therapy.
Patients with functional deficits had lower mean QoL scores: dependence in IADLs (66.3 vs. 79.9, P = .001) and recent falls (56.7 vs. 76.8, P = .001). Patients ≤6 months from diagnosis had lower QoL (66.7) than those ≥2 years from diagnosis (77.3, P = .03). However, patients on later lines of therapy (≥4th-line) had lower QoL (62.2) than those on 1st-line treatment (76.0, P = .04).
Conclusions
Patients with physical impairments and more advanced PCDs had lower QoL than those without deficits or earlier in their disease course. Early identification of physical impairments may facilitate interventions that mitigate these deficits and thereby improve QoL for patients with PCDs.
Keywords: plasma cell disorders, quality of life, physical function, geriatric assessment
The plasma cell disorders (PCDs) multiple myeloma (MM) and light chain amyloidosis (AL) are disproportionately diseases of older adults, whose care may be complicated by frailty associated with advancing age. This article evaluates the prevalence of functional deficits and symptoms in a cohort of persons with PCDs and associations of demographic, disease-related, functional, and psychosocial measures with quality of life.
Implications for Practice.
The plasma cell disorders multiple myeloma and light chain amyloidosis are disproportionately diseases of older adults, whose care may be complicated by comorbidity and frailty associated with advancing age. In a cohort of patients treated for plasma cell disorders, patients with physical impairments and more advanced diseases reported lower quality of life, findings that support early identification of physical impairments to facilitate interventions aimed at mitigating the impact of these deficits for those receiving treatment for these disorders.
Introduction
Plasma cell disorders (PCDs) encompass many related conditions driven by abnormal clonal plasma cell proliferation and are typically characterized by the secretion of dysfunctional immunoglobulins or immunoglobulin fragments.1 One can conceptualize PCDs as existing on a “cell-to-protein” spectrum, wherein organ injury is mediated by invasive plasma cells, toxic immunoglobulins (proteins), or both. The end organs involved, and thus the symptoms experienced by patients, vary across this spectrum. Multiple myeloma (MM) sits toward the cellular end of this spectrum, while light-chain amyloidosis (AL) exists on the protein end. In all symptomatic PCDs, therapy aimed at killing toxic plasma cells is the mainstay of treatment.2,3
Survival in many PCDs is improving, but they remain incurable.4,5 Patients with PCDs consequently have a longer “opportunity” to experience what is frequently a substantial degree of morbidity.6 Understanding the symptom burden and functional impairments that patients along the PCD spectrum experience is therefore vital for supporting patient well-being and quality of life (QoL).
Prior studies have suggested that preserved performance status (a subjective composite measure of global health and anticipated chemotherapy tolerance) is a strong predictor of QoL among patients with PCDs, a relationship most thoroughly evaluated in the setting of MM.7-10 Among patients with AL, both patient-reported QoL and physical impairments have been linked to healthcare utilization, although the relationship between physical function and QoL was not assessed.11
Isolated assessments of performance status have notable limitations. PCDs are disproportionately diseases of older adults. The median age at diagnosis for patients with MM is 69,12,13 and estimates for those with AL range from 6514 to 76.15 Prior studies have indicated that routine assessments of performance status do not adequately evaluate for the presence of important functional deficits, particularly among older adults.16,17 Therefore, guidelines from professional organizations including the National Comprehensive Cancer Network and the International Society of Geriatric Oncology advocate for incorporating more comprehensive evaluations into oncology practice, with Geriatric Assessment (GA) methodologies generally highlighted as an optimal approach.18-21 These assessments consist of evaluations of the older adult’s physical capabilities, function, nutrition, comorbidities, cognition, and psychosocial status.19,22
In this study, we report findings from a multidimensional assessment of function and symptoms, as elicited by a brief GA and supplementary questionnaires, among a cohort of patients participating in an observational registry. The primary aims of our study were (1) to evaluate the prevalence of clinically relevant functional deficits and symptoms in this cohort encompassing the spectrum of PCDs and (2) to evaluate associations of demographic, disease, treatment, physical function, and psychosocial measures with QoL.
Methods
Setting and Patients
Study participants receiving systemic therapy for a PCD were recruited into an observational PCD Registry (ClinicalTrials.gov identifier NCT03717844) from 2018 to 2020 at our institution. The current analysis included assessments from the time of enrollment for each participant. Recruitment was initially restricted to individuals aged ≥65. Criteria were expanded in 2019 to include adults of all ages. Enrollment paused for 6 months during 2020 due to the COVID-19 pandemic (part of an institution-wide accrual hold) and subsequently resumed with a remote assessment option. Non-English-speaking individuals were excluded due to the use of English-language instruments. Those not seen longitudinally at our institution were not recruited due to incomplete follow-up. Recruitment was otherwise sequential. All participants provided written informed consent. The university institutional review board approved the study protocol.
Demographic and Clinical Variables
We collected demographic data including age, self-identified race, sex, educational attainment, employment, and marital status. Disease- and treatment-related variables included diagnosis per published criteria,1 stage at diagnosis (International Staging System23 for MM and Mayo 2012 stage24 for AL), time from diagnosis, number of lines of therapy,25 and therapy intensity. We deemed therapy “de-intensified” if treatment dosing was empirically reduced from full-dose regimens as published due to concerns for patient frailty, very advanced age, comorbidities, or other factors, as documented in the treating provider’s notes. Standard dose reductions for impaired metabolism (due to renal or hepatic function) were not considered “de-intensified.”
