Abstract
Background:
Air pollution epidemiological studies usually rely on estimates of long-term exposure to air pollutants, which are difficult to ascertain. This problem is accentuated in settings where sources of personal exposure differ from those of ambient concentrations, including household air pollution environments where cooking is an important source.
Objective:
The objective of this study was to assess the feasibility of estimating usual exposure to PM2.5 based on short term measurements.
Methods:
We leveraged three types of short-term measurements from a cohort of mother-child pairs in 26 communities in rural Ghana: (A) personal exposure to PM2.5 in mothers and age four children, ambient PM2.5 concentrations (B) at the community level and (C) at a central site. Baseline models were linear mixed models with a random intercept for community or for participant. Lowest root-mean-square-error (RMSE) was used to select the best performing model.
Results:
We analyzed 240 community-days and 251 participant-days of PM2.5. Medians (IQR) of PM2.5 were 19.5 (36.5) μg/m3 for the central site, 28.7 (41.5) μg/m3 for the communities, 70.6 (56.9) μg/m3 for mothers, and 80.9 (74.1) μg/m3 for children. The ICCs (95% CI) for community ambient and personal exposure were 0.30 (0.17, 0.47) and 0.74 (0.65, 0.81) respectively. The sources of variability differed during the Harmattan season. Children’s daily exposure was best predicted by models that used community ambient compared to mother’s exposure as a predictor (log-scale RMSE: 0.165 vs 0.325).
Conclusion:
Our results support the feasibility of predicting usual personal exposure to PM2.5 using short-term measurements in settings where household air pollution is an important source of exposure. Our results also suggest that mother’s exposure may not be the best proxy for child’s exposure at age four.
Keywords: Personal exposure, PM2.5, LMICs, Harmattan
Introduction
Air pollution is a leading cause of morbidity and mortality worldwide. The Global Burden of Disease study estimates that approximately 4 million deaths are attributable to ambient air pollution (1). PM2.5, particulate matter less than 2.5 micrometers in diameter, is the most studied form of air pollution and is linked to a wide range of diseases in several organ systems. The strongest causal associations are seen between PM2.5 pollution and pulmonary and cardiovascular disease (2). Importantly, children are particularly vulnerable to the effects of air pollution because their lungs are still maturing (3). They also tend to be outside for longer periods and are usually more active when outdoors (4). Exposure to air pollution in infancy and early childhood causes lung damage, impairs lung growth, and can increase risk for pneumonia, asthma, and chronic pulmonary disease later in life (5–8). Recent evidence also suggests associations with neurodevelopmental outcomes, further underscoring the disproportionate vulnerability of children to fine particulate matter exposure (9,10).
Epidemiological analyses and data fusion approaches aimed at quantifying the health burden of air pollution typically rely on exposure estimates provided by central-site monitors or satellite-based models, which are rarely available in low- and middle-income countries (LMICs) (11,12). Exposures common in LMICs, such as solid fuel combustion for cooking and heating, result in large variability in individual-level exposures over time that ambient measures will likely miss (7). This complicates the estimation of true exposure (13–15). Further, seasonal phenomena such as the Harmattan season - a yearly trade wind that blows from the Sahara towards the Gulf of Guinea and carries with it a large amount of dust - are an additional significant driver of temporal variability in exposure (16).
As a result, air pollution epidemiologists frequently encounter the challenge of estimating health effects of long-term exposures that vary widely over time (17). Continuous measurements of personal exposures for all study subjects over the study period might be considered the “gold standard” for exposure assessment, but is rarely feasible (18). Short-term surrogate measures, which are imprecise estimates of longer-term average exposure, are typically used, leading to potentially biased exposure-response relations (19). In addition, in LMICs, when children’s exposure has been of interest, the use of their mother’s exposure as a proxy has been considered. Two factors underlie this approach. First, measuring children’s personal PM2.5 exposure used to be particularly difficult because previous generations of PM2.5 monitors were bulky and heavy, making it difficult for a small child to carry a monitor for many hours (20). This explains, in part, why direct measurements of personal exposure to air pollution among children in settings with prevalent biomass combustion are scarce (21). Second, it has been suggested that children often spend most of their time in close proximity to their mothers. This widespread reliance on surrogates of long-term exposure suggests that alternative approaches could prove beneficial for air pollution epidemiology studies, especially in LMICs.
Given these limitations, we leveraged personal and community exposure measurements from a well-characterized longitudinal cohort to estimate long-term exposure to PM2.5. Specifically, we concurrently measured: 1) personal exposure to PM2.5 in mother-child pairs, 2) ambient PM2.5 concentration at the community level, and 3) ambient PM2.5 concentration at a central site. Using these measurements, we assessed sources of variability in community ambient concentration of and personal exposure to PM2.5, and developed prediction models to estimate long-term exposure to be used in epidemiological studies of the health impacts of PM2.5.
Methods
Study population
We used data collected through the Child Lung Function study, an extension of the Ghana Randomized Air Pollution and Health Study (GRAPHS). GRAPHS is a cluster-randomized cookstove intervention trial in rural Ghana that has been described elsewhere (22). Briefly, between August 2013 and March 2016, a total of 1,414 nonsmoking, pregnant women were recruited from communities in the Kintampo North Municipality and Kintampo South District of Ghana and were followed through the first year of life of their children. In 2017, additional funding was obtained to continue longitudinal follow-up of N = 700 mother-child pairs with continued exposure and lung phenotyping assessments (Child Lung Function Study). The GRAPHS study objectives dictated 48-hr PM2.5 exposure assessment in mothers after birth, which was expanded to include their age four children as part of the Child Lung Function Study. Thus, mothers could have undergone exposure assessment twice whereas children only participated in one exposure assessment session. In the current analyses, we included participants who are enrolled in the Child Lung Function Study, underwent personal exposure assessment at the time of community exposure assessment, and who had at least two, valid 24-hr personal exposure monitoring sessions. Therefore, the analyses in this manuscript include 121 Child Lung Function Study mothers and children (up to age four) living across 26 communities whose personal exposure to PM2.5 was ascertained between October 2016 and April 2019. Table S1 illustrates the impact of exclusion decisions on sample size in greater detail (Table S1). Procedures were approved by human studies and institutional ethics committees at Kintampo Health Research Centre (KHRC), the Icahn School of Medicine at Mount Sinai, and the Columbia University Mailman School of Public Health, and written consent was obtained from all mothers.
