Abstract
Reactions that lead to destruction of aromatic ring systems often require harsh conditions and, thus, take place with poor selectivities. Selective partial dearomatization of fused arenes is even more challenging but can be a strategic approach to creating versatile, complex polycyclic frameworks. Herein we describe a general organophotoredox approach for the chemo- and regioselective dearomatization of structurally diverse polycyclic aromatics, including quinolines, isoquinolines, quinoxalines, naphthalenes, anthracenes and phenanthrenes. The success of the method for chemoselective oxidative rupture of aromatic moieties relies on precise manipulation of the electronic nature of the fused polycyclic arenes. Mechanistic studies show that the addition of a hydrogen atom transfer (HAT) agent helps favor the dearomatization pathway over the more thermodynamically downhill aromatization pathway. We show that this strategy can be applied to rapid synthesis of biologically valued targets and late-stage skeletal remodeling en route to complex structures.
Subject terms: Synthetic chemistry methodology, Photocatalysis
The synthesis of polycyclic structures bearing partially saturated (hetero)arenes is important as many of these structures have biological relevance. Here the authors develop a one-step method for selective dearomative functionalization of feedstock (hetero)arenes via organophotocatalysis.
Introduction
Polycyclic scaffolds bearing partially dearomatized fused arenes are commonly encountered in natural products, pharmaceuticals and bioactive molecules1–5. While these molecular frameworks lead to great structural diversity and intriguing biological properties, they engender synthetic challenges as their assembly often requires prefunctionalized substrates and multi-step sequences. Direct dearomatization of fused arenes constitutes an efficient approach for the construction of polycyclic scaffolds due to its high atom and step economy1–5. However, selective dearomatization and functionalization of fused arenes is difficult because harsh conditions are often required to disrupt aromaticity, leading to poor selectivities. As a result, dearomative functionalization of fused arenes has been largely limited to activated arenes such as indoles6–15 and naphthols16–20.
Issues of chemo- and regioselectivity also add to the difficulty of dearomative functionalizations of unactivated fused arenes such as quinolines and naphthalenes. Selective dearomatization of pyridine moieties in fused arenes is feasible21–25 owing to the electron deficient nature of pyridine and assistance provided by Lewis acid complexation. Selective dearomatization of phenyl moieties in fused arenes, on the other hand, is significantly more challenging. To our knowledge, only one example has been described in a recent report by Brown, Houk and Glorius involving a photochemical [4 + 2] cycloaddition between quinolines and alkenes (Fig. 1b)26 enabled by energy transfer and Lewis acid activation. A similar [4 + 2] cycloaddition strategy initiated by energy transfer was also employed in the dearomatization of naphthalene by Sarlah et al. (Fig. 1c)27–32.
Fig. 1. Methods for selective dearomative functionalization of fused arenes.
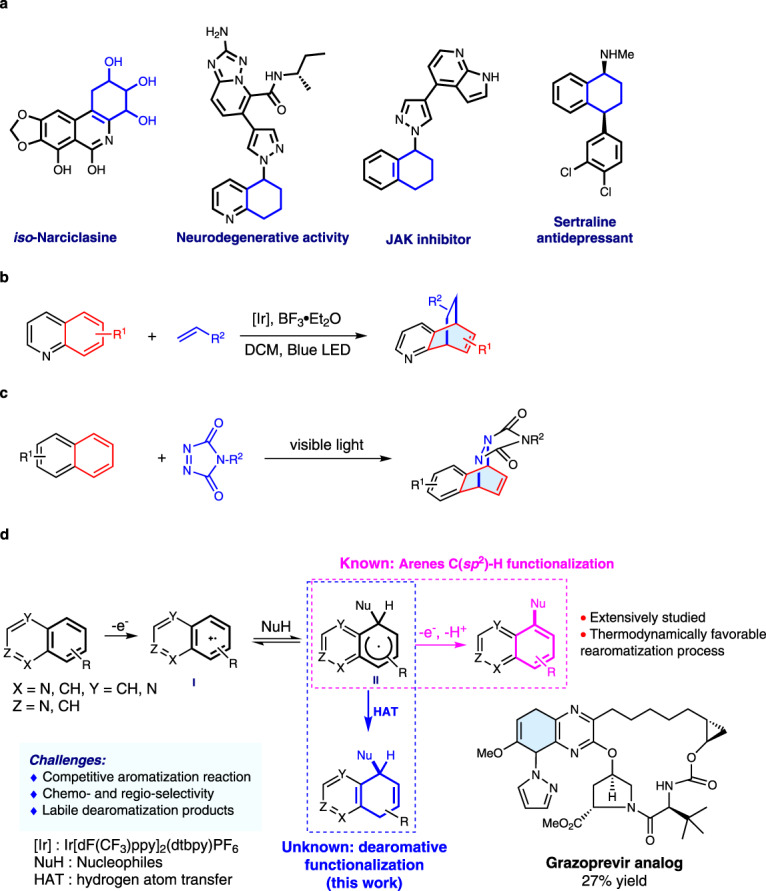
a Examples of partially dearomatic natural products and bioactive compounds. b Selective dearomatization of quinolines. c Selective dearomatization of naphthalenes. d Selective dearomatization of structurally diverse fused arenes (this work).
We wondered if a photoredox strategy could be used to selectively dearomatize the phenyl moieties in quinolines, isoquinolines, quinoxalines, naphthalenes and other fused arenes. In reported photoredox processes, radicals add selectively to the pyridine ring in quinolines33,34, and indiscriminately to the phenyl rings in naphthalenes. In contrast to Sarlah’s [4 + 2] cycloaddition strategy, in which regioselectivity is governed primarily by steric effects27,32, we envisaged taking advantage of the relative electron richness of the phenyl ring to achieve selective dearomative functionalization (Fig. 1d). Photocatalytically promoted single electron transfer (SET) oxidation of quinolines would produce radical cation I, in which the positive charge is mostly localized on the phenyl ring. We anticipated nucleophiles to add to intermediate I selectively to form radical II. Direct arene C(sp2)-H functionalization with nucleophiles under photoredox catalysis or electrophotochemical conditions have been elegantly leveraged by Nicewicz35–38, Lambert39, Hu40, and Wickens41 to generate aromatic functionalized products. We postulated that it would be possible to direct the reaction toward the less thermodynamically stable dearomatized products by capturing radical II with a sufficiently activated hydrogen atom transfer (HAT) agent.
