Abstract
Primary CNS Vasculitis (PCNSV) is a rare inflammatory disorder affecting the blood vessels of the central nervous system. Patients present with a combination of headaches, seizures, and focal neurological deficits. There is usually a diagnostic delay. Treatment is based on observational studies and expert opinion. Our objective was to identify clinical, laboratory, neuroimaging, pathologic or management-related associations with 2 year outcome in patients with primary CNS vasculitis. We conducted a cohort study at a single tertiary care referral centre of prospectively (2018-2019) and retrospectively (2010-2018) identified individuals with primary CNS vasculitis (diagnosis was proven by either brain biopsy or cerebral digital subtraction angiography). Clinical, imaging and histopathologic findings, treatment, and functional outcomes were recorded. Univariate and stepwise multiple logistic regression were applied. P-value<0.05 was considered statistically significant. The main outcome measures were documentation of clinical improvement or worsening (defined by mRS scores) and identification of independent predictors of good functional outcome (mRS 0-2) at 2 years. We enrolled eighty-two biopsy and/or angiographically proven PCNSV cases. The median age at presentation was 34 years with a male predilection and a median diagnostic delay of 23 months. Most patients presented with seizures (70.7%). All patients had haemorrhages on MRI. Histologically lymphocytic subtype was the commonest. Corticosteroids with cyclophosphamide was the commonest medication used. The median mRS at follow-up of 2 years was 2 (0-3), and 65.2% of patients achieved a good functional outcome. Myelitis and longer duration of illness before diagnosis were associated with poorer outcomes. The presence of hemorrhages on SWI sequence of MRI might be a sensitive imaging marker. Treatment with steroids and another immunosuppressant probably reduced relapse rates in our cohort. We have described the third largest PCNSV cohort and multi-centre randomised controlled trials are required to study the relative efficacy of various immunosuppressants.
Study registration: CTRI/2018/03/012721.
Subject terms: Neuroscience, Neurology
Introduction
Primary CNS Vasculitis (PCNSV) is a rare, heterogeneous and polymorphic inflammatory disorder affecting the blood vessels of the central nervous system, often resulting in significant morbidity and mortality1,2. It has a male preponderance with a median onset of 50 years1,3,4. Patients usually present with a combination of headaches, seizures and focal neurological deficits, and there is a long time lag between symptom onset and eventual diagnosis3–5. The diagnosis is made by the Calabrese and Mallek criteria6, where an unexplained acquired neurological deficit with either an angiogram or CNS biopsy shows features of vasculitis, with other causes ruled out and requires a high index of suspicion.
Even though a plausible diagnosis of PCNSV may be made on MRI correlating with clinical findings and cerebral DSA, meningo-cortical/spinal cord biopsy remains the reference standard to certify the diagnosis. The treatment of PCNSV is based on retrospective studies and expert opinions since there have been no RCTs. Till date, only a few adult cohorts have been reported, the largest ones by Salvarani et al. from Mayo clinic4 and de Boysson et al. from the French cohort5. We describe the third largest cohort of adult PCNSV, including clinical demographics, management and predictors of a good functional outcome at 2 years.
Methods
Patient recruitment
From January 1, 2010, through July 31, 2019, patients at AIIMS, New Delhi who were diagnosed with PCNSV were included in the study. We initiated the PCNSV registry after approval by the All India Institute of Medical Sciences Institutional Ethics Committee in February 2018, and the protocol is registered in the Clinical Trial Registry of India (CTRI/2018/03/012721). Informed consent was obtained from all subjects or their legal guardians. All cases before the registry were retrieved retrospectively from the files and electronic hospital records. All clinical data were verified by two neurologists. The age of onset was considered to be the age at which the first clinical features were apparent. We followed the EQUATOR reporting guidelines and the study was conducted in accordance with the declaration of Helsinki and principles of good clinical practice.
