Abstract
Central giant cell granuloma (CGCG) is a benign non-neoplastic intraosseous lesion mainly found in the anterior mandible. It is characterized by multinucleated giant cells, representing osteoclasts or macrophages. Central odontogenic fibroma (COF) is an uncommon benign lesion of the jaws. It originates from the odontogenic ectomesenchyme. In rare cases, COF may accompany a CGCG. To date, 49 cases of COF accompanied by CGCG-like lesions have been reported in the literature. In this paper, we present another case of COF-CGCG in a 46-year-old female. The lesion was located in the posterior mandible. Excisional biopsy was carried out, and histopathological analysis revealed multinucleated giant cells with numerous strands of odontogenic epithelium. A literature review of previously reported cases was also performed.
Key Words: Granuloma, Giant Cell; Fibroma; Odontogenic Tumors
Introduction
Central giant cell granuloma (CGCG) is a benign non-neoplastic intraosseous lesion found mainly in the anterior mandible, and often crossing the midline [1]. Although there is controversy about the nature of this lesion, some theories describe it as a reactive lesion, a developmental anomaly, or a benign neoplasm. The World Health Organization considers CGCG as a bone-related lesion [2]. Extragnathic CGCG can occur mainly in the craniofacial region and small long bones of the hands and feet [3]. Most of the reported cases have occurred in patients between 10 to 25 years, and it is more common in females than males with a 2:1 ratio [4]. Based on the radiographic features, CGCG can be divided into non-aggressive and aggressive types with non-aggressive lesions making up most of the cases [1]. They usually present as an asymptomatic, painless, slow-growing swelling in the jaw, and can often cause tooth displacement [5]. Histopathologically, CGCG is composed of giant cells that are believed to represent osteoclasts while some others suggest that they might be macrophages. These lesions are similar to brown tumors of hyperthyroidism and giant cell lesions in cherubism and Noonan syndrome and neurofibromatosis type 1 [4,6].
Radiographically, CGCG is a unilocular or multilocular well-defined radiolucency. Large lesions may cause tooth displacement, root resorption, or cortical perforation [5]. These lesions are often treated by curettage or en bloc resection [7]. Central odontogenic fibroma (COF) is an uncommon benign lesion of the jaws. It originates from the odontogenic ectomesenchyme. The maxilla and mandible are affected almost equally. Most maxillary lesions tend to occur in the anterior region; however, mandibular lesions are mostly located posterior to the first molar [8]. An unerupted tooth is involved in one-third of the lesions [8]. COF lesions that are associated with unerupted teeth are believed to originate from the dental follicle; while, those that are not associated with an unerupted tooth arise from the periodontal ligament [7]. The occurrence of COF with CGCG is quite rare. Such a case was first reported in 1985 by Wangerin and Harms [9].
Over the years, more reports of this lesion were documented. In addition to the published cases, several cases have been presented at professional meetings. In 1993, Fowler et al. [10] reported an associated giant cell reaction in 3 out of 24 cases of COF. Kruse-Lösler et al. [11] presented a case diagnosed with COF accompanied by CGCG in the 2006 Meeting of the Western Society of Teachers of Oral Pathology. In 2008, Hassan et al. [12] presented 7 cases of the hybrid lesion at the 62nd Annual Meeting of the American Academy of Oral and Maxillofacial Pathology.
Two cases were presented at the 71st Annual Meeting of the American Academy of Oral and Maxillofacial Pathology in 2017 [13,14]. To date, 49 cases of COF with CGCG have been reported, considering the national conferences and reports (Table 1). Allen et al. [15] presented three cases, all in women and in the mandibular region, and suggested that “this pathological process does not represent a "collision lesion" but instead, is a unique presentation of a central odontogenic fibroma” [15]. Herein, we report a case of COF with CGCG in a 46-year-old female and also perform a literature review of the previous cases.
Table 1.
