Abstract
Objectives: This study aimed to compare the colonization of Enterococcus faecalis (E. faecalis), Escherichia coli (E. coli), Streptococcus mutans (S. mutans) and Staphylococcus aureus (S. aureus) isolated from the oral cavity on different suture materials used in oral implantology.
Materials and Methods: Patients scheduled for implant surgery were included in this study. After flap approximation, the surgical site was sutured using silk, nylon, polyglactin 910 (Vicryl®) and triclosan-coated polyglactin 910 (Vicryl® Plus) sutures in a randomized order. Seven days after surgery, the sutures were removed and incubated in bile esculin agar (for E. faecalis), MacConkey agar (for E. coli), mitis salivarius agar (for S. mutans), and mannitol salt agar (for S. aureus) at 37°C for 24 h. The colonies were then counted. Data were analyzed using the Kruskal-Wallis and Mann-Whitney U tests.
Results: Vicryl® sutures showed the highest accumulation of E. faecalis, followed by Vicryl® Plus, nylon, and silk. There was no significant difference between nylon and silk (P=0.5) or between Vicryl® and Vicryl® Plus (P=0.4). Vicryl® Plus sutures showed the highest accumulation of E. coli followed by Vicryl®, silk and nylon (P<0.01). Vicryl® sutures showed the highest accumulation of S. mutans, followed by Vicryl® Plus, silk, and nylon. Vicryl® Plus sutures showed the highest accumulation of S. aureus, followed by Vicryl®, nylon, and silk.
Conclusion: Nylon sutures showed the least microbial accumulation. Vicryl® and triclosan-coated Vicryl® Plus sutures had no advantage over the commonly used silk sutures in decreasing the number of bacteria.
Key Words: Sutures, Silk, Nylons, Polyglactin 910, Bacteria, Escherichia coli, Enterococcus faecalis, Staphylococcus, Streptococcus, Dental Implants
Introduction
Delayed surgical wound healing is a major concern for patients and dental clinicians. Wound infection and dehiscence are two common postsurgical complications that prolong the course of wound healing and increase the treatment costs [1,2]. In dentoalveolar surgery, wound closure is achieved by the use of suture threads. However, suture threads are potential risk factors for impairment of wound healing since they can enhance bacterial adhesion and colonization, and development of infection at the surgical site [3,4] . Thus, many studies have evaluated strategies to prevent bacterial colonization on suture threads in the first place. Suture threads coated with antibacterial agents were introduced to achieve this goal [5, 6] . Peri-implantitis can occur due to the activity of aerobic and anaerobic bacteria. Fusobacteria, Peptostreptococcus species, Prevotella intermedia, Actinomyces species, Capnocytophaga, Enterococci, Streptococci, and Staphylococcus aureus (S. aureus) are among the commonly isolated bacteria from peri-implantitis and postoperative infections [7-10] . Recently, enteric and non-oral bacteria were detected in the peri-implant environment of diseased implants, amongst which Entero-coccus faecalis (E. faecalis) and Escherichia coli (E. coli) were more significant [11]. Also, Streptococcal species including Streptococcus mutans (S. mutans), Streptococcus mitis, Streptococcus sanguinis, and Streptococcus oralis are amongst the primary periodontal colonizers which have been demonstrated to colonize titanium surfaces and provide new receptors for putative periodontal and peri-implant pathogens [12,13].
Evidence shows that the risk of infection depends on bacterial adhesion and physical and chemical properties of suture materials [14,15]. Multifilament suture materials have a higher tendency to attract bacteria than monofilament suture materials due to the capillary effect and higher porosities of the former sutures [16]. Silk braided sutures are non-absorbable multifilament sutures made of organic threads. Due to easy use and low cost, they are commonly used in oral surgical procedures [17,18]. Vicryl sutures are synthetic, multifilament absorbable sutures made of Polyglactin 910. Vicryl Plus is a recently introduced Vicryl suture with triclosan coating [19]. Triclosan is an antiseptic material with confirmed antibacterial effects on Gram-positive and Gram-negative bacteria [20-22]. Nylon sutures are synthetic monofilament sutures with no reported bacterial contam-ination [23].
Studies on different suture materials and comparing their effects on colonization of both Gram-positive and Gram-negative bacteria are lacking. Considering the known complications of delayed wound healing and the existing controversy regarding an ideal suture material, this study aimed to assess bacterial colonization on four types of suture materials used in implant surgery.
