Abstract
Avian pox is a highly contagious poultry disease that causes significant economic losses. Mosquitoes belonging to the genus Culex (Diptera: Culicidae) have a fundamental role in disseminating Avipoxvirus (Poxviridae). This study proposes investigating the presence of Avipoxvirus (APV) DNA in Culex spp. from Rio de Janeiro to determine its frequency and perform a phylogenetic analysis based on the core like the 4b protein (p4b) gene. The detection of APVs was conducted individually on four hundred Culex spp. mosquitoes. A total of 12.23% (47/384) of the Culex spp. were positive in the PCR. Sequencing the p4b gene revealed that this study’s sequences displayed 98.8–99% identity with Fowlpoxvirus (FWPW) sequences available in GenBank. In the phylogenetic analysis, these APVs were clustered in the A1 subclade together with FWPW sequences from several countries. The evolutionary distance of the p4b gene was 0.61 ± 0.21% in rural areas and 0.38 ± 0.16% in peri-urban areas. The current investigation is the first study to report the detection of APVs in field-caught mosquitoes. Moreover, a high frequency of APV DNA was observed in Culex spp. captured in domestic areas, where backyard poultry is present. This data demonstrates the importance of implementing control measures for Culex spp. to mitigate the transmission of APVs in backyard poultry in Rio de Janeiro.
Subject terms: Environmental microbiology, Parasitology, Virology
Introduction
Avian pox is an important viral disease with a high incidence in tropical and subtropical countries1. Caused by a double-stranded DNA virus of the genera Avipoxvirus from the Poxviridae family, which infects and produces clinical signs in numerous domestic and wild birds2,3. Avipoxviruses (APVs) have a cosmopolitan distribution and can affect any avian species, with no predilection for gender or age, despite being more common and deadly in young birds4,5. In domestic avian species, particularly in commercial poultry production, APVs have a relevant health impact6. Furthermore, APVs can also have relevant impacts on the health of wild bird species. In some cases, APV infections put the conservation of the affected avian species at risk, especially in outbreaks where the virus is introduced in non-adapted ecosystems7–9.
The means of transmission includes arthropods (such as biting midges, flies, mosquitoes, and mites) acting as mechanical vectors5,10–12. APV propagation can also be carried out by aerosols generated by infected birds, direct contact with injuries, and the ingestion of contaminated food or water13. Previous studies have reported the vital role of the cosmopolitan species Culex quinquefasciatus (Say, 1823) and Aedes aegypti (Linnaeus, 1762) as mechanical vectors in APV transmission5,14, which takes place due to the permanence of viable viral particles in the mosquitoes’ proboscis, remaining for up to 14 days15. Furthermore, global warming could increase arthropod-borne diseases, both in humans and wildlife16,17. This tendency might be even worse in regions suffering from a history of deforestation, such as the Southeastern region of Brazil18,19.
APV diversity is primarily classified into three phylogenetical groups (A, B, C), characterized by Fowlpoxvirus (FWPW) (clade A; subdivisions A1–A7), Canarypoxvirus (clade B; subdivisions B1-B3), and Psittacinepoxvirus (clade C)20. However, two other clades (D, E) have been proposed21,22. Clade D includes a unique APV strain, QP-241, isolated from a Japanese quail collected in Italy21. The sequences included in clade E were earlier recorded from outbreaks in turkey herds in Hungary22 and layer chickens in Mozambique23. Ribeiro et al.24 also reported fowls from the Southern region of Brazil presenting APV sequences clustering in clade E. However, no investigation as to the viral agent in mosquitoes has been performed in Brazil. Moreover, few studies have targeted the circulation of APVs and their identification in mosquito species with vectoring capacities, such as mosquitoes of the Culex genus. Furthermore, it presents an analysis of the phylogenetic relationship and the genetic variability of APVs from Culex spp. collected in rural and peri-urban areas nearby backyard poultry located in the municipality Seropedica, Rio de Janeiro, Brazil.
Results
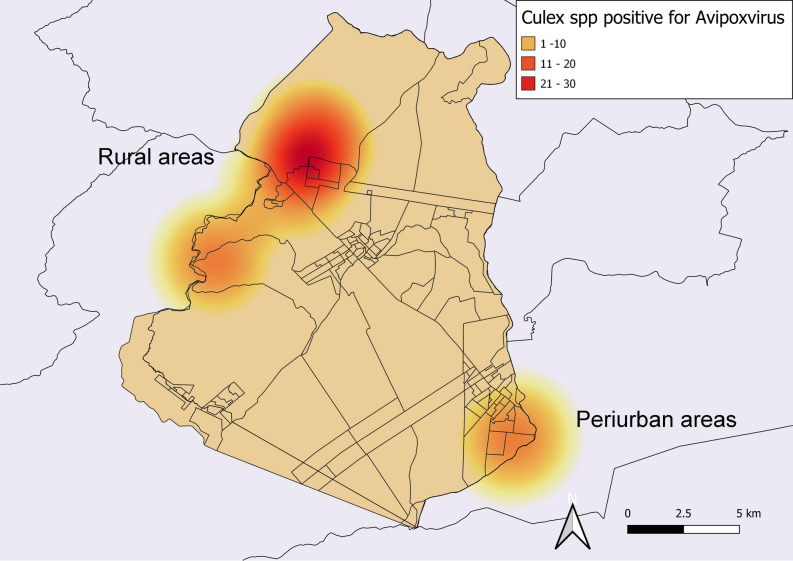
The PCR applied in the current study, which targets APVs, presented a detection limit of 100 copies of the APV p4b gene fragment. The molecular screening resulted in a frequency of 12.23% (47/384) in Culex mosquitoes. Of these positive samples, 13.63% (21/154) were from peri-urban areas, and 11.30% (26/230) were from rural areas (Fig. 1). There was no significant statistical association between the frequency of APVs in Culex mosquitoes and the analyzed areas (p > 0.05). However, the kernel map identified a hot zone, demonstrating a higher concentration of APV-positive mosquitoes in rural areas of the municipality of Seropedica (Fig. 2).