Inclusion criteria
All adult patients (age ≥ 18 years) with a diagnosis of PCNSV, satisfying the diagnostic criteria below:
Recent history or presence of an acquired unexplained neurological deficit,
Evidence of vasculitis in a CNS biopsy specimen, or
Cerebral DSA with changes characteristic of vasculitis.
Exclusion criteria
Evidence of systemic vasculitis or other PCNSV mimickers.
Hypercoagulable state.
Findings explained by other causes.
Blood investigations
Serum vasculitis profile (ANA, lupus anticoagulant, anti-cardiolipin antibody, anti-dsDNA and ANCA) was done in all patients to exclude a systemic vasculitis. Common chronic infectious inflammatory aetiologies were ruled out by testing for HIV, hepatitis B and C, and syphilis (VDRL). Other tests like HLA-B51, RA factor, anti-SSA and SSB, cryoglobulins and 2D-echocardiography were done based on patient’s clinical profile. Serum NMO and MOG antibody testing was done in all patients with spinal cord manifestations.
Imaging studies
MRI and DSA were reviewed and analysed by two neuroradiologists. MRIs were reviewed for abnormalities in the form of grey and white matter changes, haemorrhages, and enhancing lesions in the different CNS regions7–9.
DSAs were evaluated for arterial (steno-occlusive changes, irregularity, beaded/pearlescent appearance, aneurysms) or venous phase abnormalities (dilatation and tortuosity of small veins, puddling and staining of contrast, parenchymal venous phase abnormalities).
Histopathology
Biopsies were either targeted (sampled from regions of the brain that demonstrated abnormalities on imaging) or blind (non-dominant frontal or temporal pole). They were reviewed and reported by the neuropathologist, and a PCNSV diagnosis required the presence of transmural inflammation of small- or medium-sized meningo-cortical blood vessels. It was further classified into granulomatous, lymphocytic and necrotizing subtypes. Patients with both biopsy and angiography positive PCNSV were considered as biopsy positive cases.
Cerebrospinal fluid (CSF)
CSF examination was considered abnormal if cells > 5/µL or protein > 45 mg/dL.
Management strategies and functional outcome
The treatment received was documented and good clinical outcome was defined as an mRS score of 0–2. Relapse was defined as a new clinical deficit, an increase in the pre-existing symptoms, or evidence of a new lesion on follow up MRI. However, seizure or headache recurrence without new MRI lesions did not qualify as relapse.
Statistical analysis
Categorical variables were reported as proportions, whereas continuous variables as medians with interquartile range. Wherever applicable, differences in categorical variables were assessed by chi-square or Fisher’s exact test, whereas continuous variables were assessed by Mann Whitney test. Univariate and step-wise multiple logistic regression was applied to find independent predictors of good functional outcome (mRS 0–2), and adjusted Odds ratio were calculated. P-value < 0.05 was considered statistically significant. All statistical analyses were done using SPSS version 27.0.
Results
A total of 271 patients with suspected PCNSV were screened, and 82 cases were identified as per inclusion and exclusion criteria. Fifty had a positive biopsy, forty-five had a positive DSA, and thirteen had dual positivity (Fig. 1).
Figure 1.
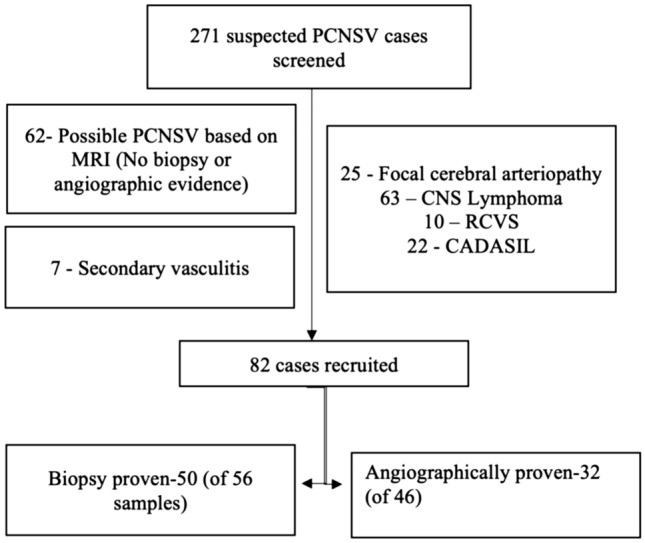
Patient screening and selection for our study.