In this work, we describe the successful development of a general chemo- and regioselective method for the dearomative functionalization of diverse fused arenes using this strategy.
Results and discussion
Reaction model design
In order to undergo selective photoredox promoted dearomative functionalization, fused arenes must have oxidation potentials that enable them to be oxidized by excited states of photocatalysts (PCs) through SET. The oxidation potential of unsubstituted quinoline (Eox = 2.23 V vs SCE, Supplementary Fig. 2) suggests that its oxidation would be very difficult using common PCs. However, introduction of an OMe group onto the phenyl moiety of quinoline lowers the oxidation potential sufficiently (e.g., 6-methoxyquinoline 1a, Eox = 1.83 V vs SCE, Table 1 and Supplementary Fig. 2), making it possible to participate in thermodynamically driven SETs with excited states of conventional organophotoredox catalysts such as acridinium and triphenylpyrylium (TPT) salts that have excited state reduction potentials (E*red) greater than 2.0 V (Table 1).
Table 1.
Exploration and optimization of reaction conditions
 | ||
|---|---|---|
| Entry | Variation from the “Standard Conditions”a | Yieldb (%) |
| 1 | None | 81, 76c (<5 of 4a)b |
| 2 | Mes-Acr1 instead of Mes-Acr2 | 75 |
| 3 | Mes-Acr3 instead of Mes-Acr2 | 67 |
| 4 | DCA instead of Mes-Acr2 | 34 |
| 5 | TPT instead of Mes-Acr2 | 18 |
| 6 | 4CzIPN instead of Mes-Acr2 | trace |
| 7 | DCM (0.5 M) with 2,6-lutidine (0.2 equiv) | 20 |
| 8 | DCM (0.2 M) with 2,6-lutidine (0.2 equiv) | 49 |
| 9 | DCM (0.1 M) with 2,6-lutidine (0.2 equiv) | 63 |
| 10 | DCM (0.05 M) with 2,6-lutidine (0.2 equiv) | 76 |
| 11 | DCM (0.025 M) with 2,6-lutidine (0.2 equiv) | 74 |
| 12 | without Ph3SiSH | 53 (23 of 4a)b |
| 13 | without Mes-Acr2 | trace |
| 14 | without light | trace |
| 15 | 390 nm light | 40 |
| 16 | O2 instead of N2 | trace (56 of 4a)b |
aStandard conditions: unless otherwise specified, a mixture of 6-methoxyquinoline 1a (0.3 mmol, 0.05 M), pyrazole 1b (0.2 mmol), Ph3SiSH (0.04 mmol), and Mes-Acr2 (0.005 mmol) in DCM under a N2 atmosphere at room temperature was irradiated with 40 W Kessil blue LEDs for 48 h.
bYield determined by 1H NMR spectroscopy using 1,3,5-trimethoxybenzene as an internal standard.
cIsolated yield.
Reaction optimization
We assessed the viability of the proposed dearomative functionalization protocol by reacting 6-methoxyquinoline 1a with pyrazole 2a as a nucleophile under irradiation with blue LEDs in DCM solution containing HAT agents and commercially available organic photosensitizers (Table 1). The desired dearomatization product 3a is generated in the highest yield using 1.5 equiv of 1a, 1.0 equiv of 2a (0.05 M), 2.5 mol% N-phenylmeso-acridinium tetrafluoroborate (Mes-Acr2), and 0.2 equiv of the HAT agent. Nucleophile addition occurred exclusively at the 5-position. The efficiency of the reaction is dependent on the HAT agent (Supplementary Table 1). Among the 17 different thiols and benzeneselenols screened, Ph3SiSH was found to be the superior HAT agent, leading to regioselective formation of 3a in 81% yield (Table 1, entry 1). While photocatalysts such as TPT (E*red = 2.55 V vs SCE, 18%, entry 4) with excited state reduction potentials higher than the oxidation potential of 1a were found to promote this reaction, Mes-Acr2 (2.5 mol%, E*red = 2.2 V vs SCE) was identified as the best photocatalyst (3a formed 81% 1H NMR yield, entry 1). Solvent has a significant effect on the reaction (Supplementary Table 2). Furthermore, substrate ratio (Supplementary Table 3) and concentration have a marked impact on the yield (entries 7–11) with low concentrations of 1a being more beneficial (entries 7 vs 10). The absence of 2,6-lutidine does not affect the reaction outcome (entries 1 vs 10) as quinoline 1a can serve as the base. Lastly, control experiments confirm that the HAT agent, light and photocatalyst are all required for selective dearomatization. In particular, the HAT agent is critical for minimizing the formation of the aromatization product 4a (entries 1 vs 12).
