Abstract
Heart disease is the leading cause of death among men and women. Women have a unique phenotype of ischemic heart disease with less calcified lesions, more nonobstructive plaques, and a higher prevalence of microvascular disease as compared to men, which may explain in part why current risk models to detect obstructive coronary artery disease (CAD) may not work as well in women. This paper summarizes the sex differences in the functional and anatomical assessment of CAD in women presenting with stable chest pain and provides an approach for using multimodality imaging for the evaluation of suspected ischemic heart disease (IHD) in women in accordance to the recent published AHA/ACC guidelines for the evaluation and Diagnosis of Chest Pain.[1]. A paradigm shift in the approach to imaging ischemic heart disease women is needed including updated risk models, a more profound understanding of CAD in women where nonobstructive disease is more prevalent, and algorithms focused on the evaluation of ischemia with nonobstructive CAD (INOCA) and Myocardial infarction with nonobstructive CAD (MINOCA).
Keywords: Cardiovascular disease, Heart disease in women, Imaging, Ischemia
Introduction
Heart disease is the leading cause of death among men and women. In 2017, of the one million deaths among women in the United States, over 400,000 were related to cardiovascular disease. Recent reports document declines in cardiovascular disease (CVD) mortality for females, but reductions are lower when compared with men.[2] Women have a unique phenotype of ischemic heart disease with less calcified lesions, more nonobstructive plaques, and a higher prevalence of microvascular disease as compared to men, which may explain in part why current models to detect obstructive coronary artery disease (CAD) may not work as well in women.[3] This paper summarizes the sex differences in the functional and anatomical assessment of CAD in women presenting with stable chest pain and provides an approach for using multimodality imaging for the evaluation of suspected ischemic heart disease (IHD) in women in accordance to the recent published AHA/ACC guidelines for the evaluation and Diagnosis of Chest Pain[1]. A paradigm shift in the approach to imaging ischemic heart disease women is needed including updated models of risk; a more profound understanding of CAD in women where nonobstructive disease is more prevalent; algorithms focused on the evaluation of ischemia with nonobstructive CAD (INOCA) and Myocardial infarction with nonobstructive CAD (MINOCA) should be included.
Sex Differences
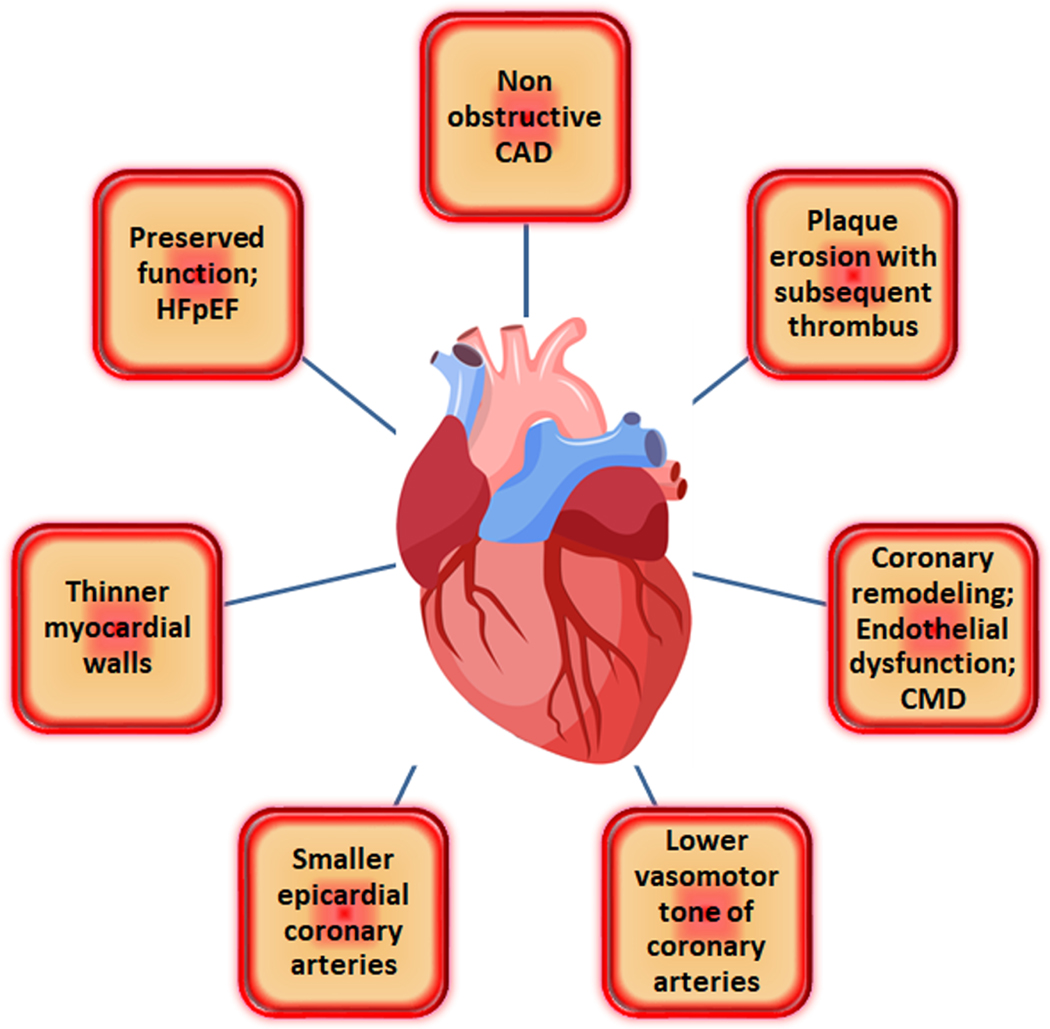
There is growing evidence demonstrating sex and gender differences in risk factors, coronary anatomy, and clinical presentation of IHD.[4] There are significant gender differences related to cardiac and coronary anatomy. Women have smaller epicardial coronary arteries than men, even after adjustment for age, body mass index (BMI), body surface area (BSA), and left ventricular (LV) mass, which complicates accurate assessment of distal coronary arteries by coronary computed tomographic angiography (CTA) [5]. Women have thinner myocardial walls, which is challenging for evaluation of non-transmural ischemia by Cardiac MRI (CMR). Positron emission tomographic (PET) imaging studies have shown that, compared to men, women have higher coronary blood flow at both rest and peak stress, but similar coronary flow reserve (CFR). The mechanisms underlying the observed differences are likely to be multifactorial. Vasomotor tone is lower in coronary arteries in women than in men, which could be partially related to sex hormone effects[6].It is hypothesized that higher coronary blood flow in women, coupled with their smaller coronary arteries, may result in clinically significantly higher endothelial shear stress, potentially contributing to sex differences in susceptibility to coronary atherosclerosis. [7] Figure 1 summarizes how IHD presents differently in women as compared to men due to a spectrum of distinctive characteristics.
Figure 1.
Ischemic heart disease presents differently in women as compared to men due to a spectrum of distinctive characteristics.
More women than men presenting with symptoms and signs suggestive of ischemia are found to have no obstructed coronary arteries (INOCA). In a large study of patients undergoing elective angiography [8], 58.4% had nonobstructive CAD, and female sex was the strongest predictor of nonobstructive disease.
