Abstract
CoronaVirus disease-2019 has changed the delivery of health care worldwide and the pandemic has challenged oncologists to reorganize cancer care. Recently, progress has been made in the field of precision medicine to provide to patients with cancer the best therapeutic choice for their individual needs. In this context, the Foundation Medicine (FMI)-Liquid@Home project has emerged as a key weapon to deal with the new pandemic situation. FoundationOne Liquid Assay (F1L) is a next-generation sequences-based liquid biopsy service, able to detect 324 molecular alterations and genomic signatures, from May 2020 available at patients’ home (FMI-Liquid@Home). We analyzed time and costs saving for patients with cancer, their caregivers and National Healthcare System (NHS) with FMI-Liquid@Home versus F1L performed at our Department. Different variables have been evaluated. Between May 2020 and August 2021, 218 FMI-Liquid@Home were performed for patients with cancer in Italy. Among these, our Department performed 153 FMI-Liquid@Home with the success rate of 98% (vs. 95% for F1L in the hospital). Time saving for patients and their caregivers was 494.86 and 427.36 hours, respectively, and costs saving was 13 548.70€. Moreover, for working people these savings were 1084.71 hours and 31 239.65€, respectively. In addition, the total gain for the hospital was 163.5 hours and 6785€, whereas for NHS was 1084.71 hours and 51 573.60€, respectively. FMI-Liquid@Home service appears to be useful and convenient allowing time and costs saving for patients, caregivers, and NHS. Born during the COVID-19 pandemic, it could be integrated in oncological daily routine in the future. Therefore, additional studies are needed to better understand the overall gain and how to integrate this service in different countries.
Keywords: liquid biopsy, cfDNA, precision medicine, home, cost saving
The Foundation Medicine (FMI)-Liquid@Home project has emerged as a key weapon to deal with the pandemic. FoundationOne Liquid Assay (F1L) is a next-generation-sequences-based liquid biopsy service, able to detect 324 molecular alterations and genomic signatures. This article analyzes time and cost savings for patients with cancer, their caregivers, and the National Healthcare System with FMI-Liquid@Home versus F1L.
Implications for Practice.
This study demonstrates that liquid biopsy at the homes of patients’ with cancer could help to identify the best treatment, with the same specificity, sensibility, and accuracy than in hospital, with a gain of time and economic resources for all figures implicated in the oncological care (patients with cancer, their caregiver, hospital, and National Healthcare System) during the pandemic. In the future, it could be integrated into the oncological daily routine as a useful tool to identify the best treatment for each patient, with clinical and psychological benefits for patients, in the era of precision medicine.
Introduction
In December 2019, in Wuhan, China, infection by the Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) was detected1,2 and then the CoronaVirus Disease 2019 (COVID-19) rapidly spread worldwide becoming a global health emergency.3,4 On March 11, 2020 the World Health Organization (WHO) declared COVID-19 pandemic4,5 and since then, a large number of countries worldwide have applied unprecedented restrictive measures to prevent the disease from overwhelming National Health Systems (NHS).6,7 Italy was the first country in Europe to impose a generalized lockdown on March 11, 2020, allowing its citizens to leave their homes only in selected circumstances.8 These interventions have successfully limited the spread of the disease.
The pandemic heavily affected the public health system, which not only retarded social development but also the diagnosis and treatment of other diseases. COVID-19 patients required hospitalization and intensive care, so there was a reassignment of beds, health workers, hospital wards, and resources to effort the medical emergency, at the expense of medical and surgery provision, elective procedures, and daily clinical care.9,10
This situation had challenged oncologists to reorganize cancer care in order to strikingly reduce hospital visits and admissions.11 Patients with cancer were a vulnerable population to SARS-CoV-2,12 and they had an increased risk of developing severe illness and complications compared with the general community.13-17
COVID-19 had spread rapidly worldwide, challenged and changed the health care systems, which faced a radical reorganization.18 In particular, standard cancer screening and follow-up dropped drastically, such as new cancer diagnoses and surgical procedures.19-21
In this prospective, the effort of the oncology world was to respond to the needs of patients with cancer in the pandemic contest trying to guarantee primary care in the safest manner.22-25 Moreover, precision medicine continuing to provide to the patients the best therapeutic strategy was an objective to be pursued.
In the era of next-generation sequences (NGS)-based tumor comprehensive genomic profiling (CGP), many commercial panels have spread to analyze cancer genome for developing tailored treatments.26,27 Identification of specific molecular alterations, both actionable and resistance-conferring ones, in different tumors type and in each patient ensured a personalized management and treatment.28 Although some limitations, liquid biopsy had widely spread in the context of precision medicine, analyzing circulating tumor DNA (ctDNA) in blood cancer samples in order to obtain a real-time molecular profiling.29-31
FoundationOne Liquid Assay (F1L) is a target-specific NGS-based service for liquid biopsy of Foundation Medicine (FMI) to sequence circulating cell-free DNA (cfDNA) in peripheral whole blood plasma from patients with cancer. F1L detects 324 molecular alterations: substitutions and indels, copy number alterations, selected genomic rearrangements, and genomic signatures including tumor fraction (TF), blood tumor mutational burden (bTMB), and microsatellite instability (MSI) status.32
In the COVID-19 pandemic period, Roche Foundation Medicine tried to meet the new needs of oncological community. For the first time, F1L was made available as a home service (FMI-Liquid@Home project) at the same cost of F1L in the hospital.33
The aim of this paper was to ensure liquid biopsy at the patients’ home, avoiding the risk of COVID-19 infection on the way to the hospital or at hospital, also helping needs of patients who lived far away from the hospital.