The clinical characteristics are mentioned in Table 1. The median age at presentation was 34 years with a male predilection (4.8:1), and there was a median diagnostic delay of 23 months from the first symptom to diagnosis. The most common presenting symptom was seizures (70.7%), followed by headache (59.8%). Most of our patients suffered from progressive deficits. Concomitant hypertension was present in 13 patients and diabetes in 4 patients. ESR was elevated in 27 patients (32.9%).
Table 1.
Clinical characteristics.
| PCNSV cohort (n = 82) | |
|---|---|
| Sex (male:female) | 68:14 (4.8:1) |
| Median age at presentation (IQR) | 34 (27.8–42) years |
| Median age at onset (IQR) | 28.6 (22.9–38.2) years |
| Median age at diagnosis (IQR) | 32 (25–39.2) years |
| Diagnostic delay | 23 (6.8–48) months |
| Symptoms [number(%)] |
Seizures—58 (70.7%) Headache—49 (59.8%) Hemiparesis—45 (54.9%) Cognitive impairment—24 (29.3%) Visual impairment—18 (21.9%) Ataxia—17 (20.7%) Paraparesis—11 (13.4%) Fever—0 |
| Clinical course_1 |
Static—9 (10.9%) Progressive—73 (89.1%) |
| Clinical course_2 |
Monophasic—15 (18.3%) Multiphasic—67 (81.7%) |
| Relapse | 10 (12.2%) |
| Lost to follow up | 36 (43.9%) |
| Favourable mRS 0–2 | Yes—30/46 (65.2%) |
| mRS distribution | |
| mRS0 | 19 |
| mRS1 | 3 |
| mRS2 | 8 |
| mRS3 | 7 |
| mRS4 | 3 |
| mRS5 | 2 |
| mRS6 | 4 |
| Mortality | 4/46 (8.7%) |
| Initial treatment |
Steroids only—5 (6.2%) Steroids + cyclophosphamide—40 (48.8%) Steroids + azathioprine—24 (29.3%) Steroids + methotrexate—3 (3.6%) Steroids + mycophenolate—3 (3.6%) Steroids + rituximab—7 (8.5%) |
| Treatment at last follow up |
Steroids +/− ASD—3 (6.5%) Steroids + immunosuppresants +/− ASD—7 (15.2%) Immunosuppressants +/− ASD—13 (28.3%) Antiseizure drugs (ASD) only—13 (28.3%) No treatment—6 (13%) Dead—4 (8.7%) |
CSF examination was done in 48/82 patients and was abnormal in 42 (87.5%); pleocytosis in 21 and increased protein in 42 (median value-82.5 mg/dl; range: 29-444 mg/dl). Gram’s stain, TB-PCR, VDRL, Cryptococcal antigen, and cultures were negative in all patients (Supplementary Table 1).
Imaging
The brain as the first CNS region involved was seen in 85.4% cases, spinal cord in 6.1%, and both in 8.5% cases (Supplementary Table 1). Microhemorrhages and/or macrohemorrhages were seen in all the patients. Spinal cord involvement was commonly multifocal and dorsal cord was most frequently involved.
Cerebral DSA was done in 46/82 patients, and was abnormal in 45 patients (97.8%). Most patients (41/45) with abnormal DSA had venous phase abnormality. Four patients had arterial abnormalities (2 had multiple arterial irregularities with vessel occlusions, and 2 had multiple arterial irregularities only).
Histopathology
A meningo-cortical biopsy was done in 56 out of the 82 patients (targeted:49, blind:7) and showed features of PCNSV in fifty (89.3%) patients. Though targeted biopsy had a higher positivity rate than blind biopsy, the difference was not statistically significant.