Conventional noninvasive stress testing
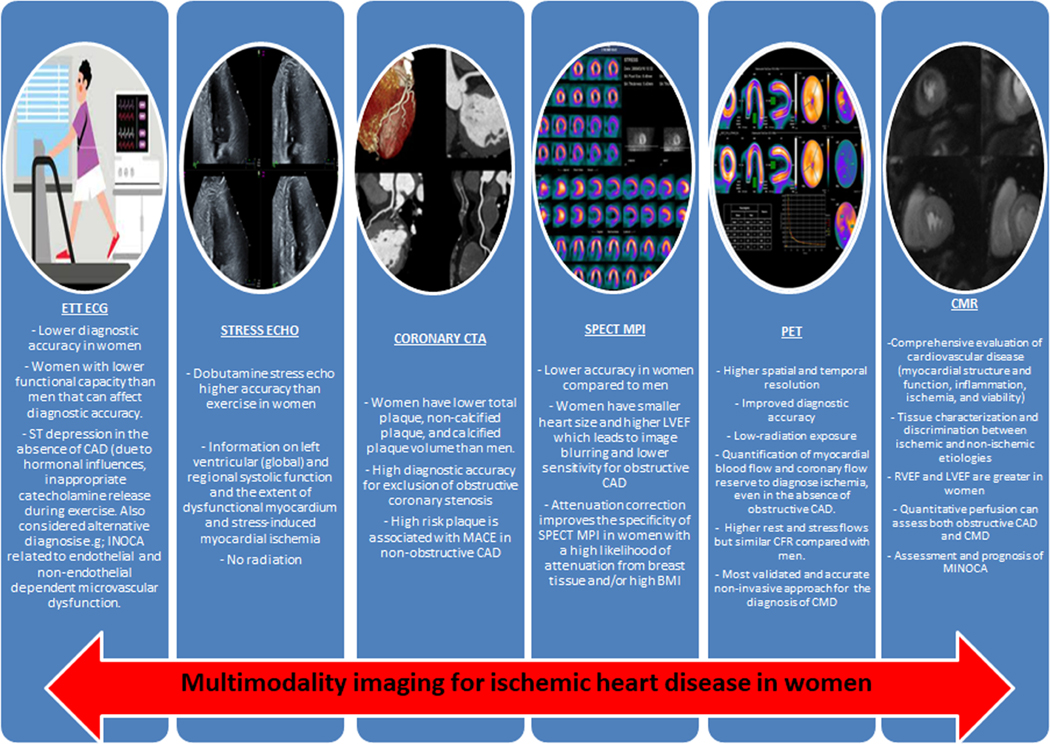
Noninvasive imaging plays a critical role in the diagnosis and management of patients with ischemic heart disease (IHD). There are a number of imaging modalities available for diagnosing CAD in symptomatic women. Women have more often nonobstructive CAD in the presence of evidence of ischemia that could be explained by the presence of microvascular dysfunction, defined as epicardial, microvascular endothelial, or nonendothelial dysfunction [9]. The different imaging modalities and their specifications regarding limitations and advantages, specifically in women are discussed below. Central Illustration. It is important to consider that the accuracy of standard noninvasive diagnostic testing can vary significantly when assessed against a gold standard of documented anatomic obstructive CAD, especially in women.
Testing choice will be influenced by local expertise and availability, but knowledge regarding which test may be preferable is helpful when selecting between different modalities. The following section provides a brief overview of various noninvasive tests available in the evaluation of symptomatic patients.
Exercise treadmill testing (ETT) with or without imaging
A treadmill exercise electrocardiogram (ECG) is one of the most commonly used noninvasive tests for the assessment of IHD. However, the diagnostic accuracy of exercise ECG for detecting obstructive CAD is lower in women than men, if ST segment response alone is used in predicting obstructive disease. A meta-analysis of 29 studies that included 3,392 women reported a sensitivity and specificity of 62% and 68%, respectively, with a positive predictive value of only 47%[10]. A variety of factors have been postulated to interfere with the ECG response to exercise in women. Hormonal influences, inappropriate catecholamine release during exercise, and estrogen, with molecular similarities to digitalis can cause a digitalis-like false-positive ECG changes[13]. Moreover, women less often attained adequate exercise stress and they have less exercise-induced angina than men[11].The positive predictive value of ST-segment depression with exercise testing is significantly lower in symptomatic women than in symptomatic men, while the negative predictive value of ST-segment depression is similar. Among women, exercise-induced ST depression in the absence of obstructive CAD has been described. Results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) Trial, supports that in low risk women, who can exercise, a diagnostic strategy that uses ETT vs. exercise MPI yields similar post-test outcomes while providing significant diagnostic cost savings.[12] In patients with high pre-test risk, the addition of stress imaging may improve cardiovascular risk assessment and better guide clinical management. Therefore, an initial strategy of ETT without imaging is appropriate for low-intermediate risk women who can exercise and have a normal resting electrocardiogram (ECG) as supported by recent consensus statements [13].
Stress Echocardiography
There is limited data regarding gender differences in stress echocardiography, but the diagnostic performance is thought to be similar. The absence of radiation with stress echocardiography makes it an attractive technique, particularly for younger women. The AHA Consensus Statement recommends the addition of imaging to exercise in the evaluation of intermediate-risk women who have an abnormal ECG or an abnormal baseline ECG that would interfere with the interpretation of ST-segment depression with exercise[14]. Stress echocardiography may be limited due to patient-dependent factors leading to inability to obtain adequate acoustic windows at peak stress. Although there are not many studies examining gender differenes in the performance of stress echocardiography, evidence indicates that this modality provides significantly higher specificity and accuracy than exercise ECG alone with no significant differences between men and women.[15]
In a head to head comparison of dobutamine stress echocardiography vs. exercise electrocardiography for the detection of CAD in women[16], dobutamine stress echocardiography showed higher accuracy in female patients with chest pain with a sensitivity of 70.4 % vs. 53.7%, respectively and a specificity of 94.6% vs. 73.6% for detection of >50% coronary artery stenosis. The higher accuracy of DSE was maintained after exclusion of the patients who could not achieve over 85% age-predicted heart rate before ischemia induction.
SPECT myocardial perfusion imaging (MPI) in women
SPECT MPI provides a more sensitive and specific prediction of the presence of IHD than exercise ECG alone. The reported sensitivity of exercise MPI has ranged from 78% to 88% and the specificity from 64% to 91% in women.[17] Studies have reported sex differences in the diagnostic accuracy of SPECT MPI, with lower accuracy for SPECT MPI in women compared to men[13]. SPECT MPI has a number of sex-specific challenges in women. Women have a smaller heart size than men, potentially resulting in a lower sensitivity to detect obstructive CAD due to the low resolution of conventional gamma cameras [18]. Furthermore, variable breast attenuation can result in false positive studies. Attenuation correction represents an important consideration to improve the specificity of SPECT MPI in women with a high likelihood of attenuation from breast tissue and/or high body mass indices (BMI) [13].
The Women trial [19] showed an improvement in the diagnostic accuracy of standard treadmill exercise ECG when combined with MPI in women with intermediate-high pre-test IHD risk. The diagnostic accuracy in detecting obstructive CAD was greater for ETT with SPECT MPI than for ETT alone (MPI, sensitivity 78% [95% CI 72% to 83%] versus ETT only, sensitivity 61% [95% CI 54% to 68%]).[20]
The prognostic value of a normal SPECT MPI among women is excellent with 99% event-free survival and similar to that of men in a large meta-analysis. [21]. Additionally, studies have confirmed the excellent prognostic value of SPECT MPI in women, including older women [22] and women of diverse racial and ethnic subsets.[23]
Cardiac Coronary Tomography Angiography
A high diagnostic accuracy of CT angiography (CTA) for detection of obstructive coronary stenosis at both thresholds of 50% and 70% stenosis has been reported with a sensitivity, specificity, and positive and negative predictive value of 95%, 83%, 64%, and 99%, respectively and 94%, 83%, 48%, 99%, respectively.[24] This high accuracy is preserved in women as demonstrated in sex-specific analyses.[25, 26] The strength of CTA is its high sensitivity and negative predictive value. However, one study demonstrated lower sensitivity in women in distal coronary segments (56% vs 85%, p <0.05) and side branches (54% vs 89%, p <0.001). The smaller epicardial size likely also impacts the diagnostic specificity, which was shown to be lower in women compared to (75% vs. 90%, p < 0.05) [27].
The PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) randomized 10,003 outpatients with stable symptoms suggestive of CAD to a strategy of either functional or anatomic (CTA) testing [28]. Over a median follow-up period of 25 months, there was no difference between testing arms in clinical events overall or by sex. A substudy of PROMISE [29] assessed the relationship between sex and noninvasive testing results and the relationship between sex and a composite of death, MI, and unstable angina hospitalization. Women were significantly less likely to have a significantly abnormal CTA (≥70% stenosis) as compared to a positive stress test (8% vs. 12%, adjusted OR 0.67 [95% CI 0.55–0.82]).Specifically,CTA was less likely to be positive compared with exercise ECG (OR: 0.39;95% CI: 0.25 to 0.61; p<0.001) and nuclear stress testing (OR: 0.66; 95% CI: 0.53 to 0.82; p<0.001) but not compared with stress echocardiography (OR:0.90;95%CI:0.63 to 1.30; p = 0.58) In addition, in women, a positive CTA was more strongly associated with subsequent clinical events than a positive stress test (CTA adjusted HR 5.86 [95% CI 3.32–10.35]; stress adjusted HR 2.27 [95% CI 1.21–4.25]; adjusted p=0.028)
CTA in women has the additive value of detection of atherosclerotic plaque as compared to stress testing. From a large cohort from the CONFIRM registry (N=23,853), with 2.3 ±1.1 year follow-up, nonobstructive CAD by CTA conferred increased mortality risk compared with patients without CAD (H.R.: 1.60; 95% CI: 1.18 to 2.16; p = 0.002). Five year follow up of the CONFIRM registry showed after adjustment, that there was a strong association between increased MACE risk and nonobstructive CAD (H.R.: 2.16 for women, 2.56 for men; p < 0.001 for both).[30]. Data from the CAC Consortium[31], revealed women with larger sized and more numerous CAC lesions had 2.2-fold higher CVD mortality (P < 0.0001) as compared to men. This findings suggest that nonobstructive plaque carries a higher relative risk in women than in men. CTA clearly offers a unique opportunity to make recommendations for preventive care and initiate appropriate therapy in women.
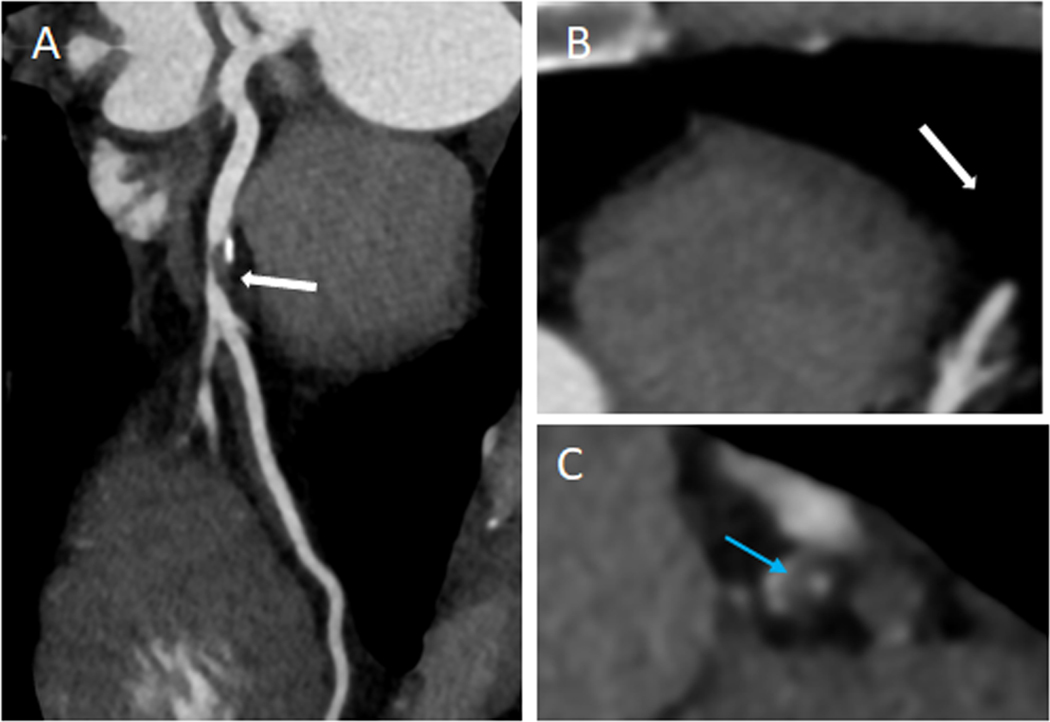
CTA also offers the opportunity to assess plaque characteristics. CTA studies have shown that women have lower total plaque volume and both calcified and noncalcified plaque volume than men [32], which was maintained across age subgroups[33]. A post-hoc analysis of the PROMISE trial [34] aimed to determine whether high-risk plaque (low attenuation plaque, positive remodeling, spotty calcification, and the napkin ring sign) was associated with incident MACE independently of significant stenosis and cardiovascular risk factors. High-risk plaque was associated with a higher MACE rate (6.4% vs. 2.4%; hazard ratio, 2.73; 95% CI, 1.89–3.93). High-risk plaque features were a stronger predictor of MACE in women (aHR, 2.41; 95% CI, 1.25–4.64) vs men (aHR, 1.40; 95% CI, 0.81–2.39)( Figure 2).
Figure 2.
Central Illustration. Advantages and disadvantages of different Stress modalities in women
While coronary artery calcium scoring (CACS) in asymptomatic patients has proven to be an effective risk stratifier for adverse CVD events, particularly in patients who are low-intermediate risk, [35, 36] the use of CACS in symptomatic women is more controversial. From the CAC Consortium data[31], it has been shown that women have fewer calcified lesions, fewer calcified vessels, and lower CAC volumes. While a calcium score of 0 carried a similar low risk of CVD events for both men and women, women with any detectable CAC had a 1.3 fold relative hazard for CVD mortality as compared to men. In the CONFIRM registry [37] the absence of CAC effectively ruled out obstructive CAD (NPV 96.5% for ≥50% stenosis; 98.6% for ≥70% stenosis) and major adverse cardiovascular events occurred in <1%. In the CRESCENT trial patients with stable angina were prospectively randomized to either cardiac CT or stress testing. [38] Patients randomized to CT first underwent CAC scanning. If CAC was absent, participants did not undergo additional testing unless the pre-test probability for obstructive CAD was determined to be >70%. Notably, in this study, cardiac CTA led to a more efficient diagnosis in both sexes when compared with functional testing, and this effect was more prominent in women (p interaction=0.01). Furthermore, the reduced need for further testing after CTA, compared with functional testing, was most evident in women (p interaction=0.009).
Using zero CAC as a gatekeeper in symptomatic patients will need more long-term and prospective outcome data. There has been interest in integrating anatomical and physiologic testing to obtain the most complete assessment. In this aspect, the combination of CAC with stress MPI has demonstrated significant promise in improving prognostic and diagnostic value[39]. The newly released ACC guidelines provide a Class 2a recommendation for consideration of CAC in low risk symptomatic patients[1].
Positron Emission Tomography (PET) MPI
PET has excellent diagnostic performance, with a sensitivity of 90–92% and specificity of 81–88% for detecting angiographically significant stenoses [40, 41]. These studies suggest the superior diagnostic performance of PET compared to SPECT MPI.
Extensive data support the diagnostic value of PET MPI in women and men with known or suspected CAD. A multicenter registry of 7,601 patients showed that in patients with known or suspected CAD, the extent and severity of ischemia and scar on PET MPI provided robust and incremental risk estimates of cardiac death and all-cause death compared with traditional coronary risk factors, with a predicted annual cardiac event rate of <1% for a normal scan vs. >4.2% for an abnormal scan.[42] Hybrid or sequential PET/CT imaging protocols add the capability to evaluate anatomical disease with quantification of CAC scoring or with CCTA, thereby increasing test sensitivity for the diagnosis of CAD, including nonobstructive disease. [43]
In women, stress myocardial perfusion PET has several advantages over the more commonly performed SPECT imaging, including higher spatial resolution, improved diagnostic accuracy, lower radiation exposure, and the ability to quantify myocardial blood flow and coronary flow reserve (CFR) to diagnose INOCA. In addition, there is an increasing recognition that coronary microvascular dysfunction (CMD) with or without epicardial CAD is prevalent in women. [44] [45] (Figure 3)
Figure 3.