Reported cases of hybrid central odontogenic fibroma-central giant cell granuloma in the literature
| Author | Age (y) | Sex | Location | Year | Associated features | Radiographic findings |
Treatment | Recurrence | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Wangerin & Harms [9] | 7 | N/A | L Mandible (M) | 1985 | Unerupted molars | MRL | N/A | Yes, after one year of FU |
| 2 | Allen et al, [15] | 66 | F | R Mandible (PM-M) | 1992 | RCT tooth | MRL | Curettage | None after 6 months of FU |
| 3 | Allen et al, [15] | 14 | F | L Mandible (PM-M) | 1992 | Vital teeth, no expansion |
URL, 3.5cm | Curettage | None after 48 months of FU |
| 4 | Allen et al, [15] | 30 | F | L Mandible (PM-M) | 1992 | Orthodontic treatment, some expansion |
MRL 1.5×2cm | Curettage, curettage of recurrent lesion |
Yes, after 14months of FU |
| 5-7 | Fowler et al, [10] (3 cases) |
N/A | N/A | N/A | 1993 | N/A | N/A | N/A | N/A |
| 8 | Odell et al, [16] | 5 | F | Anterior maxilla | 1997 | Buccal expansion | N/A | Curettage | None |
| 9 | Odell et al, [16] | 11 | M | Posterior maxilla | 1997 | Buccal expansion | URL | Curettage, conservative excision of recurrent lesion |
Yes, after 36 months |
| 10 | Odell et al, [16] | 20 | F | Mandible (PM-M) | 1997 | N/A | URL 1.5×1cm | Curettage | None |
| 11 | Odell et al, [16] | 21 | F | Posterior Mandible | 1997 | Buccal expansion | URL, 3×2 cm | Curettage | None |
| 12 | Odell et al, [16] | 22 | F | Mandible (PM-M) | 1997 | Buccal expansion, cortical perforation |
N/A | Curettage and extraction of involved teeth |
None |
| 13 | Odell et al, [16] | 39 | F | Mandible (PM-M) | 1997 | Expansion, mobile teeth |
N/A | Curettage | None |
| 14 | Odell et al, [16] | 43 | F | Mandible | 1997 | N/A | N/A | Curettage, curettage of recurrent lesion |
Yes, after 36 months |
| 15 | Odell et al, [16] | 50 | F | Mandible PM | 1997 | N/A | URL | Curettage | None |
| 16 | Taylor et al, [19] | 17 | F | R Mandible (C-PM) | 1999 | Buccal expansion | MRL 2.5 × 2cm | Curettage | None after 72 months of FU |
| 17 | Kruse-Losler et al, [11] |
22 | F | R Mandible (LI-M) | 2006 | Lingual & inferior expansion |
Mostly URL with scalloped edge, with hint of MRL in post. area |
Surgical excision | None after 24 months of FU |
| 18-24 | Hassan et al, [12] | Average 49 |
5 M 2 F | Mandible | 2008 | N/A | N/A | N/A | Yes, 3 cases |
| 25 | Younis et al, [25] | 57 | F | R Mandible (PM-M) | 2008 | Buccal expansion | URL 2×2.5cm | Curettage | None after 18 months of FU |
| 26 | Tosios et al, [23] | 18 | M | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | Lost to FU |
| 27 | Tosios et al, [23] | 20 | F | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | None after 117 months of FU |
| 28 | Tosios et al, [23] | N/A | N/A | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | None after 28 months FU |
| 29 | Tosios et al, [23] | N/A | N/A | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | None after 43 months of FU |
| 30 | Tosios et al, [23] | N/A | N/A | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | None after 76 months of FU |
| 31 | Tosios et al, [23] | N/A | N/A | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | None after 39 months of FU |