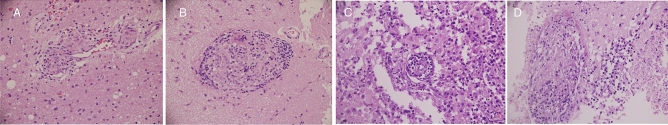
Lymphocytic vasculitis was the most common PCNSV subtype (54%), followed by granulomatous vasculitis (44%) and necrotizing vasculitis (2%) (Fig. 2).
Figure 2.
Photomicrographs showing non-necrotizing granulomas involving the vessel wall on Hematoxylin and Eosin stain at 200 × (A) and 400 × (B) respectively. Photomicrographs showing lymphocytic vasculitis (C) and thrombus formation in the vessels (D).
Management
The most common treatment modality used was steroids with cyclophosphamide [intravenous 750 mg/m2, once a month for 6 doses] (n = 40) followed by steroids with azathioprine [2–3 mg/kg/day] (n = 24). Other immunosuppressants used were mycophenolate mofetil (2–3 gm/day) (n = 3), methotrexate (15–25 mg/week) (n = 3), and rituximab (375 mg/m2 intravenous infusion once a week for four weeks followed by a repeat infusion at six months, if required) (n = 7). Two patients required additional treatment with intravenous immunoglobulin. The 2 year mRS scores were available in 46 patients with the median mRS score being 2 (IQR 0–3). Thirty patients (65.2%) achieved a good functional outcome (mRS 0–2); 10 cases had a relapse, while four of our patients died (Table 1).
The presence of delay in diagnosis of PCNSV (p—0.007), paraparesis (p—0.000) and spinal cord involvement by disease (p—0.001) were adversely associated with functional outcomes on univariate analysis (Table 2). Multivariate analysis revealed spinal cord involvement (OR 0.04, CI 0.004–0.392) and delay in diagnosis (OR 0.31, CI 1.15–10.92) to be associated with worse outcomes (Table 2).
Table 2.
Predictors of favourable functional outcome (mRS 0–2).
| Univariate analysis | Patients with mRS 0–2 (n = 30) | Patients with mRS 3–6 (n = 16) | p-value |
|---|---|---|---|
| Sex (M:F) | 26:4 | 13:3 | 0.626 |
| Median age (IQR) | 35.5 (29–41) years | 33 (29–44) years | 0.431 |
| Median symptom onset age (IQR) | 34 (25–39) years | 28.4 (26.4–38.7) years | 0.336 |
| Age at diagnosis | 34.8 (28–40) years | 32.1 (29.1–41.7) years | 0.455 |
| Delay in diagnosis | 11 (6–24) months | 39 (21–51.5) months | 0.007 |
| Headache | 21 | 8 | 0.181 |
| Cognitive impairment | 9 | 4 | 0.720 |
| Hemiparesis | 15 | 9 | 0.686 |
| Progressive course | 28 | 16 | 0.291 |
| Multiphasic course | 25 | 13 | 0.859 |
| Paraparesis | 0 | 7 | 0.000 |
| Ataxia | 5 | 5 | 0.253 |
| Seizure | 21 | 8 | 0.181 |
| Visual impairment | 5 | 6 | 0.115 |
| Abnormal CSF protein | 19 | 13 | 0.805 |
| CSF leucocytosis | 12 | 5 | 0.214 |
| Spinal cord involvement by disease | 2 | 9 | 0.001 |
| Hemorrhages | 30 | 16 | 0.702 |
| Granulomatous PCNSV | 9 | 2 | 0.075 |
| Lymphocytic PCNSV | 9 | 6 | 0.081 |
| Multivariate analysis | Odds ratio | p-value | Confidence interval |
|---|---|---|---|
| Spinal cord involvement | 0.04 | 0.006 | 0.004–0.392 |
| Delay in diagnosis | 0.31 | 0.031 | 1.15–10.92 |
Discussion
The median age of onset was 28.6 years, significantly younger than the other major cohorts with male predominance (82.9%)4,5,10. Our patients were younger as compared to other Western cohorts4,5 and Europe but was similar to the series from the southern part of India (Sundaram et al.)10. Vasculitis may affect an individual of any age. The reason for this, although not clearly understood, probably reflects an interaction between an unknown environmental trigger or a genetic predisposition. The sex ratio was skewed towards males probably because of a region-specific male bias towards seeking tertiary care.
