Abstract
Short-chain fatty acids (SCFA) can regulate appetite by stimulating the secretion of satiety hormones. However, the impact of short-chain fatty acid propionate on the release of gut satiety hormones and appetite regulation in pigs is not completely understood. In this study, 16 pigs were infused with saline or sodium propionate through a fistula in the caecum during a 28-day experimental period. We characterized the effects of propionate administration on peptide YY (PYY) and glucagon-like peptide 1 (GLP-1) secretion from colonic tissue, and investigated the role of propionate infusion on the expression of appetite-related genes in the colon and hypothalamus. Further, the direct impact of propionate administration on the expression of orexigenic neuropeptide agouti-related protein (AgRP) in hypothalamic N38 cells was also examined. The results showed that intra-cecal infusion of propionate reduced the short-term feed intake (P < 0.05) but not the long-term feed intake in pigs (P > 0.05). Propionate administration stimulated PYY and GLP-1 release from colon tissue in vivo and ex vivo (P < 0.05). It also upregulated PYY expression in the colonic mucosa (P < 0.05). Meanwhile, the GLP-1 and PYY levels in the blood were increased after intra-cecal infusion of propionate at d 28 (P < 0.05). Additionally, intra-cecal infusion of propionate upregulated the mRNA and protein expression of free fatty acid receptor 2/3 (FFAR2/FFAR3) in the colonic mucosa (P < 0.05). Propionate infusion also downregulated the orexigenic AgRP mRNA expression (P < 0.05) and upregulated the anorexigenic cocaine-and amphetamine-regulated transcript (CART) mRNA expression (P = 0.09) in the hypothalamus. Moreover, propionate administration directly downregulated AgRP expression in hypothalamic N38 cells in a dose-dependent manner (P < 0.05). Collectively, these findings demonstrated that cecal propionate stimulated colonic secretion of satiety hormones and suppressed appetite to reduce the short-term feed intake in pigs. This study highlights that microbial-derived propionate exerts an important role in regulating the physical functions of the host.
Keywords: Propionate, Gut hormones, Appetite, Hypothalamus, Pig
1. Introduction
Over the past decades, increasing evidence has shown that gut microbiota influences energy metabolism and the development of obesity. Subsequently, researchers have found that microbiota-generated metabolites, such as short-chain fatty acids (SCFA), partly determine the effects of gut microbes on host metabolism (Krautkramer et al., 2021; Van der Hee and Wells, 2021). SCFA are a class of the most important metabolites, mainly derived from microbial fermentation of dietary fibers in the large intestine. The total concentration of SCFA in the proximal colon of humans ranges from 70 to 140 mmol/L based on the diet type (Mortensen and Clausen, 1996). The molar ratio of acetate, propionate, and butyrate is approximately 60:25:15 (Cummings et al., 1996; Mortensen and Clausen, 1996). Recently, several studies have reported that SCFA can modulate energy homeostasis by stimulating gut hormone secretion to suppress appetite in humans (Chambers et al., 2015) and rodents (Frost et al., 2014). However, the effects of SCFA on pig appetite and gastrointestinal hormone secretion are still lacking.
Gastrointestinal (GI) hormones, such as peptide YY (PYY), glucagon-like peptide 1 (GLP-1), cholecystokinin (CCK) and ghrelin, are secreted by different enteroendocrine cells, which play a key role in modulating feed intake (Han et al., 2021). Interestingly, several studies have demonstrated that gut hormone secretion correlates with the fermentation of dietary fibers, especially satiety hormones, such as PYY and GLP-1 (Cani et al., 2004; Wanders et al., 2014; Koh et al., 2016). Further studies have also indicated that microbial-derived SCFA stimulate the secretion of gut hormones both in vitro and in vivo (Tolhurst et al., 2012; Psichas et al., 2015).