MATERIALS AND METHODS
Ethical approval:
This study complied with the principles stated in the Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964, and approved by the Faculty of Dentistry, Islamic Azad University of Tehran (IR.IAU.DENTAL.REC.1397,21) and registered in the Iranian Registry of Clinical Trials with regis-tration numbers IRCT20180617040117N1 and IRCT20180617040119N2.
Study population :
A single-blind, randomized clinical trial was designed. The study sample was drawn from the population of patients presenting to the Implant Research Center of Dental Faculty of Islamic Azad University of Tehran between September 22, 2017 and July 21, 2018; 160 specimens from 20 patients were evaluated. The patients had been scheduled to receive two or three adjacent dental implants on each side of the jaw and signed informed consent forms prior to participation in the study. The inclusion criteria consisted of severely decayed, non-restorable or fractured teeth scheduled for extraction. The exclusion criteria were history of periodontal disease, signs of periodontal destruction, any systemic condition affecting the wound healing or osseointegration, and intake of medications that could interfere with uneventful healing. Patients who required hard or soft tissue augmentation were also excluded.
Patients who required bone augmentation due to thread exposure during implant placement were also excluded from the study. The sample size was calculated to be a minimum of 10 [5] and a maximum of 20 [6] in each group considering α=0.05, and β=0.2, using one-way ANOVA power analysis feature of PASS 11. Thus, 20 samples of each suture material were used for each type of microorganism.
Surgical procedure and laboratory tests:
Each surgical site (including the space required for placement of two adjacent dental implants measuring 18mm) was randomly divided into four sections. One type of suture was used in each section. Prior to surgery, patients were evaluated for any sign of infection (periodontal and non-periodontal) in the oral cavity and patients with no infection and inflammation underwent surgery. Patients rinsed their mouth with 0.2% chlorhexidine mouthwash before dental implant placement and were instructed to continue using it twice daily until suture removal. A total of 160 samples of the four suture materials and four types of microorganisms were evaluated. Vicryl Plus®, Vicryl®, silk, and nylon sutures (all suture materials were produced by Ethicon Inc., Johnson & Johnson Company, Somerville, NJ, USA) were used with 3 mm distance from each other. Allocation of suture material to the site was random and selected by a different individual other than the surgeon using a computer-generated randomization list such that two nylon sutures were applied on both sides of the Vicryl Plus® suture. These nylon sutures were not included in the study and were only applied to prevent the effect of triclosan coating of Vicryl Plus® suture on bacterial accumulation on other suture threads. Thus, each Vicryl Plus® suture had 6 mm distance from other sutures (Fig. 1). For postsurgical care, patients were instructed to use an icepack in the first 24 h after the surgery and due to the simplicity of the surgery and use of a delicate infection control protocol during implant placement, no antibiotics were prescribed for any of the participants. The sutures were removed one week after surgery and placed in brain heart infusion broth (Himedia, India) to allow the proliferation of all aerobic bacteria.
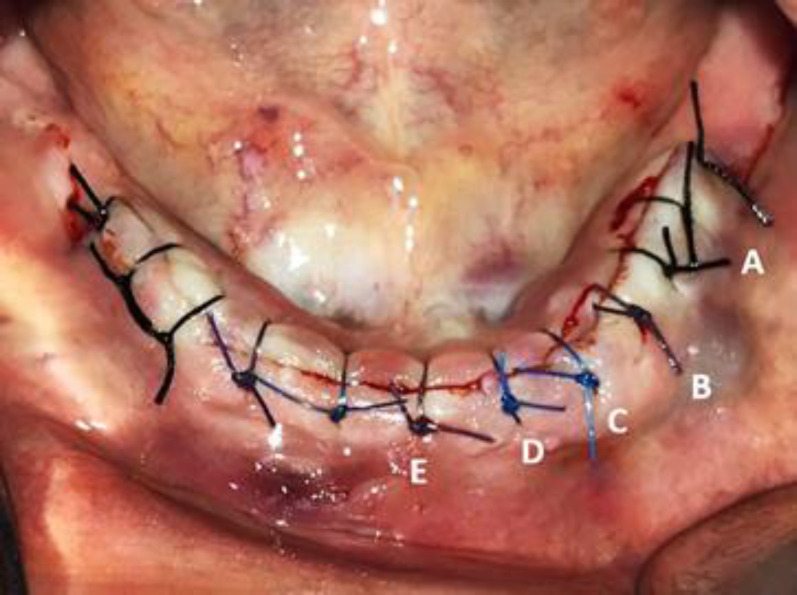
Fig. 1.
