Abstract
Despite its proven efficacy, adherence to adjuvant endocrine therapy remains a significant challenge around the world and in sub-Saharan Africa. This commentary discusses the results of the study by Getachew and colleagues, which examined the use of a multi-pronged nurse-led intervention to improve adherence to endocrine therapy in Ethiopia.
Epidemiologic studies from sub-Saharan Africa (SSA) indicate that most patients with breast cancer have tumors that express estrogen and/or progesterone receptors. Endocrine therapy is a cornerstone in the management of these patients, particularly for patients with non-metastatic cancers that are potentially curable. Adjuvant endocrine therapy (AET) remains one of the most effective interventions to prevent cancer recurrence and prolong survival. Seminal international meta-analyses conducted in 2005 showed a yearly reduction in breast cancer mortality of over 30% with 5-years of AET.1 And more recent international studies highlight that longer duration of AET, up to 10 years, provides even greater survival benefit for patients with more advanced disease.2,3 Commonly used AET options, such as tamoxifen and non-steroidal aromatase inhibitors, are administered orally on a daily schedule that makes them well-suited for low- and middle-income countries (LMICs) and other limited resource settings where access to health systems is constrained. Despite its proven efficacy, adherence to AET remains a significant challenge around the world and in SSA, with studies showing that less than 50% of patients complete intended duration of therapy.4
Recent reviews of global studies, but including none from SSA, have identified several factors that are associated with nonadherence to AET. These factors include personal demographic factors; side-effects and quality of life; knowledge, beliefs, and attitudes; socio-behavioral factors like self-efficacy (the belief in one’s own capacity to achieve a health goal through behavior modification); and structural health-system factors.5,6 A variety of strategies have been evaluated in high-resource settings to address these barriers to AET adherence.7,8 However, there are gaps in the literature regarding barriers and facilitators of AET adherence in SSA, and more importantly, there are no rigorous studies from the SSA region evaluating possible interventions to improve adherence.
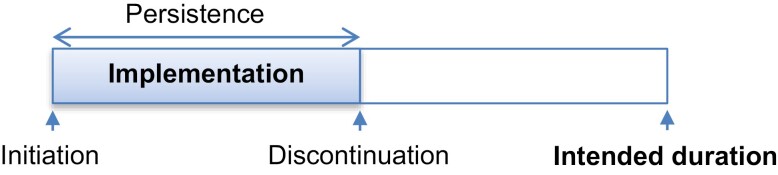
The study by Getachew et al examines the use of a multi-pronged nurse-led intervention to improve adherence to endocrine therapy in Ethiopia.9 While the concept of oral medication adherence may appear simple, it encompasses multiple components (see Fig. 1). AET medication nonadherence may refer to delay or lack of initiation, early discontinuation, or poor implementation (interruptions of daily use).10 Early discontinuation and poor implementation of AET have been linked to poor outcomes, highlighting the need for innovative and effective solutions.11,12
Figure 1.
Taxonomy of AET adherence. Adapted from a commonly used adherence taxonomy framework,10 this schematic outlines different elements of medication adherence including initiation, persistence, and implementation.
The Getachew et al study involves a cluster randomization trial of 8 hospitals in Ethiopia with ready access to tamoxifen, 4 each in the control and intervention arms. A prior study in Ethiopia including 51 patients eligible for AET showed that 25 (49%) did not initiate AET; and of the 26 patients who initiated AET, one-year persistence rate was low at 52%, with only 35% showing good adherence implementation.13 To address this AET adherence gap, the current study developed a multipronged intervention where nurses were trained to deliver patient education, provide literacy materials, give counseling, make phone call reminders, and actively monitor medication refills of patients at the intervention hospitals. The investigators assessed the efficacy of the intervention on AET adherence implementation and persistence at 6 and 12 months. The outcome measures were medication possession ratio (MPR) of 80% or higher; compliance with daily use as determined by a simplified medication adherence scale (SMAQ) score of over 80% or higher; and rates of medication persistence (proportion of eligible patient remaining on therapy). Of the evaluable patients at 12 months, the authors show relatively higher rates of adherence by MPR at 90% and 79% in the intervention and control groups, respectively (P = .302). The rates of adherence implementation by SMAQ were lower, but statistically significantly different at 70% and 45% in the intervention and control groups, respectively (P = .036). AET persistence at 12 months was also markedly higher in the intervention group (91%) compared with the control group (78%). The authors also explored the possible impact of patient and social demographic factors on AET adherence.