High risk plaque features were a stronger predictor of MACE in women in PROMISE (aHR,2.41; 95% CI, 1.25–4.64). (A) Curved multiplanar reconstruction showing partially calcified plaque in the proximal LAD with evidence of positive remodeling (white-arrow). (B) Axial Image of the lesion. (C) Cross-sectional view of the plaque showing low attenuation plaque (blue arrow).
Another critical advantage of PET MPI is the ability to quantify rest and stress myocardial and their ratio, CFR. CFR is an integrated marker of coronary vasomotor dysfunction that measures the hemodynamic effects of focal, diffuse, and small-vessel coronary artery disease on myocardial tissue perfusion. [46] It is a well validated index that allows the assessment of blood flow impairment originating from obstructive, diffuse, or microcirculatory involvement of the coronary circulation[47]. Abnormalities in the microcirculation could explain inducible myocardial ischemia beyond the effects of epicardial coronary obstruction and identify individuals and high risk for MACE. Because the coronary microcirculation is beyond the resolution of invasive or noninvasive coronary angiography, direct interrogation of coronary microvascular function is necessary to establish the diagnosis of CMD.[43]
The accuracy and reproducibility of PET for the quantitative noninvasive measurement of myocardial blood flow and CFR has been well validated in experimental animals and humans subjects.[48].
PET studies have shown that CMD is prevalent in women and men (54% and 51%, respectively). Regardless of sex, CFR has shown to be a powerful incremental predictor of MACE; a similar trend was maintained in patients with no CAC. [43] Even in a visually normal PET MPI, impaired CFR adds essential prognostic value. In addition, abnormalities in the microcirculation could explain inducible myocardial ischemia beyond the effects of epicardial coronary obstruction and identify individuals with a high risk of MACE.[43]
In patients with very low CFR (<1.6), women showed a higher frequency of nonobstructive CAD, whereas men showed a higher frequency of severely obstructive CAD (P=0.002).[45]. A differential effect on outcomes between women and men is noted in individuals with very low CFR (CFR <1.6) (hazard estimated from the linear interaction of CFR and sex in the final model, model P<0.001, interaction P=0.04).[49]
In addition, abnormal blood flow reserve by PET is associated with diastolic dysfunction. A recent study with 64.7% of females who did not have obstructive CAD showed an independent association of impaired CFR (defined as less than 2) and diastolic dysfunction as well as an increase in cardiovascular outcome events or HFpEF hospitalization alone.[50] These findings suggest a pathophysiological link between CMD and HFpEF.
Cardiac Magnetic Resonance (CMR)
CMR has a prominent role in the assessment of women with suspected or known CAD. CMR provides a comprehensive evaluation of cardiovascular disease, including evaluation of myocardial structure and function, inflammation, ischemia, and viability.[51]
CMR has advantages in chronic coronary syndromes or stable CAD compared to other methods in women. CMR stress testing is most commonly performed using first-pass contrast-enhanced perfusion imaging during coronary vasodilator stress. Given CMR’s superior spatial resolution compared to SPECT or PET MPI, it can demonstrate non-transmural ischemia that can be missed by other modalities and is less sensitive to the technical limitations of other modalities related to breast tissue, obesity, and lung disease. Stress CMR for detecting ischemia has proven to be an effective and robust risk stratification tool in patients of both sexes presenting with suspected CAD. In the Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC) study [52], comparing stress CMR vs. conventional SPECT MPI, reported a sensitivity of 86.5% and a specificity of 83.4% for stress CMR. A sub-study of the CE-MARC study showed that diagnostic accuracy was similar in the sexes for perfusion CMR (P=1.00) but was significantly worse in women for SPECT (P<0.0001). [53]
In terms of predicting prognosis, stress CMR is an excellent tool. A meta-analysis that included nineteen studies and involved 11,636 patients with a mean follow-up of 32 months demonstrated the prognostic value of a normal stress CMR with annualized events rates of 4.9% for a positive versus 0.8% for a negative stress CMR (p < 0.0001), 2.8% versus 0.3% for cardiovascular death (p < 0.0001), and 2.6% versus 0.4% for MI (p < 0.0005).[54]
While conventional noninvasive imaging tests are often normal in coronary microvascular dysfunction, stress CMR presents a diagnostic opportunity. Panting et al.[55] initially described the role of CMR in syndrome X. In this study, semi-quantitative stress CMR could demonstrate subendocardial hypoperfusion compared to controls. To date, CMR perfusion assessment has been primarily qualitative unlike PET perfusion, because of the complexity and time needed for quantification. This is changing due to new quantitative perfusion mapping techniques which are becoming more widely available. Perfusion mapping is an approach where perfusion maps are generated automatically on the scanner with each image pixel encoding myocardial blood flow (MBF). Myocardial perfusion reserve (MPR) is calculated as a ratio of the stress perfusion divided by rest perfusion [56, 57]. Similarly, MPRI has been found to be lower in women with MI and nonobstructive CAD when compared with healthy controls.[58] These findings were confirmed in a larger cohort of patients with microvascular dysfunction confirmed by coronary reactivity testing.[59](Figure 4)
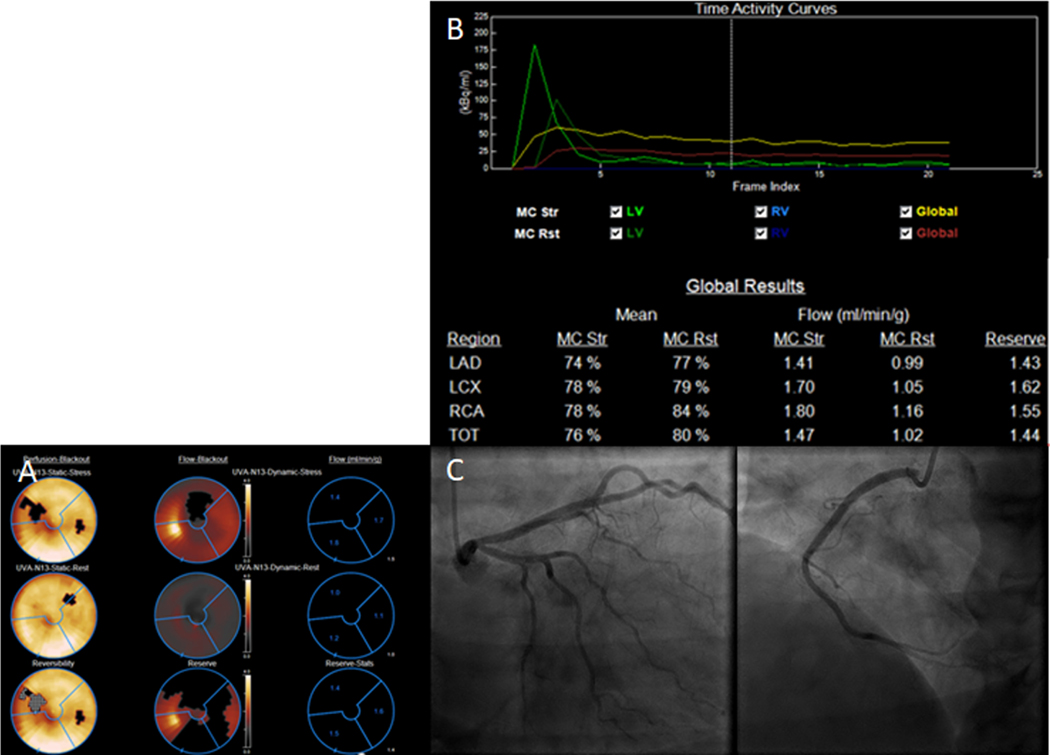
Figure 4.