Dearomatization of quinolines, isoquinolines, and quinoxalines
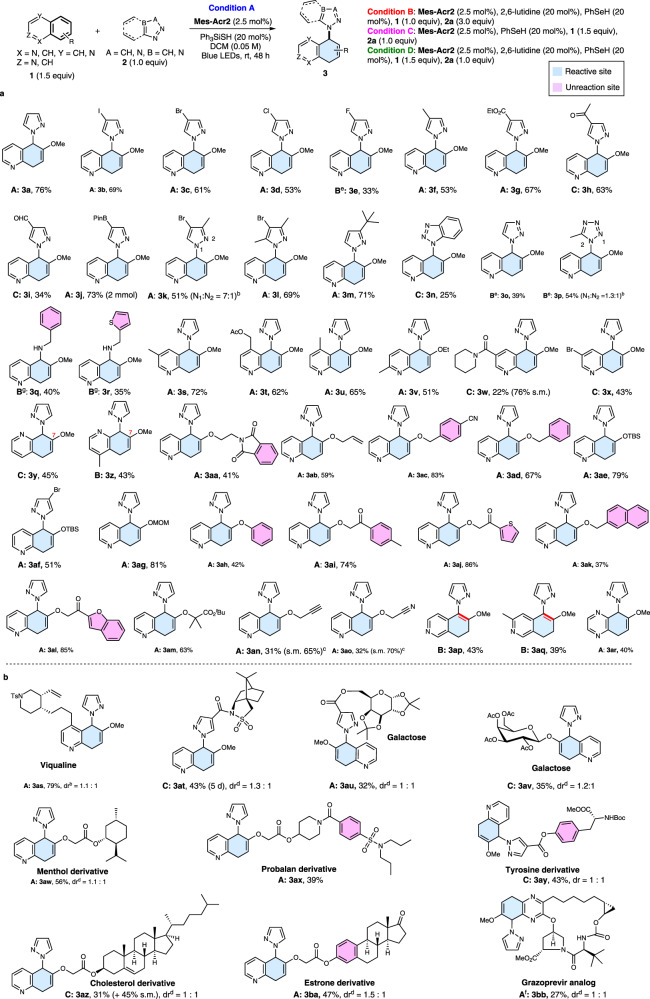
Having established optimal conditions for the photoredox promoted dearomative functionalization reaction, we next explored the scope with fused arenes as substrates and azoles as nucleophiles (Fig. 2a). Our results show that the reaction proceeds with high chemo- and regioselectivity for a wide range of quinolines and azoles. Both electron-donating (3f, 3m) and electron-withdrawing groups are tolerated on the pyrazole. Halogenated pyrazoles reacted with 6-methoxyquinoline with Ph3SiSH as the HAT agent (condition A giving 3b–3d). For 5-fluoropyrazole, benzeneselenol, which is 10-fold more reactive than thiols in HAT42,43, is required along with 2,6-lutidine (condition B) to yield 3e. Other electron-deficient pyrazoles also reacted in the presence of benzeneselenol to produce 3h and 3i (condition C, without 2,6-lutidine). Notably, the dearomatization method can be applied to a pyrazole boronate, which generates 3j (73%) containing a useful handle for subsequent coupling reactions. Di- and trisubstituted pyrazoles also reacted smoothly to form 3k (51%) and 3l (69%), as did other common azoles including benzotriazole (3n), 1,2,3-triazoles (3o), and tetrazole (3p) under condition B or C. Alkyl amines are also good nucleophiles for the dearomatization of quinolines, generating 3q (40%) and 3r (35%).
Fig. 2. Scope of photoredox dearomative functionalization of quinolines, isoquinolines, and quinoxalines with azoles.
a Scope of quinolines and quinoxalines with azoles. b Late-stage functionalization. aReaction conditions: unless otherwise specified, see footnote a of Table 1 and supplementary information; bRatio of isomers was determined by using 1H NMR analysis of the crude reaction mixture; cs.m.: starting materials; ddr value was determined by 1H NMR analysis of the crude reaction mixture; eCondition B without 2,6-lutidine; fCondition A using 3,6-di-tert-butyl-9-mesityl-10-phenylacridin-10-ium tetrafluoroborate as photocatalyst for 5 d. gCondition B but using amines (2.0 equiv) and 3,6-di-tert-butyl-9-mesityl-10-phenylacridin-10-ium tetrafluoroborate as photocatalyst for 2 d.
6-Alkoxy-substituted quinolines are particularly effective substrates for the dearomative functionalization reaction, producing diverse substituted 5,8-dihydroquinolines as products (Fig. 2a). A wide range of alkoxy substituents, including those containing methyl/ethyl (3s, 3u, 3v, and 3z), ester (3t, 3am), bromo (3x), amide (3w), phthalimide (3aa), alkene (3ab), benzyl (3ac, 3ad), TBS (3ae, 3af), MOM (3ag), phenyl (3ah), ketone (3ai), alkyne (3an), nitrile (3ac, 3ao), thiophene (3aj) and benzofuran (3al) moieties, are well tolerated. Moreover, the process promotes selective dearomatization and functionalization of quinoline ring systems even when other arene moieties including benzene (3q, 3aa, 3ac, 3ad, 3ah, 3ai), thiophene (3r, 3aj) and benzofuran (3al) are present. Notably, the protocol enables highly selective dearomatization of a quinoline (Eox = 1.86 V vs SCE) over a naphathalene (Eox = 1.92 V vs SCE) ring (3ak) despite their similar oxidation potentials. In addition to controlling chemoselectivity, 6-alkyloxy groups also direct site selective formation of 5-nucleophile-substituted-5,8-dihydroquinolines. Furthermore, dearomatization of 7-methoxyquinolines is also highly regioselective generating only 8-substituted 5,8-dihydroquinoline products (3y, 3z). Isoquinolines can participate in the process (3ap, 3aq). However, regioisomeric 7,8-dihydroisoquinolines are formed. Finally, quinoxalines are also viable substrate for the dearomatization process (3ar).
Owing to the mild conditions required, the organophotoredox-promoted dearomative functionalization process is applicable to late-stage functionalizations of complex pharmaceutically relevant quinoline structures including viqualine (3as), Oppolzer’s camphorsultam (3at), galactose (3au, 3av), menthol (3aw), probalan (3ax), tyrosine (3ay), cholesterol (3az), and estrone (3ba) groups (Fig. 2b). It is particularly impressive that a grazoprevir analog (3bb) containing various functional groups was selectively dearomatized utilizing this process. These results underscore the mildness, high chemo- and regioselectivity, and practicality of this protocol.