Study context
The communities included in our study were located in the Bono East Region of Ghana, which is formerly known as the Brong-Ahafo Region (Figure 1). A pilot study in the GRAPHS study population demonstrated that 99% of households cooked with biomass (wood or charcoal) as their primary cooking fuel (23). Further, a recent nationally representative survey indicated that, in 2017, 91% of rural households and 73% of all households relied on biomass fuels for cooking. This region of Ghana experiences both a tropical wet and dry savanna climate. In particular, the Harmattan season (December to March) is characterized by episodes of dry and dusty winds blowing from the Sahara Desert. Annual bushfires are common during the Harmattan season (24). After data cleaning (Table S2), 29 out of 36 communities were included in the final ambient monitoring sample.
Figure 1. Map of study area.
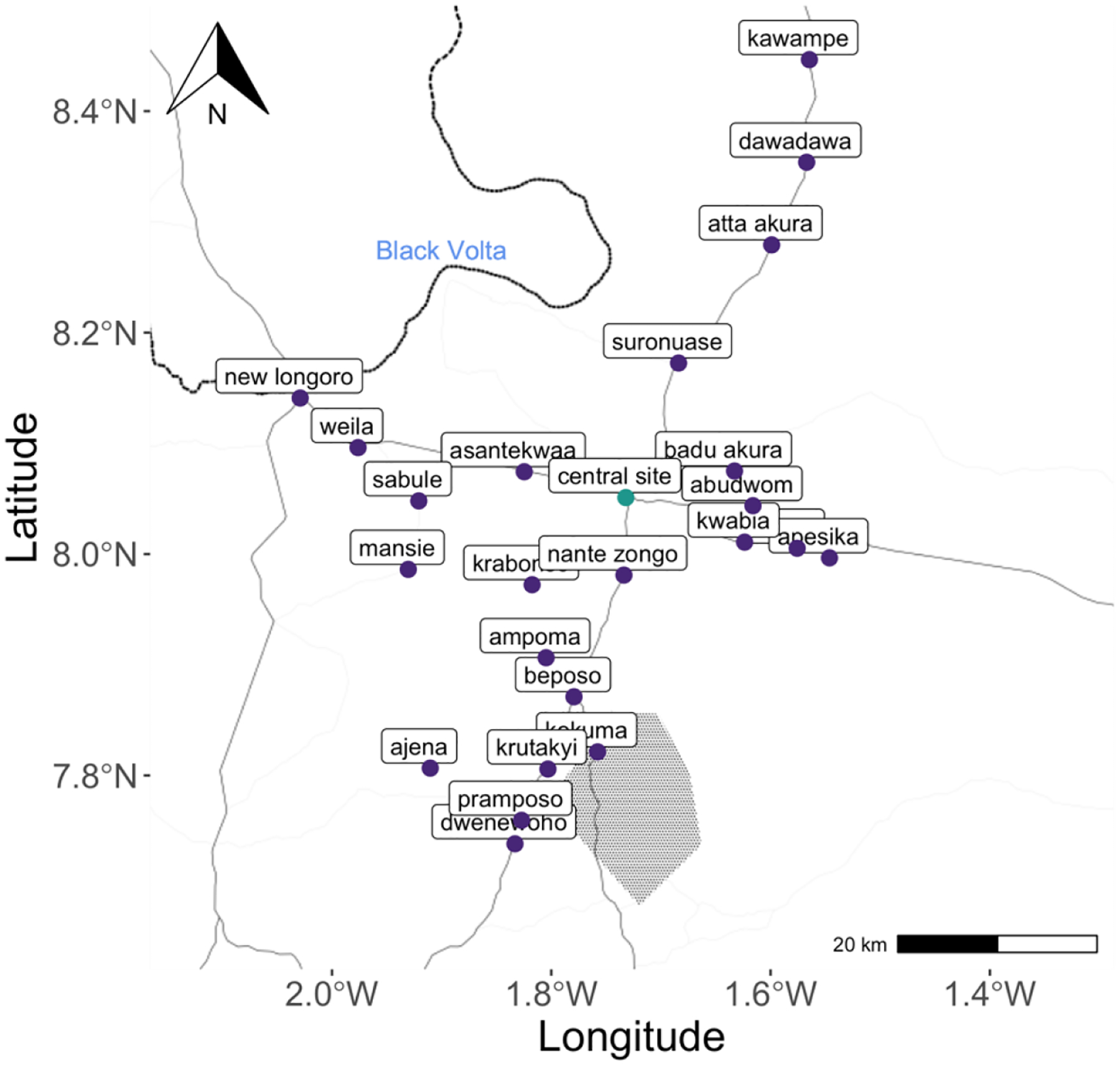
The light grey lines represent main roads. The dark black line is the Black Volta River. The shaded area is a monkey sanctuary.