This study is an important contribution highlighting some critical aspects of maintaining AET adherence. First, measuring several components of adherence are crucial to obtain a comprehensive picture as well as to better tailor interventions. In addition, in many LMICs such as Ethiopia, where there are limited numbers of trained oncologists, empowering, training, and utilizing nurses can be a valuable way to extend oncology services and improve care delivery.14 Medication adherence, toxicity monitoring, and counseling are important care aspects that can be led by nurses. There are other emerging examples of how these task-shifting models can meaningfully improve care delivery in rural, resource limited settings.15 The study also highlights the importance of using a multi-pronged approach to improving medication adherence; studies on adherence in other disease have shown that isolated interventions tend to be less effective than multi-faceted and contextually driven solutions. Finally, the study is a good example of using a pragmatic trial approach—in this case cluster randomization—to examine the efficacy of embedding interventions within routine care practices that are suitable for application in LMICs.
While the results showing improvements in adherence in the intervention group are promising, there are some important study limitations and caveats that highlight the many challenges of conducting behavioral interventional studies in LMICs:
-
•
The results report adherence at the 1-year mark, which is less than the currently recommended 5-10 year duration of AET therapy. Randomized trials of adjuvant tamoxifen taken for 1-year, 2-years, and 5-years, have demonstrated reduced breast cancer recurrence rates at 10 years by 21%, 29%, and 47%, which corresponds to proportional mortality reductions of 12%, 17%, and 26%.16 Thus, while 1-year of endocrine therapy reduces the rates of both recurrence and death, these benefits can be more than doubled by continuing treatment for a full 5-year course.
-
•
Continued attrition and therapy discontinuation over the course of AET therapy is typical, and some interventions that have shown efficacy in short-term adherence trials have not translated to longer-term adherence. Further follow-up beyond one year is warranted.
-
•
Some differences between the control and intervention groups and the hospitals through which they were treated may partly explain the higher adherence rates in the intervention group. These differences included stage distribution (71% having advanced disease in the control group vs 46% in the intervention group), differences in prior use of endocrine therapy (5% vs 18%); differences in care processes at the hospitals, eg, use of fine needle aspiration cytology for diagnosis (95% vs 68%) and more patients being “advised to go to other places” in the control group (87% vs 45%). The investigators were unable to control for these differences on multivariable modeling due to small sample size.
-
•
Finally, there was low participation rate in adherence measurements, and a notable differential in participation rates in the control (38%) compared with the intervention group (52%); these may limit the validity and internal generalizability of the study results.
This study sets the stage for future exploration of AET adherence interventions in SSA. While multi-pronged interventions are valuable, they can be a challenge to deliver consistently, and with high fidelity. Not all the components of the intervention are equally valuable, hence employing even more innovative trial approaches such as stepped-wedge cluster randomized trials and factorial trial designs would be valuable to tease out what components of a complex AET adherence intervention are truly valuable and essential.17,18 In addition, rigorous use and integration of implementation science approaches and frameworks into future AET adherence studies may help to tease out multi-layer effects and influences. Systematic assessment of intervention costs and cost-effectiveness compared with usual care are also important components that need to be incorporated into future trial, both to identify high-value components of intervention as well as to enhance the likelihood of uptake by care systems and policy makers.19
With rising rates of breast cancer in LMICs, optimal utilization of effective treatment options is paramount. There is an urgent need to identify low-hanging fruits, such as improving the utilization of effective generic oral AET agents. Investment and improvement of pathology capacity for breast cancer molecular classification to identify eligible patients with hormone receptor positive cancers, and implementation science-informed AET trials are important pillars in addressing this need.