Intraoral photograph of a patient scheduled for implant surgery. Two dental implants were inserted in each side of the mandible. Wound approximation was achieved with (A) black 4-0 silk, (B) 4-0 Vicryl®, (C) 4-0 Nylon, and (E) 4-0 Vicryl Plus® sutures. Additional nylon sutures were used to prevent the Vicryl Plus® suture from affecting other suture materials (D). The left mandible was sutured with black 4-0 silk (not included in the study)
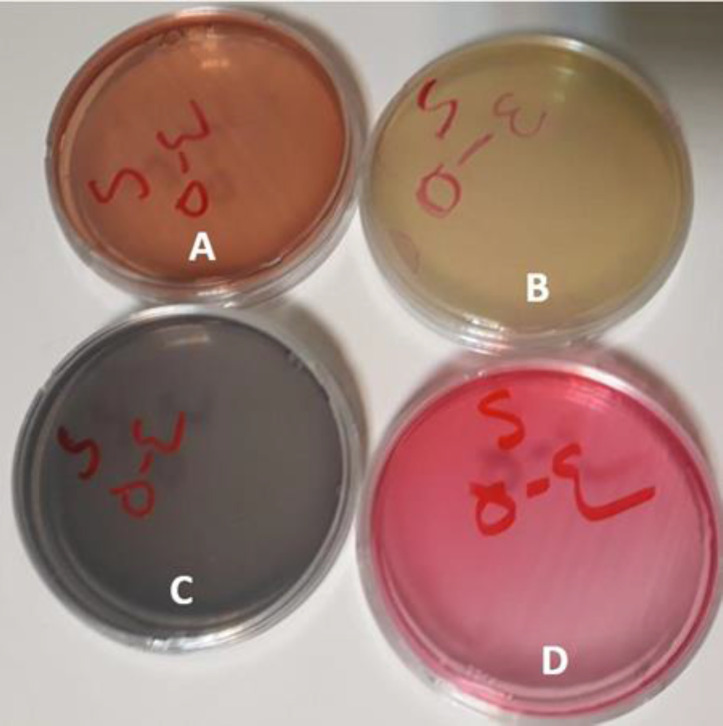
They were then transferred to a laboratory within 12 h [24]. Next, 1ml of each suspension was transferred to the culture media namely bile esculin agar for the culture of E. faecalis, MacConkey agar for the culture of E. coli, Mitis salivarius agar for the culture of S. mutans, and mannitol salt agar for the culture of S. aureus (all from Himedia, India) (Fig. 2).
Fig. 2.
Different bacterial culture media used in the study: (A) MacConkey agar for Escherichia coli, (B) esculin agar for Enterococcus faecalis, (C) Mitis salivarius agar for Streptococcus mutans, and mannitol salt agar for Staphylococcus aureus culturing.
After culture, the plates were incubated at 37°C for 24 h to allow bacterial proliferation. The number of colony forming units (CFUs) was then counted. The colleagues responsible for performing the laboratory tests were not aware of the type of suture materials used (single-blind design). The mean and standard deviation of colony counts of all four tested microorganisms were calculated and analyzed using the Kruskal-Wallis test. Pairwise comparisons were performed by the Mann-Whitney U test with Bonferroni correction. Level of significance was set at 0.05.
Results
Table 1 shows the count of microorganisms on the four suture materials. Regarding the count of E. faecalis, maximum accumulation was noted on Vicryl® sutures followed by Vicryl Plus®, nylon, and silk. According to the Kruskal-Wallis test, the difference between the four groups regarding E. faecalis count was significant (P<0.05). The Mann Whitney test showed no significant difference between the silk and nylon sutures (P=0.5). The difference between the Vicryl® and Vicryl Plus® sutures in this respect was not significant either (P=0.4).
Table 1.