Personal exposure assessment
Personal exposure was measured via the MicroPEM v3.2b, a small aerosol exposure monitor that measures PM2.5 in both real time and on a filter. Specifically, the MicroPEM includes a nephelometer for real-time monitoring at 5 second resolution, a Teflon filter for analysis of integrated concentrations at a 0.5 LPM flow rate over a 48-hour sampling period, and an accelerometer for assessing wearing compliance of subjects. As previously done as part of GRAPHS, each deployment was calibrated by a gravimetric correction factor (field blank adjusted filter mass to nephelometer average) (25). To do so, Teflon filters were pre- and post-weighed on a microbalance after equilibration in an environmentally controlled glovebox, following established protocols at Columbia University (25). Filters were installed in and removed from the MicroPEM in a clean air hood at the KHRC laboratory. During the first and last 5-min periods of each deployment, a low back pressure HEPA filter was attached to the MicroPEM to aid in correction of the nephelometer baseline drift. A constant baseline drift was assumed, leading to a linear interpolation between the pre- and post-deployment HEPA readings. Negative values and values less than 0.5 μg/m3 were replaced by 0.5 μg/m3 for statistical analysis. Further details on exposure validation procedure have been described elsewhere (25). After cleaning and adjustment by gravimetric correction of the continuous data, daily averages were isolated by splitting each 48-hr session into two observations: the first 24 hours were averaged to obtain the first daily average whereas the second daily average was computed by averaging the next 20–24 hours to allow inclusion of runs that had been cut short due to battery issues or field pick up schedules. If the duration of the second session did not meet the 20 hours threshold, that session was excluded from further analysis and modeling.
Ambient air pollution
Central site monitoring:
Continuous monitoring of PM2.5 was implemented from September 2016 to July 2019 at Kintampo Health Research Center, which is the study’s central site. Throughout the study period, an ambient continuous (nephelometer) and integrated (filter) air sampler (E-Sampler by Met-One) measured PM2.5 ambient concentrations at the central site. Specifically, an E-Sampler was set at a flow rate of 2.0 LPM to collect PM2.5 data every 10 minutes. A 47-mm Teflon filter sample was collected every month for gravimetric correction. Data points with flow > 2.2 or < 1.9 LPM and showing alarm statuses with typically zero PM2.5 values were discarded for further analysis. In parallel, a meteorological station (Onset HOBO RX3000), located next to the central site E-sampler, provided continuous data on meteorological variables including wind speed and direction, temperature, and relative humidity.
Community ambient monitoring:
Similarly to central site monitoring, PM2.5 ambient concentrations in study communities were assessed with the Met-One E-Sampler. Due to limited equipment availability, E-Samplers were rotated between communities with each event spanning two to ten days. In each community, the E-Sampler location was determined by identifying central coordinates relative to the study participants’ addresses. Figure S1 illustrates the overlapping PM2.5 measurements.
Covariates
Information on mother’s age, education level, secondhand smoke exposure, defined as a smoking household member, and primary cooking fuel was collected through questionnaires at baseline (22). Following methodology adapted from Gunnsteinsson et al (26), questionnaires determined household assets that were enumerated as counts when possible (eg, number of livestock, number of electronic devices) and were used to determine the wealth index, a household-level socioeconomic status measure relative to other households in the study. Child sex and date of delivery were recorded at birth. To potentially capture air pollution emissions coming from neighboring households, we estimated population density by calculating the number of compounds located and individuals living within a 50 m radius of each study household using local census data (27).
Statistical analysis
Variance component of community ambient and personal exposure:
A linear mixed model with only a random intercept per community - referred to as community baseline model – was used to partition community ambient PM2.5 variability into within community and between community variability components. Similarly, a linear mixed model with a random intercept per participant (participant baseline model) was used to decompose personal exposure variability into within-participant and between-participant variability components. Using the variance components from a one-way ANOVA, intra-class correlation coefficients (ICC) were computed to determine the proportion of total variability attributable to between-community (or between-participant) variability (28). Subgroups analyses were performed by 1) stratifying the results by season and 2) stratifying the results between mothers and children.
Prediction models of community ambient and personal exposure:
For modeling purposes, all PM2.5 measurements were log-transformed to ensure normal distribution of residuals. Our modeling strategy relied on a two-step approach (Figure S2). In Step 1, we developed linear mixed models that could predict 24-hr community ambient PM2.5 concentration based on central site ambient concentration. In Step 2, we developed linear mixed models that could predict 24-hr personal exposure to PM2.5 based on community ambient concentration. This was done separately for mothers and children. In both Step 1 and Step 2, we started with the following base (intercept-only) models:
where ln(Yik) is the kth measurement of log-transformed PM2.5 concentration for community i (Step 1) or participant j (Step 2), bi (or bj) is the community (or participant) random effect, and εik (or εjk) is the remaining error.
Predictors of community ambient PM2.5 included factors that could affect pollutant transport such as meteorological variables. We included seasonality (Harmattan vs no Harmattan), wind speed, wind direction, temperature, and relative humidity. For personal exposure (Step 2) models, we evaluated participant characteristics, household characteristics and seasonality as predictors of mothers’ personal exposure. Fuel type, kitchen type, ventilation, and geographical location of the household have all been shown to be strong predictors of household level PM2.5 concentrations (29). Based on data available through baseline data collection surveys, we selected similar covariates that have the potential to influence individual exposure. These variables included wealth index (categorical variable), secondhand smoking status (dichotomous variable), primary cooking fuel type (categorical variable), primary cooking location (categorical variable) and neighboring density (continuous variable). For children’s models, we additionally compared mother’s exposure to community ambient concentration as potential predictors of personal exposure. Model parameters were estimated using restricted maximum likelihood. For each model, the proportion of variance explained by the fixed effects alone (marginal R2) and the proportion of variance explained by the fixed and random effects jointly (conditional R2) were calculated. All models were evaluated using leave-one-out cross validation and the lowest RMSE was used to select the best performing Step 1 and Step 2 models.
Analyses were conducted with R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). Models were implemented and evaluated using “lme4”, “modelr”, and “mgcv”.
Results
The mean (sd) age among mothers was 28.8 (7.01) years old (Table 1). Most of them (59.5 %) did not receive formal education. The primary cooking fuel most commonly used was wood (96.2%). Secondhand smoking in the household or compound was not a common occurrence (16.4%). On average, 54 people resided within a 50m radius of a given study household.
Table 1.