In conclusion, Getachew et al have shown that nurse-driven multi-layered interventions can be a valuable tool for improving medication adherence as well as in all aspects of the cancer care continuum—prevention, early detection, active treatment delivery, and palliative care. While nurses have long been undervalued and poorly integrated into oncology programs, fundamental strengthening of SSA health systems to deliver oncology high-quality care calls for empowering nurses to fulfill a variety of roles beyond direct care delivery, such as educators, patient advocates, navigators, and researchers.14,20 In SSA and other settings with limited numbers of physicians and other oncology care providers, the importance of capacity strengthening and scope expansion for oncology nursing cannot be overemphasized.
Contributor Information
Temidayo A Fadelu, Center for Global Cancer Medicine, Dana-Farber Cancer Institute, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
Lori Buswell, Center for Global Cancer Medicine, Dana-Farber Cancer Institute, Boston, MA, USA.
Benjamin O Anderson, World Health Organization, Geneva, Switzerland; Department of Surgery and Global Health-Medicine, University of Washington, Seattle, WA, USA.
Funding
TAF is supported by a 2021 Conquer Cancer Breast Cancer Research Foundation Career Development Award for Diversity, Inclusion and Breast Cancer Disparities in honor of Susan Hirschhorn and in memory of her mother, supported by Breast Cancer Research Foundation.
Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer, or Breast Cancer Research Foundation or any other funders.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Manuscript writing: All authors. Final approval of manuscript: All authors.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717. 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784. 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816. 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hershman DL, Kushi LH, Shao T, et al. Early Discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120-4128. 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambert LK, Balneaves LG, Howard AF, Gotay CC.. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat. 2018;167(3):615-633. 10.1007/s10549-017-4561-5. [DOI] [PubMed] [Google Scholar]
- 6. Kidwell KM, Harte SE, Hayes DF, et al. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120(16):2403-2411. 10.1002/cncr.28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurtado-de-Mendoza A, Cabling ML, Lobo T, Dash C, Sheppard VB.. Behavioral interventions to enhance adherence to hormonal therapy in breast cancer survivors: a systematic literature review. Clin Breast Cancer. 2016;16(4):247-255.e3. 10.1016/j.clbc.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ekinci E, Nathoo S, Korattyil T, et al. Interventions to improve endocrine therapy adherence in breast cancer survivors: what is the evidence?. J Cancer Surviv. 2018;12(3):348-356. 10.1007/s11764-017-0674-4. [DOI] [PubMed] [Google Scholar]
- 9. Getachew S, Addissie A, Seife E, et al. Breast nurse intervention to improve adherence to endocrine therapy among breast cancer patients in South Ethiopia. Oncologist. 2022;27:e650-e660. 10.1093/oncolo/oyac081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691-705. 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C.. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108(7):1515-1524. 10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529-537. 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reibold CF, Tariku W, Eber-Schulz P, et al. Adherence to newly implemented tamoxifen therapy for breast cancer patients in Rural Western Ethiopia. BRC. 2021;16(5):484-490. 10.1159/000512840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Challinor JM, Galassi AL, Al-Ruzzieh MA, et al. Nursing’s potential to address the growing cancer burden in low- and middle-income countries. J Glob Oncol. 2016;2(3):154-163. 10.1200/JGO.2015.001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shulman LN, Mpunga T, Tapela N, et al. Bringing cancer care to the poor: experiences from Rwanda. Nat Rev Cancer. 2014;14(12):815-821. 10.1038/nrc3848. [DOI] [PubMed] [Google Scholar]
- 16. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. The Lancet. 1998;351(9114):1451-1467. 10.1016/S0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- 17. Montgomery AA, Peters TJ, Little P.. Design, analysis and presentation of factorial randomised controlled trials. BMC Med Res Methodol. 2003;3(1):26. 10.1186/1471-2288-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ.. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015;350:h391. 10.1136/bmj.h391. [DOI] [PubMed] [Google Scholar]
- 19. Erfani P, Bhangdia K, Stauber C, et al. Economic evaluations of breast cancer care in low- and middle-income countries: a scoping review. Oncologist. 2021;26(8):e1406–e1417. 10.1002/onco.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galassi A, Challinor J.. Strengthening the oncology nurse workforce in low-income and middle-income countries. Lancet Oncol. 2015;16(8):887-888. 10.1016/S1470-2045(15)00144-8. [DOI] [PubMed] [Google Scholar]