Comparison of microbial count (CFUs/ml) on different types of suture threads
| Bacterial count (CFUs/ml) ×103 | |||||
|---|---|---|---|---|---|
| Thread type | E. faecalis | E. coli | S. mutans | S. aureus | |
| Silk | ≈ 4 | ≈ 9.1 | ≈ 7.2 | ≈ 0.1 | |
| Nylon | ≈ 4 | ≈ 6.3 | ≈ 4.7 | ≈ 1.5 | |
| Vicryl ® | ≈ 10 | ≈ 9.1 | ≈ 11.39 | ≈ 4 | |
| Vicryl Plus ® | ≈ 9.1 | ≈ 21.2 | ≈ 8.03 | ≈ 4.57 | |
| P-value | 0.06 | 0.01 | 0.2 | 0.6 | |
Regarding the count of E. coli, maximum accumulation was noted on Vicryl Plus® followed by Vicryl®, silk, and nylon. The Kruskal-Wallis test showed that the difference in this regard was significant among the four groups (P=0.01). The Mann-Whitney U test showed a significant difference in this respect between the Vicryl Plus® and other sutures (P=0.01). The difference between the silk and Vicryl Plus® sutures was not significant in this regard (P=0.8). Nylon sutures had significant differences with all other sutures in this respect (P=0.01).
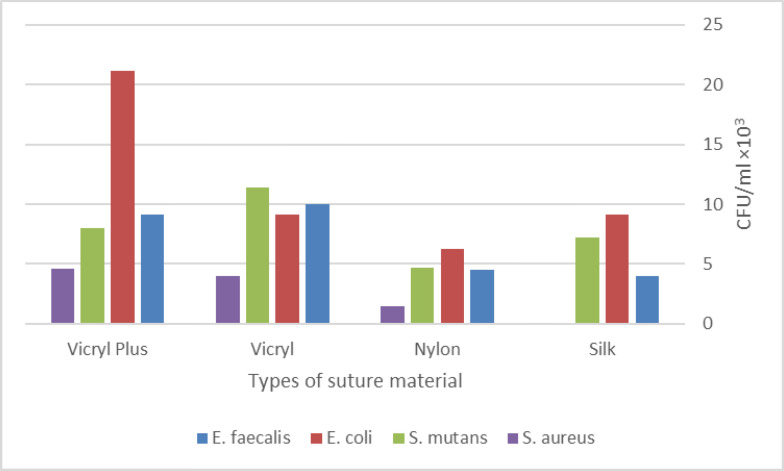
With regards to the count of S. mutans, maximum accumulation was noted on Vicryl® sutures followed by Vicryl Plus®, silk, and nylon. Adhesion of bacteria to Vicryl® sutures was over 2.4 times the rate of their adhesion to nylon; however, the Kruskal-Wallis test showed no significant difference in adhesion of microorganisms (P=0.2). Regarding the count of S. aureus, maximum accumulation was noted on Vicryl Plus® sutures followed by Vicryl®, nylon, and silk. The adhesion of bacteria to Vicryl Plus® was around 4 times their adhesion rate to nylon; the Kruskal-Wallis test showed no significant difference in adhesion of microorganisms (P=0.6; Fig. 3).
Fig. 3.
Comparison of microbial count (CFUs/ml ×103) among different types of suture threads
Discussion
This study compared the colonization of E. faecalis, E. coli, S. mutans, and S. aureus on different suture materials used in oral implantology. The results showed lower accumulation of E. faecalis on silk and nylon sutures. E. coli had the lowest accumulation on nylon sutures. Also, the lowest accumulation of S. mutans was noted on nylon sutures while the lowest accumulation of S. aureus was noted on silk and nylon sutures.
Asher et al. [24] evaluated the accumulation of aerobic and anaerobic bacteria on silk, nylon, Vicryl Plus® and polyester sutures in 50 patients who had undergone different types of oral surgical procedures such as implant surgery and flap surgery. All four types of sutures were applied in the oral cavity of all patients. The results showed equal accumulation of bacteria on silk, Vicryl Plus®, and polyester sutures; however, nylon suture showed significantly lower accumulation of aerobic and anaerobic bacteria.