Descriptive characteristics of the participants and study households, N = 79
| Mean (SD) or N (%) | |
|---|---|
| Participant characteristics | |
| Mother’s age, Mean (SD) | 28.8 (7.0) |
| Child’s age, Mean (SD) | 4.13 (0.32) |
| Mother’s educational level, N (%) | |
| None | 47 (60) |
| Primary school | 12 (15) |
| Middle/Junior High School | 15 (19) |
| Technical/Commercial/Senior High School and above | 5 (6) |
| Wealth index, Mean (SD) | −0.13 (1.68) |
| Primary cooking fuel type, N (%) | |
| Wood | 76 (96) |
| Charcoal | 2 (3) |
| Other | 1 (1) |
| Primary cooking location, N (%) | |
| Totally open | 40 (51) |
| Roof only | 9 (11) |
| Veranda | 9 (11) |
| Partially enclosed (2–3 walls with roof) | 5 (6) |
| Fully enclosed | 16 (20) |
| Household characteristics | |
| Secondhand smoking, N (%) | 13 (16) |
| Count of compounds within a 50m radius, Mean (SD) | 7.4 (3.9) |
| Count of residents within a 50m radius, Mean (SD) | 54 (33) |
We analyzed 240 community-days and 251 participant-days of PM2.5. On average ambient daily concentrations of PM2.5 were lower than personal exposure to PM2.5 on the same day (Table 2). Medians (IQR) of PM2.5 were 19.5 (36.5) μg/m3 for the central site, 28.7 (41.5) μg/m3 for the communities versus 70.6 (56.9) μg/m3 for mothers, and 80.9 (74.1) μg/m3 for children. Wearing compliance was high among both groups (Figure S3). Further, PM2.5 ambient concentrations and personal exposure were higher during the Harmattan season than throughout the rest of the year. Seasonality appeared to have a greater influence on the distribution of community ambient concentrations than on that of personal exposure (Figure 2).
Table 2.
Summary of air pollution measurements, N = number of daily PM2.5 averages*
| N | Mean | SD | Median | Min | Max | IQR | |
|---|---|---|---|---|---|---|---|
| Central site – community observations | |||||||
| Central Site Daily Average PM2.5 (μg/m3) | 240 | 30.2 | 24.9 | 19.5 | 3.17 | 106 | 36.5 |
| Community Daily Average PM2.5 (μg/m3) | 240 | 43.5 | 36.2 | 28.7 | 4.72 | 173 | 41.5 |
| Central Site Temperature (°C) | 240 | 27.1 | 1.7 | 27.4 | 22.8 | 31.0 | 2.13 |
| Central Site Relative Humidity (%) | 240 | 67.9 | 18.7 | 74.1 | 14.6 | 95.5 | 22.7 |
| Central Site Wind Speed (m/s) | 240 | 0.226 | 0.117 | 0.203 | 0.0121 | 0.652 | 0.148 |
| Community – personal observations (mothers) | |||||||
| Community Daily Average PM2.5 (μg/m3) | 167 | 35.5 | 32.2 | 22.5 | 5.2 | 177 | 25.9 |
| Personal Daily Average PM2.5 (μg/m3) | 167 | 83 | 53.2 | 70.6 | 10.3 | 390 | 56.9 |
| Community – personal observations (children) | |||||||
| Community Daily Average PM2.5 (μg/m3) | 84 | 49.9 | 43.6 | 34.4 | 5.2 | 189 | 51.6 |
| Personal Daily Average PM2.5 (μg/m3) | 84 | 82.3 | 43.6 | 80.9 | 21.6 | 185 | 74.1 |
Measurements were matched based on the date and time they were taken.
Figure 2.
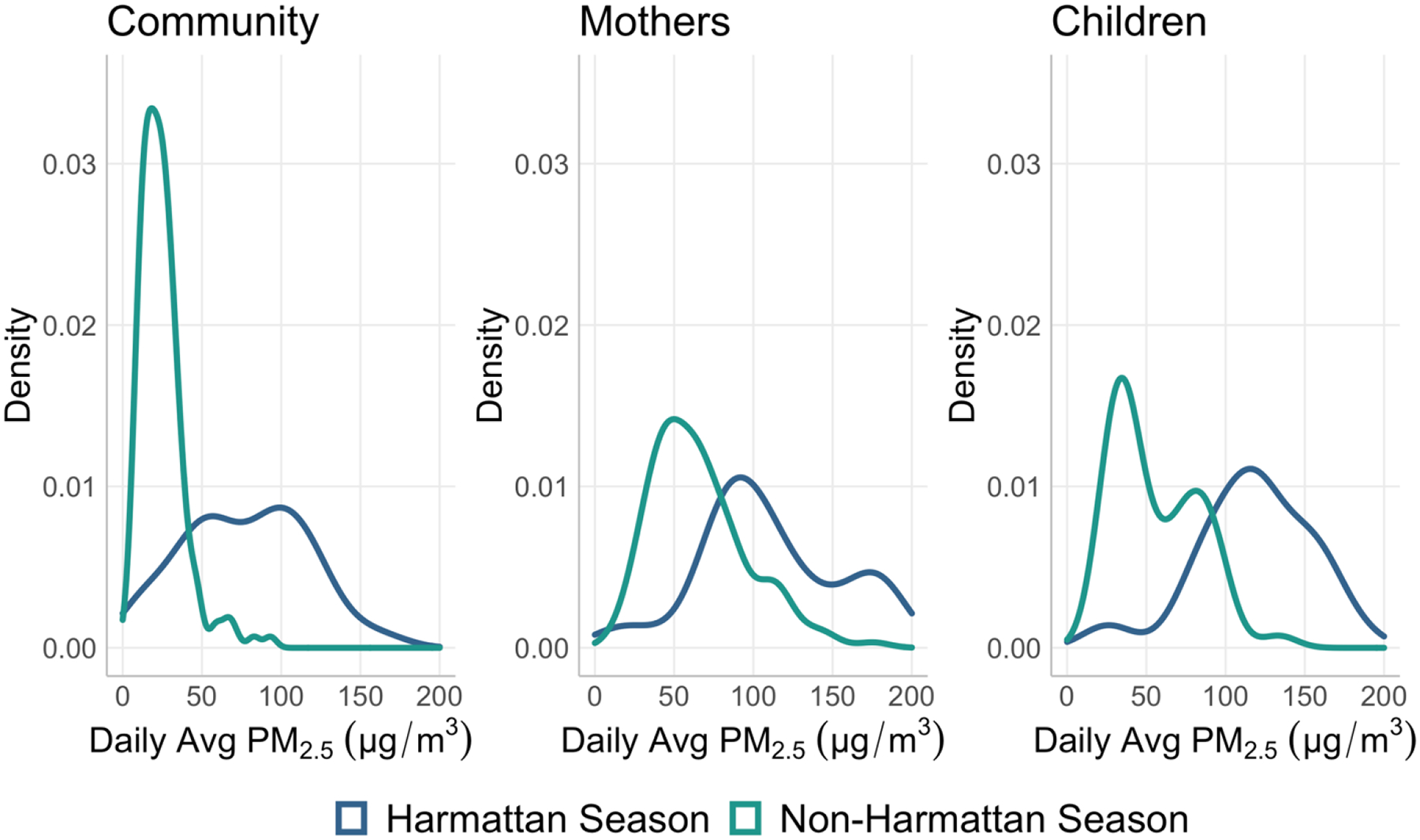
Probability density of measured daily PM2.5 ambient concentration and personal exposure.