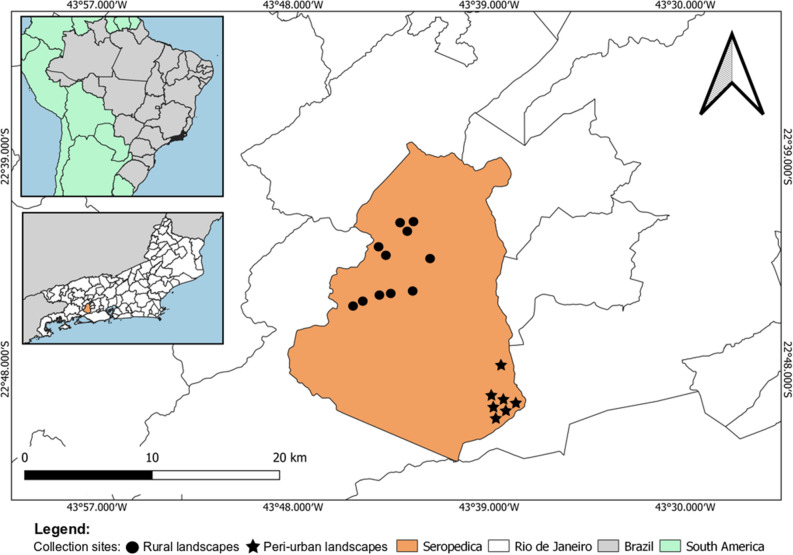
Figure 1.
The geographic location of Seropedica, Rio de Janeiro, highlighting the capture points of Culex mosquitoes in subsistence breeding of Gallus gallus located in rural and peri-urban areas. Map created in QGIS software 3.22.8 'Białowieża' (https://qgis.org/pt_BR/site/).
Figure 2.
Kernel map showing the concentration of Culex spp. positive for Avipoxvirus in rural and peri-urban areas in Seropedica, RJ. Map created in QGIS software 3.22.8 'Białowieża' (https://qgis.org/pt_BR/site/).
The qPCR targeting the chicken mitochondrial gene revealed that 91.49% (43/47) of the p4b-positive APV samples were also positive for chicken blood, and 8.51% (4/47) of the p4b-positive APV samples were negative for chicken blood.
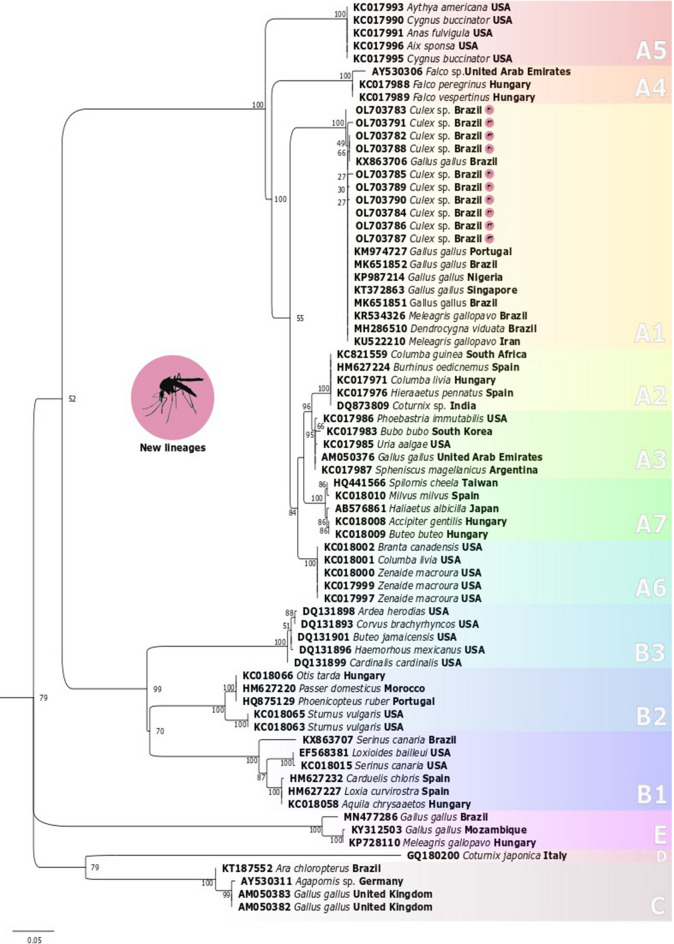
A total of ten positive samples were sequenced for phylogenetic reconstruction based on the pb4 gene. The identity percentage of the sequences in this study ranged from 98.8 to 99%, with FWPW sequences available in the GenBank database. All sequences recovered from Culex mosquitoes in this study were clustered within clade A, subclade A1, and denoted as part of the FWPW category. In the same group, the A1 subclade isolates were recovered in Gallus gallus from Brazil, Nigeria, Portugal, and Singapore; Meleagris gallopavo from Iran and Brazil; and Dendrocygna viduata from Brazil (Fig. 3). The global evolutionary distance of the FWPW p4b gene was 0.48 ± 0.14% in the targeted region. The evolutionary distance between FWPW sequences from mosquitoes collected in rural and peri-urban areas was 0.47 ± 0.13%. The evolutionary distance was compared within each evaluated area, obtaining a value of 0.61 ± 0.21% in the rural area and 0.38 ± 0.16% in the peri-urban area (Table 1).