Dearomatization of fused arenes
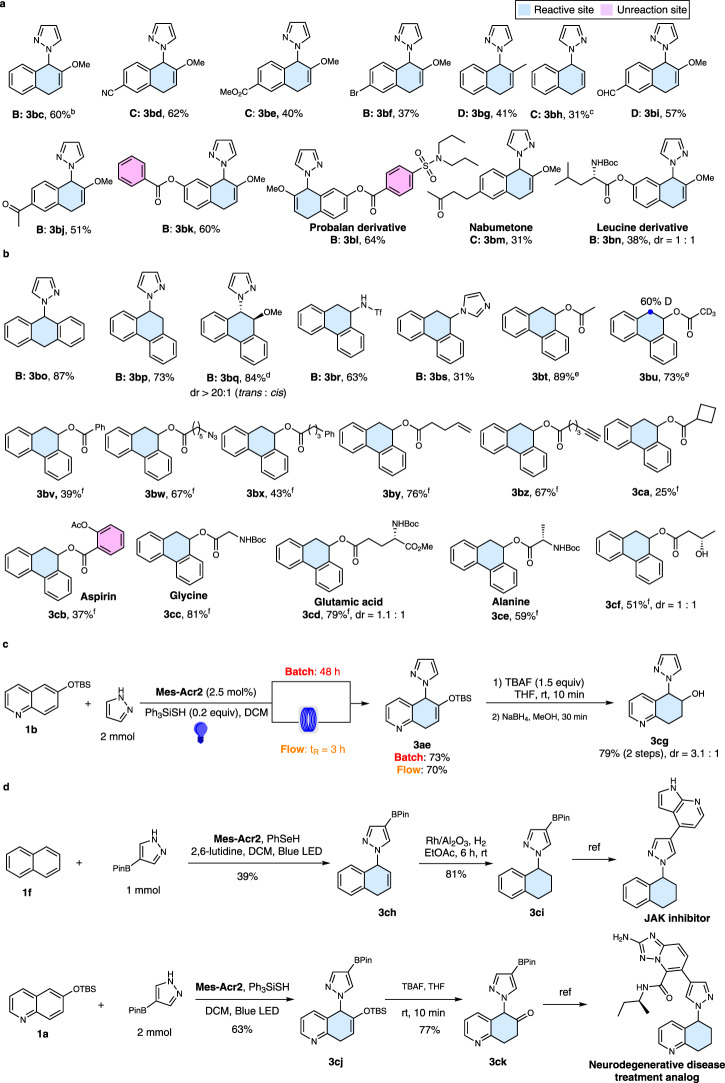
Encouraged by the success of the photoredox reaction of quinolines, we explored its utility for the selective dearomatization of fused non-heteroaromatics. The results show that selective dearomatization of fused arenes is more challenging. As with quinolines, electron-donating substituents can be used to lower the oxidation potentials and enhance reactivity. A 2-methoxy substituent in naphthalene, for instance, lowers the oxidation potential to 1.8 V (vs SCE, supplementary Fig. 2) and enables selective oxidation by the excited state of photocatalyst Mes-Acr2. Selective dearomatization and C-1 functionalization of 2-methoxynaphthalene by pyrazole using condition B led to 3bc (Fig. 3a). The efficiency of this process is also dependent on the base and the HAT agent, as are shown in 3bd, 3be, and 3bm (condition C), 3bf, 3bj, 3bk, 3bl, and 3bn (condition B) and 3bi (condition D). The dearomatization strategy can be applied to methyl substituted naphthalene (3bg). Finally, unsubstituted naphthalene (3bh) also participates in the process to produce the 1-substituted product regioselectively.
Fig. 3. Dearomatization of fused arenes.
a Scope of naphathalenes. b Scope of anthracenes and phenanathrenes. c Large scale synthesis. d Application in synthesis of valuable targets. aReaction condition: unless specified, see footnote a of Table 1 and the ESI; bRatio of 6-methoxynaphthalene to pyrazole: 1: 2; cRatio of naphthalene to pyrazole is 2:1; dReaction time: 4 d; eCondition B using 10.0 equiv of acid; fCondition B using 5.0 equiv of acid.
UV-mediated photochemcial dearomative functionalization of naphthalene and phenanthrene with excess amines and cyanide (10–25 equiv) were reported by Yasuda and Pac in 1970s and 80s44–47. However, these classic methods showed very limited substrate scopes and poor functional group tolerance due to the harsh activation method. Furthermore, it is challenging to employ these methods for dearomatization of fused heteroaromatic systems such as quinoline, isoquinoline, and quinaxoline because they cannot be activated by UV light. In contrast, our mild, visible-light-mediated photoredox reaction can be applied to the dearomative functionalization of polycyclic aromatic hydrocarbons with a broad substrate scope, in addition to highly-valued fused heteroaromatics. High yields with azoles, which are challenging nucleophiles in Yasuda and Pac’s UV method, are obtained for simple anthracene (3bo, 87%) and phenanthrene (3 bp, 73%) (Fig. 3b). Notably, 6-methoxyphenanthrene reacts to form the trans substitution product 3bq (84%). Moreover, nucleophiles other than azoles, such as trifluoromethanesulfonamide (3br) and imidazole (3bs), can also participate in the process. Furthermore, carboxylic acids, which are often used as radical precursors in photoredox reactions48, serve as effective nucleophiles for dearomative functionalization of phenanthrenes. These reactions proceed in moderate to high yields (3bt–3cf, 25–89%) and display broad functional group tolerance including phenyl (3bv, 3bx), azide (3bw), alkenyl (3by), alkynyl (3bz), cyclobutyl (3ca), and hydroxyl acid groups (3cf). Due to their weaker nucleophilicity and steric effects, benzoic acid (3cb) and secondary carboxylic acids (3ca) are less effective nucleophiles for this process. In addition, the reaction can be utilized for the direct modification of biologically relevant structures such as aspirin (3cb) and amino acids (3cc–3ce).
Synthetic applications
Studies with the silyloxyquinoline 1b show that the synthetic protocol can be scaled up without loss of yield (Fig. 3c). Moreover, the reaction can be adapted for use in a flow system, an emerging technology in organic synthesis49. Using the flow system, the reaction time is reduced dramatically to 3 h without compromising the yield. The product silylenol ether 3ae can be converted to the corresponding alcohol 3cg. The preparative power of this method was also demonstrated by its use in the cost-effective synthesis of important biologically active targets, including a JAK inhibitor (Fig. 3d)50. Our synthetic route starts from naphthalene ($0.04/g) instead of the more expensive 1-bromo-1,2,3,4-tetrahydronaphthalene ($370/g) used in the earlier preparative pathway50. Dearomatization of naphthalene with pyrazol-4-ylboronic acid pinacol ester produces key intermediate 3ch, which is then reduced using Rh/Al2O3 catalyzed hydrogenation to form 3ci. The JAK inhibitor is then readily prepared from 3ci using reported procedures50. For synthesis of the compound with neurodegenerative activity shown in Fig. 3d, our protocol employed 6-hydroxyquinoline ($1.6/g) as starting material, which is much cheaper than the previously used precursor, 5-hydroxy-5,6,7,8-tetrahydroquinoline ($416/g)51. These studies clearly demonstrate the synthetic value of the our dearomative functionalization protocol, which provides direct and efficient access to polycyclic partially dearomatized frameworks that were previously inaccessible or required strenuous synthetic efforts.