Other major comparatives between our study and these 3 cohorts are listed in Table 3. CSF was abnormal in approximately 90% of our patients which advocates for CSF analysis in suspected PCNSV cases, especially to rule out infectious causes since remaining findings are non-specific and may non-contributory.
Table 3.
Comparison between the largest cohorts of PCNSV.
| Mayo cohort4 | French cohort5 | Sundaram et al.10 | AIIMS cohort | |
|---|---|---|---|---|
| Total cases | 163 | 112 | 45 | 82 |
| Median age (years) | 48 | 47 | 36 | 28.6 |
| Sex (M:F) | 78:89 | 60:52 | 31:14 | 68:14 |
| Most common symptom | Headache (59.5%) | Motor deficit | Hemiparesis | Seizures (70.7%) |
| Abnormal DSA | 113/163 (69.3) | 74/95 (78) | 30/42 (71) | 45/46 (97.8) |
| Abnormal biopsy (%) | 58/81 (72) | 33/53 (62) | 19/29 (66) | 50/56 (89.3) |
| Abnormal CSF (%) | 117/126 (93) | 73/104 (70) | 25/45 (60) | 42/48 (87.5) |
| Median mRS | 2 | 2 | 2 | |
| Good functional outcome | mRS 0–3 |
mRS 0–2: 63/112 56% |
mRS 0–2: 33/45 77% |
mRS 0–2: 30/46 65.2% |
| Mortality (%) | 25/163 (15) | 9/112 (8) | 7/45 (16) | 4/46 (8.5%) |
MRI was abnormal in all cases. In a systematic review of published cases of PCNSV, cerebral MRI was anomalous in 93% of patients11 and CSF was abnormal in 74%12. In our study, SWI showed hemorrhages in 100% PCNSV cases, which is significantly high compared to the existing literature. This could be due to selection bias as these patients might have been selectively taken up for DSA or brain biopsies. The most frequent presentation in PCNSV is brain hemorrhage with an incidence of 10.8–63.6% on MRI8,13,14. Hemorrhages have been reported to be commoner in women and associated with necrotizing vasculitis subtype14.Our results might indicate that hemorrhages are underestimated in other studies and the increased incidence in our study might be due to the use of SWI in all cases. Post-treatment with steroids, the white matter FLAIR hyperintensities may disappear, but the SWI hemorrhages remain15.
We found evidence of spinal cord involvement in 38.3% which was significantly higher than 5% documented by Salvarani et al. (2008d). We concurred that the thoracic cord was most frequently involved. This was probably due to the institute policy of imaging the spinal cord in all patients with suspected PCNSV.
DSA was done in suspected cases based on MRI findings. It showed a high sensitivity to detect vasculitis and was positive in 98% cases. Lack of a high prevalence of arterial abnormalities in our cohort could be due to predominant small vessel vasculitis in our cohort (all small-vessel vasculitis are not detectable by DSA because its resolution cannot discern vessel diameters < 0.2 mm), secondary to case selection and lack of DSA in all cases. DSA has a sensitivity of 50–90% in the diagnosis of PCNSV16–19. CTA/MRA, though not well studied with histopathological confirmation, appear even less sensitive20–22. Other inflammatory, metabolic, malignant or vascular pathologies can simulate PCNSV like ICAD, RCVS, radiation vasculopathy and should always be ruled out.
CNS biopsy was positive in approximately 90% of our cases which is the highest amongst cohorts reported. Most of these were targeted biopsies. Lymphocytic subtype was the most common (54%). The lymphocytic subtype was the most common in the series by Sundaram et al.10 and Oon et al.23, while granulomatous subtype was the most common in the Mayo cohort4. None of our patients had findings of amyloid angiopathy on histopathology.