The hypothalamus is the major neural center for sensing energy status and controlling feeding behavior, which receives, integrates, and transmits appetite signals. The neuronal appetite core of the hypothalamus consists of anorexigenic and orexigenic neurons. They co-express the pro-opiomelanocortin precursor/cocaine amphetamine-regulated transcript neuropeptide (POMC/CART) and the neuropeptide Y/agouti-related protein (NPY/AgRP), respectively, to suppress and activate appetite. Several previous studies have revealed that gut hormones trigger neuropeptide expression associated with appetite in the hypothalamus (Larsen et al., 1997; Acuna-Goycolea and van den Pol, 2005; Ghamari-Langroudi et al., 2005). For instance, PYY and GLP-1 upregulated POMC/CART and downregulated NPY/AgRP expression in the hypothalamus through the peripheral circulatory system or vagal nerves (Abbott et al., 2005; Koda et al., 2005). Therefore, SCFA may stimulate gut hormone secretion to act on the hypothalamus, thereby regulating appetite. Additionally, a previous study has reported that colonic acetate can cross the blood–brain barrier to upregulate POMC expression and downregulate AgRP expression, which led to reduce appetite in rats, suggesting that acetate has a direct role in central appetite regulation (Frost et al., 2014). The concentration of intestinal propionate is the second highest among SCFA, only lower than the concentration of acetate. Whether propionate directly acts on the hypothalamic appetite neurons is unclear.
In this study, we investigated the impact of intra-cecal infusion of propionate on gut hormone secretion and appetite in a pig model. Feed intake and gastrointestinal hormone levels in the colon and plasma were also evaluated. The expression of appetite-related genes in the colonic mucosa and hypothalamus were analyzed using qPCR. Lastly, we presented a potential model of propionate regulation of pig appetite.
2. Materials and methods
2.1. Animal ethics
This experiment followed the Chinese Experimental Animal Care and Use guidelines and was approved by the Animal Care and Use Committee of Nanjing Agricultural University.
2.2. Animals, housing, and sampling
Sixteen Duroc × Landrace × Large White barrows (aged 8 wk, weighing approximately 16 kg) were raised on an experimental farm in Jiangsu Province, China. The pigs were fed on a commercial diet according to National Research Council (2012) during the experimental periods. The diet formula was as described in the previous study (Zhang et al., 2018). A simple “T”-type fistula was fitted at the cecum of each pig via surgery for perfusion. A jugular vein intubation was fixed in all pigs for the collection of dynamic blood samples. The pigs were given a 2-wk recuperation period after surgery. During the recuperation period, the pigs were sprayed using a tincture of iodine on the incision sites twice a day. Additionally, they were hypodermically injected with ceftriaxone sodium to prevent possible infection. The pigs (aged 10 wk, weighing approximately 20 kg) were randomly divided into 2 groups based on body weight with a randomized complete block design. Pigs in the control group (n = 8) were given saline solution, while the treatment group (n = 8) were given propionate solution (2 mmol/L, 25 mL, pH 5.8). The solutions were administered twice a day (at 07:00 and 18:00) through a “T”-type fistula. All the pigs were individually housed in concrete pens and had free access to diet and water under a controlled temperature of 22 ± 2 °C, humidity of 60% ± 5%, and natural light. Mental status, feed intake, and shape of feces were observed and recorded to assess the health status of each pig. The animal houses were cleaned 3 times per week.
Dynamic blood samples were collected through jugular vein intubation at 0, 15, 30, 60, and 120 min after seven days of propionate infusion at 07:00. The blood samples were then stored at room temperature for 4 h, and centrifuged at 3,000 × g for 15 min to obtain the blood serum. The serum was stored at −20 °C for the determination of hormone concentration. All the pigs were slaughtered and weighed at d 28. The gastrointestinal tract was immediately isolated from enterocoelia, and a 2-cm segment of colonic tissue was obtained, washed, and rinsed using phosphate buffer solution, then stored in liquid nitrogen. The colonic mucous membrane was scraped off from colon tissue with a clean glass slide, collected in a microtube, and stored in liquid nitrogen. The skull of pigs was cut open using an electric saw to remove the hypothalamus, which was store in liquid nitrogen.
2.3. Measurement of feed intake
All the pigs were fed on a commercial diet after propionate administration. Before feeding, the weight of the initial feed was recorded, and the remaining feed was weighed at 22:00 to calculate the intraday feed intake, which was used to determine the average weekly and daily feed intake. Feed efficiency was expressed as the average daily feed intake and average daily gain ratio. The remaining feed was weighed at 1, 2, and 4 h after feeding and used to calculate feed intake at different time intervals.