Mechanistic studies
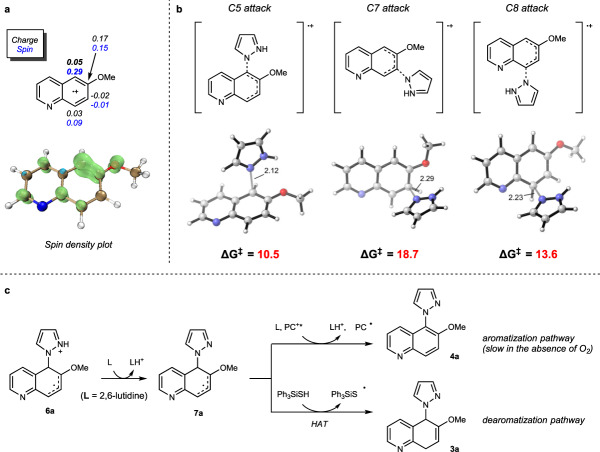
To gain insight into the reaction mechanism, we conducted additional experimental and computational studies. The excited state PC+* Mes-Acr2* possesses strong oxidizing power (Ered* = 2.2 V) and can oxidize the 6-methoxyquinoline (1a) (Eox = 1.83 V vs SCE, supplementary Fig. 2) to give radical cation 5a (Fig. 4a and Stern-Volmer quenching experiments, supplementary Figs. 3 and 4). The oxidation potential of the fused arene substrate is crucial for the success of this process, as demonstrated by the high reactivity of 1a in contrast to the lack of reactivity of unsubstituted quinoline (Eox = 2.23 V vs SCE, Supplementary Fig. 2). In addition, when a mixture of 1a and 6-acetoxyquinoline (1c) (Eox = 2.2 V vs SCE, supplementary Fig. 2) is subjected to the reaction conditions, only 1a undergoes selective dearomatization to form 3a, indicating that electron-withdrawing substituents inhibit the reaction. In contrast, 1a and 2-methoxynaphthalene (1e, Eox = 1.8 V vs SCE, Supplementary Fig. 2), which have similar oxidation potentials, both react under the same conditions to produce the corresponding dearomatized products 3a and 3bc (Fig. 4b). Likewise, phenanthrene 1d (Eox = 1.91 V vs SCE, Supplementary Fig. 2) is an effective substrate for this process (Fig. 4b). Notably, the pyrazole nucleophile (Eox = 2.15 V vs SCE, Supplementary Fig. 2) is not oxidized by Mes-Acr2*. This is supported by luminescence quenching study of Mes-Acr2 with varying concentrations of 6-methoxyquinoline 1a and pyrazole 2a (Supplementary Fig. 4).
Fig. 4. Mechanistic studies.
a Proposed catalytic cycle. b Chemoselectivity studies. c Study of roles of HAT reagent. d Study of regioselectivity of isoquinoline engaged reaction.
Following the oxidation of 1a through SET, pyrazole reacts with the electrophilic radical cation 5a to form the radical cation 6a (Fig. 4a). Calculated Hirshfeld charge and spin densities (Fig. 5a; see Supplementary Fig. 6 for calculated charge and spin densities for other substrates) indicate that C5 has the highest spin density (bold face) in 5a and is therefore the preferred site for nucleophilic addition. The calculated free energies of the key transition states also support the observation that pyrazole attack at the C5 position is kinetically favored over C8 or C7 by at least 3 kcal/mol (Fig. 5b).
Fig. 5. Computational investigations.
a Calculated Hirshfeld charge and spin densities of radical cation 5a at the ωB97X-D/def2-TZVPP, SMD (CH2Cl2)//ωB97X-D/def2-SVP level of theory. b Calculated C–N forming transition states for the reaction between 5a and pyrazole at the ωB97X-D/def2-TZVPP, SMD (CH2Cl2)//ωB97X-D/def2-SVP level of theory. Free energies are in kcal/mol, and interatomic distances are in Å. c Possible fates of radical cation 6a.
Subsequent exothermic deprotonation of 6a by 2,6-lutidine gives a neutral radical 7a (Fig. 5c). Calculated free energies suggest that sequential SET oxidation and deprotonation of 7a to form the aromatic substitution product 4a is more thermodynamically favorable (−62.5 kcal/mol from 7a with the excited state PC+* Mes-Acr2* as the oxidant, Figs. 5c and S7). This is reflected in the outcomes of processes leading to fully aromatic products developed by Nicewicz35–38, Lambert39, Hu40 and Wickens41. However, the aromatization pathway requires an oxidant (such as the excited-state photocatalyst or oxygen) to be present in sustained concentrations. Because this pathway does not regenerate the photocatalyst, the aromatization process has been previously noted to be slow in the absence of oxygen, despite its favorable thermodynamics.
Given the apparent hurdles in the aromatization pathway, we reasoned that the presence of a reactive HAT agent (e.g., Ph3SiSH) could intercept 7a and redirect the course of the reaction toward the dearomatized product 3a rather than the more stable aromatic product 4a (Fig. 5c). We elected to employ thiol and selenol HAT agents that have weak S-H and Se-H bonds (S-H: 82 kcal/mol, Se-H: 73 kcal/mol) and correspondingly high H-atom transfer rates (PhSH: K20 = 9.0 × 107 M−1S−1 and PhSeH: K20 = 1.3 × 109 M−1S−1)42,43,52. Indeed, the addition of the HAT agent had a marked effect on the yield of the dearomatized product. Control experiments showed that in the absence of the HAT agent Ph3SiSH, the yield of the dearomatized product 3ae dropped from 76% to 31% (Fig. 4c). The effect of the HAT agent is particularly noticeable in reactions of isoquinolines and naphthalenes with pyrazole. Only when PhSeH is present do these processes produce the desired products 3ap and 3bc (Fig. 4c). In these cases, the HAT process might have been further facilitated by the polarity match48 between the protic S-H/Se-H and the nucleophilic carbon radical 7a. Calculations on the HAT step also confirm that PhSeH decreases the barrier of the HAT step by about 9 kcal/mol relative to Ph3SiSH (Supplementary Fig. 7). After the HAT step, the Ph3SiS• radical is reduced to the Ph3SiS− anion by the PC• Mes-Acr2 radical. Protonation of the Ph3SiS− anion regenerates both the HAT agent Ph3SiSH and the ground-state photocatalyst Mes-Acr2 (Fig. 4a). Further computational efforts are underway in our laboratories to elucidate the full mechanistic picture (see discussion accompanying Supplementary Fig. 8).