Sala-Perez et al, [25] in their split-mouth study evaluated patients who underwent bilateral surgical extraction of maxillary third molars. They used silk sutures at one side and Monocryl Plus® sutures with triclosan coating on the other side. The sutures were removed after 3 and 7 days. They showed significantly lower accumulation of aerobic and anaerobic bacteria on Monocryl Plus® sutures compared with silk sutures on day 3; however, this difference was no longer significant on day 7. Thus, it was concluded that the antibacterial properties of triclosan lasted for up to 3 days postoper-atively, and significantly decreased thereafter.
Pelz et al, [26] in a similar study compared Vicryl® and Vicryl Plus® sutures and observed equal accumulation of aerobic and anaerobic bacteria on both suture types one week after surgery. Venema et al. [20] exposed Vicryl® and Vicryl Plus® sutures to human saliva for 4 h. They divided the samples into two groups. One group served as the control group and did not undergo any intervention. In the intervention group, the sutures were immersed in 0.12% chlorhexidine for 30 s. Bacteria were cultured on plates. The difference between the intervention and control groups was not significant irrespective of suture type. However, a significant difference was noted in the accumulation of bacteria on the same suture type between the control and chlorhexidine groups.
They concluded that the antibacterial efficacy of continuous use of chlorhexidine was higher than that of triclosan coating of Vicryl Plus® sutures. Masini et al. [27] immersed Prolene (polypropylene), Monocryl® (polyglycaprone), silk, Vicryl® and Vicryl Plus® sutures directly in a standard suspension of S. aureus for 12 h and then rinsed them with saline. Bacterial accumulation was inspected under an electron microscope. They found that bacterial accumulation on Vicryl Plus® was significantly higher than that on other suture types. No significant difference was noted in microbial accumulation on other suture types. Edmiston et al. [28] immersed Vicryl® and Vicryl Plus® sutures in a standard suspension of S. aureus, Staphylococcus epidermidis and E. coli for 24, 48, 72, and 96 h. They were rinsed, and bacterial contamination was evaluated under an electron microscope. The results showed that the accumulation of all three bacterial types on Vicryl Plus® sutures was significantly lower than that on Vicryl® suture. They concluded that triclosan maintains its antibacterial effect for a minimum of 96 h after placement in the oral cavity [28]. It seems that the triclosan coating of Vicryl Plus® and Monocryl Plus® maintains its antibacterial properties maximally for up to 3 days after surgery [5]. Thus, since in oral surgery the sutures need to remain in the oral cavity for a minimum of one week, these sutures have no advantage over other suture materials [29].
Also, it seems that multifilament sutures absorb a higher number of microorganisms than monofilament sutures. Nylon sutures, which are synthetic monofilament sutures, have the lowest accumulation of bacteria followed by silk sutures, which have a relatively lower accumulation of micro-organisms compared with Vicryl® and Vicryl Plus®. Thus, Vicryl® and Vicryl Plus® sutures have no superiority to silk sutures, which is the most commonly used suture type in oral surgery in terms of reduction of bacterial accumulation.
This study had several strengths. Twenty patients were evaluated in each group and microorganisms were isolated from the oral cavity of patients, which is an advantage since previous studies used standard strains of bacteria [20, 27, 28] . Also, the most commonly used suture materials in implant surgery were evaluated in this study. The main limitation of this study was the high standard deviation values. However, it should be noted that a wide variability exists in the accumulation of microorganisms, and the use of chlorhexidine can affect bacterial accumulation on sutures.
Despite the advantages of nylon sutures in decreasing the accumulation of micro-organisms, studies on nylon sutures are limited. Thus, further studies are warranted in this respect. Also, future studies are recommended to focus on nylon sutures and daily use of chlorhexidine on the accumulation of oral microorganisms especially anaerobic bacteria and periopathogenic microorganisms.
CONCLUSION
Nylon sutures showed the lowest microbial accumulation compared with other suture materials. Vicryl® and triclosan-coated Vicryl Plus® sutures had no advantage over the commonly used silk sutures in decreasing the bacterial count.
ACKNOWLEDGMENTS
The authors would like to thank Parto Abzar Pasargard Company for providing us with the surgical sutures. There is no conflict of interests related to this study.