Participants completed a modified Cancer and Aging Research Group GA26,27 along with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life of Cancer Patients Core 30 (QLQ-C30) questionnaire,28,29 a validated 30-item questionnaire of self-reported symptoms and function. The QLQ-C30 includes sub-scores for 9 individual symptoms (fatigue, pain, appetite loss, etc.), 5 functional domains (physical, role, social, emotional, and cognitive functioning), and overall QoL. Each score ranges from 0 to 100. Higher functional scores indicate superior function, while higher symptom scores indicate more intense symptoms.
Physical function was evaluated via study team assessments (performed by trained Study Coordinators) and patient-reported items. Study team assessments included Karnofsky Performance status (KPS) and Timed Up and Go (TUG) test. KPS scores of ≥80 are considered functionally normal.30-32 TUG times ≥14 s are prognostic of worse health outcomes.33,34 Self-assessed items included patient-reported KPS,35 falls in the preceding 6 months,36 an inventory of instrumental activities of daily living (IADLs),37 and QLQ-C30 Physical Function score. IADL scores <14 indicate dependence in at least one IADL, and QLQ-C30 Physical Function scores <83 indicate impairment.38
Comorbidity was captured via self-reported presence/absence of 14 conditions drawn from the Older Americans Resources and Services health assessment37 and a number of daily medications. Psychological health was evaluated using the Mental Health Index-13 (MHI) Depression and Anxiety scales, with scores of ≥12 and ≥6 indicating significant depression or anxiety, respectively.39 Psychosocial functioning was further assessed via the QLQ-C30 Role functioning, emotional functioning, social functioning, and cognitive functioning scales, which have thresholds for impairment of <58, <71, <58, and <75, respectively.38
Outcome Variable
The primary outcome was the QLQ-C30 global health and QoL score. Items used to calculate this composite score are unique from items used for the function/symptom scales.
Statistical Analysis
Descriptive statistics are provided as means and ranges or as frequencies and percentages. Diagnostic group distributions and proportions were compared via Wilcoxon rank-sum test or Fisher’s exact test, respectively, with uncorrected P values reported. Analyses were conducted for the overall cohort and the 2 largest diagnostic subgroups (MM and AL). For comparisons between diagnostic groups, subjects meeting diagnostic criteria for both MM and AL were grouped based on their dominant clinical phenotype (whether symptoms and pattern of organ injury better fit MM or AL). For measures scored based on multi-item responses (IADLs, QLQ-C30, and MHI), only patients with responses for all items received a score. Univariate linear regression was used to evaluate associations of various disease characteristics, comorbidities, and the presence of functional deficits with QoL. Uncorrected P values are reported.
Results
Patient Demographics and Diagnoses
From 2018 to 2020, 121 patients enrolled. The mean age was 68.6% and 66% were 65 or older (Table 1). The cohort was 72% white and 46% female. Most individuals had some college education or greater (78%) and were married (71%). The majority (74%) had MM, 14% had AL, 7% had concurrent MM and AL, and 5% had another PCD prompting treatment. These diagnoses included plasma cell leukemia (n = 1), smoldering MM on investigational protocols (n = 3), smoldering MM and AL (n = 1), and polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes (POEMS) syndrome (n = 1). Demographic characteristics were similar across diagnostic groups. The mean number of comorbidities was 2.3; most commonly hypertension (51%) and arthritis (42%).
Table 1.
Characteristics of study participants treated for plasma cell disorders.
| Variable | Full cohort (n = 121) | MM without AL(a) (n = 89) | AL without MM(a) (n = 21) | P (b) |
|---|---|---|---|---|
| Demographics | ||||
| Age—mean (SD) | 68.6 (10.9) | 69.1 (11.1) | 68.1 (9.2) | .60 |
| Age group—number of participants (%) | ||||
| <55 years | 14 (12%) | 11 (12%) | 1 (5%) | |
| 55-64 years | 27 (22%) | 17 (19%) | 6 (28%) | |
| 65-74 years | 48 (40%) | 36 (40%) | 9 (43%) | |
| ≥75 years | 32 (26%) | 25 (28%) | 5 (24%) | .65 |
| Race—N (%) | ||||