In addition to promoting the formation of the dearomatized product 3a, the HAT agent Ph3SiSH also retards the conversion of 3a (Eox = 1.60 V vs SCE, Supplementary Fig. 2) back to the starting material 1a via PC+*(Mes-Acr2*)-mediated oxidation under the standard reaction conditions (Fig. 4c). Specifically, preliminary experiments show that the photoinduced reaction of 3a in the presence of Ph3SiSH leads to 61% recovery of 3ae and formation of 28% of 1b. In contrast, in the absence of the HAT agent, 1b was produced in 49% yield.
It is also worth noting that the dearomatization pathway selectively produced the 5,8-dihydroquinoline regioisomer 3a. We did not observe the formation of the 7,8-dihydroquinoline 3a’, which was calculated to be 3.8 kcal/mol less stable than 3a due to the steric repulsion between the alkene substituents (Supplementary Fig. 8). However, reactions between isoquinolines and pyrazole yielded the regioisomeric 7,8-dihydroisoquinolines. Our experiments suggest that the reaction initially forms 5,8-dihydroisoquinoline 3ap’, which is then converted to the more stable 7,8-dihydroisoquinoline 3ap (Fig. 4d). The 5,8-dihydroisoquinoline 3ap’ was obtained in the presence of H2O. Two possible pathways could lead to 5,8-dihydroisoquinoline 3ap. The deprotonation/C=C isomerization pathway was ruled out as we did not observe the formation of 3ap when 3ap’ was treated with 2,6-lutidine, a base used in the dearomatization process. In contrast, 3ap was formed under the photoredox reaction conditions through SET oxidation of the C=C bond, deprotonation and HAT processes. This pathway was also supported by calculated reaction energies (Supplementary Scheme 1).
In this work, we develop a conceptually unique organophotoredox catalytic dearomative functionalization strategy for the selective disruption of aromaticity in fused arenes. A reactive HAT agent is utilized to help direct the reaction toward the dearomatized product over the thermodynamically more favorable aromatizatized product. The preparative power of the protocol is demonstrated by applications to structurally diverse fused arenes including quinolines, isoquinolines, quinoxalines, naphthalenes, anthracenes and phenanthrenes. The method can also be applied to the synthesis and late-stage skeletal editing of complex pharmaceutically valued structures. We anticipate that this process will enable facile access to a wide range of synthetically versatile frameworks and accelerate the construction of new molecular architectures for drug discovery.
Methods
Representative procedures for the dearomatization of heteroarenes
To an oven-dried 20 mL-Schlenk tube equipped with a stir bar, was added pyrazole (0.2 mmol), quinoline derivatives (0.3 mmol), Ph3SiSH (0.04 mmol), and Mes-Acr2 (0.005 mmol). The tube was evacuated and back-filled with N2 for three times, then sealed with rubber stopper and parafilm. Subsequently, the degassed dichloromethane (4 mL) was added. The reaction was irradiated by the two 40 W Kessil Blue LEDs, cooling by the electronic fan. After the completion of reactions (usually 48 h), the resulted solution was purified by flash column chromatography on silica gel eluting with hexane/ethyl acetate or DCM/ethyl acetate in indicated ratio.
Representative procedures for the dearomatization of arenes
To an oven-dried 20 mL-Schlenk tube equipped with a stir bar, was added pyrazole (0.6 mmol), naphthalene derivatives (0.2 mmol), PhSeH (0.04 mmol), Mes-Acr2 (0.005 mmol), 2,6-lutidine (0.04 mmol) as the base, will be added. The tube was evacuated and back-filled with N2 for three times, then sealed with rubber stopper and parafilm. Subsequently, the degassed dichloromethane (4 mL) was added. The reaction was irradiated by the two 40 W Kessil Blue LEDs, cooling by the electronic fan. After the completion of reactions, the resulted solution was purified by flash column chromatography on silica gel eluting with hexane/ethyl acetate or DCM/ethyl acetate in the indicated ratio.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
W.W. acknowledges the NIH (5R01GM125920) and University of Arizona for financial support and the NSF MRI for acquisition of 500 MHz NMR spectrometer (1920234). S.C. is grateful to Oberlin College for financial support. K.N.H thanks the National Science Foundation (Grant CHE-1764328). DFT calculations were performed using the SCIURus, the Oberlin College HPC cluster (NSF MRI 1427949).
Author contributions
P.J., F.G., J.C., and X.M. conducted and analyzed the synthetic experiments. C.C.D. performed DFT calculations under the direction of S.C. K.N.H. and W.W. planned, designed and directed the project. P.J., S.C., K.N.H. and W.W. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks Lisa Roy, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Data availability
Experimental procedures and characterization data are given in the Supplementary Information. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files. For Cartesian coordinates, see Supplementary Data 1 file. Further data can be obtained from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kendall N. Houk, Email: houk@chem.ucla.edu
Shuming Chen, Email: shuming.chen@oberlin.edu.
Wei Wang, Email: wwang@pharmacy.arizona.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-32201-7.