Most patients were treated with steroids in combination with cyclophosphamide or azathioprine. A small proportion of patients were also treated with a combination of steroids with rituximab, methotrexate, mycophenolate or steroids alone. This highlights the lacunae of PCNSV treatment which till date is guided by expert opinion and retrospective data since RCTs are lacking. Ten of our patients had a relapse on treatment. However, no variable was significantly associated with relapse occurrence. Relapses may affect between 30 and 50% cases4,24,25 and the lesser relapses in our cohort confirms that an induction strategy with steroids and a potent immunosuppressant is beneficial in supressing the initial inflammatory process and preventing subsequent flare-ups. Cyclophosphamide was the most commonly used immunosuppressant (48.8%) as has been recommended by the European League Against Rheumatism for small and medium vessel vasculitis26. Rituximab was also used in a substantial number of our patients (8.5%) as it is a good alternative to cyclophosphamide in patients with contraindications, toxicity or reservations for use27,28. No treatment modality was associated with a significantly better outcome at follow-up.
Most of our patients were off steroids on follow-up (69.6%), requiring either no treatment (13%), antiseizure drugs alone (28.3%), or an immunosuppressant (28.3%). There was no significant difference between the biopsy-proven and angiographically proven cases (Supplementary Table 2).
A good functional outcome was taken as an mRS between 0 and 2. A 2 year follow-up data was available for 46 of our patients. The median mRS was 2, and good functional outcome was achieved by 65.2% of our cases. We lost four patients (8.7%). These outcome measures were similar to other previously published PCNSV cohorts4,5,10 (Table 3). Spinal cord involvement and delay in diagnosis were associated with poorer outcomes.
The strength of present study is the large number cases with long follow-up which helped to confirm the diagnosis and rule out mimics. Our cohort of PCNSV is the third-largest in the world and adds to literature of this rare disease entity. We included only those cases which were diagnosed angiographically or by CNS biopsy, with all potential mimickers excluded. We describe the largest number of PCNSV cases affecting the spinal cord. Compared with individual case reports or smaller previous series, our study cohort is largest in which SWI has been done.
Our study has various limitations. Its partial retrospective nature binds it to the shortcomings of data collection, possible selection bias and potential confounding factors. However, data was collected prospectively after initiation of the PCNSV registry in February 2018. The imaging studies were performed on different scanners of variable strength and heterogeneous protocols. All of our patients didn’t undergo DSA, which is taken as the reference-standard for the prebiopsy diagnosis of PCNSV29. CNS biopsy is considered the gold standard for PCNSV diagnosis but was not done in all cases. A significant number of patients were lost to follow up after first discharge.
Conclusion
We describe a cohort PCNSV patients which showed the utility of DSA and brain biopsy in achieving final diagnosis. The presence of micro and macrohemorrhages in SWI sequence on MRI Brain might be a good imaging marker for PCNSV. Treatment with steroids and another immunosuppressant probably reduced relapse rates in our cohort. Multi-centre RCTs are required to find the relative efficacy of various immunosuppressants.
Supplementary Information
Acknowledgements
The study was supported by funding from Indian Council of Medical Research (File No. 5/3/8/49/2020-ITR).