| White | 87 (72%) | 62 (70%) | 17 (81%) | |
| Black or African-American | 33 (27%) | 26 (29%) | 4 (19%) | |
| Other | 1 (1%) | 1 (1%) | 0 (0%) | .53 |
| Sex—N (%) | ||||
| Female | 56 (46%) | 42 (47%) | 9 (43%) | |
| Male | 65 (54%) | 47 (53%) | 12 (57%) | .81 |
| Education—N (%) | ||||
| High school graduate or less | 26 (22%) | 21 (24%) | 3 (15%) | |
| Some college or greater | 94 (78%) | 68 (76%) | 17 (85%) | .55 |
| Used (≥32 h/week)—N (%) | 14 (12%) | 7 (8%) | 4 (21%) | .11 |
| Married—N (%) | 86 (71%) | 63 (71%) | 16 (76%) | .79 |
| Live alone—N (%) | 22 (19%) | 16 (19%) | 3 (14%) | .76 |
| Disease and treatment | ||||
| Diagnosis—N (%) | ||||
| Multiple myeloma without amyloidosis | 89 (74%) | ----- | ----- | |
| MM and AL | 9 (7%) | ----- | ----- | |
| Light chain amyloidosis without MM | 17 (14%) | ----- | ----- | |
| Other PCD warranting chemotherapy | 6 (5%) | ----- | ----- | |
| ISS stage at diagnosis (for patients with MM)—N (%) | ||||
| Stage I | 37 (38%) | ----- | ----- | |
| Stage II | 24 (24%) | ----- | ----- | |
| Stage III | 25 (26%) | ----- | ----- | |
| Unable to calculate | 12 (12%) | ----- | ----- | |
| Mayo stage at diagnosis (for patients with AL amyloidosis)—N (%) | ||||
| Stage I | 3 (12%) | ----- | ----- | |
| Stage II | 5 (19%) | ----- | ----- | |
| Stage III | 5 (19%) | ----- | ----- | |
| Stage IV | 9 (35%) | ----- | ----- | |
| Unable to calculate | 4 (15%) | ----- | ----- | |
| Time since diagnosis | ||||
| 0-6 months | 31 (26%) | 15 (17%) | 12 (57%) | |
| 6-12 months | 9 (7%) | 6 (7%) | 1 (5%) | |
| 1-2 years | 13 (11%) | 8 (9%) | 5 (24%) | |
| >2 years | 68 (56%) | 60 (67%) | 3 (14%) | <.0001 |
| Current line of therapy | ||||
| 1st | 60 (49%) | 41 (46%) | 12 (57%) | |
| 2nd-3rd | 48 (40%) | 36 (40%) | 8 (38%) | |
| ≥4th line | 13 (11%) | 12 (13%) | 1 (5%) | .55 |
| Current treatment program—N (%) | ||||
| Full intensity induction | 52 (43%) | 39 (44%) | 7 (33%) | |
| Empirically de-intensified regimen | 22 (18%) | 12 (13%) | 7 (33%) | |
| Maintenance program | 38 (31%) | 36 (40%) | 2 (10%) | |
| No current therapy | 9 (8%) | 2 (3%) | 5 (24%) | .0002 |
| Medical comorbidities | ||||
| Number of comorbid conditions- mean (SD) | 2.3 (1.7) | 2.3 (1.7) | 2.6 (1.5) | .43 |
| Other cancers or leukemia—N (%) | 19 (16%) | 15 (17%) | 1 (5%) | .30 |
| Arthritis or rheumatism—N (%) | 50 (42%) | 37 (43%) | 8 (38%) | .81 |
| High blood pressure—N (%) | 60 (51%) | 49 (57%) | 7 (33%) | .087 |
| Heart disease—N (%) | 32 (27%) | 17 (20%) | 11 (52%) | .0044 |
| Circulation trouble in arms or legs—N (%) | 17 (14%) | 13 (15%) | 4 (19%) | .74 |
| Diabetes—N (%) | 18 (15%) | 16 (19%) | 1 (5%) | .18 |
| Stomach or intestinal disorders—N (%) | 17 (15%) | 10 (12%) | 5 (24%) | .17 |
| Osteoporosis—N (%) | 19 (16%) | 15 (17%) | 3 (14%) | 1.0 |
| Chronic liver or kidney disease—N (%) | 17 (15%) | 8 (9%) | 9 (43%) | .0008 |
| Depression—N (%) | 18 (15%) | 11 (13%) | 4 (19%) | .49 |
| Daily medications—Mean (SD) | 8.4 (4.3) | 8.3 (4.3) | 8.9 (4.5) | .53 |
| Taking 0-4 | 24 (20%) | 17 (19%) | 5 (24%) | |
| Taking 5-9 | 52 (43%) | 41 (47%) | 6 (29%) | |
| Taking ≥10 | 44 (37%) | 30 (34%) | 10 (48%) | .31 |
| Physical function | ||||
| Self-reported KPS—N (%) | ||||
| 80 | 100 (83%) | 74 (83%) | 17 (81%) | |
| <80 | 21 (17%) | 15 (17%) | 4 (19%) | .76 |
| Independently-assessed KPS—N (%) | ||||
| ≥80 | 89 (80%) | 64 (78%) | 17 (85%) | |
| <80 | 22 (20%) | 18 (22%) | 3 (15%) | .76 |
| Timed up and go—N (%) | ||||
| <14 seconds | 85 (70%) | 61 (69%) | 16 (76%) | |
| ≥14 seconds | 36 (30%) | 28 (31%) | 5 (24%) | .60 |
| IADL score—N (%) | ||||
| 14 | 76 (64%) | 57 (66%) | 12 (57%) | |
| <14 | 42 (36%) | 29 (34%) | 9 (43%) | .45 |
| Falls in the past 6 months—N (%) | ||||
| 0 falls | 103 (87%) | 73 (83%) | 19 (95%) | |
| ≥ 1 falls | 16 (13%) | 15 (17%) | 1 (5%) | .30 |
| QLQ-C30 physical functioning—mean (SD) | ||||
| Score <83 (impaired) | 56 (49%) | 39 (46%) | 12 (60%) | |
| Score ≥83 | 59 (51%) | 45 (54%) | 8 (40%) | .33 |
| Psychosocial | ||||
| MHI-13 depression—N (%) | ||||
| Not depressed (<12) | 93 (82%) | 72 (86%) | 16 (84%) | |
| Depressed (≥12) | 21 (18%) | 12 (14%) | 3 (16%) | 1.0 |
| MHI-13 anxiety—N (%) | ||||
| Not anxious (<6) | 86 (73%) | 64 (74%) | 15 (75%) | |
| Anxious (≥6) | 32 (27%) | 23 (26%) | 5 (25%) | 1.0 |
| QLQ-C30 role functioning—N (%) | ||||
| Percentage ≥58 | 91 (79%) | 70 (83%) | 14 (70%) | |