References
- 1.Pape AR, Kaliappan KP, Kündig EP. Transition-metal-mediated dearomatization reactions. Chem. Rev. 2000;100:2917–2940. doi: 10.1021/cr9902852. [DOI] [PubMed] [Google Scholar]
- 2.López Ortiz F, Iglesias MJ, Fernández I, Andújar Sánchez CM, Ruiz Gómez G. Nucleophilic dearomatizing (DNAr) reactions of aromatic C-H systems. A mature paradigm in organic synthesis. Chem. Rev. 2007;107:1580–1691. doi: 10.1021/cr030207l. [DOI] [PubMed] [Google Scholar]
- 3.Roche SP, Porco JA. Dearomatization strategies in the synthesis of complex natural products. Angew. Chem. Int. Ed. 2011;50:4068–4093. doi: 10.1002/anie.201006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng C, You S-L. Advances in catalytic asymmetric dearomatization. ACS Cen. Sci. 2021;7:432–444. doi: 10.1021/acscentsci.0c01651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huck CJ, Sarlah D. Shaping molecular landscapes: recent advances, opportunities, and challenges in dearomatization. Chem. 2020;6:1589–1603. doi: 10.1016/j.chempr.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche SP, Tendoung J-JY, Tréguier B. Advances in dearomatization strategies of indoles. Tetrahedron. 2015;71:3549–3591. doi: 10.1016/j.tet.2014.06.054. [DOI] [Google Scholar]
- 7.Zheng C, You SL. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 2019;36:1589–1605. doi: 10.1039/C8NP00098K. [DOI] [PubMed] [Google Scholar]
- 8.Gentry EC, Rono LJ, Hale ME, Matsuura R, Knowles RR. Enantioselective synthesis of pyrroloindolines via noncovalent stabilization of indole radical cations and applications to the synthesis of alkaloid natural products. J. Am. Chem. Soc. 2018;140:3394–3402. doi: 10.1021/jacs.7b13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An J, Zou Y-Q, Yang Q-Q, Wang Q, Xiao W-J. Visible light-induced aerobic oxyamidation of indoles: a photocatalytic strategy for the preparation of tetrahydro-5h-indolo[2,3-b]quinolinols. Adv. Synth. Catal. 2013;355:1483–1489. doi: 10.1002/adsc.201300175. [DOI] [Google Scholar]
- 10.Zhu M, Zheng C, Zhang X, You S-L. Synthesis of cyclobutane-fused angular tetracyclic spiroindolines via visible-light-promoted intramolecular dearomatization of indole derivatives. J. Am. Chem. Soc. 2019;141:2636–2644. doi: 10.1021/jacs.8b12965. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, et al. Gadolinium photocatalysis: Dearomative [2+2] cycloaddition/ring-expansion sequence with indoles. Angew. Chem. Int. Ed. 2020;59:9639–9645. doi: 10.1002/anie.202001200. [DOI] [PubMed] [Google Scholar]
- 12.Liu, K. et al Electrooxidation enables highly regioselectivedearomative annulation of indole and benzofuran derivatives. Nat. Commun. 11, 10.1038/s41467-019-13829-4 (2020). [DOI] [PMC free article] [PubMed]
- 13.Wu J, Dou Y, Guillot R, Cyrille Kouklovsky C, Vincent G. Electrochemical dearomative 2,3-difunctionalization of indoles. J. Am. Chem. Soc. 2019;141:2832–2837. doi: 10.1021/jacs.8b13371. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Organophotocatalytic dearomatization of indoles, pyrroles and benzo(thio)furans via a Giese-type transformation. Commun. Chem. 2021;4:20. doi: 10.1038/s42004-021-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Photoredox asymmetric nucleophilic dearomatization of indoles with neutral radicals. ACS Catal. 2021;11:998–1007. doi: 10.1021/acscatal.0c04696. [DOI] [Google Scholar]
- 16.Pouységu L, Deffieux D, Quideau S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron. 2010;66:2235–2261. doi: 10.1016/j.tet.2009.12.046. [DOI] [Google Scholar]
- 17.Dhineshkumar J, Samaddar P, Prabhu KR. A copper catalyzed azidation and peroxidation of β-naphthols via an oxidative dearomatization strategy. Chem. Commun. 2016;52:11084–11087. doi: 10.1039/C6CC04894C. [DOI] [PubMed] [Google Scholar]
- 18.Kulish K, Boldrini C, Reis MC, Pérez JM, Harutyunyan SR. Lewis acid promoted dearomatization of naphthols. Chem. Eur. J. 2020;26:15843. doi: 10.1002/chem.202003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, W., Li, G., Hong, L., & Wang, R. Asymmetric dearomatization of phenols. Org. Biomol. Chem. 2164-2176 (2016) [DOI] [PubMed]
- 20.Zhu G, et al. Chiral phosphoric acid catalyzed asymmetric oxidative dearomatization of naphthols with quinones. Org. Lett. 2016;18:5288–5291. doi: 10.1021/acs.orglett.6b02609. [DOI] [PubMed] [Google Scholar]
- 21.Takamura M, Funabashi K, Kanai M, Shibasaki M. Asymmetric Reissert-type reaction promoted by bifunctional catalyst. J. Am. Chem. Soc. 2000;122:6327–6328. doi: 10.1021/ja0010352. [DOI] [Google Scholar]
- 22.Zurro M, Asmus S, Beckendorf S, Mück-Lichtenfeld C, Mancheño OG. Chiral helical oligotriazoles: new class of anion-binding catalysts for the asymmetric dearomatization of electron-deficient N-heteroarenes. J. Am. Chem. Soc. 2014;136:13999–14002. doi: 10.1021/ja507940k. [DOI] [PubMed] [Google Scholar]
- 23.Pappoppula M, Cardoso FSP, Garrett BO, Aponick A. Enantioselective copper-catalyzed quinoline alkynylation. Angew. Chem. Int. Ed. 2015;54:15202–15206. doi: 10.1002/anie.201507848. [DOI] [PubMed] [Google Scholar]