The correlation between daily central site ambient PM2.5 and daily community ambient PM2.5 (r = 0.86) was stronger than that between daily community ambient PM2.5 and daily personal exposure to PM2.5 (Figure 3). In general, personal exposure to PM2.5 was higher than community ambient PM2.5 (Figure 3B).
Figure 3. Correlations between daily averages of (A) central site and community ambient PM2.5 concentrations and (B) community ambient PM2.5 concentration and personal exposure.
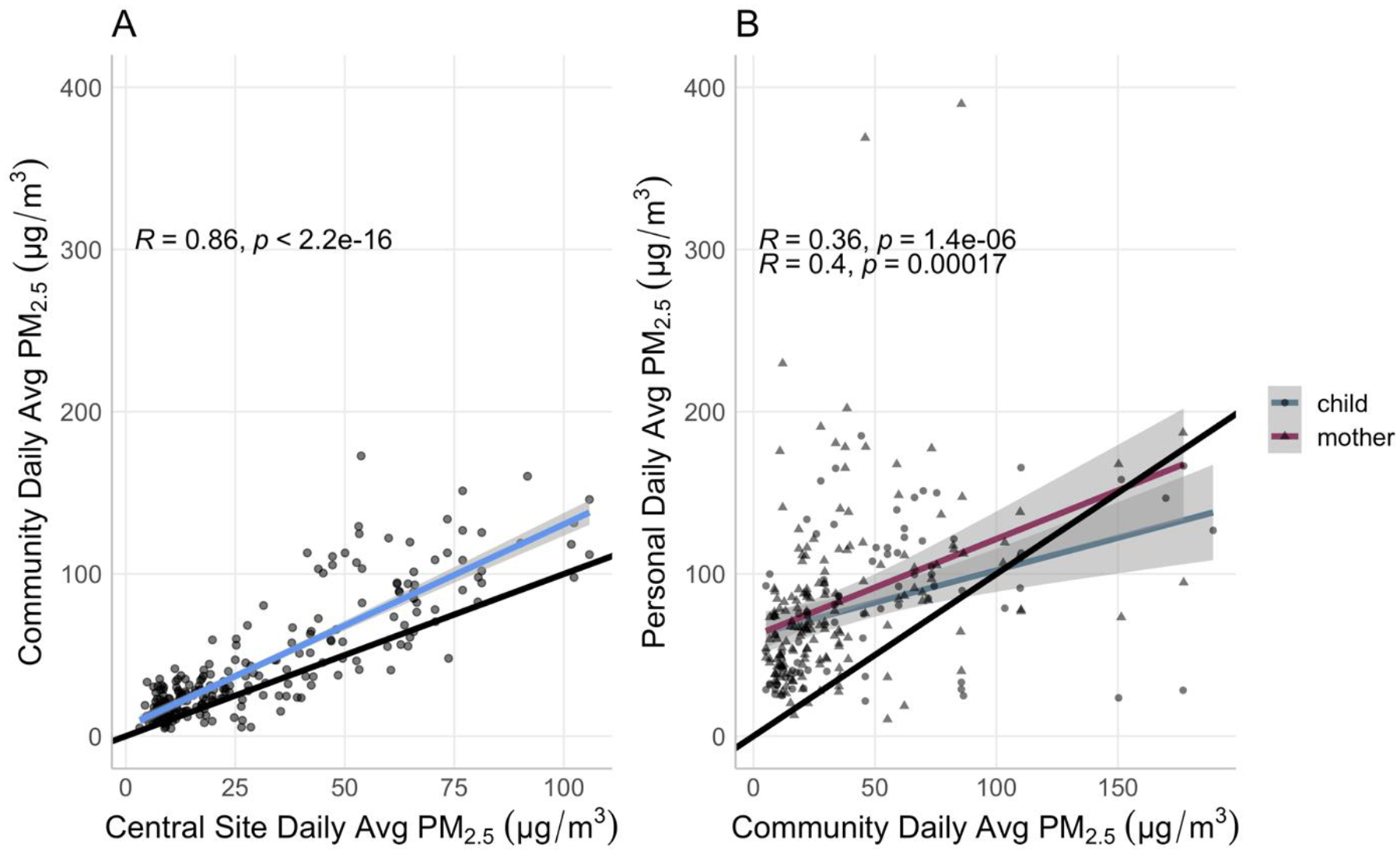
The black line represents the 1:1 ratio line. The colored lines represent the respective linear regression lines.