| Percentage <58 (impaired) | 24 (21%) | 14 (17%) | 6 (30%) | .21 |
| QLQ-C30 emotional functioning —N (%) | ||||
| 71 | 92 (81%) | 69 (83%) | 16 (80%) | |
| <71 (impaired) | 22 (19%) | 14 (17%) | 4 (20%) | .75 |
| QLQ-C30 social functioning—N (%) | ||||
| ≥58 | 96 (84%) | 73 (88%) | 15 (75%) | |
| <58 (impaired) | 18 (16%) | 10 (12%) | 5 (25%) | .16 |
| QLQ-C30 cognitive functioning—N (%) | ||||
| ≥75 | 91 (80%) | 69 (83%) | 13 (65%) | |
| <75 (impaired) | 23 (20%) | 14 (17%) | 7 (35%) | .12 |
| Global health/quality of life | ||||
| EORTC C30 Global health status QoL—mean (SD) | 74.4 (22.2) | 74.0 (22.7) | 72.1 (21.3) | .62 |
| Symptoms | ||||
| Fatigue—N (%) | ||||
| Score ≤39 | 83 (73%) | 62 (75%) | 13 (65%) | |
| Score >39 (clinically significant symptoms) | 31 (27%) | 21 (25%) | 7 (35%) | .41 |
| Nausea/vomiting—N (%) | ||||
| Score ≤8 | 86 (75%) | 66 (80%) | 11 (55%) | |
| Score >8 (clinically significant symptoms) | 28 (25%) | 17 (20%) | 9 (45%) | .042 |
| Pain—N (%) | ||||
| Score ≤25 | 76 (66%) | 51 (61%) | 18 (90%) | |
| Score >25 (clinically significant symptoms) | 39 (34%) | 33 (39%) | 2 (10%) | .016 |
| Dyspnea—N (%) | ||||
| Score ≤17 | 72 (64%) | 58 (71%) | 7 (35%) | |
| Score >17 (clinically significant symptoms) | 41 (36%) | 24 (29%) | 13 (65%) | .0043 |
| Insomnia/sleep disturbance—N (%) | ||||
| Score ≤50 | 86 (75%) | 64 (77%) | 15 (75%) | |
| Score >50 (clinically significant symptoms) | 28 (25%) | 19 (23%) | 5 (25%) | 1.0 |
| Appetite loss—N (%) | ||||
| Score ≤50 | 98 (87%) | 71 (87%) | 18 (95%) | |
| Score >50 (clinically significant symptoms) | 14 (13%) | 11 (13%) | 1 (5%) | .45 |
| Constipation—N (%) | ||||
| Score ≤50 | 100 (88%) | 73 (89%) | 17 (85%) | |
| Score >50 (clinically significant symptoms) | 13 (12%) | 9 (11%) | 3 (15%) | .70 |
(a) For comparisons between disease groups, subjects meeting diagnostic criteria for both MM and AL were grouped based on their dominant clinical phenotype on clinical review (4 patients were included in the AL group and 0 in the MM group). Those deemed to have an overlapping clinical phenotype were excluded from these comparisons. (b) P-value for comparison between diagnostic groups. Missing data reflect incomplete questionnaire responses by individual patients (1 for education, 1 for daily medications, 3 for IADLs, 6-7 for EORTC C30 items, 7 for MHI depression, and MHI depression, and 3 for MHI Anxiety) or lack of in-person assessment of KPS by study team during COVID-19 pandemic (n=10).
Abbreviations: MM, Multiple myeloma; AL, immunoglobulin light chain amyloidosis; PCD, plasma cell disorder; ISS, International Staging System; KPS, Karnofsky Performance Status; IADL, Instrumental activity of daily living; QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life of Cancer Patients Core 30 questionnaire; MHI, Mental Health Index.
The median time from diagnosis was 34.9 months. Recently diagnosed patients (≤6 months from diagnosis) accounted for 26%, while a majority (56%) were >2 years from diagnosis. Despite this relatively long time from diagnosis, 49% of individuals had received only one line of therapy, with 29% having received 2 lines, 11% three lines, and 11% four or more lines of therapy. The vast majority were on some form of plasma-cell-directed therapy, with only 8% receiving no therapy at the time of assessment. Patients with AL were more likely than those with MM to be off treatment.
Physical Function
Among the full cohort, KPS was impaired (score <80) in 17% by self-assessment and 20% by study-team assessment (Table 1). Prolonged TUG times were observed in 30%. Falls were reported by 13%. Impairment in at least one IADL was noted by 36%. Impaired QLQ-C30 physical function scores (score <83)38 were observed in 49%. Rates of physical function impairments were similar across diagnostic groups.
Psychosocial Assessment
Significant depression and anxiety based on the MHI-13 were noted among 18% and 27% of patients, respectively. Significant impairments in role functioning, emotional functioning, and social functioning based on QLQ-C30 responses were described by 21%, 19%, and 16% of study participants. Subjective difficulties with cognitive functioning were reported by 20% of the overall cohort as part of the QLQ-C30. The prevalence of these impairments was similar across the diagnostic groups.