Figure 3.
Phylogenetic tree estimated by the maximum likelihood method from partial p4b gene sequences of Avipoxvirus isolated in this study (highlighted) compared to sequences available in GenBank. The numbers on the branches indicate the bootstrap value out of 1000 replicates. The bar represents five substitutions per 100 nucleotide positions.
Table 1.
Evolutionary distance (%) between p4b gene sequences of Avipoxvirus obtained from Culex spp. in rural and peri-urban areas in the municipality of Seropedica, Rio de Janeiro, Brazil.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| OL703782—Rural area | |||||||||
| OL703783—Rural area | 0.38 | ||||||||
| OL703784—Rural area | 0.19 | 0.19 | |||||||
| OL703785—Peri-urban area | 0.76 | 0.76 | 0.57 | ||||||
| OL703786—Peri-urban area | 0.19 | 0.19 | 0.00 | 0.57 | |||||
| OL703787—Peri-urban area | 0.19 | 0.19 | 0.00 | 0.57 | 0.00 | ||||
| OL703788—Peri-urban area | 0.00 | 0.38 | 0.19 | 0.76 | 0.19 | 0.19 | |||
| OL703789—Peri-urban area | 0.38 | 0.38 | 0.19 | 0.76 | 0.19 | 0.19 | 0.38 | ||
| OL703790—Rural area | 0.38 | 0.38 | 0.19 | 0.76 | 0.19 | 0.19 | 0.38 | 0.38 | |
| OL703791—Rural area | 1.15 | 1.15 | 0.95 | 1.54 | 0.95 | 0.95 | 1.15 | 1.15 | 1.15 |
Discussion
Brazil is the second leader in the world’s poultry production and the first leader in chicken exportation25. Therefore, any negative impact on poultry health can result in substantial commercial losses. In this context, it is of utmost interest to investigate all aspects of poultry health. Avian pox is a viral disease with a high incidence in tropical and subtropical countries1. Avipoxviruses have no predilections in terms of host species, sex, or age, targeting more than 374 domestic and wild bird species and making it difficult to achieve environmental eradication3. Furthermore, arthropods perform APV transmission, which further exacerbates the potential of dispersion. Mosquitoes are one of many arthropods playing the role of mechanical vector in the Avian pox epidemiological chain, which is a potent threat given that mosquitoes populations in the tropics are high26.
Considering the substantial economic loss caused by Avian pox, it is vital to elucidate the transmission chain of APVs by identifying potential transmission sources5. Although investigations targeting APVs in mosquitoes are scarce, prior studies have demonstrated that APV detection in potential vectors is possible and can become an essential tool for monitoring the pathogen circulation in areas where the disease occurrence may increase due to climatic factors. In addition, APV detection in potential vectors can further clarify the mosquitoes’ participation in the APV transmission chain in a determined area, which could improve the strategic measures to control the Avian pox disease. Furthermore, the present study can contribute to the clarification of the biology of the vectors involved in and strategic measures for vector control, which is of veterinary interest5,27.
The frequency of APVs found in field-caught Culex spp. collected in the present investigation (12.23%; 47/384) was higher than previously recorded by Yeo et al.5, who observed a positivity of 2.60% (4/154) in mosquito pools collected in areas where outbreaks of Avian pox occurred in Singapore. This divergence may be related to several factors, such as differences in the sampling approaches adopted in these studies, sample conservancy, and the chosen collection sites. Although the studies by Yeo et al.5 and Lee et al.12 chose the PCR method for the detection of Avipoxvirus in mosquito and biting midge pools, respectively, the present study applied a distinct sampling method by individually testing Culex specimens. Individual sampling proved to be sufficient to obtain the total amount of DNA after performing the modified extraction technique outlined by Ayres et al.28. This was demonstrated by quantification through spectrophotometry and by checking the quality of genomic DNA targeting the mitochondrial cytochrome oxidase subunit I (COI) gene, which was of good quality at the expected height28. Moreover, the DNA extracted from the samples obtained in the current investigation was preserved in a DNA stabilization solution and maintained in an ultra-freezer at − 80 °C until molecular detection.
Notably, the selected spots were located in areas where outbreaks of Avian pox had occurred in previous years. However, during the present investigation, no Avian pox case was observed or recorded close to the studied points by the owners or local veterinarians. Despite the absence of the disease’s clinical signs in backyard chickens, the frequency of APVs in Culex spp. was relatively high in the target area, raising questions about the involvement of other domestic birds species participating in the maintenance of virus circulation. The presence of four Culex spp. that were positive for APVs and negative for the chicken mitochondrial gene reinforced this hypothesis. Therefore, more studies must be performed to elucidate the transmission chain of APVs in the targeted area.
Prior studies targeting APVs-p4b obtained of avian species from Brazil demonstrate the presence of several APVs lineages of distinct clades circulating throughout the country. Studies conducted in the Southeastern region of Brazil, the same area of the current research, have reported the presence of APV sequences clustered in subclade A1 and isolated from both turkeys and chickens29,30. Moreover, in the Northeastern region of Brazil, Braga et al.31 isolated APVs with lineage clustering in the A1 subclade coming from a Brazilian native duck (Dendrocygna viduata) (Fig. 3). The present phylogenetic reconstruction exposes the lineages of APVs recovered from Culex mosquitoes from the state of Rio de Janeiro, also grouped in the A1 subclade (APVs-A1) (Fig. 3).