- 24.Yan X, Ge L, Castiñeira Reis M, Harutyunyan SR. Nucleophilic dearomatization of N-heteroaromatics enabled by Lewis acids and copper catalysis. J. Am. Chem. Soc. 2020;142:20247–20256. doi: 10.1021/jacs.0c09974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitch JA, Rogova T, Duarte F, Dixon DJ. Dearomative photocatalytic construction of bridged 1,3-diazepanes. Angew. Chem. Int. Ed. 2020;59:4121–4130. doi: 10.1002/anie.201914390. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, et al. Photochemical intermolecular dearomative cycloaddition of bicyclic azaarenes with alkenes. Science. 2021;371:1338–1345. doi: 10.1126/science.abg0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Southgate EH, Pospech J, Fu J, Holycross DR, Sarlah D. Dearomative dihydroxylation with arenophiles. Nat. Chem. 2016;8:922–928. doi: 10.1038/nchem.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okumura M, Shved AS, Sarlah D. Palladium-catalyzed dearomative syn−1,4-carboamination. J. Am. Chem. Soc. 2107;139:17787–17790. doi: 10.1021/jacs.7b11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez LW, Klöckner U, Pospech J, Hauss L, Sarlah D. Nickel-catalyzed dearomative trans−1,2-carboamination. J. Am. Chem. Soc. 2018;140:4503–4507. doi: 10.1021/jacs.8b01726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang C, Okumura M, Deng H, Sarlah D. Palladium-catalyzed dearomative syn−1,4-oxyamination. Angew. Chem. Int. Ed. 2019;58:15762–15766. doi: 10.1002/anie.201909838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wertjes WC, Okumura M, Sarlah D. Palladium-catalyzed dearomative syn-1,4-diamination. J. Am. Chem. Soc. 2019;141:163–167. doi: 10.1021/jacs.8b13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiqi Z, Wertjes WC, Sarlah D. Chemical equivalent of arene monooxygenases: dearomative synthesis of arene oxides and oxepines. J. Am. Chem. Soc. 2020;142:10125–10131. doi: 10.1021/jacs.0c02724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncton MAJ. Minisci reactions: Versatile CH-functionalizations for medicinal chemists. Med. Chem. Commun. 2011;2:1135–1161. doi: 10.1039/c1md00134e. [DOI] [Google Scholar]
- 34.Proctor RSJ, Phipps RJ. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 2019;58:13666–13699. doi: 10.1002/anie.201900977. [DOI] [PubMed] [Google Scholar]
- 35.Romero NA, Margrey KA, Tay NE, Nicewicz DA. Site-selective arene C-H amination via photoredox catalysis. Science. 2015;349:1326–1330. doi: 10.1126/science.aac9895. [DOI] [PubMed] [Google Scholar]
- 36.Margrey KA, McManus JB, Bonazzi S, Zecri F, Nicewicz DA. Predictive model for site-selective aryl and heteroaryl C–H functionalization via organic photoredox catalysis. J. Am. Chem. Soc. 2017;139:11288–11299. doi: 10.1021/jacs.7b06715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McManus JB, Nicewicz DA. Direct C–H cyanation of arenes via organic photoredox catalysis. J. Am. Chem. Soc. 2017;139,:2880–2883. doi: 10.1021/jacs.6b12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margrey KA, Levens A, Nicewicz DA. Direct aryl C−H amination with primary amines using organic ohotoredox catalysis. Angew. Chem. Int. Ed. 2017;56:15644–15648. doi: 10.1002/anie.201709523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang H, et al. Electrophotocatalysis with a trisaminocyclopropenium radical dication. Angew. Chem. Int. Ed. 2019;58:13318–13322. doi: 10.1002/anie.201906381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, et al. Photoelectrocatalytic arene C–H amination. Nat. Catal. 2019;2:366–373. doi: 10.1038/s41929-019-0231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Targos K, Williams OP, Wickens ZK. Unveiling Potent Photooxidation Behavior of Catalytic Photoreductants. J. Am. Chem. Soc. 2021;143:4125–4132. doi: 10.1021/jacs.1c00399. [DOI] [PubMed] [Google Scholar]
- 42.Newcomb M, Choi S-Y, Horner JH. Adjusting the Top End of the Alkyl Radical Kinetic Scale. Laser Flash Photolysis Calibrations of Fast Radical Clocks and Rate Constants for Reactions of Benzeneselenol. J. Org. Chem. 1999;64:1225–1231. doi: 10.1021/jo981930s. [DOI] [Google Scholar]
- 43.Newcomb M, Varick TR, Ha C, Manek MB, Yue X. Picosecond radical kinetics. Rate constants for reaction of benzeneselenol with primary alkyl radicals and calibration of the 6-cyano-5-hexenyl radical cyclization. J. Am. Chem. Soc. 1992;114:8158–8163. doi: 10.1021/ja00047a026. [DOI] [Google Scholar]
- 44.Yasuda M, Yamashita T, Shima K, Pac C. Photochemical reactions of aromatic compounds. 43. Direct photoamination of arenes with ammonia and primary amines in the presence of electron acceptors. J. Org. Chem. 1987;51:753–759. doi: 10.1021/jo00381a009. [DOI] [Google Scholar]
- 45.Yasuda M, Matsuzaki Y, Shima K, Pac C. Photochemical reactions of aromatic compounds. Part 44. Mechanisms for direct photoamination of arenes with ammonia and amines in the presence of m-dicyanobenzene. J. Chem. Soc., Perkin. Trans. 1988;2:745. doi: 10.1039/p29880000745. [DOI] [Google Scholar]
- 46.Yasuda M, Watanabe Y, Tanabe K, Shima K. Regiochemistry on photoamination of methoxy-substituted phenanthrenes via electron transfer. J. Photochem. Photobiol. A: Chem. 1994;79:61–65. doi: 10.1016/1010-6030(93)03750-B. [DOI] [Google Scholar]
- 47.Mizuno, K., Pac, C. & Sakura, H. Photochemical cyanation of aromatic hydrocarbons with cyanide anion. J. Chem. Soc. Chem. Commun. 553 (1972).
- 48.Xuan J, Zhang Z-G, Xiao W-J. Visible-light-induced decarboxylative functionalization of carboxylic acids and their derivatives. Angew. Chem. Int. Ed. 2015;54:15632–15641. doi: 10.1002/anie.201505731. [DOI] [PubMed] [Google Scholar]
- 49.Plutschack MB, Pieber B, Gilmore K, Seeberger PH. The Hitchhiker’s guide to flow chemistry. Chem. Rev. 2017;17:11796–11893. doi: 10.1021/acs.chemrev.7b00183. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers, J. D., et al. Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as Janus kinase inhibitors. US2009181959A1.
- 51.Schirok, H., et al. Substituted 2-amino-pyrazolyl-[1,2,4]triazolo[1,5A]pyridine derivatives and use thereof. WO 2020/215094.
- 52.Mayer JM. Understanding hydrogen atom transfer: from bond strengths to Marcus theory. Acc. Chem. Res. 2011;44:36–46. doi: 10.1021/ar100093z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Experimental procedures and characterization data are given in the Supplementary Information. The authors declare that all other data supporting the findings of this study are available within the article and its Supplementary Information files. For Cartesian coordinates, see Supplementary Data 1 file. Further data can be obtained from the corresponding author upon request.