Quality of Life
The mean QLQ-C30 QoL score for the full cohort was 74.4. No significant differences were noted by age, race, education, marital status, or cohabitation (Table 2). Female patients reported somewhat greater QoL scores than males (78.9 vs. 70.4, P =.042). The presence of most individual comorbidities was not associated with QoL scores (data not shown). Lower QoL scores were seen among those with high blood pressure (69.9 vs. 79.8, P = .013) and depression (63.9 vs. 76.7, P = .018).
Table 2.
Univariate associations between patient characteristics/disease factors/functional deficits and quality of life among patients treated for plasma cell disorders
| Variable | QLQ-C30 global health/QoL score (mean) | SD | P |
|---|---|---|---|
| Demographics | |||
| Age | |||
| <55 years | 79.2 | 17.2 | (referent) |
| 55-64 years | 72.2 | 22.0 | .35 |
| 65-74 years | 74.6 | 24.0 | .51 |
| 75 years | 73.9 | 22.2 | .47 |
| Race | |||
| White | 72.8 | 23.8 | (referent) |
| Black/African-American | 78.0 | 17.9 | .26 |
| Other | 83.3 | -- | .64 |
| Sex | |||
| Female | 78.9 | 21.0 | |
| Male | 70.4 | 22.7 | .04 |
| Education | |||
| High school or less | 69.0 | 19.0 | |
| Some college or greater | 76.0 | 23.0 | .16 |
| Marital Status | |||
| Married | 72.4 | 24.0 | |
| Not married | 79.2 | 16.4 | .14 |
| Living alone | |||
| Living alone | 77.4 | 16.3 | |
| Not living alone | 73.7 | 22.2 | .47 |
| Disease and treatment | |||
| Diagnosis | |||
| Multiple myeloma | 74.0 | 22.7 | (referent) |
| AL amyloidosis without MM | 72.1 | 21.3 | .73 |
| Other diagnosis | 81.8 | 19.7 | .28 |
| Time since diagnosis | |||
| 0-6 month | 66.7 | 20.8 | (referent) |
| 6-12 months | 83.3 | 20.8 | .049 |
| 1-2 years | 70.5 | 25.1 | .60 |
| >2 years | 77.3 | 21.7 | .033 |
| Current line of therapy | |||
| 1st | 76.0 | 20.2 | (referent) |
| 2nd-3rd | 75.9 | 21.5 | .99 |
| ≥4th line | 62.2 | 29.8 | .04 |
| Current treatment program | |||
| Full intensity induction | 70.7 | 22.4 | .32 |
| Empirically de-intensified regimen | 70.2 | 25.0 | .34 |
| Maintenance program | 80.4 | 17.6 | .84 |
| No current therapy | 78.7 | 28.9 | (referent) |
| Physical function | |||
| Self-reported KPS | |||
| ≥80 | 78.5 | 19.9 | |
| <80 | 55.0 | 22.7 | <.0001 |
| Independently-assessed KPS | |||
| ≥80 | 79.0 | 19.8 | |
| <80 | 58.7 | 24.3 | <.0001 |
| Timed up and go (TUG) | |||
| <14 seconds | 76.3 | 22.7 | |
| ≥14 seconds | 70.1 | 20.5 | .18 |
| IADL score | |||
| <14 | 66.3 | 22.9 | |
| 14 | 79.9 | 18.6 | .001 |
| Falls in the past 6 months | |||
| 0 | 76.8 | 20.8 | |
| 1 or more falls | 56.7 | 24.0 | .001 |
| EORTC C30 physical functioning | |||
| vScore ≥83 | 84.5 | 17.6 | |
| Score <83 (impaired) | 64.0 | 21.8 | <.0001 |
| Psychosocial | |||
| MHI-13 depression | |||
| Not depressed (<12) | 76.7 | 21.2 | |
| Depressed (≥12) | 62.7 | 24.8 | .01 |
| MHI-13 anxiety | |||
| Not anxious (<6) | 75.8 | 22.0 | |
| Anxious (≥6) | 68.9 | 22.5 | .15 |
| QLQ-C30 role functioning | |||
| Score ≥58 | 79.4 | 19.1 | |
| Score <58 (impaired) | 55.6 | 23.3 | <.0001 |
| QLQ-C30 emotional functioning | |||
| Score ≥71 | 75.9 | 22.4 | |
| Score <71 (impaired) | 68.2 | 20.7 | .14 |
| QLQ-C30 social functioning | |||
| Score ≥58 | 78.8 | 19.9 | |
| Score <58 (impaired) | 50.9 | 18.9 | <.0001 |
| QLQ-C30 cognitive functioning | |||
| Score ≥75 | 77.7 | 20.9 | |
| Score <75 (impaired) | 61.2 | 22.6 | .001 |
Abbreviations: QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life of Cancer Patients Core 30 questionnaire; QoL, quality of life; AL, immunoglobulin light chain amyloidosis; MM, multiple myeloma; KPS, Karnofsky Performance Stat us; IADL, instrumental activity of daily living; MHI, Mental Health Index.
Quality-of-life scores did not differ significantly between diagnostic groups, although symptoms were notable for greater pain among patients with MM and more frequent dyspnea among those with AL. Quality-of-life scores were similar among those receiving full intensity induction treatment (70.7) and empirically de-intensified regimens (70.2). Individuals receiving maintenance therapy (80.4) or no current treatment (78.7) had higher QoL scores, although these differences were not statistically significant.