FMI-Liquid@Home pilot project born by the partnership between Roche Italy and Egg s.r.l., a society that offers an articulated system of services to make patient care more efficient and effective.
It consisted in relying on national network of specialized nurses that perform blood draws for F1L directly at patients’ home.
This project started in May 2020 and the first patient benefited from the project services on May 11 in Italy. In this country, FMI-Liquid@Home had the greatest diffusion and it is currently still spreading.
The aim of this work was to compare costs and time saved by patients with cancer, their caregiver and hospital between FMI-Liquid@Home and F1L performed in our Department.
Different variables have been included in the analysis both for patients, caregivers and for NHS. In particular, for patients and caregivers average travel time saved, average waiting time avoided at the hospital, cost avoided for commuting and reduction work absence were considered. For NHS time saved for process, hospital workload and its economic impact were taken into account.
Material and Methods
Patient Selection
FMI-Liquid@Home Patients
FMI-Liquid@Home project started in May 2020. Two hundred and nine patients with cancer (≥18 years) were included in the analysis. From May 2020 to August 2021, 218 home blood draws were performed with 8 patients (3.83% of the population) undergoing to more than one home blood draw due to analysis failure. All patients provided written informed consent.
Liquid Biopsy Patients Afferent to Our Department
From May 1, 2020 to August 31, 2021 in our Oncological Department were collected 399 liquid biopsies for FoundationOne Liquid Assay from 399 oncological patients (≥18 years) affected by solid tumors. Among these, 246 blood samples were collected at the Oncological Department. 153 samples were collected at patients’ home (70% of all FMI-Liquid@Home project patients’). All patients provided written informed consent.
The multidisciplinary Molecular Tumor Board of the Institution chose all the patients who needed the analysis. Moreover, the patient’s referring oncologist chose the type of analysis (at home or at hospital) according to the clinical conditions and the patient’s preference. Defeated or elderly patients, patients with an increased risk for COVID-19 infection or complications, patients that lived far away from the hospital were candidate to FMI-Liquid@Home; on the other hand, patients fit for a blood draw in hospital could choose between at home or in hospital sampling according to the referring oncologist.
FoundationOne Liquid Home Assay (FMI-Liquid@Home)
FMI-Liquid@Home project was the result of the partnership between Roche Italy and Egg s.r.l., a society with the mission to maximize patient’s diagnostic pathway improving quality of life and offering innovative services. The physician reported to Roche/Egg platform33 the Test Requisition Form with patient consent, home data, and the phone number. After a contact between nurse and patient, a specialized nurse performed a blood draws for FoundationOne Liquid Analysis with F1L kit tubes (two anti-coagulated peripheral whole blood tubes—8.5 mL per tube) at the patient’s home. The courier picked up the F1L box prepared by nurse directly at patient home, sending it to Cambridge (USA) or Penzberg Lab (Germany) for analysis.
The FMI-Liquid@Home used the same technologies of F1L in hospital. The test required about ≥25 ng cfDNA. Extracted cfDNA undergoes to whole-genome library construction, and the libraries were sequenced with deep coverage using the NovaSeq 6000 platform. F1L identified 309 genes with complete exonic coding coverage and 15 genes with only select non-coding coverage; the genomic signatures bTMB, MSI-H status, TF32. FMI-Liquid@Home had the same cost of F1L in hospital and if the analysis failed, it was repeated for free.
Data Collection and Analysis
FMI-Liquid@Home data have been collected in order to evaluate economic impact of this initiative for patients and NHS. Data protection and privacy regulations have been observed in capturing, forwarding, processing, and storing subject data. In particular, personal and sensitive patients’ data have been deleted, keeping only anonymous data necessary for the analysis.
Different variables have been included in the analysis both for patients, caregivers and for NHS. In particular:
Average Travel Time Saved
To calculate the average travel time saved for a patient to reach the hospital and to calculate the kilometers, not having retained the patient’s home address, the nurses who performed the blood draws were used as a reference.
For each province in which there was a hospital adhering to the service, a nurse located within the same province of the center was trained and used for all home draws. In cases there was a high number of patients outside the province of the center, additional nurses were inserted to reduce the kilometers needed to reach the patients who referred to the hospital. In these cases, the kilometers traveled by the nurses (round trip) were considered.
For patients requiring home blood draws in geographic region different from that of the hospitals, a new nurse in that region was trained. In these cases, kilometers indicated by Google Maps (fastest route) for the trip from the hospital to the new locality have been considered.
This calculation method leaded to an inevitable approximation. In particular, there was an underestimation of the kilometers that patients would have to travel to reach the hospital.
Among 209 patients, 185 patients were within the same region as the hospital center, whereas 24 were in outside regions. For 218 blood draws performed, we estimated:
Average distance patient—center (round trip): 34 737.6 km/218 = 159.35 km (A)
To calculate the average time of traveling, it was estimated how many kilometers were traveled on average in 1 hour. Moreover, it was taken as a reference the maximum speed in an extra-urban road, 90 km/hour, since mixed urban/extra-urban routes have been travelled. The error due to the approximation will lead to an underestimation of the real value.