| 32 | Tosios et al, [23] | N/A | N/A | Mandible (PM-M) | 2008 | N/A | RL | Surgical excision | Lost to FU |
| 33 | Marina de Deus Moura de et al, [17] |
24 | F | Mandible (R M-L M) | 2008 | Cortical Expansion | N/A | Curettage | None after 8 months of FU |
| 34 | Mosqueda-Taylor et al, [21] |
14 | M | L Mandible (M) | 2011 | Buccal & lingual swelling |
URL 4×3.2cm | Surgical excision | None after 16 months of FU |
| 35 | Mosqueda-Taylor et al, [21] |
14 | M | L Mandible (PM-M) | 2011 | Buccal expansion | MRL 4.5×3cm | Surgical excision | None after 24 months of FU |
| 36 | Eversole [24] | 42 | F | Mandible, body | 2011 | N/A | RL | Enucleation/ Curettage |
None |
| 37 | Eversole [24] | 27 | F | Mandible, ramus | 2011 | Impaction | RL | Enucleation/ Curettage |
None |
| 38 | Bologna-Molina et al, [27] |
14 | M | L Mandible (PM-M) | 2011 | Asymptomatic | Panoramic: URL in the body of the mandible, CT: MRL vestibular cortical expansion |
Curettage with milling of the bone walls |
None after 2 years of FU |
| 39 | Castillo et al, [20] | 14 | M | Mandible (M) | 2011 | Expansion and tenderness |
URL | Curettage | None |
| 40 | Damm [18] | 75 | F | Ant Mandible | 2013 | N/A | URL | Curettage | None |
| 41 | Eliot & Kessler [28] | 22 | F | R Mandible (PM-M) | 2014 | Expansion & swelling |
MRL | Surgical excision | None |
| 42 | Schultz & Rosebush [14] |
12 | F | Ant Mandible | 2017 | Asymptomatic | RL | N/A | N/A |
| 43 | Leite et al, [13] | 42 | F | L Mandible (M) | 2017 | Edentulous area | Surgical excision | None after12 months of FU |
|
| 44 | Upadhyaya et al, [7] | 10 | M | Ant Mandible (C-I) | 2018 | Buccal and lingual expansion, impaction |
URL 1.9×1.8cm | Curettage | None after 72 months of FU |
| 45 | Upadhyaya et al, [7] | 63 | F | L Mandible (M) | 2018 | Buccal expansion | URL 1.7×1cm | N/A | Awaiting treatment |
| 46 | Upadhyaya et al, [7] | 62 | M | R Mandible (PM) | 2018 | Asymptomatic | URL | Curettage | None after 12 months of FU |
| 47 | Vijintanawan et al, [26] |
27 | M | L Mandible (PM) | 2019 | Asymptomatic | URL | Curettage | None after 6 months of FU |
| 48 | Flores-Hidalgo et al, [22] |
65 | F | L Mandible (PM) | 2019 | Paresthesia | MRL | Excisional biopsy |
Yes, after 9 months |
| 49 | Ramadan & Essawy [29] |
33 | F | L Mandible (PM-M) | 2020 | Buccal expansion | URL | Curettage | None after 12 months of FU |
| 50 | Our case | 46 | F | L Mandible (PM-M) | 2020 | Buccal expansion and perforation |
URL | Excisional biopsy |
None after 25 months of FU |
N/A: not available; Ant: anterior; R: Right, L: Left, RL: Radiolucent, URL: Unilocular radiolucency, MRL: Multilocular radiolucency, PM: Premolar, M: Molar, FU: Follow-up
Table 1 shows all the reported cases of this hybrid lesion.
CASE REPORT
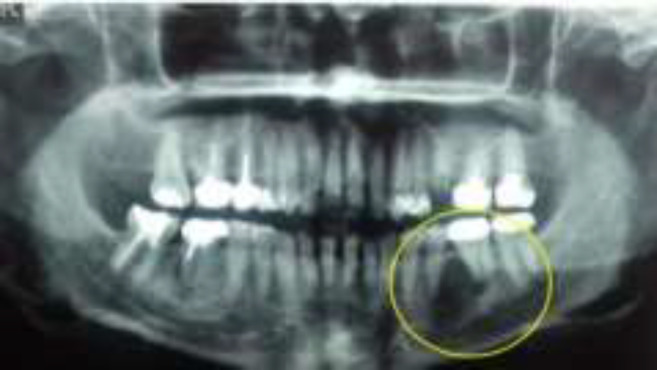
A 46-year-old female was referred to an oral surgeon for evaluation of a radiolucent lesion in her left lower jaw which was accidentally found on radiographic examination by her dentist. On radiographic examination, the lesion was a well-defined radiolucency located between the premolar and molar area (i.e., teeth #19-20) (Fig.1).