Historically, the lineages of APVs from subclade A1 were strongly associated with Galliform hosts (Gallus gallus and Meleagris gallopavo) according to the results published by Gyuranecz et al.20, Jarmin et al.32, and Manarolla et al.21. Nevertheless, recent records of the APVs-A1 sequence infecting a Brazilian native duck (Order Anseriformes) reported by Braga et al.31 have raised questions about this subclade’s specificity within the species of the Galliformes order. Furthermore, given the fact that the most common APV host species of subclade A1 (Gallus and Meleagris gallopavo) are exotic species introduced by humans as food resources in most regions of the globe, such as in Brazil, it is possible to suggest that the lineages of APVs of the A1 subclade accompany their hosts in species introduction around the globe. However, given the record of an APVs-A1 infecting a native Brazilian species31, it is possible to suggest a community APVs-A1 transmission between different avian host species.
The species-level identification in Culex spp. requires genitalia dissection, making such samples unviable for molecular analysis. Cytochrome oxidase I (COI) DNA barcoding contains information for identifying mosquitoes of the Culex genus33. However, a criticism of using the COI barcode for specification is the ambiguous identification or the absence of clusters in phylogenetic trees of recently diverged species34–36. Algorithms were developed by Meier et al.37 and van Velzen et al.38 to improve COI barcoding identification at the species level. However, Laurito et al.39 employed a COI barcode and BCM algorithm to identify Culex spp. from Brazil and Argentina and concluded that the COI barcode does not contain enough information to distinguish Culex spp. Thus, the present study could not determine the real prevalence of APVs associated with a particular Culex species. Nevertheless, most Culex species found in peri-urban and rural areas in the state of Rio de Janeiro are highly ecologically similar40–45. Therefore, the differences do not substantially impact the control measures applied to Culex species and the transmission of APVs.
The present research reports on the genetic diversity of APVs. The most divergent APV sequences were obtained in mosquito samples from rural areas. This result may indicate pathogen adaptation to highly anthropized areas since significant genetic divergence is present in areas with lower anthropization levels due to more significant interactions and an abundance of host species found in these regions. Giraudeau et al.46 demonstrated for the first time that the highest rates of Avipoxvirus infection in finches (Haemorhous mexicanus) were in urban areas, where human activities were more often present.
Methods
Study area
The present study was conducted in the municipality of Seropedica (22° 44′ 38″ south latitude; 43° 42′ 27″ west longitude) in Rio de Janeiro as represented in the Fig. 1, from June 2016 to July 2017. Mosquito collections were carried out in properties with backyard poultry from rural and peri-urban areas.
Mosquito collections and identification of Culex spp
The parameters of an infinite population were considered based on the desired level of prevalence to determine the sample size47, with a sample error of 5%, a confidence level of 95%, and an expected prevalence of 50%, resulting in a total of 384 mosquitoes in sampling. The collections were carried out using CDC light traps in peri-domestic areas, which operated for 12 h, three times a week, totaling 864 h of operation of the traps from July 2016 to July 2017. A total of 2839 mosquitoes of the Anopheles, Aedes, and Culex genera were collected, with the Culex genus being the most abundant and comprising 96.23% (2732/2839). The identification of mosquitoes at the genus level was performed by morphological structures common to the Culex spp. according to Forattini43 and Berlin and Belkin48. Characteristics such as sex and engorgement were considered. The specimens were individually placed in tubes containing RNAlater® solution and stored at – 20 °C until molecular analysis was performed.
DNA extraction
DNA extraction was performed for each specimen of Culex spp. Before the extraction protocol, each specimen stored in RNAlater® (ThermoFisher Scientific) had its eyes dissected and discarded to avoid possible inhibitory action in molecular analysis caused by pigmentary components from insect eyes49. Each body was washed three times with 500 μL of sterile PBS and centrifuged at 14,500×g for 3 min to remove the RNAlater® solution. A protocol published by Ayres et al.28 was performed for DNA extraction. All samples were quantified by Nanodrop® ND-2000 spectrophotometry (Nanodrop Technologies, DE, USA), and samples were standardized at 30 ng/μL28.
Amplification of cytochrome C oxidase subunit I (COI) gene in Culex spp. DNA
A polymerase chain reaction (PCR) based on the mitochondrial COI gene, considered barcoding for mosquito identification33, was performed to verify DNA quality. The assay was performed using the universal primers LCO1490 (5'-GGTCAACAAATCATAAAGATATTGG-3') and HCO2198 (5'-TAAACTTCAGGGTGACCAAAAAATCA-3'). A final volume of 12.5 μL, containing 1 × PCR buffer, 2.5 mM of MgCl2, 0.2 mM of each dNTP, 0.5 mM of each primer, 1 U of Platinum Taq DNA Polymerase (Invitrogen®), and 3 μL of Culex spp. DNA. The thermocycling conditions were as follows: 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min on the ProFlex™ 3 × 32-well PCR System (ThermoFisher Scientific). The amplified products were subjected to electrophoresis on a 1.5% agarose gel in a 1 × TAE Buffer. Then, the gels were stained by immersion in ethidium bromide (5 mg/μL) and visualized under ultraviolet light on UV Transilluminator, E-gel electrophoresis system (Invitrogen, ThermoFisher Scientific).