Distance covered in 1 hour= 90 km (B)
The average travel time necessary for a patient to reach the hospital and back to home was calculated as follows:
Average time for reaching hospitals (round trip): A/B= 1.77 hours (C)
Average waiting time avoided at the hospital: To estimate the time saved by a patient for each blood draw taken at home instead of at hospital, the wait time before the patient undergoes to blood draw was also considered. To estimate this time, a sample of 35 patients, from May 2021 to June 2021, who accessed to our Department and joined the F1L service, has been evaluated. For these patients, it has been considered the average time to be identified at the entrance of the Department; the time to wait for the access in the building; the time to perform the triage for Sars-Cov-2 and to identify the patient by the nurse (example: label the tubes).
Average time spent for patient waiting for the blood sample at the hospital= 0.50 hour (D)
Percentage of Patient with Caregivers
Caregivers were necessary for some patients to go to the hospital for drawing blood samples. To consider the impact of caregivers, it was necessary to calculate the percentage of patients who would had needed the caregiver. To calculate this percentage, a small sample of 22 patients was taken, who joined the service from June 2021 to July 2021. For patients with caregiver, the related age was asked in order to calculate if the caregiver was in working age. In particular, 19 out of 22 patients needed a caregiver. To calculate the percentage of caregivers in working age, the age range used was 18-65.
Percentage of patients with caregivers: 19/22 = 86.36% (E)
Percentage of caregivers in working age = 84.21% (F)
Cost Avoided for Traveling
To estimate the costs saved by patients for the trip to the hospital, it was used the average kilometers calculated to find the average travel time. To calculate the travel cost, average cost to travel 1 km was identified assuming the patient would had traveled by car. It was taken as the cost per kilometer the contractually recognized rate for the home service.
Cost per kilometer= 0.39€/km (G)
It was possible to calculate the travel cost that patients who used the service would had incurred if they had to go to the hospital for blood drawing.
Average cost for commute patient-center for one blood draw: A*G= 62.15€ (H)
Patients and Caregiver in Working Age
To estimate the number of working hours saved by caregivers and patients, it was necessary to identify the average working hours lost to perform blood draw in hospitals and the percentage of patients and caregiver (84.21%) in working age.
To calculate the percentage of patients in working age (age range 18-65), the date of birth of 209 patients for 218 blood draws was recovered from the F1L test requisition form.
Percentage of patients in working age = 51.67% (I)
Average Working Hours Lost for Performing Blood Draw in Hospitals
To estimate the average of lost working hours, it was considered the average time needed to carry out the blood draw at the hospital (excluding waiting times) and round trip.
To calculate the average time to carry out the blood draw at the hospital, a sample of 35 patients who joined the service in our Department from May 2021 to June 2021 was taken and the time necessary for the sampling activity was measured. It was considered the average time taken by our nurses for the measurement of vital sign, the preparation of the kit tubes for the test and the blood sampling with a blood draw.
Average hours for performing a blood draw in hospital (without waiting time)= 0.25 hour (J)
The average time to carry out the blood draw at the hospital and by going to the center:
Average hours for blood draw in hospital: C + D + J = 2.52 hours (K)
For the average hourly cost, the value of 28.8€/hour was assumed.34
Time and Cost Saved for Process Workload Reduction for NHS
To estimate the time saved by the hospital, it was necessary to consider the average time for hospital, to manage patient’s appointment and to perform blood sample logistics (preparation and sending blood samples to the laboratory).
For this calculation, a sample of 35 blood draws collected in our Department from May 2021 to June 2021 has been evaluated. To calculate the overall savings of the hospital, it was considered the working hours saved by the hospital to make and manage the blood samples and it was considered the hourly Health Care Professionals (HCP) wage of 41.5€/hour.35
Patient Satisfaction Survey
After carrying out a home blood draw, 209 patients were asked to answer a few questions to measure their degree of satisfaction with the service through a telephone interview about the preference between liquid biopsy at home or in hospital and about recommending home service to another patient.
Results
Population Characteristics
From May 2020 to August 2021, 209 patients with cancer benefited from FMI-Liquid@Home service and 218 home blood draws were performed. Among these patients, 153 have been treated to our Department. Characteristics of the patient population are shown in Table 1.
Table 1.
Characteristics of the patient population.
| Total N (%) | Liquid Assay in hospital (%) | Liquid Assay at home (%) | |
|---|---|---|---|
| Age | |||
| Median | 63 | 63 | 63 |
| Mean | 61.5 | 61.3 | 61.8 |
| Gender | |||
| Female | 168 (42%) | 101 (41%) | 67 (44%) |
| Male | 231 (58%) | 145 (59%) | 86 (56%) |
| Race | |||
| Caucasian | 399 | 246 | 153 |
| ECOG PS | |||
| 0 | 146 (36.6%) | 107 (43.6%) | 39 (25.5%) |
| 1 | 210 (52.6%) | 116 (47.1%) | 94 (61.5%) |
| 2 | 38 (9.5%) | 21 (8.5%) | 17 (11.1%) |
| 3 | 5 (1.3%) | 2 (0.8%) | 3 (1.9%) |
| Systemic anti-cancer therapies at the time of the test | |||
| Before/after radical surgery/adjuvant | 30 (7.5%) | 24 (10%) | 6 (4%) |
| First line | 188 (47.1%) | 107 (43%) | 81 (53%) |
| Second line | 50 (12.6%) | 27 (11%) | 23 (15%) |
| Advanced lines | 131 (32.8%) | 88 (36%) | 43 (28%) |
| Tumor samples types | |||
| CRC | 150 (37.6%) | 90 (37%) | 60 (39.2%) |
| NSCLC | 103 (25.8%) | 52 (21%) | 51 (33.3%) |
| GC | 52 (13%) | 41 (16.5%) | 11 (7.2%) |
| PC | 15 (3.7%) | 8 (3.2%) | 7 (4.5%) |
| BTC | 3 (0.7%) | 2 (0.8%) | 1 (0.6%) |
| BC | 19 (4.7%) | 11 (4.5%) | 8 (5.4%) |
| Others | 57 (14.5%) | 42 (17%) | 15 (9.8%) |
Abbreviations: CRC, colorectal cancer; NSCLC, non-small cell lung cancer; GC, gastrointestinal cancer; PC, pancreatic cancer; BTC, biliary tract cancer; BC, breast cancer; ECOG PS: Eastern Cooperative Oncology Group Performance Status; N, number.