2.4. In vitro stimulation of PYY and GLP-1 release from colonic tissue after propionate administration
2.4.1. Preparation of dose solutions
SCFA propionate was obtained from Sigma–Aldrich (Sigma Inc, MO, USA). The propionate powder was dissolved in warm Krebs' Ringer bicarbonate (KRB, 37 °C) buffer (NaCl, 118 mmol/L; KCl, 4.75 mmol/L; KH2PO4, 1.18 mmol/L; MgSO4, 1.18 mmol/L; CaCl2, 2.5 mmol/L; NaHCO3, 25 mmol/L; Glucose, 11 mmol/L) with 25 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES, adjusted pH 7.2 to 7.4). The propionate solution was then diluted to 0, 5, 20, and 100 mmol/L solutions. The pH of all the solutions was adjusted to 7.2 to 7.4 before analysis.
2.4.2. Colonic tissue collection and sample preparation
The fresh colonic tissues were obtained from pigs and stored in ice-cold KRB/HEPES solution for further use. The colonic tissues were cut longitudinally and washed using KRB/HEPES. Colonic tissue (2 cm) was obtained from the proximal colon and cut into pieces into a sterile culture vessel as described by Wang et al. (2018). Finally, a sample of the diced colonic tissues weighing 0.4 g was used to perfusion system.
2.4.3. Perfusion technique and process
The perfusion system established in the previous study (Zhao et al., 2018) consisted of a media reservoir, a peristaltic pump, a tissue chamber, an oxygen bomb (a mixture of 95% O2 and 5% CO2), a water bath, silicone hose (inside diameter; 1 mm), and collection tubes. The tissue chambers were incubated in a water bath at 37 °C for 30 min before perfusion. KRB/HEPES solution with the mixture of 95% O2 and 5% CO2 was pumped into the tissue chamber using a peristaltic pump. The colonic tissue samples were randomly put into the chamber to start the test. During the perfusion stages, the tissue chambers were put in a water bath at 37 °C, and the solution was gassed with a mixture of 95% O2 and 5% CO2.
All the samples were perfused with KRB/HEPES solution for 60 min before propionate stimulation to obtain stable physiology. The samples were then perfused with different propionate concentrations (0, 5, 20, and 100 mmol/L). The speed of the peristaltic pump was adjusted to 6 mL/h. The perfusate samples were collected at 30 and 60 min to analyze PYY and GLP-1 concentrations.
2.5. Cell culture
A N38 cell line encoding AgRP gene was isolated from an embryonic mouse hypothalamus and cultured in a low-glucose DMEM solution (Gibco, CA, USA) with 10% fetal bovine serum (FBS) (Gibco, CA, USA) and 1% penicillin-streptomycin solution (Gibco, CA, USA). The cells were cultured thrice at 37 °C in a humidified atmosphere containing 5% CO2, then treated with 0, 10, 50, 100, and 500 μmol/L propionate for 4 and 12 h to explore the dose effect of propionate on AgRP expression. The cells were washed thrice using phosphate-buffered saline before sampling. Then, the total RNA of the cells was extracted for qPCR analysis.
2.6. Gastrointestinal hormone analysis
The levels of GI hormones (PYY, GLP-1, CCK, Ghrelin, and Insulin) in the blood and perfusate samples were measured using the enzyme-linked immunosorbent assay (ELISA) kits (Function Bio Co Ltd, Beijing, China) for pigs following the manufacturer's instructions. PYY, GLP-1, CCK, ghrelin, and insulin levels were 10 to 600 pg/mL, 2 to 100 pg/mL, 10 to 200 ng/L, 50 to 3,000 ng/L, and 1.5 to 40 mIU/L, respectively. For GI hormone levels in colonic tissue, 1 g of colonic tissue was added to 9 mL PBS (0.1 mol/L, pH 7.4), homogenized using grinders, centrifuged at 3,000 × g for 5 min to obtain supernatant. Similarly, ELISA kits were used to determine the GI hormone levels in colonic tissue following the manufacturer's instructions.
2.7. Total RNA extraction and quantitative real-time PCR
Total RNA was extracted from the colonic mucosa and hypothalamus of pigs and N38 cells using an RNeasy kit (Aidlab Bio Co., Ltd, Beijing, China) following the manufacturer's protocols. Total RNA concentration and purity were detected by Nano-Drop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). All RNA samples were performed to electrophoresis through a 1% agarose-formaldehyde gel to verify integrity. PrimerScript TM RT Reagent kit (Takara Bio, Dalian, China) was used to convert RNA to cDNA for quantitative real-time PCR. Quantitative real-time PCR was performed to analyze the mRNA expression of free fatty acid receptor 2 (FFAR2), free fatty acid receptor 3 (FFAR3), glucagon (GCG), PYY, and glucagon-like-peptide-1 receptor (GLP-1R) in colonic mucosa and AgRP, NPY, POMC, CART, GCG, and GLP-1R in the hypothalamus of pigs. In addition, the mRNA expression of AgRP in N38 cells was quantified by qPCR. Beta-actin was used as the control gene to standardize the results. The relative expression levels were analyzed using the 2−ΔΔCt method. The sequences of primers are listed in Table 1.