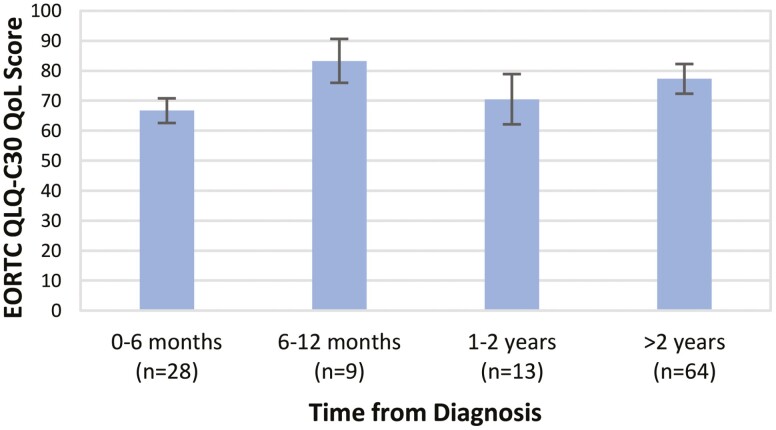
Those further from diagnosis had higher QoL than recently diagnosed individuals: 77.3 for those greater than 2 years from diagnosis vs. 66.7 within 6 months of diagnosis (P = .033) (Fig. 1). The mean QoL score trended higher for those 6-12 months from diagnosis (83.3), although this group was relatively small (n = 9). Patients on later lines of therapy (4th-line or greater) had lower quality of life scores (62.2) compared with those on first-line treatment (76.0, P = .043) (Fig. 2). Those on second- to third-line treatment (75.9) had similar scores to those on first-line treatment.
Figure 1.
Composite quality of life score versus time from diagnosis among patients with plasma cell disorders. Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life of Cancer Patients Core 30 questionnaire; QoL, Quality of life.
Figure 2.
Composite quality of life score versus line of systemic therapy among patients with plasma cell disorders. Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life of Cancer Patients Core 30 questionnaire; QoL: Quality of life.
Patients with self-assessed KPS of <80 had lower QoL scores (55.0) compared with those with self-assessed KPS of ≥80 (78.5, P < .0001). Similar findings were seen for study-team-assessed KPS (58.7 vs. 79.0, P < .0001). Patients with physical functional deficits per QLQ-C30 (physical function score <83) also reported lower QoL scores compared to those without similar physical deficits (64.0 vs. 84.5, P < .0001). Those with dependence in ≥1 IADL (66.3 vs. 79.9, P = .0009) or ≥1 fall in the prior 6 months (56.7 vs. 76.8, P = .0009) had lower QoL compared with those without these deficits. There was no significant association between TUG results and QoL.
Mental Health Index scores positive for depression (≥12) were associated with lower QoL scores (62.7 vs. 76.7, P = .0099). Mental Health Index-identified anxiety did not demonstrate a significant association with QoL. Impairments in role functioning (QoL score 55.6 vs. 79.4 for those without impairment, P < .0001), social functioning (50.9 vs. 78.8, P < .0001), and cognitive functioning (61.2 vs. 77.7, P = .0012) were each associated with lower QoL scores.
Discussion
This study used a brief but systematic evaluation of function, symptoms, and comorbidity to assess associations of functional and disease-related factors with QoL among a cohort of adults with PCDs. Overall QoL scores were high compared to both population samples40,41 and other cohorts with MM.9,10,42 In fact, the mean QoL score (74.4) was higher than previously published US general population norms (63.9).43 This discrepancy may reflect selection bias among individuals able to seek care at an academic center.
With this observation in mind, several results are noteworthy. First, overall QoL scores were similar between the 2 largest diagnostic subgroups: individuals with MM (74.0) and AL (72.1). This finding is particularly interesting given differences in symptom patterns between groups, namely, the greater frequency of pain among those with MM versus dyspnea among those with AL. This finding highlights the differential symptom burden in patients with PCDs, as well as the importance of probing more deeply than just global “how is your health” questions in research and clinical assessments.
Second, the diversity of our cohort is a relative strength of the study. Our study’s inclusion of 27% of subjects identifying as Black or African-American is notable, particularly given the higher incidence of MM among this population in the US.13 Prior studies of QoL among individuals with PCDs have either not reported race/ethnicity9,10 or have had samples that were >90% White.7,8,11,44
Third, several prior studies have demonstrated a relationship between performance status and health-related QoL,7-10 although prior research has also demonstrated the inadequacy of unidimensional performance status assessment in capturing function among adults with cancer.16 The present study features a more detailed functional assessment than prior studies. As part of this more detailed assessment, lower KPS, lower QLQ-C30 physical function scores, and IADL impairments were each associated with lower QoL scores. This study therefore further validates previously described relationships between function and QoL and reinforces the conclusions voiced by other authors that further research is needed regarding strategies for intervening upon functional deficits among patients with PCDs.42 Moreover, while functional impairments were associated with lower QoL, age was not associated with QoL, highlighting the importance of considering both “physiologic age” and chronologic age in treatment decision-making.