Fig. 1.
Panoramic radiograph showing a radiolucent lesion in the left posterior mandible, between second premolar and first molar
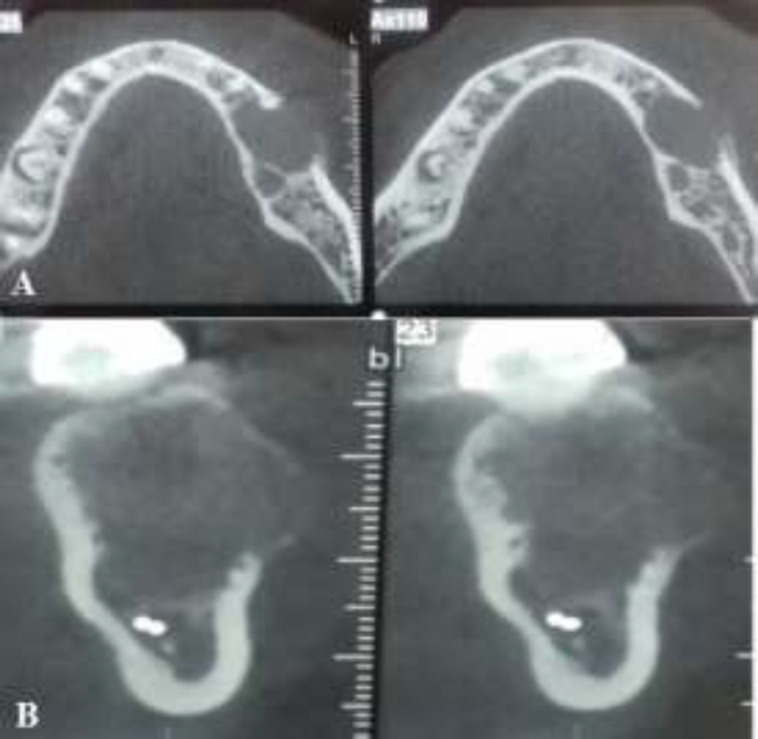
The patient did not report any pain or numbness in the area. However, expansion and perforation of the buccal cortical plate were noted on cone-beam computed tomography scan (Fig. 2A and 2B).
Fig. 2.
Cone-beam computed tomography scan demonstrating a unilocular radiolucency with buccal expansion and perforation: (A) axial and (B) sagittal views
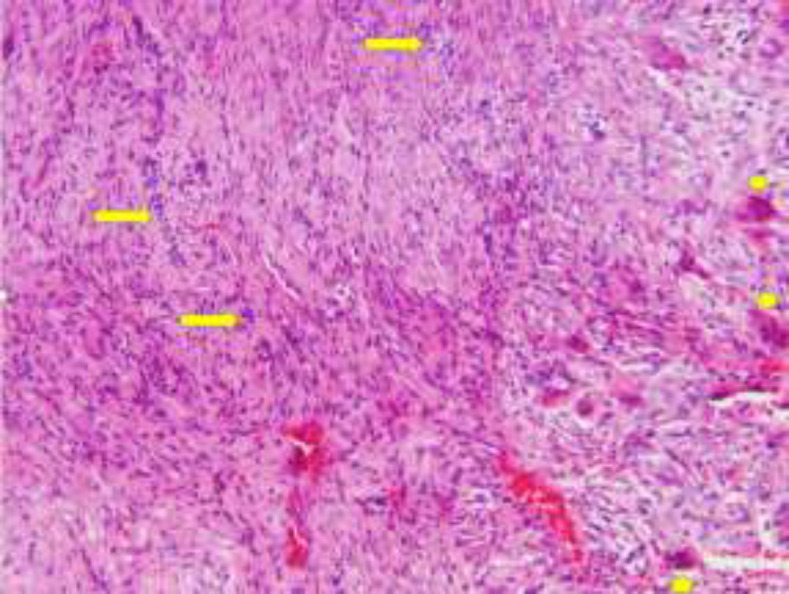
The greatest diameter of the lesion was 1 cm. The differential diagnosis included CGCG and aneurysmal bone cyst. An excisional biopsy was performed. Microscopic examination revealed hypercellular connective tissue and plump spindle-shaped cells in a hemorrhagic background admixed with numerous multinucleated giant cells. Also, nests and strands of bland odontogenic epithelium were evident (Fig. 3).