Case of a 47 year-old female. (A) Stress PET study demonstrated a reversible perfusion defect of apical to mid anterior and inferoseptum segments. (B) Quantitative perfusion values show globally decreased perfusion during stress in the 3 coronary distributions, and globally decreased CFR in all 3 coronary distributions (C) Coronary angiogram demonstrated normal coronary arteries. The findings are consistent with INOCA/CMD
Studies have shown the incremental prognostic value of quantitative myocardial perfusion mapping based on the signal conversion to gadolinium concentration to visual assessment independently of clinical and imaging risk factors, including ischemia extent, ejection fraction, and LGE size in men and women.[60, 61] A multicenter study of 1049 patients with suspected and known CAD showed that lower myocardial stress MBF and MPR by CMR perfusion mapping were associated with death and MACE independent of other clinical risk factors,[62] even in patients who had normal qualitative perfusion and no known obstructive CAD. Furthermore, a recent investigation demonstrated females have higher myocardial perfusion, myocardial blood volume (MBV), and extracellular volume (ECV) compared to males during adenosine stress CMR, and that sex is an independent contributing factor to myocardial perfusion and ECV; with no differences in myocardial perfusion reserve (MPR).[63] Similarly, Zorach et al[64] observed that MPR was similar between men and women despite rest and stress flows being higher in females than males. In this prospective study of 46 subjects with angina and no obstructive coronary artery disease, where 74% were women, it was shown that stress MBF and MPR were lower when compared to healthy controls suggesting CMD being the likely cause of typical angina symptoms.[65]
Patients with evidence of myocardial injury without evidence of obstructive CAD are another group in whom CMR has an important diagnostic role. Potential underlying mechanisms include coronary disorders (i.e. coronary dissection, plaque disruption, coronary spasm, microvascular dysfunction, coronary embolism); myocardial disorders (myocarditis, takotsubo cardiomyopathy). Identification of the underlying etiology is important for risk stratification and treatment decision-making. Studies[51, 66, 67] have shown that CMR identifies the underlying etiology in 74–87%of cases. Similar results were presented in a recent trial, specifically in women presenting with MINOCA where 74.1% had an identifiable cause by CMR. [67] Identification of the cause of MINOCA has the potential to guide medical therapy as it was shown in a study of 134 patients presented with MINOCA [68] where CMR had a significant impact in 66% of subjects, either by a new diagnosis (54%) or change in management (41%). The use of CMR in the diagnosis of myocarditis, and quantitative CMR for the assessment of MINOCA are supported in the recent ACC Chest Pain Guidelines with a class 1 recommendation[1].
Women’s stable ischemic heart disease: A Paradigm Shift
When evaluating women for suspected CAD, the pre-test probability must be considered, and testing should be chosen wisely according to appropriateness. There are several pre-test probability scores for use in symptomatic patients with suspected CAD. Older pre-test scores estimate the probability of obstructive CAD, resulting in significant overestimation in contemporary patients referred for noninvasive testing, particularly women[69]. It is preferable to use current estimates, as emphasized in the recently published Chest Pain Guidelines[1], such as the pre-test probability proposed by Juarez-Orozco et al.[70] In the absence of these models, low-risk patients may include those <40 years of age or who have symptoms that have a low likelihood of myocardial ischemia. In women with relatively low risk and with normal resting ECG that can exercise, a treadmill exercise testing is reasonable as the first step for evaluation. Exercise stress testing provides valuable information about exercise capacity, hemodynamic response to exercise, and association of angina with exercise. Stress imaging with echocardiography may also be reasonable, particularly in younger women, as it will provide information about wall motion abnormalities, assess ventricular function, and increase specificity without exposing the patient to radiation exposure. Coronary artery calcium scoring (CAC) has a potential role in this group, especially if risk-enhancing factors are present. CAC is its high sensitivity to detect atherosclerotic disease that has been shown to impact treatment strategies and guide lifestyle modifications.[71]
Furthermore among lower risk patients, a CAC of zero has a very high negative predictive value for both the presence of obstructive CAD and adverse CVD events. The role of CAC testing has been addressed in the recent Chest pain Guidelines as reasonable as a first-line test for excluding calcified plaque and identifying patients with a low likelihood of obstructive CAD[1]. In women at intermediate-risk, the first step should include reviewing results of prior cardiac testing and any prior chest CT images to detect coronary calcium burden. An anatomical strategy vs. functional stress testing are both reasonable as initial strategy given similar clinical outcomes[28]. Patient-specific factors should drive the decision-making process when choosing between anatomic and functional strategies.
An initial strategy with coronary CTA is favored in patients with no known coronary artery disease, absence of significant coronary calcification on prior chest CT, prior normal, mildly abnormal, or inconclusive stress test results, low likelihood of high-quality stress testing. In addition to a very high negative predictive value for the presence of obstructive CAD, coronary CTA has the unique ability to detect nonobstructive plaque which can guide preventative strategies. The presence of nonobstructive disease in women should prompt intensive medical therapy with aspirin and statin which is often overlooked.
An initial strategy with a functional stress testing is favored over an anatomical approach in patients with known CAD (prior MI, coronary revascularization, or stenosis>50% on prior invasive angiogram or CTA), significant coronary calcification on prior chest CT, high risk for iodinated contrast, low likelihood of high-quality coronary CTA, or lack of timely access to CTA. Functional testing is also reasonable in women without known CAD to assess the relationship between anginal symptoms and objective evidence of ischemia. Functional testing with quantification of flow reserve predicts hard events and MACE and improves risk stratification for patients being investigated for ischemia in patients without and in the group with obstructive CAD; this was also demonstrated specifically for women.[45, 72]. A normal CFR has a high NPV for excluding high-risk CAD on angiography.[73]. For this reason when available PET or CMR with quantification of myocardial perfusion should be preferred over conventional stress testing modalities.
In the absence of high risk features by anatomical or functional imaging, most women with stable CAD can initially be treated medically. However in women with medically refractory symptoms, or frequent symptoms additional testing may be necessary. Given the low prevalence of obstructive CAD in women, in patients initially undergoing a functional test, proceeding with an anatomical test such as CTA prior to angiography may be a reasonable approach. Use of CTA after stress testing can diagnose or exclude obstructive CAD and identify patients who may benefit from referral to invasive coronary angiography. The ISCHEMIA (International Study of Comparative Health Effectivenesss with Medical and Invasive approaches) trial used CTA after site-determined moderate to severe ischemia to exclude patients with nonobstructive CAD and identifying those with significant left main stenosis. In women who initially undergo a CTA showing nonobstructive disease, but with ongoing symptoms, particularly among the subgroup of patients with cardiometabolic disease (i.e., obesity, metabolic syndrome, and diabetes mellitus), chronic kidney disease, or HfpEF, a quantitative functional study (PET or CMR) should be pursued to establish the diagnosis of CMD.
Approximately 20–30% of patients with nonobstructive CAD will demonstrate ischemia[69]. When documented myocardial ischemia occurs without a coronary stenosis ≥50%, the diagnosis of INOCA is established. This condition has been associated with an increased risk of death, myocardial infarction (MI), and stroke, compared to the general population [8, 9], especially in those who remain symptomatic [10] or have any associated amount of atherosclerotic disease [11].
In WISE[10] persistent symptoms at 1-year follow-up predicted MACE among those with INOCA. Signs and symptoms of ischemia in INOCA patients may be related to abnormalities in flow within the microvasculature. Hence, many symptomatic patients without obstructive CAD will benefit from assessment of coronary microvascular dysfunction (CMD). Patients at higher risk for CMD include women, especially those with concomitant hypertension, diabetes, and related insulin-resistant states.[69]
Evidence suggests that testing for CMD can lead to the diagnosis of microvascular angina, resulting in improving risk stratification. In symptomatic women, especially in the presence of a positive exercise test for myocardial ischemia in the absence of obstructive CAD, an invasive coronary reactivity reactivity testing to assess vasospasm and the nonendothelial-dependent and endothelium-dependent microvascular reactivity should be considered[74].