The ICCs (95% CI) for community ambient and personal exposure were 0.3 (0.17, 0.47) and 0.74 (0.65, 0.81) respectively (Table 3). Most of the ambient PM2.5 variability came from within community (i.e., temporal) whereas most of the variability in personal exposure to PM2.5 came from between individuals, which could be due to a mixture of behavioral, temporal and spatial variations. The partitioning of variability differed during the Harmattan season, especially for community ambient concentrations (Figure S4). It is important to note that, by design, community ambient monitoring was asynchronous between communities. As a result, temporal variability is also captured by the community level ICC. Similarly, the daily averages were always consecutive for children since they only participated in one round of exposure measurement. For the mothers, however, the ICC captures additional temporal variability due to the time elapsed between the two potential round of measurements that they could have partaken in. ICC results stratified between mothers and children are presented in Table S3.
Table 3.
Decomposition of variability in ambient and personal PM2.5 (non-simultaneous measurements)
| ICC* | LCI (5th perc.) | UCI (95th perc.) | ||
|---|---|---|---|---|
| Community ambient PM 2.5 | Overall | 0.3 | 0.17 | 0.47 |
| During Harmattan season | 0.64 | 0.44 | 0.82 | |
| During non-Harmattan season | 0.096 | −0.012 | 0.27 | |
| Personal PM 2.5 | Overall | 0.74 | 0.65 | 0.81 |
| During Harmattan season | 0.85 | 0.73 | 0.92 | |
| During non-Harmattan season | 0.69 | 0.56 | 0.79 |
ICC: intraclass correlation coefficient
We present results for the baseline model and the corresponding model that yielded the lowest RMSE for community ambient (Table 4), mothers (Table 5) and children (Table 5) separately. In community ambient models, windspeed and seasonality lowered the baseline model’s RMSE from 0.339 to 0.336 (Table 4). Mother’s daily exposure to PM2.5 was best predicted by models that included seasonality and primary cooking fuel type (Table 5). The lowest RMSE was obtained for children’s daily exposure, which was best predicted by models that used community ambient compared to mother’s exposure as the main predictor (log-scale RMSE: 0.165 vs 0.385). For both participant groups, the models with the lowest RMSEs also explained the highest proportion of variance; the conditional R2 values were 0.63 and 0.92 for mothers and children respectively.
Table 4.
Prediction models of community ambient PM2.5 (Step 1)
| Model | Regression equation | R2m | R2c | Log-scale RMSE |
|---|---|---|---|---|
| Baseline community model | 0.65 | 0.72 | 0.339 | |
| Baseline community model + seasonality + wind speed | 0.68 | 0.74 | 0.336 |
R2m represents the proportion of the variance explained by the fixed effects alone (marginal R2).
R2c represents the proportion of the variance explained by the fixed and random effects jointly (conditional R2).
Harmattan is a dry season characterized by dusty winds.
Table 5.
Prediction models of personal exposure to PM2.5 (Step 2)
| Model | Regression equation | R2m* | R2c** | Log-scale RMSE | |
|---|---|---|---|---|---|
| Mothers | Baseline model | 0.13 | 0.58 | 0.334 | |
| Baseline model + seasonality + primary cooking fuel | 0.20 | 0.63 | 0.325 | ||
| Children | Baseline model (with mother exposure as predictor) | 0.16 | 0.80 | 0.402 | |
| Baseline model (with mother exposure as predictor) + seasonality | 0.23 | 0.81 | 0.385 | ||
| Baseline model (with community ambient as predictor) + seasonality | 0.41 | 0.92 | 0.165 |
R2m represents the proportion of the variance explained by the fixed effects alone (marginal R2).
R2c represents the proportion of the variance explained by the fixed and random effects jointly (conditional R2).
Harmattan is a dry season characterized by dusty winds.
Discussion
We analyzed community level ambient and personal exposure to PM2.5 in a cohort of mother-child pairs in rural Ghana. We identified sources of variability in exposure that are specific to the region (ie, Harmattan season). We leveraged concurrent measurements of PM2.5 at the central site and at the community level as well as personal exposure to PM2.5 to develop predictions of long-term exposure. The models performed best for children exposure to PM2.5; the log RMSE for the best performing model was 0.165 and the proportion of variance explained was 0.92. Our results support the feasibility of using predicted long-term personal exposure for epidemiological studies in rural Ghana and similar contexts. The applicability of this approach to other exposure settings awaits assessment.
Few studies have attempted to predict long-term exposure to PM2.5 from short-term personal exposure measurements in LMICs. The findings of the only other study that, to our knowledge, also uses a combination of ambient concentrations and short-term personal exposure measurements to predict long-term exposure support ours. Using a cohort of the general population in peri-urban South India, Sanchez et al. developed long-term exposure models for men and women separately. Similarly to our results, the models that performed best included time invariant predictors such as primary stove type. The lowest log RMSEs were 0.43 and 0.53 for men and women respectively. The authors concluded that these models performed moderately well (30). Using a simulation approach allowing them to compare multiple study designs, Keller et al. found that the addition of a time adjustment (eg, temporal splines) to a linear mixed model yielded the best performing modeling approach to combine short-term measurements into predictions of long-term exposure (31). Notably, the improvement in model performance was greater for study designs, such as ours, in which baseline measurements do not occur simultaneously (31). These results suggest that future studies employing designs similar to ours should consider a time adjustment to increase their predictive ability.
Compared to other studies that evaluated variance components of exposure metrics in LMICs populations, our results showed higher between participant variation. In an analysis of repeated 48-hr personal exposure to CO measurements in Guatemala, McCracken et al. reported lower ICCs than we did; 33% in children and 29% in adult women (17). The authors pointed to this large within-participant variability to suggest that short-term personal exposure measurements present low reliability as a measure of long-term exposure. Similarly, in a study conducted in The Gambia, Dionisio et al. reported an ICC of 39% for personal exposure to CO for children, which decreased to 27% in the complete model (20). Further, studies from high-income countries have also reported between-participant variation in PM2.5 personal exposure lower than our results (32). In a study of the general adult population in Sweden, Johannesson et al. found that 84% of the total variance in personal exposure to PM2.5 was attributable to within-individual variability (32). The comparatively high between participant variance that we obtained could be explained by the fact that our study area included 26 different communities with potentially different microenvironments (Table S4).