Notes:
Cite this article as: Nadafpour N, Montazeri M, Moradi M, Ahmadzadeh S, Etemadi A. Bacterial Colonization on Different Suture Materials Used in Oral Implantology: A Randomized Clinical Trial. Front Dent. 2021:18:25.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1.Wikesjo UM, Nilveus R. Periodontal repair in dogs: effect of wound stabilization on healing. J Periodontol. 1990 Dec;61(12):719–24. doi: 10.1902/jop.1990.61.12.719. [DOI] [PubMed] [Google Scholar]
- 2.Jager DHJ, Maarse F, Klausch T, Karagozoglu KH, Ten Bruggenkate CM, Sandor GK, et al. Wound dehiscences following pre-implant bone augmentation with autogenous iliac crest bone grafts: A retrospective cohort study. Int J Oral Implantol (Berl) 2019 Jan;12(2):227–36. [PubMed] [Google Scholar]
- 3.Otten JE, Wiedmann-Al-Ahmad M, Jahnke H, Pelz K. Bacterial colonization on different suture materials--a potential risk for intraoral dentoalveolar surgery. J Biomed Mater Res B Appl Biomater. 2005 Jul;74(1):627–35. doi: 10.1002/jbm.b.30250. [DOI] [PubMed] [Google Scholar]
- 4.Koyuncuoglu CZ, Yaman D, Kasnak G, Demirel K. Preference of suture specifications in a selected periodontal and implant surgeries in Turkey. Eur J Dent. 2019 Feb;13(1):108. doi: 10.1055/s-0039-1688732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabrizi R, Mohajerani H, Bozorgmehr F. Polyglactin 910 suture compared with polyglactin 910 coated with triclosan in dental implant surgery: randomized clinical trial. Int J Oral Maxillofac Surg. 2019 Oct;48(10):1367–71. doi: 10.1016/j.ijom.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Dhom J, Bloes DA, Peschel A, Hofmann UK. Bacterial adhesion to suture material in a contaminated wound model: Comparison of monofilament, braided, and barbed sutures. J Orthop Res. 2017 Apr;35(4):925–33. doi: 10.1002/jor.23305. [DOI] [PubMed] [Google Scholar]
- 7.Lafaurie GI, Sabogal MA, Castillo DM, Rincon MV, Gomez LA, Lesmes YA, et al. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J Periodontol. 2017 Oct;88(10):1066–89. doi: 10.1902/jop.2017.170123. [DOI] [PubMed] [Google Scholar]
- 8.Rakic M, Grusovin MG, Canullo L. The microbiologic profile associated with peri-implantitis in humans: A systematic review. Int J Oral Maxillofac Implants. 2016 Mar;31(2):359–68. doi: 10.11607/jomi.4150. [DOI] [PubMed] [Google Scholar]
- 9.Albertini M, Lopez-Cerero L, O'Sullivan MG, Chereguini CF, Ballesta S, Rios V, et al. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin Oral Implants Res. 2015 Aug;26(8):937–41. doi: 10.1111/clr.12387. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang LF, Watt RM, Mattheos N, Si MS, Lai HC, Lang NP. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin Oral Implants Res. 2016 Jan;27(1):13–21. doi: 10.1111/clr.12508. [DOI] [PubMed] [Google Scholar]
- 11.Canullo L, Rossetti PH, Penarrocha D. Identification of Enterococcus faecalis and Pseudomonas aeruginosa on and in implants in individuals with peri-implant disease: A cross-sectional study. Int J Oral Maxillofac Implants. 2015 May;30(3):583–7. doi: 10.11607/jomi.3946. [DOI] [PubMed] [Google Scholar]
- 12.Narendrakumar K, Kulkarni M, Addison O, Mazare A, Junkar I, Schmuki P, et al. Adherence of oral streptococci to nanostructured titanium surfaces. Dent Mater. 2015 Dec;31(12):1460–8. doi: 10.1016/j.dental.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Meza-Siccha AS, Aguilar-Luis MA, Silva-Caso W, Mazulis F, Barragan-Salazar C, Del Valle-Mendoza J. In Vitro evaluation of bacterial adhesion and bacterial viability of Streptococcus mutans, Streptococcus sanguinis, and Porphyromonas gingivalis on the abutment surface of titanium and zirconium dental implants. Int J Dent. 2019 Jun;2019:4292976. doi: 10.1155/2019/4292976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermeier A, Schneider J, Harrasser N, Tubel J, Muhlhofer H, Pforringer D, et al. Viable adhered Staphylococcus aureus highly reduced on novel antimicrobial sutures using chlorhexidine and octenidine to avoid surgical site infection (SSI) PLoS One. 2018 Jan;13(1):e0190912. doi: 10.1371/journal.pone.0190912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Yang SB, Wang YG, Zhang SH, Yu ZF, Tang TT. Bacterial inhibition potential of quaternised chitosan-coated VICRYL absorbable suture: An in vitro and in vivo study. J Orthop Translat. 