It is important to mention that the recently published Guideline for the Evaluation and Diagnosis of Chest Pain [1], give a 2a recommendation for patients with INOCA to consider stress PET MPI and stress CMR with MPR measurement to diagnose CMD and enhance risk stratification. To date, appropriately designed outcome trials investigating therapeutic strategies targeting women with INOCA and persistent symptoms are lacking. The WARRIOR trial [75] will potentially provide data necessary to determine how to best manage symptoms in this challenging population. The recently published CIAO-ISCHEMIA (Changes in Ischemia and Angina over One year in ISCHEMIA Trial Screen Failures With INOCA) trial highlights the complex nature of INOCA pathophysiology and the possible multifactorial nature of angina. Patients with moderate or severe ischemia on stress echocardiography without obstructive disease by CTA were more often female, but had largely similar ischemia compared to patients with obstructive CAD. Half of 1-year INOCA stress echocardiograms were normal and 23% had moderate or severe ischemia at 1 year. Angina improved in 43% and worsened in 14%. Ischemia and angina were not correlated in those with INOCA or with obstructive CAD, and the change in ischemia was not correlated with the change in angina in patients with INOCA. Results will suggest that management should be focus on symptom control to maximize patients quality of life[76].
Another important entity is myocardial infarction without coronary occlusion (MINOCA) in which patients present with symptoms of ACS but without coronary obstruction. Women with ACS are less likely to have significant obstructive CAD than men but are more likely to have thrombus formation and plaque erosion[77]. Among women presenting with potential MINOCA, different etiologies such as spontaneous coronary artery disection, coronary thromboembolism, coronary vasospasm, microvascular dysfunction, takotsubo stress-induced cardiomyopathy and myocarditis should be considered. As mentioned above, recent evidence support that CMR has a crucial role to identify the cause that may alter therapeutic strategies”.The recent published ACC/AHA Guideline for the Evaluation and diagnosis of Chest Pain recognizes the value of CMR in patients with MINOCA and gives class 1 recommendations in this population as an effective tool to distinguish myopericarditis from other causes, incluiding myocardial infarction[1].
Sex-specific differences in the performance of noninvasive testing for IHD necessitate a sex-based diagnostic work-up. A multimodality approach is fundamental to understand women’s chest pain symptoms. Whether the initial strategy is an anatomical or functional test, considering clinical presentation and persuing further evaluation for CMD in patients with refractory symptoms is essential. (Figure 5). In accordance with the recent AHA/ACC New guidelines to Evaluate Chest Pain, invasive coronary angiography (ICA) is recommended for guiding treatment decision-making in patients with obstructive CAD and stable chest pain despite guideline-directed medical therapy and moderate-severe ischemia. [1]
Figure 5.
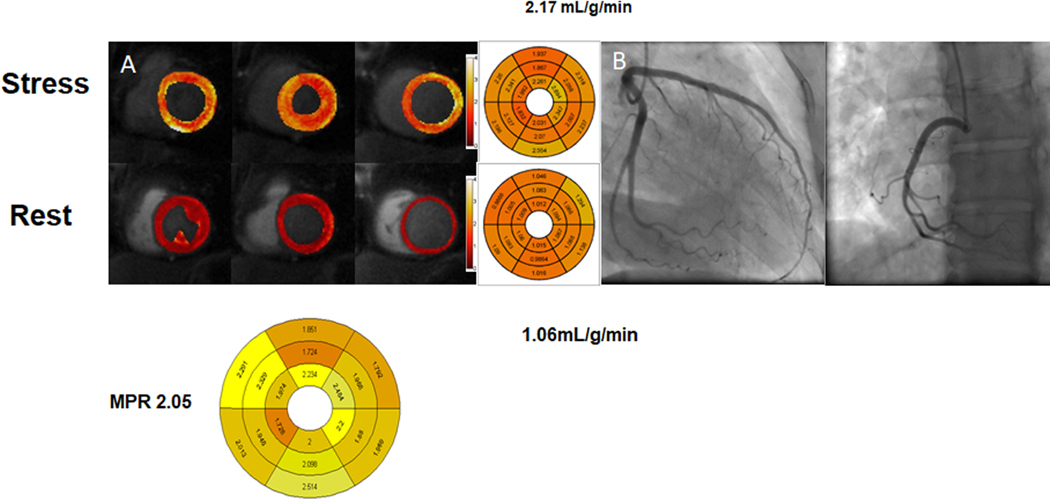
Stress CMR quantification with MVD. In patients with risk factors for MVD, both MPR (2.21 [1.95,2.69] vs. 2.93 [2.763.19], p < 0.001) and stress myocardial perfusion (2.65 ± 0.62 ml/min/g, vs. 3.17 ± 0.49 ml/min/g p < 0.002) are reduced as compared to controls*.
A case of a 52 year old female with chest pain. (A) Adenosine-stress perfusion cardiac magnetic resonance imaging showing evidence of globally reduced coronary flow at stress, consistent with coronary microvascular dysfunction. Myocardial perfusion reserve index decreased at 2.05. (B) Coronary angiogram demonstrated normal coronary arteries.
Zorach B. Shaw PW, Bourque J, et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson. 2018;20(1):14. Published 2018 Feb 22. doi: 10.1186/s12968-018-0435-1.
A paradigm shift which considers sex-specific difference in risk factors, coronary physiology and pathophysiology, and clinical symptoms is needed to provide optimal care for women presenting with angina. A greater focus on primary prevention in women with non-obstructive CAD, and evaluation of INOCA and MINOCA is needed and supported with the recent chest pain guidelines. With the current armamentarium of non-invasive cardiovascular imaging tools, we are well poised to meet the challenges of this paradigm shift.
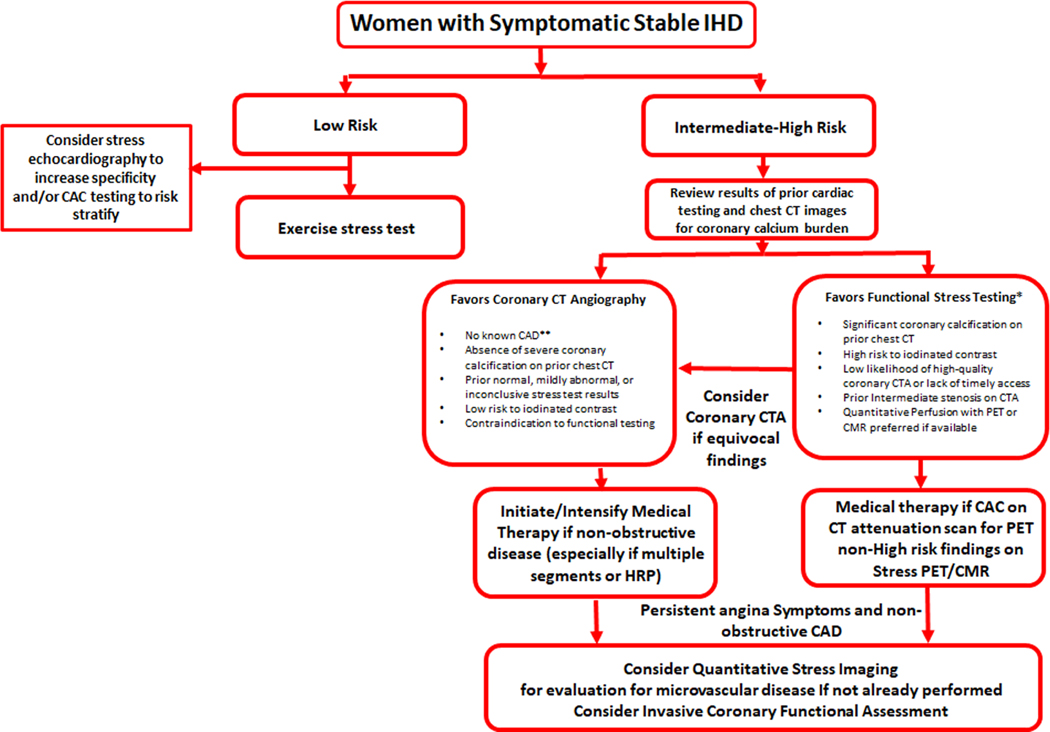
Figure 6.