The median age was 63 years; all patients were Caucasian, 58% were male and 42% were female. The population distribution for F1L in hospital and FMI-Liquid@Home was similar. The Performance Status according to ECOG was 2 or 3 in 9.3% of patients who performed F1L in hospital and in 13% of patients who performed FMI-Liquid@Home. Majority of F1L (47.1%), both in Hospital (43%) and at home (53%), was performed at baseline of first-line treatment. The most frequent tumor subtypes were ColoRectal Cancer (CRC) and non–small cell lung cancer (NSCLC) both in hospital (37% and 21%, respectively) ad at home (39.2% and 33.3%, respectively).
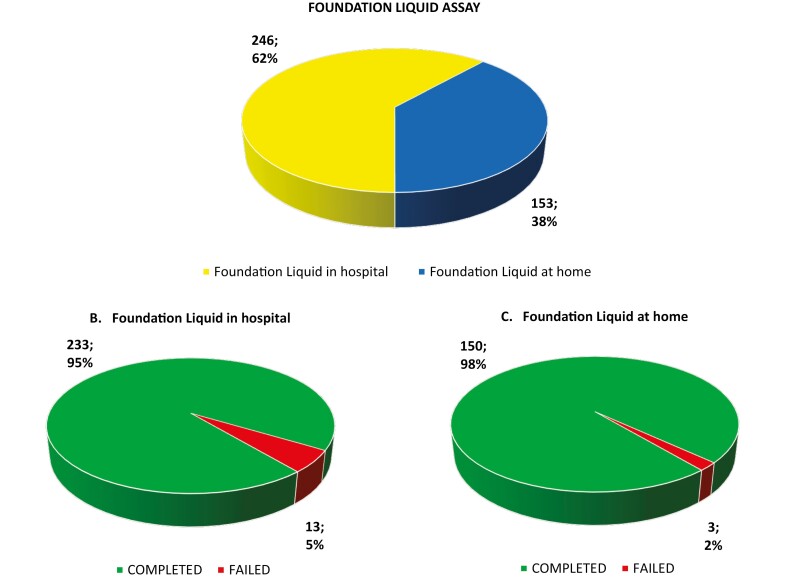
The success rate of the F1L was 96% (failed rate 4%); in fact, 383 samples out of 399 completed the analysis and 16 failed. Among the total number of 399 liquid biopsies performed in our Department, 62% were performed in hospital with a success rate of 95%, and 38% were performed at the patient home with a success rate of 98% (Fig. 1). Regarding failure reasons, all samples failed for suboptimal cfDNA amount.
Figure 1.
Foundation liquid assay versus foundation liquid home assay: number of samples and percentage of samples with completed or failed analysis. (A) Total liquid samples: 399 liquid samples, both in hospital and at home. (B) Liquid analysis in hospital: 246 liquid samples, both completed and failed analysis. (C) Liquid analysis at home: 153 liquid samples, both completed and failed analysis.
Cost and time effectiveness of FMI-Liquid@Home service for patients and their caregiver
It was possible to calculate the average travel time saved by a patient to reach the hospital and back to home (1.77 hours) and the average of waiting time avoided for all procedures once the patient arrived at the hospital (0.50 hour). Subsequently, we calculated the total hours saved for the patient for one blood draw and the total hours saved for 209 patients for 218 blood draws as described in Material and Methods.
Total hours saved for the patient for one blood draw (C + D): 2.27 hours (L)
Total hours saved for the patients for 218 blood draws (L*218): 494.86 hours (M)
In addition, we calculated the average travel time saved by a caregiver to reach the hospital and back to home (1.77 hours) and the average of waiting time avoided for all procedures at the hospital (0.50 hour).
Then we calculated the total hours saved for the caregiver for one blood draw (same time of the patient) and the total hours saved for the caregivers for 209 patients for 218 blood draws as described in Material and Methods.
Total hours saved for the caregiver for one blood draw (C + D): 2.27 hours (L)
Total hours saved for the caregivers for 218 blood draws (M*E): 427.36 hours (N)
Regarding cost, we calculated the average cost for commute patient-center for one blood draw as 62.15€. Then we calculated the total costs saved for commute patient-center for 209 patients for 218 blood draws as 13 548.70€ as described in Material and Methods.
Total costs saved for the patients and caregivers for 218 blood draws (H*218): 13 548.70€ (O)
According to the average hours for blood draw in hospital as 2.52 hours, it was therefore logical to assume that a patient or a caregiver should lose at least half a day’s work to go to the hospital in order to carry out the blood draw. We calculated the total working hours saved thanks to the home blood draw service for patients and caregivers both for one blood draw as 4 hours and for 209 patients for 218 blood draws as 1084.71 hrs. Considering the total of working hours saved, we estimated the total work cost saved as 31 239.65€ as described in Material and Methods.