PCR assay targeting p4b gene of Avipoxvirus in Culex spp
A pair of primers was selected to detect the Avipoxvirus based on the p4b gene, which encodes the viral nucleocapsid protein. These primers are widely used for the molecular detection of Avipoxvirus amplifying a PCR fragment of 578 bp50,51. The positive control was obtained from the total DNA extracted from the lyophilized vaccine Bouba Aviária Suave Biovet®. The vaccine content aliquots were resuspended in 1 × sterile phosphate saline buffer (1 × PBS) with pH 7.2 and extracted using the Invitrogen™ PureLink™ Genomic DNA Mini Kit. All extracted aliquots were quantified by Nanodrop® ND-2000 spectrophotometry (Nanodrop Technologies), standardized at a concentration of 30 ng/μL of total DNA for molecular analysis.
The PCR assay was optimized to achieve the greatest analytical sensitivity. All samples were submitted for Avipoxvirus detection assay on the ProFlex™ 3 × 32-well PCR System (ThermoFisher Scientific), with a final volume of 12 μL containing the following: 1 × PCR buffer (10 mM Tris–HCl; pH = 8.3; 50 mM KCl) (Invitrogen®), 1.5 mM Magnesium Chloride (MgCl2 50 mM, Invitrogen®), 0.2 mM of each nucleotide (dATP, dGTP, dTTP, and dCTP-100 mM Invitrogen®), 0.4 mM of primers, 1.2 U of Platinum Taq DNA Polymerase (Invitrogen®) and 1.5 µL of total DNA at 30 ng/µL. The thermocycling conditions were 94 °C for 2 min; 35 cycles of 94 °C for 60 s; 60 °C for 30 s; 72 °C for 60 s; and a final extension at 72 °C for 2 min. The amplified products were submitted to electrophoresis on a 2.0% agarose gel in a 1 × TAE buffer. The PCR products were purified using the Wizard® SV Gel Kit and PCR Clean-Up System (Promega™, Madison, WI, USA) following the manufacturer’s recommendations for sequencing.
Analytical sensitivity of Avipoxvirus-p4b-PCR
The analytical sensitivity of the PCR was assessed through the limit of detection (LOD), which was determined according to serial decimal dilutions carried out in triplicate of the p4b-PCR amplicon. The PCR amplicon was purified and quantified by the Qubit fluorometer (ThermoFisher Scientific). This amplicon concentration was employed to calculate the copy number using the following equation: copy number = (6.02 × 1023 [copies per mole] × p4b-PCR amplicon concentration [g])/(578 bp [target size] × 660 [g/mol/bp]). The number of copies ranged from 106 (one million) to 10–1 (zero) in eight different dilutions performed for further evaluation.
qPCR assay targeting chicken mitochondrial gene
The qPCR assay employed the primers targeting the chicken mitochondrial gene designed by Lahiff et al.52 and was performed with 1 × Meltdoctor buffer, 0.6 uM of each primer, and 1.5 µL of total DNA at 30 ng/µL. The thermocycling conditions were as follows: 95 °C for 10 min, 95 °C for 15 s, 57 °C for 30 s, 72 °C for 30 s for 40 cycles, and 72 °C for 10 min.
Sequencing and phylogenetic analysis of Avipoxvirus
The sequencing of positive samples for Avipoxvirus was performed using the Applied Biosystems 3730XL DNA Sequencer. The quality of the sequences was analyzed using the CLC Main Workbench Version 7.2 software (Qiagen®, Hilden, Germany) through the evaluation of the chromatogram. The contigs were assembled in the same software, and the similarity of each sequence was obtained using the BLASTn tool available at https://blast.ncbi.nlm.nih.gov/. The phylogenetic reconstruction was performed with 61 sequences available in GenBank belonging to subclades A1 to A7, B1 to B3, clades C, D, and E were compared to the samples obtained in this study. Sequences were aligned in MAFFT software using default options, and then the matrix was visually inspected for inconsistencies53. After removing misaligned positions with GBlocks, according to Talavera and Castresana54, a final matrix was obtained. The best replacement model was determined using JModelTest software55. The inference of the Avipoxvirus phylogeny was performed using the Maximum Likelihood (ML) method. The clade bootstrap values were evaluated using the RaxML self-convergence criterion with the best pseudo-replica values56.
Georeferencing
The Brazilian state cartographic base was collected in shapefile format from the electronic database of the Brazilian Institute of Geography and Statistics57 and uploaded in the GIS software [QGIS 3.22.8 'Białowieża' (https://qgis.org/pt_BR/site/], maintaining the coordinates in degrees in the Geocentric Reference System for the Americas (SIRGAS 2000, previously established by IBGE.
Thematic maps were created by applying the geographical coordinates of the collected samples in the cartographic base. A kernel map was also designed to visually distinguish infection clusters through interpolating the Avipoxvirus-positive samples and the study sites. The colored scale in the obtained raster was proportional to the frequency, ranging from 1 to 9.
Statistical analysis
The frequency of Avipoxvirus in Culex spp. captured in the municipality of Seropedica in the state of Rio de Janeiro was compared using the Chi-Square (χ2) test, admitting an error of 5% using R software version 3.6.158.
Conclusion
This study reported a high frequency of FWPW in Culex spp. in Seropedica, indicating a risk in implementing an organic system for poultry production in Seropedica, Rio de Janeiro. The FWPW circulation in this area reinforces the importance of vaccination as a preventive measure. In addition, this is the first study to report FWPW sequences obtained from Culex mosquitoes collected in the southwestern region of Rio de Janeiro, Brazil.
Acknowledgements
We would like to thank Josemar Cesar Gonçalves and Letícia Machado for supporting the completion of this work.