PROPOSED DIAGNOSTIC ALGORYTHM SPECIFIC TO WOMEN WITH SUSPECTED STABLE ISCHEMIC HEART DISEASE
*Stress CMR, Stress echocardiography, Stress PET, Stress SPECT
**Prior myocardial infarction, coronary revascularization, or stenosis >50% on prior ICA/CTA
Highlights.
Ischemic heart disease is a leading cause of morbidity and mortality for women around the world.
Sex-specific differences in both the pathophysiology of ischemic heart disease (IHD) and the performance of different noninvasive tests necessitate a sex-based diagnostic work-up.
Whether the initial strategy is an anatomical or functional test, in patients with refractory symptoms, assessment for coronary microvascular disease (CMD) should be performed.
Future algorithms must include functional, anatomical, and physiologic assessments of endothelial and microvascular function.
Acknowledgments
Disclosures: Dr. Salerno receives grant support from NIH R01 HL131919-01A1, R01 HL155962-01 and research support from Siemens Healthineers, Patricia Rodriguez and Dr. Elona Rrapo are supported by 5T32EB003841. Dr. Kramer is supported by R01 HL075792
Abbreviations:
- CAD
Coronary artery disease
- IHD
Ischemic Heart Disease
- CCTA
Coronary computed tomographic angiography
- CMR
Cardiac magnetic resonance imaging
- PET
Positron emission tomographic imaging
- CFR
coronary flow reserve
- INOCA
Ischemia with nonobstructive Coronary Artery Disease
- MPI
Myocardial Perfusion Imaging
- MACE
Major adverse cardiovascular events
- CACS
coronary artery calcium scoring
- MPR
Myocardial Perfusion Reserve
- MINOCA
Myocardial Infarction with non obstructive Coronary Artery Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gulati M, et al. , 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain. Journal of the American College of Cardiology, 2021. 78(22): p. e187–e285. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, et al. , Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 2020. 141(9): p. e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Kunadian V, et al. , An EAPCI Expert Consensus Document on Ischaemia with NonObstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. European Heart Journal, 2020. 41(37): p. 3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal NR, et al. , Sex Differences in Ischemic Heart Disease: Advances, Obstacles, and Next Steps. Circ Cardiovasc Qual Outcomes, 2018. 11(2): p. e004437. [DOI] [PubMed] [Google Scholar]
- 5.Hiteshi AK, et al. , Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol, 2014. 37(10): p. 605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mericli M, et al. , Estrogen replacement therapy reverses changes in intramural coronary resistance arteries caused by female sex hormone depletion. Cardiovascular Research, 2004. 61(2): p. 317–324. [DOI] [PubMed] [Google Scholar]
- 7.Taqueti VR, Sex Differences in the Coronary System. Advances in experimental medicine and biology, 2018. 1065: p. 257–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MR, et al. , Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J, 2014. 167(6): p. 846–52.e2. [DOI] [PubMed] [Google Scholar]
- 9.Mieres JH, et al. , Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation, 2014. 130(4): p. 350–79. [DOI] [PubMed] [Google Scholar]
- 10.Dolor RJ, et al. , AHRQ Comparative Effectiveness Reviews, in Noninvasive Technologies for the Diagnosis of Coronary Artery Disease in Women. 2012, Agency for Healthcare Research and Quality (US): Rockville (MD). [PubMed] [Google Scholar]
- 11.Keteepe-Arachi T. and Sharma S, Cardiovascular Disease in Women: Understanding Symptoms and Risk Factors. European cardiology, 2017. 12(1): p. 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw LJ, et al. , Comparative Effectiveness of Exercise Electrocardiography With or Without Myocardial Perfusion Single Photon Emission Computed Tomography in Women With Suspected Coronary Artery Disease. Circulation, 2011. 124(11): p. 1239–1249. [DOI] [PubMed] [Google Scholar]
- 13.Taqueti VR, et al. , Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. J Nucl Cardiol, 2017. 24(4): p. 1402–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mieres JH, et al. , Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation, 2014. 130(4): p. 350–79. [DOI] [PubMed] [Google Scholar]
- 15.Kwok Y, et al. , Meta-analysis of exercise testing to detect coronary artery disease in women. The American Journal of Cardiology, 1999. 83(5): p. 660–666. [DOI] [PubMed] [Google Scholar]
- 16.Kim M-N, et al. , Head to Head Comparison of Stress Echocardiography with Exercise Electrocardiography for the Detection of Coronary Artery Stenosis in Women. J Cardiovasc Ultrasound, 2016. 24(2): p. 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mieres JH, et al. , Role of Noninvasive Testing in the Clinical Evaluation of Women With Suspected Ischemic Heart Disease. Circulation, 2014. 130(4): p. 350–379. [DOI] [PubMed] [Google Scholar]
- 18.Hansen CL, Crabbe D, and Rubin S, Lower diagnostic accuracy of thallium-201 SPECT myocardial perfusion imaging in women: an effect of smaller chamber size. J Am Coll Cardiol, 1996. 28(5): p. 1214–9. [DOI] [PubMed] [Google Scholar]
- 19.Shaw LJ, et al. , Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation, 2011. 124(11): p. 1239–49. [DOI] [PubMed] [Google Scholar]
- 20.Kwok Y, et al. , Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol, 1999. 83(5): p. 660–6. [DOI] [PubMed] [Google Scholar]
- 21.Metz LD, et al. , The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol, 2007. 49(2): p. 227–37. [DOI] [PubMed] [Google Scholar]
- 22.Valeti US, et al. , Exercise single-photon emission computed tomography provides effective risk stratification of elderly men and elderly women. Circulation, 2005. 111(14): p. 1771–6. [DOI] [PubMed] [Google Scholar]
- 23.Shaw LJ, et al. , Ethnic differences in the prognostic value of stress technetium-99m tetrofosmin gated single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol, 2005. 45(9): p. 1494–504. [DOI] [PubMed] [Google Scholar]
- 24.Budoff MJ, et al. , Diagnostic Performance of 64-Multidetector Row Coronary Computed Tomographic Angiography for Evaluation of Coronary Artery Stenosis in Individuals Without Known Coronary Artery Disease: Results From the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. Journal of the American College of Cardiology, 2008. 52(21): p. 1724–1732. [DOI] [PubMed] [Google Scholar]
- 25.Tsang JC, et al. , Sex comparison of diagnostic accuracy of 64-multidetector row coronary computed tomographic angiography: results from the multicenter ACCURACY trial. J Cardiovasc Comput Tomogr, 2012. 6(4): p. 246–51. [DOI] [PubMed] [Google Scholar]
- 26.Penagaluri A, et al. , Computed Tomographic Perfusion Improves Diagnostic Power of Coronary Computed Tomographic Angiography in Women: Analysis of the CORE320 Trial (Coronary Artery Evaluation Using 320-Row Multidetector Computed Tomography Angiography and Myocardial Perfusion) According to Gender. Circ Cardiovasc Imaging, 2016. 9(11). [DOI] [PubMed] [Google Scholar]
- 27.Meijboom WB, et al. , Comparison of Diagnostic Accuracy of 64-Slice Computed Tomography Coronary Angiography in Women Versus Men With Angina Pectoris. The American Journal of Cardiology, 2007. 100(10): p. 1532–1537. [DOI] [PubMed] [Google Scholar]
- 28.Douglas PS, et al. , Outcomes of anatomical versus functional testing for coronary artery disease. The New England journal of medicine, 2015. 372(14): p. 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagidipati NJ, et al. , Sex Differences in Functional and CT Angiography Testing in Patients With Suspected Coronary Artery Disease. J Am Coll Cardiol, 2016. 67(22): p. 2607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulman-Marcus J, et al. , Sex-Specific Associations Between Coronary Artery Plaque Extent and Risk of Major Adverse Cardiovascular Events: The CONFIRM Long-Term Registry. JACC Cardiovasc Imaging, 2016. 9(4): p. 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw LJ, et al. , Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. European Heart Journal, 2018. 39(41): p. 3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez K, et al. , Coronary Plaque Burden at Coronary CT Angiography in Asymptomatic Men and Women. Radiology, 2015. 277(1): p. 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kral BG, et al. , Noncalcified coronary plaque volumes in healthy people with a family history of early onset coronary artery disease. Circ Cardiovasc Imaging, 2014. 7(3): p. 446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferencik M, et al. , Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol, 2018. 3(2): p. 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gepner AD, et al. , Comparison of Coronary Artery Calcium Presence, Carotid Plaque Presence, and Carotid Intima-Media Thickness for Cardiovascular Disease Prediction in the Multi-Ethnic Study of Atherosclerosis. Circulation: Cardiovascular Imaging, 2015. 8(1): p. e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelkar AA, et al. , Long-Term Prognosis After Coronary Artery Calcium Scoring Among Low-Intermediate Risk Women and Men. Circulation: Cardiovascular Imaging, 2016. 9(4): p. e003742. [DOI] [PubMed] [Google Scholar]