Table 1.
Primer sequences used in this study.
| Item1 | Sequence (5′ to 3′) | Reference |
|---|---|---|
| For pigs | ||
| FFAR2 | Forward: CTGCCTGGGATCGTCTGTG | Li et al. (2014) |
| Reverse: CATACCCTCGGCCTTCTGG | ||
| FFAR3 | Forward: CCACGCTGCTCAACTTCCT | Li et al. (2014) |
| Reverse: GTCTCCACTCGGGGCTTTT | ||
| PYY | Forward: AGATATGCTAATACACCGAT | Haenen et al. (2013) |
| Reverse: CCAAACCCTTCTCAGATG | ||
| GLP-1R | Forward: CACAGGCTTGTTCTGCAACC | Kelly et al. (2014) |
| Reverse: AAGACGGACAGTGCTCGAAG | ||
| GCG | Forward: CAAGAGGAACAAGAATAACAT | Haenen et al. (2013) |
| Reverse: AAGAACTTACATCACTGGTA | ||
| CART | Forward: AGCCGCGAGCCCTGGACATCTACT | Óvilo et al. (2014) |
| Reverse: AGGGACTTGGCCATACTTCTTCTC | ||
| NPY | Forward: CGTACCCCTCCAAGCCCGACAAC | Óvilo et al. (2014) |
| Reverse: AACATTTTCCGTGCCTTCTCT | ||
| POMC | Forward: GTAACTTGCTGGCGTGCATC | Wang et al. (2014) |
| Reverse: GAAGTGGCCCATGACGTACT | ||
| AgRP | Forward: AGCCCCCACTGAAGAGGAC | Óvilo et al. (2014) |
| Reverse: TTGAAGAAACGGCAGTAGCAT | ||
| β-actin | Forward: ATGCTTCTAGACGGACTGCG | Lin et al. (2014) |
| Reverse: GTTTCAGGAGGCTGGCATGA | ||
| For N38 cells | ||
| AgRP | Forward: CCACGAAACTACCTTCAACTC | this study |
| Reverse: TGATCTCCTTCTGCATCCTGT | ||
| β-actin | Forward: CTTTGGCGGAGGTGCTAGAT | this study |
| Reverse: TGCGACTACAGAGGTTCGTG | ||
FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3; PYY = peptide YY; GLP-1R = glucagon-like-peptide-1 receptor; GCG = glucagon; CART = cocaine-amphetamine regulated transcript; NPY = neuropeptide Y; POMC = pro-opiomelanocortin; AgRP = agouti-related protein.
2.8. Western blot
The Western blotting of SCFA receptors (FFAR2 approximate 43 kDa and FFAR3 approximate 41 kDa) in the colonic mucosa was performed as described in the previous study (Yu et al., 2017). Briefly, the colonic mucosa samples were lysed with lysis buffer (Sigma Inc., MO, USA), following the manufacturer's instructions. Protein concentration was measured using Bradford's method. The protein samples were degenerated in boiling water. Approximate 40 μg protein sample was loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride membranes (Merck Millipore, WWLP, USA). The membrane was blocked using a 5% free-fat milk solution and then incubated with primary antibody (anti-FFAR2, or FFAR3; dilution 1:1,000) at 4 °C overnight. The membrane was then incubated with a secondary antibody (dilution 1:500). Electrochemiluminescence (Tanon, Zhejiang Province, China) was used to develop bands. ImageJ software (National Institutes of Health, MD, USA) was used to determine band density.
2.9. Statistical analysis
All data were analyzed with SPSS 20.0 software (IBM, NY, USA). The data were expressed as mean ± SEM. The student's t-test was used to determine statistical differences between groups. P < 0.05 were considered as significant differences. All data were visualized using Graphpad Prism 7.0 (Graphpad Inc, CA, USA).