Total working hours saved for patients and caregivers for one blood draw: 4 hours (P)
Total working hours saved for community for 218 blood draws (P*218*I + P*218*E*F): 1084.71 hours (Q)
Total working cost saved for patients and caregivers (28.8€/hour*Q): 31 239.65€ (R)
Cost and time effectiveness of FMI-Liquid@Home service for NHS
The time saved for hospital to blood draw was estimated to be:
Total hours saved for center for performing one blood draw: 0.25 hour (S)
Total hours saved for center to manage logistics for one blood draw: 0.50 hour (T)
Based on these parameters, we calculated the total hours saved by the center to carry out and manage 218 withdrawals as 163.5 hours. The total cost saved by the hospital was 6785.25€.
Total hours saved for center for 218 blood draws (S + T)*218: 163.5 hours (U)
Total cost saved for center for 218 blood draws (41.5€/hour*U): 6785.25€ (V)
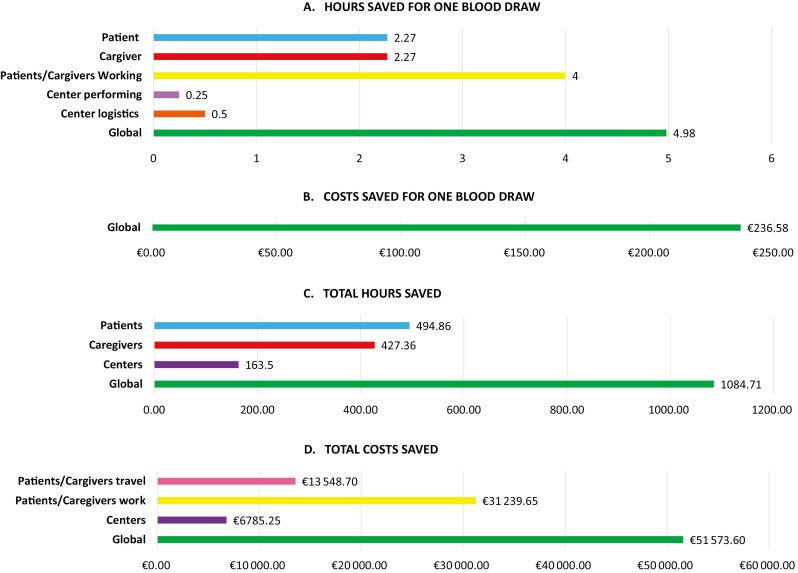
At the end, we calculated the global hours and costs saved for the community due to blood draws service at home as (Fig. 2 and Table 2):
Figure 2.
Hours saved and costs saved with foundation liquid home analysis. (A) Hours saved for one blood draw performed at home. (B) Costs saved for one blood draw performed at home. (C) Hours saved for all (218) blood draws performed at home. (D) Costs saved for all (218) blood draws performed at home.
Table 2.
Total costs saved for patients, caregivers, center, and NHS.
| Average cost for commute patient-center for one blood draw | (A*G) | 62.15€(H) |
|---|---|---|
| Total cost saved for one blood draw service (Global) | (W/218) | 236.58€(Y) |
| Total costs saved for the patients and caregivers for 218 blood draws (Travel) | (H*218) | 13 548.70€(O) |
| Total working cost saved for patients and caregivers (Work) | (28.8€/h*Q) | 31 239.65€(R) |
| Total cost saved for center for 218 blood draws | (41.5€/h*U) | 6785.25€(V) |
| Total cost saved for 218 blood draws (Global) | (O + R + V) | 51 573.60€(W) |
Total hours saved for 218 blood draws: 1084.71 hours (Q)
Total cost saved for 218 blood draws (O + R + V): 51 573.60€ (W)
Total hours saved for one blood draw service (Q/218): 4.98 hours (X)
Total cost saved for one blood draw service (W/218): 236.58€ (Y)
Patient satisfaction survey
At the interview about satisfaction with the service, 186 out of 209 patients answered, recording 99.46% of patients preferred the home service and 98.39% of patients recommended to another patient the service.
Discussion
The COVID-19 world pandemic had a detrimental impact on the oncological healthcare system in different fields, from patient with cancer care to hospitals organization. Many different studies investigated effective and potential effects of the pandemic but also the high flexibility required for oncologic teams to reorganize their daily routines.36-40 In this contest, the FMI-Liquid@Home has emerged as a key weapon to deal with the new Italian pandemic situation.
Here, 153 out of 218 liquid biopsies of FMI-Liquid@Home have been performed in our Department. Patients were assessed for molecular alterations at the diagnosis, according to the cancer subtype and tissue availability, by NGS on tissue. Tumor Molecular Board selected patients who underwent to liquid biopsy analysis.
The liquid biopsy was performed in different moments according to patients’ needs: in the case of inadequate tumor biopsy tissue at the diagnosis or impossibility to repeat biopsies; during cancer history to find druggable or resistant alterations; after surgery or adjuvant therapy to search minimal residual disease; to monitor therapeutic response.
For these reasons, the distribution of tumor subtypes could not be representative of the general population, unlike the median age and the distribution of gender.41 CRC and NSCLC emerged as the most frequent cancer subtypes among FMI-Liquid@Home (but also in hospital), according to the greater benefit they would have had by liquid biopsy in terms both of druggable identifiable mutations and in term of target available therapies.
Nearly half of patients performed the analysis at home before to start the first line of therapy and the majority of patients was selected with PS 0 or 1 according to ECOG to allow them to be enrolled in clinical trials or to benefit of experimental therapies. Anyway, the PS according to ECOG of patients performed FMI-Liquid@Home was higher than patients performed liquid biopsy in hospital in the same period (61.5% vs. 47.1% for PS 1; 11.1% vs 8.5% for PS 2 and 1.9% vs. 0.8% for PS 3). These data were in agreement with the nature and the purpose of service that guaranteed a blood draw at home for defeated patients, patients who could not reach the hospital due to distance, to the clinical conditions or at high risk of infection or complications of COVID-19.