Author contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by C.S.v.D.M., H.A.S., I.Da.C.A., P.G.P., T.H.A.J., and N.A.S. The draft preparation of the manuscript was done by D.d.S.J., T.R.A., M.P.P., and C.S.v.D.M. Funding acquisition, supervision was performed by H.A.S., and C.L.M. All authors reviewed and edited the final version of the manuscript. All authors read and approved the final manuscript.
Funding
The Coordination for the Improvement of Higher Education Personnel (CAPES, finance code 001) supported the present work. Furthermore, this research was financed by the National Council for Scientific and Technological Development (CNPq) for the fellowship granted to HAS (Research Productivity Scholarship, grant number 310819/2018-0).
Data availability
The dataset generated and analyzed during the current study is available in the GenBank repository. The sequences are deposited under the following accession numbers: OL703782, OL703783, OL703784, OL703785, OL703786, OL703787, OL703788, OL703789, OL703790, and OL703791. https://www.ncbi.nlm.nih.gov/popset/2271451140.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lebdah MA, Ola AH, Amira MI. Avipoxvirus in Egypt and African continent: A review. Zagazig Vet. J. 2019;47:364–377. doi: 10.21608/zvjz.2019.14077.1052. [DOI] [Google Scholar]
- 2.Winterfield RW, Reed W. Avian pox: Infection and immunity with quail, Psittacine, fowl, and pigeon poxviruses. Poult. Sci. 1985;64(1):65–70. doi: 10.3382/ps.0640065. [DOI] [PubMed] [Google Scholar]
- 3.Williams RAJ, Truchado DA, Benitez L. A review on the prevalence of Poxvirus disease in free-living and captive wild birds. Microbiol. Res. 2021;12:403–418. doi: 10.3390/microbiolres12020028. [DOI] [Google Scholar]
- 4.Alehegn E, Mersha C, Desalegne M. A systematic review of serological and clinicopathological features and associated risk factors of Avian Pox. Br. J. Poult. Sci. 2014;3:78–87. [Google Scholar]
- 5.Yeo G, Wang Y, Chong SM, Humaidi M, Lin XF, Mailepessov D, Chan S, How CB, Lin YN, Huangfu T, Fernandez CD, Hapuarachchi C, Yap G. Characterization of Fowlpox virus in chickens and bird-biting mosquitoes: A molecular approach to investigating Avipoxvirus transmission. J. Gen. Virol. 2019;100:838–850. doi: 10.1099/jgv.0.001209. [DOI] [PubMed] [Google Scholar]
- 6.Tripathy DN, Reed WM. Pox. In: Swayne DE, editor. Diseases of Poultry. Wiley; 2020. pp. 364–381. [Google Scholar]
- 7.Warner RE. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. The Condor. 1968;70(2):101–120. doi: 10.2307/1365954. [DOI] [Google Scholar]
- 8.Smits JE, Tella JL, Carrete M, Serrano D, López G. An epizootic of Avian pox in endemic short-toed larks (Calandrella rufescens) and Berthelot’s pipits (Anthus berthelotti) in the Canary Islands, Spain. Vet. Pathol. 2005;42:59–65. doi: 10.1038/s41590-020-0669-6. [DOI] [PubMed] [Google Scholar]
- 9.Moens MAJ, Pérez-Tris J, Milá B, Benítez L. The biological background of a recurrently emerging infectious disease: Prevalence, diversity and host specificity of Avipoxvirus in wild neotropical birds. J. Avian Biol. 2017;48:1041–1046. doi: 10.1111/jav.01240. [DOI] [Google Scholar]
- 10.Eram N, Peighambari SM, Madani SA, Razmyar J, Barin A. Sequence and phylogenetic study of two Fowlpox virus isolates obtained from layer chickens and red mite (Dermanyssus gallinae) in 2016. J. Vet. Res. 2020;75(2):218–225. [Google Scholar]
- 11.Hess C, Maegdefrau-Pollan B, Bilic I, Liebhart D, Richter S, Mitsch P. Outbreak of cutaneous form of poxvirus on a commercial turkey farm caused by the species Fowlpox. Avian Dis. 2011;55:714–718. doi: 10.1637/9771-050511-Case.1. [DOI] [PubMed] [Google Scholar]
- 12.Lee HR, Koo BS, Kim JT, Kim HC, Kim MS, Klein TA, Shin MS, Lee S, Jeon EO, Min KC, Lee SB, Bae Y, Mo IP. Molecular epidemiology of Avian poxvirus in the oriental turtle dove (Streptopelia orientalsi) and the biting midge (Culicoides arakawae) in the Republic of Korea. J. Wildl. Dis. 2017;53:749–760. doi: 10.7589/2016-10-230. [DOI] [PubMed] [Google Scholar]
- 13.Vargas GD, Albano AP, Fischer G, Hübner S, Sallis SE, Nunes CF, Raffi MB, Soares MP. Avian pox virus infection in a common barn owl (Tyto alba) in southern Brazil. Pesq. Vet. Bras. 2011;31:620–622. doi: 10.1590/S0100-736X2011000700012. [DOI] [Google Scholar]
- 14.Alley MR, Hale KA, Cash W, Ha HJ. Concurrent avian malaria and Avipoxvirus infection in translocated South Island saddlebacks (Philesturnus carunculatus carunculatus) N. Z. Vet. J. 2010;58:218–223. doi: 10.1080/00480169.2010.68868. [DOI] [PubMed] [Google Scholar]
- 15.Damassa AJ. The role of Culex tarsalis in the transmission of Fowlpox Virus. Avian Dis. 1966;10:57–66. doi: 10.2307/1588207. [DOI] [Google Scholar]
- 16.Fuller T, Bensch S, Müller I, Novembre J, Pérez-Tris J, Ricklefs RE, Smith TB, Waldenström J. The ecology of emerging infectious diseases in migratory birds: An assessment of the role of climate change and priorities for future research. EcoHealth. 2012;9:80–88. doi: 10.1007/s10393-012-0750-1. [DOI] [PubMed] [Google Scholar]