Fig. 3.
Nests of odontogenic epithelium (arrows) and multinucleated giant cells (arrowheads) with a low magnification (x20) showing the two lesions relative to each other
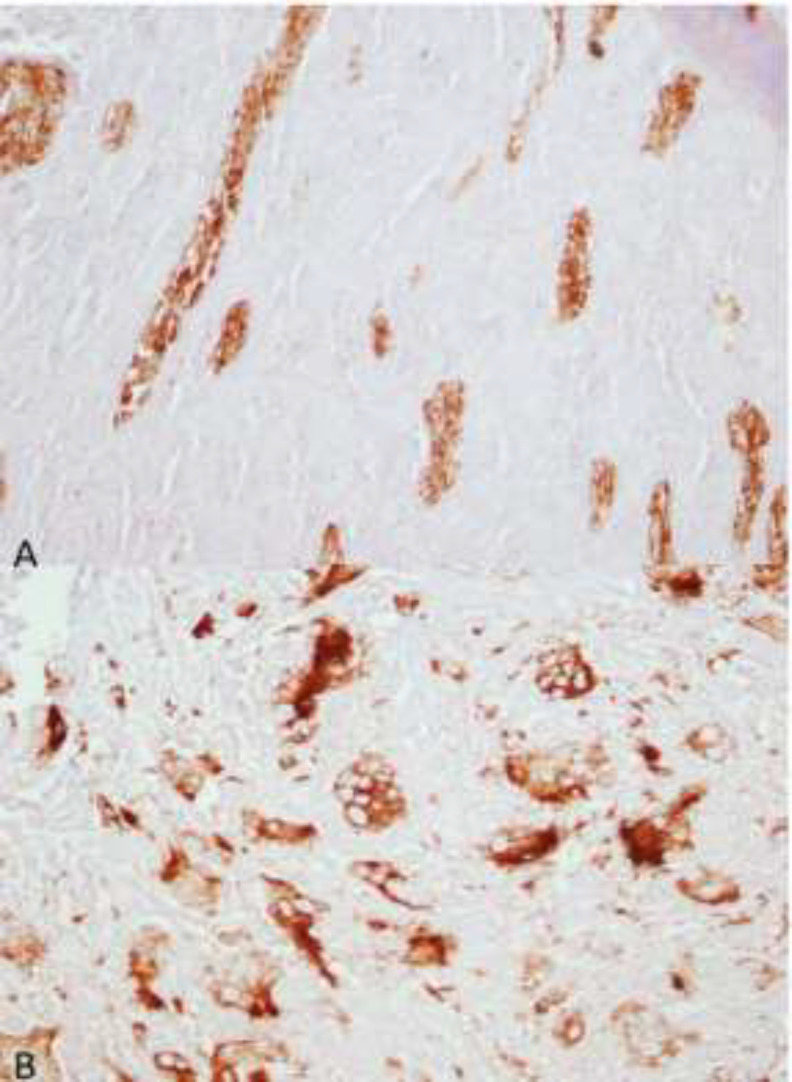
The results of immunohistochemical staining with pan-cytokeratin and CD68 confirmed the odontogenic epithelium and multinucleated giant cells (Fig. 4A and 4B). According to the histopathological features and the results of immunohistochemical assessment, the diagnosis of COF with CGCG-like lesion was made.