3. Results
3.1. Propionate reduces acute feed intake in pigs
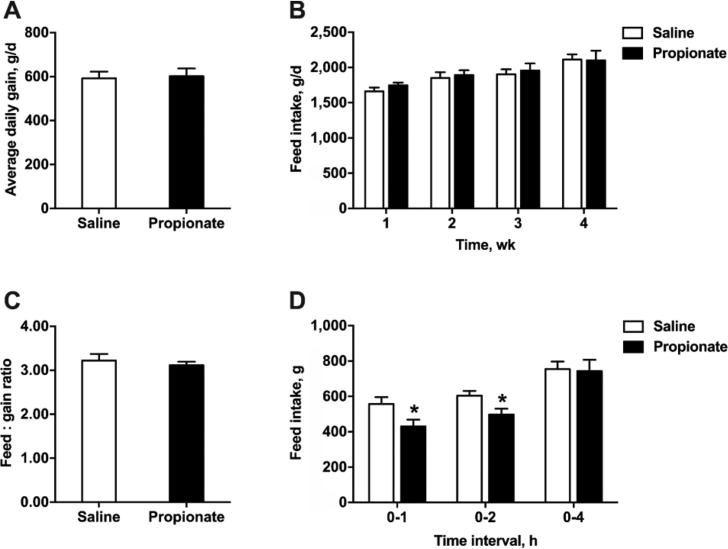
We first assessed whether propionate influences body weight gain and feed intake in pigs using a pig model with intestinal fistulas and jugular vein cannulation. Cecal infusion of propionate did not affect the average daily gain and feed conversion ratio in the 4 wk feeding experiment (P > 0.05) (Fig. 1A and C). Average weekly feed intake was not significantly different between the propionate and saline control groups (P > 0.05) (Fig. 1B). In contrast, cecal infusion of propionate significantly reduced acute feed intake at both 1 and 2 h post-injection (P < 0.05) (Fig. 1D). The feed intake of propionate-treated pigs returned to the normal level after 4 h post-infusion.
Fig. 1.
The effects of cecal infusion of propionate on (A) average daily gain, (B) feed intake, (C) feed-to-gain ratio, and (D) short-term feed intake in pigs. The data were expressed as mean ± SEM. ∗P < 0.05.
3.2. Propionate stimulates the release of GLP-1 and PYY and upregulates the expression of SCFA receptors in the colon
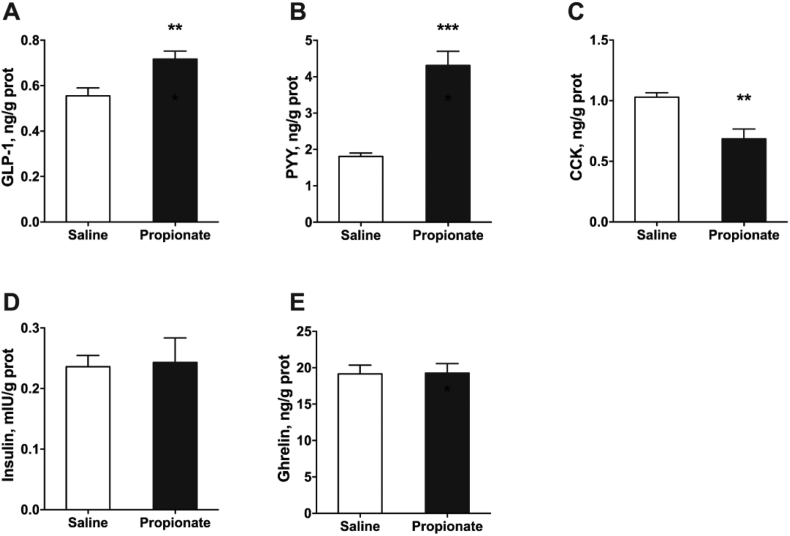
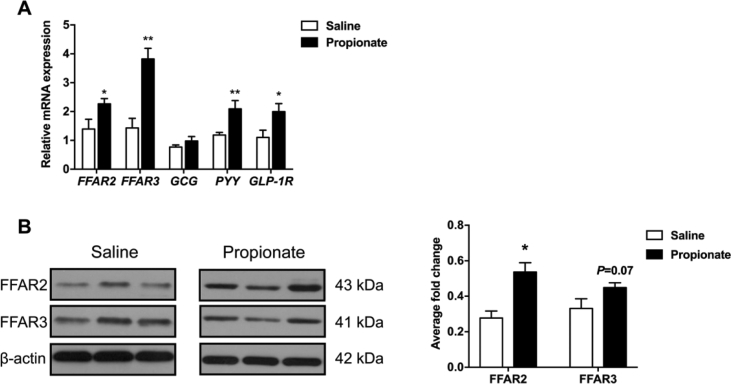
To determine whether in vivo intra-cecal administration of propionate induces gut hormone secretion in pigs, we examined GLP-1, PYY, CCK, ghrelin, and insulin levels in the colon. GLP-1 and PYY levels were significantly higher in the propionate group than in the control group (P < 0.05) (Fig. 2A and B). However, the release of CCK from the colon was suppressed after propionate treatment (P < 0.05) (Fig. 2C). Moreover, cecal infusion with propionate did not affect the release of ghrelin and insulin (P > 0.05) (Fig. 2D and E). The relative expression of PYY and GLP-1R mRNA levels were significantly upregulated in the propionate group (P < 0.05) (Fig. 3A). However, GCG (regulating the expression of GLP-1) expression was not altered in the 2 groups (P > 0.05) (Fig. 3A). qPCR analysis also showed that cecal infusion with propionate upregulated FFAR2 and FFAR3 expression in the colonic mucosa (P < 0.05) (Fig. 3A). Meanwhile, Western blot analysis showed that propionate upregulated FFAR2 and FFAR3 protein expression (P < 0.05) (Fig. 3B).