This study provided a unique opportunity to characterize exposure among children, a subgroup that is particularly vulnerable to effects of air pollution exposure but whose exposure patterns -especially in rural settings - are scarcely documented (21). Previous studies have found an association between carriage of the child on the mother’s back and pneumonia in children, and suggested that this childcare behavior is associated with higher exposures (33,34). However, our results indicate that using community ambient PM2.5 - as opposed to mother’s exposure - as a predictor of children’s exposure at age four yields better model performance. This might suggest that, in this study population, and given that the children were already four years old, the time-activity patterns of children are distinct from those of their mothers. Further, because women are often the primary cooks, their personal exposures is driven by emissions during cooking events (7). In contrast, children’s exposure is more closely related to ambient levels given the share of time spent playing outside (35). Characterizing children’s exposure with greater precision and reliability should remain the focus of future studies as evidence of the association between solid fuel use and developmental outcomes continues to emerge (36).
This study had some limitations. The feasibility of our two-step modeling approach relies on having complete subsets with overlapping central site, community ambient, and personal exposure measurements of PM2.5. As highlighted in Tables S1 and S2, participants for whom long-term exposure could be predicted must have had their personal exposure measured concurrently with community ambient level. Further, the random intercept computation required that each participant had at least two daily exposure averages. Both requirements led to a significant decrease in our sample sizes (Table S5), limiting our ability to exposure for the entire Child Lung Function Study cohort of mother-child pairs.
To accurately estimate long-term exposure, the models’ predicting ability should have been assessed against longer exposure measurements. Given the limited data availability in this study, we approximated long-term exposure using a daily exposure average measured several months apart from the daily exposure average used as a predictor, which constitutes another limitation of this study. This problem is accentuated for children for whom measurements were consecutive. In addition, ideally the cross-validation would have been performed for a subset of participants for whom we had measured long-term exposure to PM2.5 – as opposed to using a holdout approach. Finally, since the community ambient measurements did not occur simultaneously for all communities, the proportion of variability in ambient concentrations explained by between community differences cannot be fully separated from temporal variability.
Conclusion
We found that the Harmattan season -characterized by episodes of dry and dusty winds blowing from the Sahara Desert- is a significant driver of personal exposure to PM2.5 for both mothers and children in rural Ghana. Our exposure models were parsimonious but required a complete set of three overlapping short-term measurements (central site, community ambient, and personal). Our results support the feasibility of predicting usual personal exposure to PM2.5 using short-term measurements in settings similar to rural Ghana and suggest that mother’s exposure may not be the best proxy for child’s exposure at age four.
Supplementary Material
Acknowledgements
The authors acknowledge support from the National Institutes of Environmental Health Sciences grants R01 ES02699 and R01 ES019547. The authors acknowledge additional support from P30 ES009089. MD was supported by the Columbia World Project, Combating Household Air Pollution With Clean Energy and AGL was supported by K23HL135349. The authors are grateful to study participants, without whom this study would not have been possible. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. National Institutes of Health.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020. Oct 17;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu N (Nil), et al. The Lancet Commission on pollution and health. The Lancet. 2018. Feb 3;391(10119):462–512. [DOI] [PubMed] [Google Scholar]
- 3.Garcia E, Rice MB, Gold DR. Air pollution and lung function in children. Journal of Allergy and Clinical Immunology. 2021. Jul 1;148(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees N, UNICEF. Clear the air for the children: the impact of air pollution on children [Internet]. New York: UNICEF; 2016. [cited 2021 Oct 30]. Available from: http://www.unicef.org/publications/files/UNICEF_Clear_the_Air_for_Children_30_Oct_2016.pdf [Google Scholar]
- 5.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatric Respiratory Reviews. 2017. Jan 1;21:38–46. [DOI] [PubMed] [Google Scholar]
- 6.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of Improved Air Quality with Lung Development in Children. New England Journal of Medicine. 2015. Mar 5;372(10):905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AG, Kaali S, Quinn A, Delimini R, Burkart K, Opoku-Mensah J, et al. Prenatal Household Air Pollution Is Associated with Impaired Infant Lung Function with Sex-Specific Effects. Evidence from GRAPHS, a Cluster Randomized Cookstove Intervention Trial. Am J Respir Crit Care Med. 2019. Mar 15;199(6):738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The Effect of Air Pollution on Lung Development from 10 to 18 Years of Age. New England Journal of Medicine. 2004. Sep 9;351(11):1057–67. [DOI] [PubMed] [Google Scholar]
- 9.Perera F, Ashrafi A, Kinney P, Mills D. Towards a fuller assessment of benefits to children’s health of reducing air pollution and mitigating climate change due to fossil fuel combustion. Environmental Research. 2019. May 1;172:55–72. [DOI] [PubMed] [Google Scholar]