Fig. 4.
Immunoreactivity of odontogenic epithelium for pan-cytokeratin (A, x40) and giant cells for CD68 (B, x40)
It should be mentioned that all biochemical and hematological parameters of the patient including serum calcium, phosphorous, and alkaline phosphatase were within the normal range. The patient was periodically followed-up for 2 years, and no recurrence occurred during this time period.
Discussion
Hybrid COF-CGCG is a rare condition, which was first reported by Wangerin and Harms [9] in 1985. Although they introduced the case as a rare combination of two lesions, ameloblastic fibroma and CGCG, they concluded that the primary neoplastic COF induced the secondary reactive CGCG.
Most of the previously reported cases were located in the mandible (mostly in the posterior section) except for two lesions which were located in the maxilla (one in the anterior and the other in the posterior maxilla) [16].
The lesions were variable in size and rarely crossed the midline [3,17]. The age of patients has been widely variable ranging from 5 to 75 years, with a mean age of 32.5 years [16,18]. It was more common in women, with a 1.4: female-to-male ratio. Of all cases, only two were associated with pain and tenderness [19-21]. Although the clinical features often include painless swelling and buccal cortical expansion, some documented cases have reported buccal perforation [16,22,23]. According to three reports, this hybrid lesion can cause tooth displacement [17,20,24]. Due to such aggressive behavior, careful follow-up is of utmost importance [22]. Of 48 documented cases, only five showed recurrence [12,15,16].
Sufficient data are not available to determine the frequency of COF-CGCG. Younis et al. [25] stated that this hybrid lesion is associated with some reactive stimuli such as orthodontic treatment, tooth impaction, root canal therapy, and history of extraction [25]. Tosios et al. [23] reported a case that occurred in a patient with cherubism. Radiographically, the hybrid COF with CGCG can be presented as either a unilocular or a multilocular radiolucency with sharp borders. Unilocular radiolucent lesions outnumber multilocular ones with a 2.4:1 ratio. Odell et al. [16] reported a case in the maxilla that extended to the antrum. The previous cases of COF-CGCG were treated by curettage (18 cases) or surgical excision (14 cases). Curettage has shown 33% recurrence rate. Recurrence occurred in seven patients [12,15,16,22]. All the recurrent lesions occurred in patients that were initially treated by curettage except for one case that was treated by surgical excision [22]. The histopathology of six recurrent lesions was similar to that of primary lesions, containing both COF and CGCG components. One recurrent lesion consisted of CGCG components only [12].
The exact pathogenesis of the hybrid CGCG-COF is still unknown. Allen et al. [15] described this hybrid lesion as a unique presentation of COF. Odell et al. [16] postulated that clinical features such as gender, age, and site of occurrence were more suggestive of CGCG. In general, three theories have been proposed regarding the nature of this lesion [25]. The first theory describes this lesion as a “collision tumor”, which is characterized by synchronized occurrence of both COF and CGCG. Despite the unlikeliness of this theory due to the rare nature of COF and CGCG, Vijintanwan et al. [26] described their case as a collision tumor.
The second theory is about a primary CGCG which produces some growth factors and chemokines that result in formation of COF [7]. The third theory proposes that the primary lesion is COF, in which trauma or other stimuli induce a giant cell reaction. Our case was reported in a middle-aged woman, which is similar to some previously reported cases [12, 13,16,24]. The clinical and radiographic features showed no significant difference compared with other documented cases.
CONCLUSION
In this report, we added one more case to the documented cases of hybrid COF-CGCG, bringing the total to 50 cases. The recurrence rate is higher in this lesion compared with COF, indicating that the CGCG component is mainly responsible for the recurrence. Hybrid COF with CGCG-like lesion is usually treated by curettage or excision of the lesion. Due to the possibility of recurrence, close follow-up is important. The nature of this lesion is still unknown, and more studies should be carried out in order to find the exact origin and pathogenesis of this lesion.
ACKNOWLEDGMENTS
None.