- 17.Rocklöv J, Dubrow R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020;21:479–483. doi: 10.1038/s41590-020-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkett-Cadena ND, Vittor AY. Deforestation and vector-borne disease: Forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 2018;26:101–110. doi: 10.1016/j.baae.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morand S, Lajaunie C. Outbreaks of vector-borne and zoonotic diseases are associated with changes in forest cover and oil palm expansion at global scale. Front. Vet. Sci. 2021;8:230. doi: 10.3389/fvets.2021.661063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyuranecz M, Foster JT, Dán Á, Ip HS, Egstad KF, Parker PG, Higashiguchi JM, Skinner MA, Höfle U, Kreizinger Z, Dorrestein GM, Solt S, Sós E, Kim YJ, Uhart M, Pereda A, González-Hein G, Hidalgo H, Blanco J, Erdélyi K. Worldwide phylogenetic relationship of Avian poxviruses. J. Virol. 2013;87:4938–4951. doi: 10.1128/JVI.03183-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manarolla G, Pisoni G, Sironi G, Rampin T. Molecular biological characterization of avian poxvirus strains isolated from different avian species. Vet. Microbiol. 2010;140:1–8. doi: 10.1016/j.vetmic.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Bányai K, Palya V, Dénes B, Glávits R, Ivanics E, Horváth B, Farkas SL, Martona S, Adam Bálintc A, Gyuranecza M, Erdélyic K, Dán A. Unique genomic organization of a novel Avipoxvirus detected in turkey (Meleagris gallopavo) Infect. Genet. Evol. 2015;35:221–229. doi: 10.1016/j.meegid.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Mapaco LP, Lacerda Z, Monjane IVA, Gelaye E, Sussuro AH, Viljoen GJ. Identification of clade E avipoxvirus, Mozambique. Emerg. Infect. Dis. 2016;23:1602–1604. doi: 10.3201/eid2309.161981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro LC, Monteiro FL, Chagas DB, D'Ávila Vargas G, de Lima M, Fischer G, Hübner SO. Identification of clade E Avipoxvirus in Brazil. Avian Dis. 2020;64(2):223–227. doi: 10.1637/0005-2086-64.2.223. [DOI] [PubMed] [Google Scholar]
- 25.USDA. U.S. Department of Agriculture. ChooseMyPlate.gov. Washington, DC. Report Number: BR2021-0033. https://www.fas.usda.gov/data/brazil-poultry-and-products-annual-7. (2021)
- 26.Young LC, VanderWerf EA. Prevalence of avian poxvirus and effect on the fledging success of Laysan Albatross. J. Field Ornithol. 2008;79(1):93–98. doi: 10.1111/j.1557-9263.2008.00149.x. [DOI] [Google Scholar]
- 27.Garcia-Rejon JE, Blitvich BJ, Farfan-ale JA, Loroño-Pino MA, Chi-Chim WA, Flores LF, Rosado-Paredes E, Baakbaak C, Perez-Mutul J, Suarez-Solis V, Fernandez-Salas I, Beaty BJ. Host-feeding preference of the mosquito, Culex quinquefasciatus, in Yucatan State, Mexico. J. Insect Sci. 2010;32:1–10. doi: 10.1673/031.010.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayres CFJ, Romão TPA, Melo-Santos MAV, Furtado AF. Genetic diversity in Brazilian populations of Aedes albopictus. Mem. Inst. Oswaldo Cruz. 2002;97:871–875. doi: 10.1590/S0074-02762002000600022. [DOI] [PubMed] [Google Scholar]
- 29.Kunert-Filho HC, et al. Primeira análise filogenética de Avipoxvirus (APV) no Brasil. Pesq. Vet. Bras. 2016;36:357–362. doi: 10.1590/S0100-736X2016000500001. [DOI] [Google Scholar]
- 30.Esteves F, Marín SY, Resende M, Silva A, Coelho H, Barbosa M, D’Aparecida NS, Resende JS, Torres ACD, Martins NRS. Avian pox in native captive Psittacines, Brazil. Emerg. Infect. Dis. 2017;23(1):154–156. doi: 10.3201/eid2301.161133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braga JF, Couto RM, Rodrigues MC, Ecco R. Avipoxvirus detected in tumor-like lesions in a white-faced whistling duck (Dendrocygna viduata) Pesq. Vet. Bras. 2020;40:818–823. doi: 10.1590/1678-5150-PVB-6580. [DOI] [Google Scholar]
- 32.Jarmin S, Manvell R, Gough RE, Laidlaw SM, Skinner MA. Avipoxvirus phylogenetics: Identification of a PCR length polymorphism that discriminates between the two major clades. J. Gen. Virol. 2006;87:2191–2201. doi: 10.1099/vir.0.81738-0. [DOI] [PubMed] [Google Scholar]
- 33.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplifying mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994;3:294–299. [PubMed] [Google Scholar]
- 34.Meyer CP, Paulay G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005;3:422. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaila L, Stahls G. DNA barcodes: Evaluating the potential of COI to differentiate closely related species of Elachista (Lepidoptera: Gelechioidea: Elachistidae) from Australia. Zootaxa. 2006;1170:1–26. doi: 10.11646/zootaxa.1170.1.1. [DOI] [Google Scholar]