Fourth, we observed greater QoL among patients further from diagnosis (>2 years) compared to those recently diagnosed (0-6 months). These data reflect an arc in the course of disease and treatment encountered by many patients with PCDs that can be conceptualized as trichotomous. In the early phase (peri-diagnosis and early in therapy), patients commonly experience impairments in QoL related to symptoms of their recently-uncontrolled PCD and toxicity of the often-intensive treatment needed to induce a remission. Months later, after achieving remission and going onto a less-intensive maintenance program or even stopping therapy entirely (primarily in AL), many patients enter a middle “honeymoon” phase of improved PCD-related symptoms, less treatment-related toxicity, fewer clinic visits, and improved overall QoL.7 This phase was reflected among those 6-12 months from diagnosis in our cohort, who exhibited higher QoL. Unfortunately, PCDs remain incurable, and most patients eventually enter a later phase wherein the disorder becomes refractory to therapies. An accelerated cycle of relapses followed by brief remissions ensues. Therapy often becomes more intensive, and PCD-related symptoms and toxicity accumulate.8,45 Our data suggest that this phase is unsurprisingly associated with reduced QoL.
Broadly speaking, given the increasing longevity of patients with MM in particular,4,12 it behooves clinicians to pay close attention to physical, emotional, and other impairments that contribute to reduced QoL in PCD patients. The concept of “survivorship” and managing long-term sequelae of disease and therapy is arguably new in PCDs. That is of course a good problem to have, but one that clinicians need to learn to effectively address.
Our study has some limitations. This analysis does not include longitudinal assessments for individual patients and instead relies on cross-sectional comparisons of patients at different stages of the disease to make inferences regarding trajectories. Additionally, the relatively long mean duration from initial diagnosis may indicate that this cohort includes patients with a favorable prognosis compared to other cohort studies; however, the median time from diagnosis in our study (nearly 3 years) is well below the median overall survival for all patients with MM with modern therapies (>5 years).13 This study includes patients treated at a single academic medical center, which may contribute to the relatively high mean QoL scores observed.
Conclusions
The current study highlights the arc of QoL experienced by patients with PCDs receiving later lines of therapy; QoL starts lower, improves as a patient achieves remission, and again falls as the PCD enters an advanced, relapsed, and refractory stage. Functional impairment is associated with—and presumably contributes to—reduced QoL at all stages of the disease. As patients are living longer than ever before with PCDs, attention to health-related QoL and other elements of survivorship is of increasing importance. Using GA to identify specific functional impairments and then intervening to address those impairments is a vital next step for supportive care research in PCDs that may go a long way toward improving QoL among these individuals.
Contributor Information
Christopher E Jensen, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sanah N Vohra, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Kirsten A Nyrop, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Oncology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Allison M Deal, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Matthew R LeBlanc, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Shakira J Grant, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Hyman B Muss, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Oncology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Eben I Lichtman, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Samuel M Rubinstein, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
William A Wood, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Nicholas J Mangieri, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Lee Jamison, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Division of Oncology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sascha A Tuchman, Division of Hematology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Funding
The study was partially supported by the North Carolina University Cancer Research Fund. Jensen was supported by the University of North Carolina (UNC) Geriatric Oncology Program (T32-CA233419). Vohra was supported by the UNC Cancer Control Education Program (T32-CA057726). LeBlanc was supported by the UNC Cancer Care Quality Training Program (T32-CA116339).
Conflict of Interest
Matthew R. LeBlanc: Glaxo Smith Kline (C/A); Samuel M. Rubinstein: Sanofi, Roche, Janssen, Eusa pharma (C/A); William A. Wood: Genentech, Pfizer (RF), Koneksa Health (SAB, OI), Teladoc (C/A); Sascha A. Tuchman: Caelum, Janssen, Partnership for Health Analytic Research, Oncopeptides. Sanofi, Shattuck Labs (C/A); Janssen, Sanofi (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception and design: C.E.J., K.A.N., A.M.D., H.B.M., S.A.T. Provision of study material/patients: E.I.L., S.M.R., W.A.W., S.J.G., S.A.T. Collection and/or assembly of data: C.E.J., N.J.M., S.A.T. Data analysis and interpretation: C.E.J., S.N.V., K.A.N., A.M.D., M.R.L., L.J., S.A.T. Manuscript writing: C.E.J. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538-48. [DOI] [PubMed] [Google Scholar]
- 2. Kumar SK, Callander NS, Adekola K, et al. NCCN Clinical Practice Guidelines in Oncology: Multiple Myeloma. Version 7.2021. 2021. Available at: NCCN.org. Accessed July 1, 2021.
- 3. Kumar SK, Callander NS, Adekola K, et al. NCCN Clinical Practice Guidelines in Oncology: Systemic Light Chain Amyloidosis. Version 1.2022. 2021. Available at: NCCN.org Accessed July 1, 2021.