Abbreviations
- AIIMS
All India Institute of Medical Sciences
- ANA
Anti-nuclear antibody
- ANCA
Antineutrophilic cytoplasmic antibodies
- Anti-ds DNA
Anti-double stranded deoxyribonucleic acid
- Anti-SSA and SSB
Anti-Sjogren’s syndrome-related antigen A and B
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CTA
Computed tomographic angiography
- DSA
Digital subtraction angiography
- ESR
Erythrocyte sedimentation rate
- FLAIR
Fluid attenuated inversion repeat
- HIV
Human immunodeficiency virus
- ICAD
Intracranial atherosclerotic disease
- ICH
Intracerebral hemorrhage
- IQR
Interquartile range
- MOG
Myelin oligodendrocyte glycoprotein
- MRA
Magnetic resonance angiography
- MRI
Magnetic resonance imaging
- mRS
Modified Rankin scale
- NMO
Neuromyelitis optica
- PCNSV
Primary central nervous system vasculitis
- RA
Rheumatoid arthritis
- RCT
Randomised controlled trial
- RCVS
Reversible cerebral vasoconstriction syndrome
- SWI
Susceptibility weighted imaging
- TB-PCR
Tuberculosis-polymerase chain reaction
- VDRL
Venereal disease research laboratory
Author contributions
A.A., M.V.P.S., A.G. and V.V.Y. wrote the main manuscript text. Both A.G. and V.V.Y. are the corresponding authors. All authors contributed with data collection, editing and reviewing the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ajay Garg, Email: drajaygarg@gmail.com.
Venugopalan Y. Vishnu, Email: Vishnuvy16@yahoo.com
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17869-7.
References
- 1.Hajj-Ali RA, Singhal AB, Benseler S, et al. Primary angiitis of the CNS. Lancet Neurol. 2011;10:561–572. doi: 10.1016/S1474-4422(11)70081-3. [DOI] [PubMed] [Google Scholar]
- 2.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet. 2012;380:767–777. doi: 10.1016/S0140-6736(12)60069-5. [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis: analysis of 101 patients. Ann. Neurol. 2007;62:442–451. doi: 10.1002/ana.21226. [DOI] [PubMed] [Google Scholar]
- 4.Salvarani C, Brown RD, Jr, Christianson T, et al. An update of the Mayo Clinic cohort of patients with adult primary central nervous system vasculitis: Description of 163 patients. Medicine. 2015;94:e738. doi: 10.1097/MD.0000000000000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boysson H, Zuber M, Naggara O, et al. Primary angiitis of the central nervous system: description of the first fifty-two adults enrolled in the French cohort of patients with primary vasculitis of the central nervous system. Arthritis Rheumatol. 2014;66:1315–1326. doi: 10.1002/art.38340. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese LH, Mallek JA. Primary angiitis of the central nervous system: Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine. 1988;67:20–39. doi: 10.1097/00005792-198801000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Van der Flier WM, Cordonnier C. Microbleeds in vascular dementia: Clinical aspects. Exp. Gerontol. 2012;47:853–857. doi: 10.1016/j.exger.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Boulouis G, de Boysson H, Zuber M, et al. Primary angiitis of the central nervous system: Magnetic resonance imaging spectrum of parenchymal, meningeal, and vascular lesions at baseline. Stroke. 2017;48:1248–1255. doi: 10.1161/STROKEAHA.116.016194. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi U, Terae S, Ogata A, et al. MR imaging of the brain in lymphomatoid granulomatosis. Am. J. Neuroradiol. 2001;22:1283–1290. [PMC free article] [PubMed] [Google Scholar]
- 10.Sundaram S, Menon D, Khatri P, et al. Primary angiitis of the central nervous system: Clinical profiles and outcomes of 45 patients. Neurol. India. 2019;67:105–112. doi: 10.4103/0028-3886.253578. [DOI] [PubMed] [Google Scholar]
- 11.Cloft HJ, Phillips CD, Dix JE, et al. Correlation of angiography and MR imaging in cerebral vasculitis. Acta Radiol. 1999;40:83–87. doi: 10.1080/02841859909174409. [DOI] [PubMed] [Google Scholar]