Fig. 2.
The effects of cecal infusion of propionate on the secretion of gastrointestinal hormones from colon tissue in pigs. (A) Glucagon-like peptide 1 (GLP-1); (B) peptide YY (PYY); (C) cholecystokinin (CCK); (D) insulin; (E) ghrelin. The data were expressed as mean ± SEM. ∗∗P < 0.01; ∗∗∗P < 0.001.
Fig. 3.
The effects of cecal infusion of propionate on (A) the mRNA expression of genes involved in free fatty acid receptors and appetite control and (B) the protein expression of free fatty acid receptors in the colonic mucosa. The data were expressed as mean ± SEM. ∗P < 0.05; ∗∗P < 0.01. FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3; GCG = glucagon; PYY = peptide YY; GLP-1R = glucagon-like-peptide-1 receptor.
3.3. Effects of propionate on the release of GI hormones from intestinal tissue of pig ex vivo
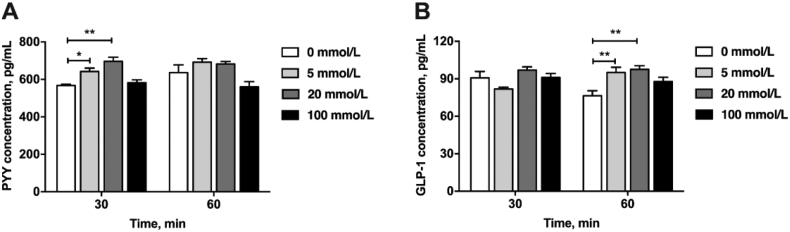
An ex vivo model of the porcine colon was developed to assess gut hormone secretion after propionate exposure. Colon tissue was incubated with propionate (0, 5, 20, and 100 mmol/L). Propionate significantly stimulated PYY secretion after 30 min of incubation (P < 0.05) (Fig. 4A, 5 and 20 mmol/L propionate). Furthermore, propionate solutions (5 and 20 mmol/L) significantly induced GLP-1 release after 60 min of incubation (P < 0.05) (Fig. 4B). However, 100 mmol/L propionate did not significantly affect GLP-1 and PYY release compared with the saline control (P > 0.05) (Fig. 4).
Fig. 4.
The different levels of propionate stimulating (A) peptide YY (PYY) and (B) glucagon-like peptide 1 (GLP-1) secretion from colonic tissue ex vivo. The data were expressed as mean ± SEM. ∗P < 0.05; ∗∗P < 0.01.