- 37.Villines TC, et al. , Prevalence and Severity of Coronary Artery Disease and Adverse Events Among Symptomatic Patients With Coronary Artery Calcification Scores of Zero Undergoing Coronary Computed Tomography Angiography: Results From the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) Registry. Journal of the American College of Cardiology, 2011. 58(24): p. 2533–2540. [DOI] [PubMed] [Google Scholar]
- 38.Lubbers M, et al. , Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J, 2016. 37(15): p. 1232–43. [DOI] [PubMed] [Google Scholar]
- 39.Rozanski A. and Berman DS, The Synergistic Use of Coronary Artery Calcium Imaging and Noninvasive Myocardial Perfusion Imaging for Detecting Subclinical Atherosclerosis and Myocardial Ischemia. Current Cardiology Reports, 2018. 20(7): p. 59. [DOI] [PubMed] [Google Scholar]
- 40.Parker MW, et al. , Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging, 2012. 5(6): p. 700–7. [DOI] [PubMed] [Google Scholar]
- 41.Mc Ardle BA, et al. , Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J Am Coll Cardiol, 2012. 60(18): p. 1828–37. [DOI] [PubMed] [Google Scholar]
- 42.Dorbala S, et al. , Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol, 2013. 61(2): p. 176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taqueti VR and Di Carli MF, Radionuclide myocardial perfusion imaging for the evaluation of patients with known or suspected coronary artery disease in the era of multimodality cardiovascular imaging. Prog Cardiovasc Dis, 2015. 57(6): p. 644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pepine CJ, et al. , Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol, 2010. 55(25): p. 2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taqueti VR, et al. , Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation, 2017. 135(6): p. 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maddox TM, et al. , Nonobstructive coronary artery disease and risk of myocardial infarction. Jama, 2014. 312(17): p. 1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Hoef TP, et al. , Diagnostic and Prognostic Implications of Coronary Flow Capacity: A Comprehensive Cross-Modality Physiological Concept in Ischemic Heart Disease. JACC Cardiovasc Interv, 2015. 8(13): p. 1670–80. [DOI] [PubMed] [Google Scholar]
- 48.Feher A. and Sinusas AJ, Quantitative Assessment of Coronary Microvascular Function. Circulation: Cardiovascular Imaging, 2017. 10(8): p. e006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taqueti VR, et al. , Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation, 2017. 135(6): p. 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taqueti VR, et al. , Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J, 2018. 39(10): p. 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucciarelli-Ducci C, et al. , Cardiovascular disease in women: insights from magnetic resonance imaging. J Cardiovasc Magn Reson, 2020. 22(1): p. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenwood JP, et al. , Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet, 2012. 379(9814): p. 453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenwood JP, et al. , Comparison of Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography in Women With Suspected Coronary Artery Disease From the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation, 2014. 129(10): p. 1129–1138. [DOI] [PubMed] [Google Scholar]
- 54.Lipinski MJ, et al. , Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol, 2013. 62(9): p. 826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panting JR, et al. , Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med, 2002. 346(25): p. 1948–53. [DOI] [PubMed] [Google Scholar]
- 56.Kellman P, et al. , Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson, 2017. 19(1): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zorach B, et al. , Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. Journal of Cardiovascular Magnetic Resonance, 2018. 20(1): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mauricio R, et al. , Stress Cardiac MRI in Women With Myocardial Infarction and Nonobstructive Coronary Artery Disease. Clin Cardiol, 2016. 39(10): p. 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomson LE, et al. , Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging, 2015. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sammut EC, et al. , Prognostic Value of Quantitative Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging, 2018. 11(5): p. 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Indorkar R, et al. , Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovasc Imaging, 2019. 12(8 Pt 2): p. 1686–1695. [DOI] [PubMed] [Google Scholar]
- 62.Knott KD, et al. , The Prognostic Significance of Quantitative Myocardial Perfusion. Circulation, 2020. 141(16): p. 1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nickander J, et al. , Females have higher myocardial perfusion, blood volume and extracellular volume compared to males – an adenosine stress cardiovascular magnetic resonance study. Scientific Reports, 2020. 10(1): p. 10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorach B, et al. , Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson, 2018. 20(1): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nickander J, et al. , Females have higher myocardial perfusion, blood volume and extracellular volume compared to males - an adenosine stress cardiovascular magnetic resonance study. Sci Rep, 2020. 10(1): p. 10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dastidar AG, et al. , Prognostic Role of CMR and Conventional Risk Factors in Myocardial Infarction With Nonobstructed Coronary Arteries. JACC Cardiovasc Imaging, 2019. 12(10): p. 1973–1982. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds HR, et al. , Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation, 2021. 143(7): p. 624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dastidar AG, et al. , Myocardial Infarction With Nonobstructed Coronary Arteries: Impact of CMR Early After Presentation. JACC Cardiovasc Imaging, 2017. 10(10 Pt A): p. 1204–1206. [DOI] [PubMed] [Google Scholar]
- 69.Genders TS, et al. , A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J, 2011. 32(11): p. 1316–30. [DOI] [PubMed] [Google Scholar]
- 70.Juarez-Orozco LE, et al. , Impact of a decreasing pre-test probability on the performance of diagnostic tests for coronary artery disease. Eur Heart J Cardiovasc Imaging, 2019. 20(11): p. 1198–1207. [DOI] [PubMed] [Google Scholar]
- 71.Budoff MJ, et al. , Long-Term Prognosis Associated With Coronary Calcification. Journal of the American College of Cardiology, 2007. 49(18): p. 1860–1870. [DOI] [PubMed] [Google Scholar]
- 72.Schindler TH and Dilsizian V, Coronary Microvascular Dysfunction: Clinical Considerations and Noninvasive Diagnosis. JACC Cardiovasc Imaging, 2020. 13(1 Pt 1): p. 140–155. [DOI] [PubMed] [Google Scholar]
- 73.Naya M, et al. , Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med, 2014. 55(2): p. 248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.AlBadri A, et al. , Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. Journal of the American College of Cardiology, 2019. 73(6): p. 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Handberg EM, et al. , Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J, 2021. 237: p. 90–103. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds HR, et al. , Natural History of Patients With Ischemia and No Obstructive Coronary Artery Disease. Circulation, 2021. 144(13): p. 1008–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graham G, Acute Coronary Syndromes in Women: Recent Treatment Trends and Outcomes. Clinical Medicine Insights: Cardiology, 2016. 10: p. CMC.S37145. [DOI] [PMC free article] [PubMed] [Google Scholar]