We also evaluated the success rate of the FMI-Liquid@Home (98%), which was slightly higher than the success rate of the liquid biopsy in the hospital (95%), demonstrating that FMI-Liquid@Home had the same quality and efficacy than F1L in hospital. FMI-Liquid@Home had the same specificity, sensibility and accuracy than F1L in hospital as well as the same cost, but with the possibility to guarantee the service everywhere in the national territory at any time, with clinical and psychologically benefits for patients. It also guaranteed no risk of COVID-19 contamination and no costs and loss of time for the patients and their caregivers but also for the hospital.
In fact, we analyzed the impact of the home assay both on time and on costs saving for different figures implicated in the oncological care, but the analysis had several limitations according to the retrospective nature of the study and the data collection method (as described in “Material and Methods”). Firstly, for the calculation of some data we had to consider small groups of patients and not the entire population; secondly, the analysis required various approximations, resulting in underestimations of the values reported in “Results”.
Our study demonstrated that time saving by patients and their caregivers due to blood drawn at home was 494.86 and 427.36 hours, respectively. Moreover, the cost savings was 13 548.70€. Interestingly, this advantage was even more evident if we considered the logistical, economic and emotional situation of patients with cancer during the pandemic. During the lockdown period, the risk of contagioun was high; it was difficult to cross different countries to go to the hospital. Patients and their families were afraid and people, more comfortable and safer in their home.
According to Patient Satisfaction Survey results (99.46% of patients preferred the home service), FMI-Liquid@Home project had a significant impact for both management of cancer patients and economic burden. Recently, progress has been made in the field of precision medicine, moving from isolated genomic analyses towards a multiomics approach in order to better understand tumor biology and to increase treatment opportunities.42-46 From oncologist side, ensuring tailored medicine to cancer patients is essential even in pandemic. On economic side, further advantages from the FMI-Liquid@Home, have been observed. For working people, time and costs saved were 1084.71 hours and 31 239.65€, respectively. The positive impact was evident for working patients and caregivers but also for the community, guaranteeing the working services in the most difficult period for national economy.
Furthermore, time and work service costs saved for the hospitals and NHS, were 163.5 hours and 6785€, respectively. This advantage positively contributed to the health system heavily affected from pandemic and in trouble due to lack of personnel, economic, and time solutions.
In addition, a total gain of 1084.71 hours and 51 573.60€ for our NHS for only 218 blood draws performed with the FMI-Liquid@Home project has been calculated. The advantage was significant both for the result and for the historical period, considering the bias of the calculation method with an unavoidable underestimation of the results and considering the pandemic a period of economic, employment, and health crisis worldwide.
The analysis of cost/time saving was conducted on 218 patients from different Oncological Departments in Italy; among them, 153 patients from our Department. We have no medical information about the remaining 65 patients according to data protection and privacy regulations.
All 153 patients of our Department chose tele-visit to discuss results of the analysis, the clinical implications, and subsequent therapeutic decision. The patients’ choice was guided by several factors including distance from the Hospital, clinical conditions, occurrence of COVID-19 positivity or isolation, anxiety about results and the impossibility to discuss with the patient and all family members at the same time (according to COVID-19 rules regarding limited accesses to the Hospital).
In addition, several patients came to our department for a second opinion or were referred to our department from other hospitals.
The cost and time saving were evident only when the blood sample for the liquid biopsy was performed at patient’s home, avoiding further access to the hospital.
In accordance with the geographic and temporal framework, the Roche around the globe launched similar programs. For example, a similar service with F1L performed at patient’s home was born in United States of America, India, and Brazil.47 Unfortunately, due to local regulation the service had spread significantly only in Italy. In Canada and Spain, Roche collaborated with local peripheral clinics to ensure patients’ blood draws near home (but not at patients’ home).47 On the other hand, Canexia Health s.r.l. offered a program similar to Roche FMI-Liquid@Home to perform liquid biopsy at cancer patients’ home, but it worked only in Canada.48
The FMI-Liquid@Home project by Roche was started during the lockdown in order to address COVID-19 pandemic needs. Then, thanks to the positive impact and advantages demonstrated, it rapidly spread demonstrating how a collaboration between industry and NHS was possible, opening new possibilities for the oncological future.
In conclusion, we analyzed the economic impact and benefit of FMI-Liquid@Home for all figures involved in the management of patients with cancer, demonstrating the benefits for the resources of NHS, hospitals, community, caregivers and patients, and offering to patients with cancer the comfort of their home and the better service in the precision oncology era. Beforehand, no similar studies were available in the literature.
In our opinion, the FMI-Liquid@Home service appeared useful and convenient, allowing for time and cost savings, and in the future, it could be integrated into the oncological daily routine. However, further studies are necessary to better understand the overall gain and to understand how to integrate this service in different countries, such as those where for laws and regulatory reasons it was not possible to reach the patient’s home.
Acknowledgment
I-CURE research project: “Identification, characterization and mining of Colorectal tumorigenesis: cause, prevention & cure (ICURE)”. Roche Foundation Medicine: Roche S.p.A., Monza, Italy.