3.4. Intra-cecal administration of propionate influences plasma hormone levels after feed intake
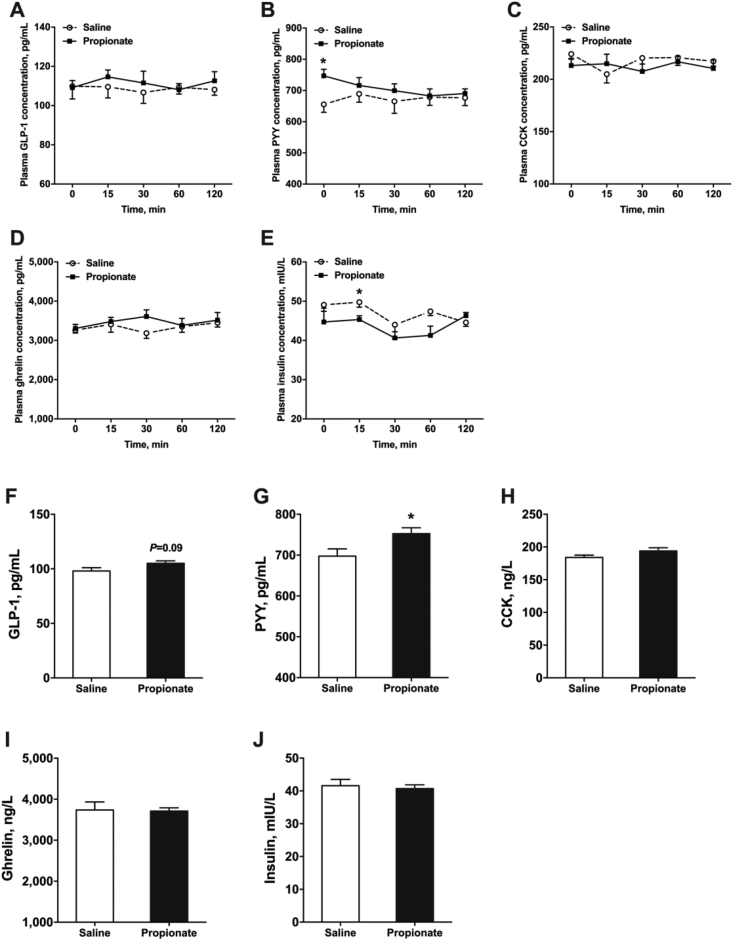
To evaluate the effects of propionate on plasma gut hormone, we determined the GLP-1, PYY, CCK, ghrelin and insulin concentration using blood samples collected on d 7 and d 28. The blood samples on d 7 were collected at different time points (0, 15, 30, 60, and 120 min) post-feeding after intra-cecal administration of propionate. The blood samples on d 28 were collected after intra-cecal administration of propionate for 30 min. Propionate treatment did not affect plasma GLP-1, CCK, and ghrelin levels at d 7 (P > 0.05) (Fig. 5A, C, and D). Nonetheless, propionate decreased plasma insulin level at 15 min after the meal (P < 0.05) (Fig. 5E). Plasma PYY level was significantly higher in the propionate group than in the control group at 0 min after the meal (P < 0.05) (Fig. 5B), possibly due to the propionate administration before d 7. Additionally, propionate significantly increased PYY concentration compared with saline treatment at d 28 (P < 0.05) (Fig. 5G). GLP-1 level showed an increasing trend in the propionate group (P = 0.09) (Fig. 5F). Similarly, plasma CCK, ghrelin, and insulin levels were not different between the 2 groups at d 28 (P > 0.05) (Fig. 5H–J).
Fig. 5.
The effects of cecal infusion of propionate on plasma gastrointestinal (GI) hormone concentration on d 7 (A to E) and d 28 (F to J). The data were expressed as mean ± SEM. ∗P < 0.05. GLP-1 = glucagon-like peptide 1; PYY = peptide YY; CCK = cholecystokinin.