- 4. Van De Donk NWCJ, Pawlyn C, Yong KL.. Multiple myeloma. Lancet. 2021;397:410-427. [DOI] [PubMed] [Google Scholar]
- 5. Palladini G, Milani P, Merlini G.. Management of AL amyloidosis in 2020. Blood. 2020;136:2620-2627. [DOI] [PubMed] [Google Scholar]
- 6. Kent EE, Ambs A, Mitchell SA, et al. Health-related quality of life in older adult survivors of selected cancers: Data from the SEER-MHOS linkage. Cancer. 2015;121:758-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramsenthaler C, Osborne TR, Gao W, et al. The impact of disease-related symptoms and palliative care concerns on health-related quality of life in multiple myeloma: a multi-centre study. BMC Cancer. 2016;16:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramsenthaler C, Gao W, Siegert RJ, et al. Symptoms and anxiety predict declining health-related quality of life in multiple myeloma: a prospective, multi-centre longitudinal study. Palliative Med. 2019;33:541-551. [DOI] [PubMed] [Google Scholar]
- 9. Despiegel N, Touboul C, Flinois A, et al. Health-related quality of life of patients with multiple myeloma treated in routine clinical practice in France. Clin Lymphoma Myeloma Leuk. 2019;19:e13-e28. [DOI] [PubMed] [Google Scholar]
- 10. Engelhardt M, Ihorst G, Singh M, et al. Real-world evaluation of health-related quality of life in patients with multiple myeloma from Germany. Clin Lymphoma Myeloma Leuk. 2021;21:e160-e175. [DOI] [PubMed] [Google Scholar]
- 11. Mccausland KL, Rizio AA, White MK, et al. Associations between health-related quality of life and self-reported emergency room department visits and inpatient hospitalizations: insights from a Secondary Data Analysis of Patients with Light-Chain (AL) Amyloidosis. Pharmacoecon Open. 2019;3:367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palumbo A, Anderson K.. Multiple myeloma. N Engl J Med. 2011;364:1046-60. [DOI] [PubMed] [Google Scholar]
- 13. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Myeloma. 2021. Available at: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 1, 2021. [Google Scholar]
- 14. Shimazaki C, Hata H, Iida S, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Int Med. 2018;57:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kyle RA, Larson DR, Kurtin PJ, et al. Incidence of AL amyloidosis in Olmsted county, Minnesota, 1990 through 2015. Mayo Clin Proc. 2019;94:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jolly TA, Deal AM, Nyrop KA, et al. Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist. 2015;20:379-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen CE, Vohra SN, Nyrop KA, et al. Geriatric-assessment-identified functional deficits among adults with multiple myeloma with normal performance status. J Geriatr Oncol. 2022;13:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dotan E, Walter LC, Browner IS, et al. NCCN Clinical Practice Guidelines in Oncology: Older Adult Oncology. Version 1.2021. Available at: NCCN.org. Accessed July 1, 2021.
- 19. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36:2326-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grant SJ, Mian HS, Giri S, et al. Transplant-ineligible newly diagnosed multiple myeloma: Current and future approaches to clinical care: a Young International Society of Geriatric Oncology Review Paper. J Geriatr Oncol. 2021;12:499-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Extermann M, Hurria A.. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824-31. [DOI] [PubMed] [Google Scholar]
- 23. Greipp PR, Miguel JS, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412-3420. [DOI] [PubMed] [Google Scholar]
- 24. Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajkumar SV, Richardson P, San Miguel JF.. Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood. 2015;126:921-2. [DOI] [PubMed] [Google Scholar]
- 26. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998-2005. [DOI] [PubMed] [Google Scholar]
- 27. Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Compr Canc Netw. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 29. Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89-96. [DOI] [PubMed] [Google Scholar]
- 30. Karnofsky DA, Burchenal JH.. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents in Cancer. Columbia University Press; 1949:191-205. [Google Scholar]
- 31. Schag CC, Heinrich RL, Ganz PA.. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187-93. [DOI] [PubMed] [Google Scholar]
- 32. Crooks V, Waller S, Smith T, Hahn TJ.. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139-44. [DOI] [PubMed] [Google Scholar]
- 33. Podsiadlo D, Richardson S.. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-8. [DOI] [PubMed] [Google Scholar]
- 34. Shumway-Cook A, Brauer S, Woollacott M.. Predicting the probability of falls in community-dwelling older adults using the timed up and go test. Phys Therapy. 2000;80:896-903. [PubMed] [Google Scholar]
- 35. Loprinzi CL, Laurie JA, Wieand HS, et al. Prospective evaluation of prognostic variables from patient-completed questionnaires. J Clin Oncol. 1994;12:601-7. [DOI] [PubMed] [Google Scholar]
- 36. Teno J, Kiel DP, Mor V.. Multiple stumbles: a risk factor for falls in community-dwelling elderly. A prospective study. J Am Geriatr Soc. 1990;38:1321-5. [DOI] [PubMed] [Google Scholar]
- 37. Fillenbaum GG, Smyer MA.. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428-34. [DOI] [PubMed] [Google Scholar]
- 38. Giesinger JM, Loth FLC, Aaronson NK, et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J Clin Epidemiol. 2020;118:1-8. [DOI] [PubMed] [Google Scholar]
- 39. Pergolotti M, Langer MM, Deal AM, et al. Mental status evaluation in older adults with cancer: Development of the Mental Health Index-13. J Geriatr Oncol. 2019;10:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scott NW, Fayers P, Aaronson NK, et al. EORTC QLQ-C30 Reference Values. 2nd ed. EORTC Quality of Life Group; 2008. [Google Scholar]
- 41. Mols F, Oerlemans S, Vos AH, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89:311-9. [DOI] [PubMed] [Google Scholar]
- 42. Boland E, Eiser C, Ezaydi Y, et al. Living with advanced but stable multiple myeloma: a study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J Pain Symptom Manage. 2013;46:671-80. [DOI] [PubMed] [Google Scholar]
- 43. Nolte S, Liegl G, Petersen MA, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153-163. [DOI] [PubMed] [Google Scholar]
- 44. Leblanc MR, Hirschey R, Leak Bryant A, et al. How are patient-reported outcomes and symptoms being measured in adults with relapsed/refractory multiple myeloma? A systematic review. Qual Life Res. 2020;29:1419-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. 2016;175:252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.