Notes:
Cite this article as: Moradzadeh Khiavi M, Karimi A, Sadeghi HMM, Derakhshan S, Tafreshi SM, Jalali S. Central Odontogenic Fibroma Accompanied by a Central Giant Cell Granuloma-Like Lesion: Report of a Case and Review of Literature. Front Dent. 2021;18:44.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1.Hosur MB, Puranik RS, Vanaki SS, Puranik SR, Ingaleshwar PS. Clinicopathological profile of central giant cell granulomas: An institutional experience and study of immunohistochemistry expression of p63 in central giant cell granuloma. J Oral Maxillofac Pathol. 2018 May;22(2):173–9. doi: 10.4103/jomfp.JOMFP_260_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadu F, Pharoah M, Lee L, Baker G, Allidina A. Central giant cell granuloma of the mandibular condyle: a case report and review of the literature. Dentomaxillofac Radiol. 2011 Jan;40(1):60–4. doi: 10.1259/dmfr/85668294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruse-Losler B, Diallo R, Gaertner C, Mischke KL, Joos U, Kleinheinz J. Central giant cell granuloma of the jaws: a clinical, radiologic, and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):346–54. doi: 10.1016/j.tripleo.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 4.de Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Nov;104(5):603–15. doi: 10.1016/j.tripleo.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Abdelkarim AZ, el Sadat SMA, Chmieliauskaite M, Syed AZ. Radiographic diagnosis of a central giant cell granuloma using advanced imaging: cone beam computed tomography. Cureus. 2018 Jun;10(6):e2735. doi: 10.7759/cureus.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lange J, van Maarle MC, van den Akker HP, Redeker EJ. DNA analysis of the SH3BP2 gene in patients with aggressive central giant cell granuloma. Br J Oral Maxillofac Surg. 2007 Sep;45(6):499–500. doi: 10.1016/j.bjoms.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Upadhyaya JD, Cohen DM, Islam MN, Bhattacharyya I. Hybrid central odontogenic fibroma with giant cell granuloma like lesion: a report of three additional cases and review of the literature. Head and Neck Pathol. 2018;12(2):166–74. doi: 10.1007/s12105-017-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neville BW, Damm DD, Allen CM, Chi AC. Oral and maxillofacial pathology. 4th ed. St. Louis: Elsevier; 2016. 676 pp. [Google Scholar]
- 9.Wangerin K, Harms D. Seltene Variationen des ameloblastischen Fibroms. Dtsch Z Mund Kiefer Gesichtschir. 1985 May-Jun;9(3):227–31. [PubMed] [Google Scholar]
- 10.Fowler C, Tomich C, Brannon R, Houston G. Central odontogenic fibroma: clinicopathologic features of 24 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1993;76:587. [Google Scholar]
- 11.Kruse-Lösler B, Diallo R, Gaertner C, Mischke KL, Joos U, Kleinheinz J. Central giant cell granuloma of the jaws: a clinical, radiologic, and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006 Mar;101(3):346–54. doi: 10.1016/j.tripleo.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 12.Hassan S, Reich R, Freedman P, editors Central odontogenic fibroma with associated central giant cell granuloma (Collision tumor?): report of 7 cases. 62nd Annual Meeting, American Academy of Oral and Maxillofacial Pathology San Francisca.2008. [Google Scholar]
- 13.Leite T, Mendes E, Abrahao A, Andrade B, Canedo N, Agostini M, et al. Central odontogenic fibroma: report of three new cases from Brazil. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2017 Sep;124(3):e229–30. [Google Scholar]
- 14.Schultz K, Rosebush M, editors Clinical pathologic conference case 3. Annual Meeting of the American Academy of Oral and Maxillofacial Pathology Newport, Rhode Island.2017. [Google Scholar]
- 15.Allen CM, Hammond HL, Stimson PG. Central odontogenic fibroma, WHO∗ type: a report of three cases with an unusual associated giant cell reaction. Oral Surg Oral Med Oral Pathol. 1992 Jan;73(1):62–6. doi: 10.1016/0030-4220(92)90156-k. [DOI] [PubMed] [Google Scholar]