Contributor Information
Stefania Napolitano, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Vincenza Caputo, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Anna Ventriglia, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Giulia Martini, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Carminia Maria Della Corte, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Vincenzo De Falco, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Stefano Ferretti, Egg srl, Innovative Health Solutions, Milano, Italy.
Erika Martinelli, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Floriana Morgillo, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Davide Ciardiello, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy; Oncology Unit, Casa Sollievo della Sofferenza Hospital, San Giovanni Rotondo, Foggia, Italy.
Ferdinando De Vita, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Michele Orditura, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Morena Fasano, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Fortunato Ciardiello, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Teresa Troiani, Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania “Luigi Vanvitelli”, Napoli, Italy.
Conflict of Interest
Stefano Ferretti: Egg srl—Innovative Health Solutions (E, OI); Erika Martinelli:Amgen, Bayer, Eisai, Merck Serono, Pierre Fabre, Roche, Servier, Incyte (H, C/A); Davide Ciardiello: Sanofi (Other—travel); Floriana Morgillo: BMS, MSD, Astrazeneca, Incyte (C/A); Ferdinando De Vita: Amgen, Lilly, Roche, Celgene (C/A); Michele Orditura: Honoraria from Epionpharma, Italfarmaco (H), Eisai (RF), Roche (Other—travel); Morena Fasano: Lilly, MSD (C/A); Fortunato Ciardiello: Merck, Roche, Amgen, Bayer, Servier, Symphogen, Pfizer (C/A), Roche, Merck, Amgen, Bayer, Ipsen (RF); Teresa Troiani: Amgen, Bayer, Merck, Novartis, Roche, Sanofi (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: S.N., V.C., S.F., F.C., T.T. Provision of study material/patients: S.N., V.C., A.V., G.M., C.M.D.C., V.D.F., S.F., E.M., F.M., D.C., F.D.V., M.O., M.F., F.C., T.T. Collection and/or assembly of data: S.N., V.C., A.V., G.M., C.M.D.C., V.DF., S.F., E.M., F.M., D.C., F.D.V., M.O., M.F., F.C., T.T. Data analysis and interpretation: S.N., V.C., S.F., F.C., T.T. Manuscript writing: S.N., V.C., S.F., F.C., T.T. Final approval of manuscript: All authors.
Data Availability
The relevant data underlying this article are available in the article. Supplementary data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Chan JFW, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microb Infect 16. 2020;9(1):221-236. https://doi.org/10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. Published online 2020;382(13):1199-1207. https://doi.org/10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Worldometer: COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/. Accessed October 2021.
- 4. World Health Organization. Coronavirus disease (COVID-19) Emergency. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed October 2021.
- 5. World Health Organization. Coronavirus disease (COVID-19) outbreak situation. https://covid19.who.int/. Accessed October 2021.
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poggio F, Tagliamento M, Maio MD, et al. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of italian oncologists toward breast cancer care and related research activities. JCO Oncology Practice Published online 2020;16(11):e1304-e1314. https://doi.org/10.1200/OP.20.00297 [DOI] [PubMed] [Google Scholar]
- 8. Remuzzi A, Remuzzi G.. COVID-19 and Italy: what next?. The Lancet 2020;395(10231):1225-1228. https://doi.org/10.1016/s0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Amrani M, Truant S, Turpin A.. COVID 19 and cancer: what are the consequences of the cancer care reorganization?. Bull Cancer. 2020;107(5):538-540. [in French] https://doi.org/10.1016/j.bulcan.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. You B, Ravaud A, Canivet A, et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21(5):619-621. https://doi.org/10.1016/S1470-2045(20)30204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang W. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. https://doi.org/10.1016/s1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Zhang L.. Risk of COVID-19 for patients with cancer. Lancet Oncol. Published online 2020;21(4):e181. https://doi.org/10.1016/S1470-2045(20)30149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Höllein A, Bojko P, Schulz S, et al. Characteristics and outcomes of patients with cancer and COVID-19: results from a cohort study. Acta Oncol. 2021;60(1):24-27. https://doi.org/10.1080/0284186X.2020.1863464 [DOI] [PubMed] [Google Scholar]
- 14. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893-903. https://doi.org/10.1016/S1470-2045(20)30309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 2020;10(7):935-941. https://doi.org/10.1158/2159-8290.CD-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brar G, Pinheiro LC, Shusterman M, et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. JCO. 2020;38(33):3914-3924. https://doi.org/10.1200/JCO.20.01580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):8. https://doi.org/10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vose JM. Delay in cancer screening and diagnosis during the COVID-19 pandemic: what is the cost?. Oncology Journal. 2020;34(9):343. https://doi.org/10.46883/ONC.2020.3409.0343 [DOI] [PubMed] [Google Scholar]