- 36.Lou M, Golding GB. Assigning sequences to species in the absence of large interspecific differences. Mol. Phylogenet. Evol. 2010;56:187–194. doi: 10.1016/j.ympev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Meier R, Shiyang K, Vaidya G, Ng PK. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst. Biol. 2006;55:715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- 38.van Velzen R, Weitschek E, Felici G, Bakker FT. DNA barcoding of recently diverged species: Relative performance of matching methods. PLoS ONE. 2012;7:30490. doi: 10.1371/journal.pone.0030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laurito M, de Oliveira TM, Almiron WR, Sallum MAM. COI barcode versus morphological identification of Culex (Culex) (Diptera: Culicidae) species: A case study using samples from Argentina and Brazil. Mem. Inst. Oswaldo Cruz. 2013;108:110–122. doi: 10.1590/0074-0276130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alencar J, Mello CF, Serra-Freire NF, Guimarães AE, Gil Santana HR, Gleiser RM. Biodiversity and temporal distribution of immature Culicidae in the Atlantic Forest, Rio de Janeiro State, Brazil. PLoS ONE. 2016;11:1–15. doi: 10.1371/journal.pone.0159240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alencar J, Mello CF, Guimarães AÉ, Gil-Santana HR, Silva JS, Santos-Mallet JR, Gleiser RM. Culicidae community composition and temporal dynamics in Guapiaçu Ecological Reserve, Cachoeiras de Macacu, Rio de Janeiro, Brazil. PLoS ONE. 2015;52:783–788. doi: 10.1371/journal.pone.0122268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Correa FF, et al. Mosquito (Diptera: Culicidae) communities in Nova Iguaçu Natural Park Rio de Janeiro, Brazil. J. Am. Mosquito Control Assoc. 2014;30:83–90. doi: 10.2987/13-6372.1. [DOI] [PubMed] [Google Scholar]
- 43.Forattini OP. Culicidologia Médica—2o volume: Identificação, Biologia, Epidemiologia. Editora da Universidade de São Paulo; 2002. [Google Scholar]
- 44.Forattini OP. Culicidologia Médica: Princípios Gerais, Morfologia, Glossário Taxonômico. Editora da Universidade de São Paulo; 1996. [Google Scholar]
- 45.Guimarães AE, Gentile C, Lopes CM, Sant'anna A, Jovita AM. Ecologia de mosquitos (Diptera: Culicidae) em áreas do Parque Nacional da Serra da Bocaina, Brasil, I-Distribuição por habitat. Rev. Saúde Públ. 2000;34:243–250. doi: 10.1590/S0034-89102000000300006. [DOI] [PubMed] [Google Scholar]
- 46.Giraudeau M, Mousel M, Earl S, McGraw K. Parasites in the city: Degree of urbanization predicts Poxvirus and coccidian infections in house finches (Haemorhous mexicanus) Open Sci. J. 2014;9(2):1–8. doi: 10.1371/journal.pone.0086747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thrusfield MV, Christley R. Veterinary Epidemiology. 4. Wiley; 2018. [Google Scholar]
- 48.Berlin GW, Belkin JN. Mosquito studies (Diptera, Culicidae): Subgenera Aedinus, Tinolestes and Anoedioporpa of Culex. Contrib. Am. Entomol. Inst. 1980;17:1–17. [Google Scholar]
- 49.Beckmann JF, Fallon AM. Decapitation improves detection of Wolbachia pipientis (Rickettsiales: Anaplasmataceae) in Culex pipiens (Diptera: Culicidae) mosquitoes by the Polymerase Chain Reaction. J. Med. Entomol. 2012;49:1103–1108. doi: 10.1603/ME12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H, Lee KW. Application of the polymerase chain reaction for the diagnosis of Fowlpoxvirus infection. J. Virol. Methods. 1997;63:113–119. doi: 10.1016/S0166-0934(96)02119-2. [DOI] [PubMed] [Google Scholar]
- 51.Binns MM, Boursnell MEG, Tomley FM, Campbell J. Analysis of the Fowlpox virus gene encoding the 4b core polypeptide and demonstration that it possesses efficient promoter sequences. Virology. 1989;170:288–291. doi: 10.1016/0042-6822(89)90380-2. [DOI] [PubMed] [Google Scholar]
- 52.Lahiff S, Glennon M, O’Brien L, Lyng J, Smith T, Maher M, et al. Species-specific PCR for the identification of ovine, porcine and chicken species in meat and bone meal (MBM) Mol. Cell. Probes. 2001;15:27–35. doi: 10.1006/mcpr.2000.0336. [DOI] [PubMed] [Google Scholar]
- 53.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization (2017) Brief. Bioinform. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56(4):564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 55.Posada D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008;25(7):1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 56.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.IBGE (Instituto Brasileiro de Geografia e Estatística). Base Cartográfica dos Estados brasileiros. Banco de Dados Eletrônico, Instituto Brasileiro de Geografia e Estatística (2018). Accessed on December 19, 2021. https://www.ibge.gov.br/.
- 58.R Core Team. A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2020). https://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated and analyzed during the current study is available in the GenBank repository. The sequences are deposited under the following accession numbers: OL703782, OL703783, OL703784, OL703785, OL703786, OL703787, OL703788, OL703789, OL703790, and OL703791. https://www.ncbi.nlm.nih.gov/popset/2271451140.