- 10.Suades-González E, Gascon M, Guxens M, Sunyer J. Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology. 2015. Oct;156(10):3473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006. Mar 15;173(6):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope CA, Ezzati M, Dockery DW. Fine-Particulate Air Pollution and Life Expectancy in the United States. New England Journal of Medicine. 2009. Jan 22;360(4):376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark ML, Peel JL, Balakrishnan K, Breysse PN, Chillrud SN, Naeher LP, et al. Health and Household Air Pollution from Solid Fuel Use: The Need for Improved Exposure Assessment. Environ Health Perspect. 2013. Oct 1;121(10):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milà C, Salmon M, Sanchez M, Ambrós A, Bhogadi S, Sreekanth V, et al. When, Where, and What? Characterizing Personal PM2.5 Exposure in Periurban India by Integrating GPS, Wearable Camera, and Ambient and Personal Monitoring Data. Environ Sci Technol. 2018. Nov 20;52(22):13481–90. [DOI] [PubMed] [Google Scholar]
- 15.Ni K, Carter E, Schauer JJ, Ezzati M, Zhang Y, Niu H, et al. Seasonal variation in outdoor, indoor, and personal air pollution exposures of women using wood stoves in the Tibetan Plateau: Baseline assessment for an energy intervention study. Environ Int. 2016. Sep;94:449–57. [DOI] [PubMed] [Google Scholar]
- 16.Adhvaryu A, Bharadwaj P, Fenske J, Nyshadham A, Stanley R. Dust and Death: Evidence from the West African Harmattan [Internet]. National Bureau of Economic Research; 2019. Jun [cited 2020 Dec 23]. Report No.: w25937. Available from: https://www.nber.org/papers/w25937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. Combining Individual- and Group-Level Exposure Information: Child Carbon Monoxide in the Guatemala Woodstove Randomized Control Trial. Epidemiology. 2009. Jan;20(1):127–36. [DOI] [PubMed] [Google Scholar]
- 18.Brauer M. How Much, How Long, What, and Where. Proc Am Thorac Soc. 2010. May 1;7(2):111–5. [DOI] [PubMed] [Google Scholar]
- 19.Özkaynak H, Baxter LK, Dionisio KL, Burke J. Air pollution exposure prediction approaches used in air pollution epidemiology studies. J Expo Sci Environ Epidemiol. 2013. Nov;23(6):566–72. [DOI] [PubMed] [Google Scholar]
- 20.Dionisio KL, Howie SRC, Dominici F, Fornace KM, Spengler JD, Donkor S, et al. The exposure of infants and children to carbon monoxide from biomass fuels in The Gambia: a measurement and modeling study. Journal of Exposure Science & Environmental Epidemiology. 2012. Mar;22(2):173–81. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz J, et al. Patterns and predictors of personal exposure to indoor air pollution from biomass combustion among women and children in rural China. Indoor Air. 2011;21(6):479–88. [DOI] [PubMed] [Google Scholar]
- 22.Jack DW, Asante KP, Wylie BJ, Chillrud SN, Whyatt RM, Ae-Ngibise KA, et al. Ghana randomized air pollution and health study (GRAPHS): study protocol for a randomized controlled trial. Trials. 2015. Sep 22;16:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Vliet EDS, Asante K, Jack DW, Kinney PL, Whyatt RM, Chillrud SN, et al. Personal exposures to fine particulate matter and black carbon in households cooking with biomass fuels in rural Ghana. Environ Res. 2013. Nov;127:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofosu FG, Hopke PK, Aboh IJK, Bamford SA. Biomass burning contribution to ambient air particulate levels at Navrongo in the Savannah zone of Ghana. J Air Waste Manag Assoc. 2013. Sep;63(9):1036–45. [DOI] [PubMed] [Google Scholar]
- 25.Chillrud SN, Ae-Ngibise KA, Gould CF, Owusu-Agyei S, Mujtaba M, Manu G, et al. The effect of clean cooking interventions on mother and child personal exposure to air pollution: results from the Ghana Randomized Air Pollution and Health Study (GRAPHS). Journal of Exposure Science & Environmental Epidemiology. 2021. Mar 3;1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunnsteinsson S, Labrique AB, West KP, Christian P, Mehra S, Shamim AA, et al. Constructing Indices of Rural Living Standards in Northwestern Bangladesh. J Health Popul Nutr. 2010. Oct;28(5):509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owusu-Agyei S, Nettey OEA, Zandoh C, Sulemana A, Adda R, Amenga-Etego S, et al. Demographic patterns and trends in Central Ghana: baseline indicators from the Kintampo Health and Demographic Surveillance System. Global Health Action. 2012. Dec 1;5(1):19033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of Chiropractic Medicine. 2016. Jun 1;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KR, Bruce N, Balakrishnan K, Adair-Rohani H, Balmes J, Chafe Z, et al. Millions Dead: How Do We Know and What Does It Mean? Methods Used in the Comparative Risk Assessment of Household Air Pollution. Annu Rev Public Health. 2014. Mar 18;35(1):185–206. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez M, Milà C, Sreekanth V, Balakrishnan K, Sambandam S, Nieuwenhuijsen M, et al. Personal exposure to particulate matter in peri-urban India: predictors and association with ambient concentration at residence. J Expo Sci Environ Epidemiol [Internet]. 2019. Jul 1 [cited 2020 May 7]; Available from: http://www.nature.com/articles/s41370-019-0150-5 [DOI] [PubMed] [Google Scholar]
- 31.Keller JP, Clark ML. Estimating long-term average household air pollution concentrations from repeated short-term measurements in the presence of seasonal trends and crossover. Environmental Epidemiology. 2022. Feb;6(1):e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johannesson S, Gustafson P, Molnár P, Barregard L, Sällsten G. Exposure to fine particles (PM 2.5 and PM 1) and black smoke in the general population: personal, indoor, and outdoor levels. J Expo Sci Environ Epidemiol. 2007. Nov;17(7):613–24. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong JR, Campbell H. Indoor air pollution exposure and lower respiratory infections in young Gambian children. Int J Epidemiol. 1991. Jun;20(2):424–9. [DOI] [PubMed] [Google Scholar]
- 34.de Francisco A, Morris J, Hall AJ, Armstrong Schellenberg JR, Greenwood BM. Risk factors for mortality from acute lower respiratory tract infections in young Gambian children. Int J Epidemiol. 1993. Dec;22(6):1174–82. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz J. Air Pollution and Children’s Health. Pediatrics. 2004. Apr 1;113(Supplement 3):1037–43. [PubMed] [Google Scholar]
- 36.Nazif-Muñoz JI, Spengler JD, Arku RE, Oulhote Y. Solid fuel use and early child development disparities in Ghana: analyses by gender and urbanicity. Journal of Exposure Science & Environmental Epidemiology. 2020. Jul;30(4):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