- 12.McVerry F, McCluskey G, McCarron P, et al. Diagnostic test results in primary CNS vasculitis: A systematic review of published cases. Neurol. Clin. Pract. 2017;7:256–265. doi: 10.1212/CPJ.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster S, Bachmann H, Thom V, et al. Subtypes of primary angiitis of the CNS identified by MRI patterns reflect the size of affected vessels. J. Neurol. Neurosurg. Psychiatry. 2017;88:749–755. doi: 10.1136/jnnp-2017-315691. [DOI] [PubMed] [Google Scholar]
- 14.Salvarani C, Brown RD, Jr, Calamia KT, et al. Primary central nervous system vasculitis presenting with intracranial hemorrhage. Arthritis Rheum. 2011;63:3598–3606. doi: 10.1002/art.30594. [DOI] [PubMed] [Google Scholar]
- 15.Hingwala D, Kesavadas C, Thomas B, et al. Clinical utility of susceptibility-weighted imaging in vascular diseases of the brain. Neurol. India. 2010;58:602–607. doi: 10.4103/0028-3886.68667. [DOI] [PubMed] [Google Scholar]
- 16.Alrawi A, Trobe JD, Blaivas M, et al. Brain biopsy in primary angiitis of the central nervous system. Neurology. 1999;53:858–860. doi: 10.1212/wnl.53.4.858. [DOI] [PubMed] [Google Scholar]
- 17.Koo EH, Massey EW. Granulomatous angiitis of the central nervous system: Protean manifestations and response to treatment. J. Neurol. Neurosurg. Psychiatry. 1988;51:1126–1133. doi: 10.1136/jnnp.51.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollmer TL, Guarnaccia J, Harrington W, et al. Idiopathic granulomatous angiitis of the central nervous system: Diagnostic challenges. Arch. Neurol. 1993;50:925–930. doi: 10.1001/archneur.1993.00540090032007. [DOI] [PubMed] [Google Scholar]
- 19.Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J. Rheumatol. 1995;22:662–667. [PubMed] [Google Scholar]
- 20.Chen SH, Sur S, Sedighim S, et al. Utility of diagnostic cerebral angiography in the management of suspected central nervous system vasculitis. J. Clin. Neurosci. 2019;64:98–100. doi: 10.1016/j.jocn.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 21.Eleftheriou D, Cox T, Saunders D, et al. Investigation of childhood central nervous system vasculitis: Magnetic resonance angiography versus catheter cerebral angiography. Dev. Med. Child. Neurol. 2010;52:863–867. doi: 10.1111/j.1469-8749.2009.03591.x. [DOI] [PubMed] [Google Scholar]
- 22.Edgell RC, Sarhan AE, Soomro J, et al. The role of catheter angiography in the diagnosis of central nervous system vasculitis. Interv. Neurol. 2016;5:194–208. doi: 10.1159/000445255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oon S, Roberts C, Gorelik A, et al. Primary angiitis of the central nervous system: Experience of a Victorian tertiary-referral hospital. Intern. Med. J. 2013;43:685–692. doi: 10.1111/imj.12038. [DOI] [PubMed] [Google Scholar]
- 24.Benseler SM. Central nervous system vasculitis in children. Curr. Rheumatol. Rep. 2006;8:442–449. doi: 10.1007/s11926-006-0040-4. [DOI] [PubMed] [Google Scholar]
- 25.de Boysson H, Parienti JJ, Arquizan C, et al. Maintenance therapy is associated with better long-term outcomes in adult patients with primary angiitis of the central nervous system. Rheumatology. 2017;56:1684–1693. doi: 10.1093/rheumatology/kex047. [DOI] [PubMed] [Google Scholar]
- 26.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann. Rheum. Dis. 2009;68:310–317. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 27.De Boysson H, Arquizan C, Guillevin L, et al. Rituximab for primary angiitis of the central nervous system: Report of 2 patients from the French COVAC cohort and review of the literature. J. Rheumatol. 2013;40:2102–2103. doi: 10.3899/jrheum.130529. [DOI] [PubMed] [Google Scholar]
- 28.Salvarani C, Brown RD, Jr, Huston J, 3rd, et al. Treatment of primary CNS vasculitis with rituximab: Case report. Neurology. 2014;82:1287–1288. doi: 10.1212/WNL.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 29.Brant-Zawadzki M, Gould R, Norman D, et al. Digital subtraction cerebral angiography by intraarterial injection: Comparison with conventional angiography. Am. J. Neuroradiol. 1982;3:593–599. doi: 10.2214/ajr.140.2.347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.