- 20. Lambertini M, Toss A, Passaro A, et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists’ perspective. ESMO Open 2020;5(2):e000759. https://doi.org/10.1136/esmoopen-2020-000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tagliamento M, Lambertini M, Genova C, et al. Call for ensuring cancer care continuity during COVID-19 pandemic. ESMO Open 2020;5(3):e000783. https://doi.org/10.1136/esmoopen-2020-000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pathania AS, Prathipati P, Abdul BAA, et al. COVID-19 and cancer comorbidity: therapeutic opportunities and challenges. Theranostics 2021;11(2):731-753. https://doi.org/10.7150/thno.51471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brunello A, Galiano A, Finotto S, et al. Older cancer patients and COVID‐19 outbreak: practical considerations and recommendations. Cancer Med 2020;9(24):9193-9204. https://doi.org/10.1002/cam4.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miaskowski C, Paul SM, Snowberg K, et al. Oncology patients’ perceptions of and experiences with COVID-19. Support Care Cancer. 2021;29(4):1941-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gyawali B, Poudyal BS, Eisenhauer EA.. Covid-19 pandemic—an opportunity to reduce and eliminate low-value practices in oncology?. JAMA Oncol 2020;6(11):1693. https://doi.org/10.1001/jamaoncol.2020.2404 [DOI] [PubMed] [Google Scholar]
- 26. Borad MJ, LoRusso PM.. Twenty-first century precision medicine in oncology: genomic profiling in patients with cancer. Mayo Clin Proc. 2017;92(10):1583-1591. https://doi.org/10.1016/j.mayocp.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 27. Yip S, Christofides A, Banerji SMR, et al. A Canadian guideline on the use of next-generation sequencing in oncology. Current Oncology. 2019;26(2):241-254. https://doi.org/10.3747/co.26.4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bewicke-Copley F, Kumar EA, Palladino G, et al. Applications and analysis of targeted genomic sequencing in cancer studies. Comput Struct Biotechnol J. 2019;17:1348-1359. https://doi.org/10.1016/j.csbj.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sato Y, Ryo M, Kato K.. Recent advances in liquid biopsy in precision oncology research. Biol Pharm Bull. 2019;42(3):337-342. https://doi.org/10.1248/bpb.b18-00804 [DOI] [PubMed] [Google Scholar]
- 30. Gambardella V, Lombardi P, Carbonell-Asins JA, et al. Molecular profiling of advanced solid tumours. The impact of experimental molecular-matched therapies on cancer patient outcomes in early-phase trials: the MAST study. Br J Cancer. 2021;125(9):1261-1269. https://doi.org/10.1038/s41416-021-01502-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Mattos-Arruda L, Siravegna G.. How to use liquid biopsies to treat patients with cancer. ESMO Open 2021;6(2):100060. https://doi.org/10.1016/j.esmoop.2021.100060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. FoundationOne Liquid CDx Label Technical Info: ‘FoundationOne_Liquid_CDx_Label_Technical_Info.Pdf’. Available: https://assets.ctfassets.net/w98cd481qyp0/3a8jFw3KUjIU3RWPdcT9Ax/cdb6d621b1d2e3baf8103af93059bce5/FoundationOne_Liquid_CDx_Label_Technical_Info.pdf. Accessed September 2021.
- 33. FoundationOne Medicine site. https://www.foundationmedicine.it/. Accessed September 2021.
- 34. Eurostat site. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Wages_and_labour_costs/it&oldid=494296. Accessed October 2021.
- 35. NEXT Project – ARAN. https://www.aranagenzia.it. Accessed October 2021.
- 36. Cuschieri S, Mamo J.. Taking care of the ordinary in extraordinary times—delayed routine care means more morbidity and pre-mature mortality. Eur J Public Health. 2021;31(Supplement_4):iv27-iv30. https://doi.org/10.1093/eurpub/ckab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamposioras K, Mauri D, Papadimitriou K, et al. Synthesis of recommendations from 25 countries and 31 oncology societies: how to navigate through Covid-19 Labyrinth. Front Oncol. 2020;10:575148. https://doi.org/10.3389/fonc.2020.575148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeegers Paget D, Allebeck P, Nagyova I.. COVID-19: what have we learned? What are the public health challenges?. Eur J Public Health. 2021;31(Supplement_4):iv1-iv2. https://doi.org/10.1093/eurpub/ckab150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodin G, Zimmermann C, Rodin D, et al. COVID-19, palliative care and public health. Eur J Cancer. 2020;136:95-98. https://doi.org/10.1016/j.ejca.2020.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Covid-19 pandemic halts cancer care and damages oncologist’s wellbeing. https://www.esmo.org/newsroom/press-office/esmo2020-covid-pandemic-halts-cancer-care-oncologist-wellbeing. Accessed October 2021.
- 41. World Health Organization cancer statisctics. https://gco.iarc.fr/today/home. Accessed October 2021.
- 42. Chakraborty S, Hosen MdI, Ahmed M, Shekhar HU.. Onco-multi-OMICS approach: a new frontier in cancer research. BioMed Res Int. 2018;2018:1-14. https://doi.org/10.1155/2018/9836256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jung HD, Sung YJ, Kim HU.. Omics and computational modeling approaches for the effective treatment of drug-resistant cancer cells. Front Genet. 2021;12:742902. https://doi.org/10.3389/fgene.2021.742902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olivier M, Asmis R, Hawkins GA, et al. The need for multi-omics biomarker signatures in precision medicine. IJMS. 2019;20(19):4781. https://doi.org/10.3390/ijms20194781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu W, Xie L, Han J, Guo X.. The application of deep learning in cancer prognosis prediction. Cancers 2020;12(3):603. https://doi.org/10.3390/cancers12030603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kolenc Z, Pirih N, Gretic P, Kunej T.. Top trends in multiomics research: evaluation of 52 published studies and new ways of thinking terminology and visual displays. OMICS J Integr Biol. 2021;25(11):681-692. https://doi.org/10.1089/omi.2021.0160 [DOI] [PubMed] [Google Scholar]
- 47. Roche site: personalized healthcare. https://www.roche.com/about/priorities/personalised_healthcare/liquid-biopsy-cancer.htm. Accessed October 2021.
- 48. Canexia Health site. https://canexiahealth.com/resources/providers/providers-access/. Accessed November 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The relevant data underlying this article are available in the article. Supplementary data underlying this article will be shared on reasonable request to the corresponding author.