- 16.Odell E, Lombardi T, Barrett A, Morgan P, Speight P. Hybrid central giant cell granuloma and central odontogenic fibroma‐like lesions of the jaws. Histopathology. 1997 Feb;30(2):165–71. doi: 10.1046/j.1365-2559.1997.d01-585.x. [DOI] [PubMed] [Google Scholar]
- 17.Marina de Deus Moura de L, de Aquino Xavier FC, Vanti LA, de Lima PS, de Sousa SC. Hybrid central giant cell granuloma and central odontogenic fibroma-like lesion of the mandible. Otolaryngol Head Neck Surg. 2008 Dec;139(6):867–8. doi: 10.1016/j.otohns.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Damm D. Interradicular radiolucency. Hybrid giant cell granuloma and odontogenic fibroma. Gen Dent. 2013 May-Jun;61(3):77,78. [PubMed] [Google Scholar]
- 19.Taylor AM, Flores VB, Franco MAD. Combined central odontogenic fibroma and giant cell granuloma-like lesion of the mandible: report of a case and review of the literature. J Oral and Maxillofac Surg. 1999 Oct;57(10):1258–62. doi: 10.1016/s0278-2391(99)90500-1. [DOI] [PubMed] [Google Scholar]
- 20.Castillo GC, Reyes RL, Mosqueda Taylor A. Lesión mandibular inusual de fibroma odontogénico central combinado con granuloma central de células gigantes mandibular. Revista Odontológica Mexicana. 2011;15(2):126–31. [Google Scholar]
- 21.Mosqueda-Taylor A, Martínez-Mata G, Carlos-Bregni R, Vargas PA, Toral-Rizo V, Cano-Valdéz AM, et al. Central odontogenic fibroma: new findings and report of a multicentric collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Sep;112(3):349–58. doi: 10.1016/j.tripleo.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Hidalgo A, Biggerstaff T, Murrah V. Hybrid central odontogenic fibroma and central giant cell granuloma lesion–A case report of an aggressive and recurrent lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2019 Nov;31(6):432–4. [Google Scholar]
- 23.Tosios KI, Gopalakrishnan R, Koutlas IG. So-called hybrid central odontogenic fibroma/central giant cell lesion of the jaws. A report on seven additional cases, including an example in a patient with cherubism, and hypotheses on the pathogenesis. Head Neck Pathol. 2008 Dec;2(4):333–8. doi: 10.1007/s12105-008-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eversole LR. Odontogenic fibroma, including amyloid and ossifying variants. Head Neck Pathol. 2011 Dec;5(4):335–43. doi: 10.1007/s12105-011-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younis RH, Scheper MA, Lindquist C, Levy B. Hybrid central odontogenic fibroma with giant cell granuloma-like component: case report and review of literature. Head Neck Pathol. 2008 Sep;2(3):222–6. doi: 10.1007/s12105-008-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijintanawan S, Chettri K, Aittiwarapoj A, Wongsirichat N. Hybrid central odontogenic fibroma with central giant cell granuloma like lesion; A case report and review of the literature. Siriraj Med J. 2019 Sep;71(5):426–31. [Google Scholar]
- 27.Bologna-Molina R, Pacheco Ruiz L, Mosqueda Taylor A, Huesca Ramirez HG, Ponce Lonato JA, González González R. Central odontogenic fibroma combined with central giant cell lesion of the mandible. Immunohistochemical profile. J Clin Exp Dent. 2011;3(4):e348–51. [Google Scholar]
- 28.Eliot C, Kessler HP. Clinical pathologic conference case 1: a multilocular radiolucency in the posterior mandible. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015 Jun;119(6):e289–92. doi: 10.1016/j.oooo.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Ramadan OR, Essawy MM. Central odontogenic fibroma with giant cell granuloma-like lesion: A report of an additional case and review of literature. Head Neck Pathol. 2021 Mar;15(1):275. doi: 10.1007/s12105-020-01153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]