2017 Jan;8:49–61. doi: 10.1016/j.jot.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler JR, Perkins TA, Buttaro BA, Truant AL. Bacteria adhere less to barbed monofilament than braided sutures in a contaminated wound model. Clin Orthop Relat Res. 2013 Feb;471(2):665–71. doi: 10.1007/s11999-012-2593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt MT, Jenkins WS. Suturing principles for the dentoalveolar surgeon. Dent Clin North Am. 2012 Jan;56(1):281–303. doi: 10.1016/j.cden.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Balamurugan R, Mohamed M, Pandey V, Katikaneni HK, Kumar KR. Clinical and histological comparison of polyglycolic acid suture with black silk suture after minor oral surgical procedure. J Contemp Dent Pract. 2012 Jul;13(4):521–7. doi: 10.5005/jp-journals-10024-1179. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, Zhang C, Fang X, Luo X, Guo J. Biomaterial suture Vicryl Plus reduces wound-related complications. Ther Clin Risk Manag. 2018;14:1417–21. doi: 10.2147/TCRM.S164658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venema S, Abbas F, van de Belt-Gritter B, van der Mei HC, Busscher HJ, van Hoogmoed CG. In vitro oral biofilm formation on triclosan-coated sutures in the absence and presence of additional antiplaque treatment. J Oral Maxillofac Surg. 2011 Apr;69(4):980–5. doi: 10.1016/j.joms.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Alfhili MA, Lee MH. Triclosan: An update on biochemical and molecular mechanisms. Oxid Med Cell Longev. 2019 May;2019:1607304. doi: 10.1155/2019/1607304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wade WG, Addy M. Antibacterial activity of some triclosan-containing toothpastes and their ingredients. J Periodontol. 1992 Apr;63(4):280–2. doi: 10.1902/jop.1992.63.4.280. [DOI] [PubMed] [Google Scholar]
- 23.Heaven CJ, Davison CR, Cockcroft PM. Bacterial contamination of nylon corneal sutures. Eye. 1995 Jan;9(1):116–8. doi: 10.1038/eye.1995.18. [DOI] [PubMed] [Google Scholar]
- 24.Asher R, Chacartchi T, Tandlich M, Shapira L, Polak D. Microbial accumulation on different suture materials following oral surgery: a randomized controlled study. Clin Oral Investig. 2019 Feb;23(2):559–65. doi: 10.1007/s00784-018-2476-0. [DOI] [PubMed] [Google Scholar]
- 25.Sala-Perez S, Lopez-Ramirez M, Quinteros-Borgarello M, Valmaseda-Castellon E, Gay-Escoda C. Antibacterial suture vs silk for the surgical removal of impacted lower third molars. A randomized clinical study. Med Oral Patol Oral Cir Bucal. 2016 Jan;21(1):e95–102. doi: 10.4317/medoral.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelz K, Todtmann N, Otten JE. Comparison of antibacterial-coated and non-coated suture material in intraoral surgery by isolation of adherent bacteria. Ann Agric Environ Med. 2015;22(3):551–5. doi: 10.5604/12321966.1167733. [DOI] [PubMed] [Google Scholar]
- 27.Masini BD, Stinner DJ, Waterman SM, Wenke JC. Bacterial adherence to suture materials. J Surg Educ. 2011 Mar;68(2):101–4. doi: 10.1016/j.jsurg.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Edmiston CE, Seabrook GR, Goheen MP, Krepel CJ, Johnson CP, Lewis BD, et al. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination? J Am Coll Surg. 2006 Oct;203(4):481–9. doi: 10.1016/j.jamcollsurg.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson RE Jr, Schuler K, Thornton BP, Vasconez HC, Rinker B. The effect of saliva and oral intake on the tensile properties of sutures: an experimental study. Ann Plast Surg. 2007 Mar;58(3):268–72. doi: 10.1097/01.sap.0000245071.98517.8c. [DOI] [PubMed] [Google Scholar]