3.5. Propionate regulates the expression of appetite neuropeptide-related genes in the hypothalamus
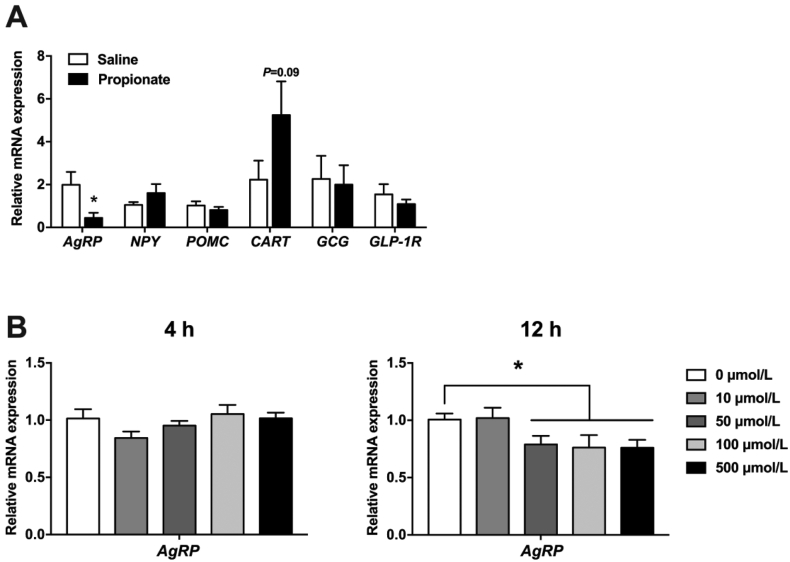
The changes in hypothalamic neuropeptide expression after intra-cecal propionate administration were also investigated. Propionate significantly decreased AgRP expression (P < 0.05) and increased CART expression (P = 0.09) (Fig. 6A). Meanwhile, NPY and POMC expressions were not significantly different between the propionate and control groups (P > 0.05) (Fig. 6A). Additionally, intra-colonic propionate administration did not affect GCG and GLP-1R expression in the hypothalamus (P > 0.05) (Fig. 6A).
Fig. 6.
The effects of propionate on the mRNA expression of (A) genes involved in appetite regulation in the hypothalamus of pigs and (B) AgRP in N38 cells. The data were expressed as mean ± SEM. ∗P < 0.05. AgRP = agouti-related protein; NPY = neuropeptide Y; POMC = pro-opiomelanocortin; CART = cocaine-amphetamine regulated transcript; GCG = glucagon; GLP-1R = glucagon-like-peptide-1 receptor.
Furthermore, hypothalamic N38 cells were used to investigate the effect of propionate administration on the mRNA expression of AgRP. In vitro (12 h) propionate treatment dose-dependently decreased the mRNA levels of AgRP in N38 cells (P < 0.05) (Fig. 6B), consistent with previous in vivo results. However, in vitro low-dose propionate treatment (10 μmol/L) did not affect the AgRP expression (P > 0.05) (Fig. 6B). Nevertheless, the AgRP mRNA expression level was not changed after propionate treatment for 4 h (P > 0.05) (Fig. 6B).
4. Discussion
Microbial-derived SCFA have been reported to regulate a variety of physical functions of the host, including appetite (Murphy and Bloom, 2006; Canfora et al., 2015; Kasubuchi et al., 2015). In this study, we explored the effect of cecal infusion with propionate on the secretion of satiety hormones and feed intake in a pig model. The results demonstrated that cecal infusion with propionate stimulated the secretion of satiety hormones from colonic tissue in vivo and ex vivo, and short-term feed intake was suppressed after intra-cecal infusion of propionate. Additionally, propionate activated the free fatty acid receptors in the colon and downregulated the expression of orexigenic genes in the hypothalamus. These results indicate that cecal infusion with propionate can directly or indirectly regulate pig appetite to suppress short-term feed intake.
Feed intake is a classic index to directly reflect the appetite of a host. In the present study, intra-cecal administration of propionate decreased short-term feed intake in pigs, indicating that propionate play a role in inhibiting pig appetite − which is consistent with previous studies in other animal models. For instance, a diet supplemented with propionate reduces the feed intake in mice (Lin et al., 2012). Additionally, Goswami et al. reported that intraperitoneal injection of propionate as well suppresses feed intake in mice in a time-dependent manner (Goswami et al., 2018). Interestingly, propionate infusion did not reduce long-term feed intake in pigs, possibly because the non-continuous infusion failed to cause long-term appetite suppression effects. Propionate in the gastrointestinal tract can be absorbed into the portal vein and transferred to the liver for gluconeogenesis (Yu et al., 2019). Thus, the rapid metabolism of propionate weakens the appetite suppressing effect. However, serval studies argued that propionate has no effect on feed intake (Darzi et al., 2012, 2016). The inconsistent results may be due to experimental conditions, propionate dose, and animal models.
PYY and GLP-1 are the major GI hormones released from enteroendocrine L cells, which enhance satiety to reduce feed intake. In this study, intra-cecal infusion of propionate stimulated the release of PYY and GLP-1 from colonic tissue in ex vivo and in vivo. Similar results have also been reported in rodents (Psichas et al., 2015). Several studies further revealed that the activation of free fatty acid receptors, including FFAR2 and FFAR3, is responsible for the secretion of PYY and GLP-1 in the colon (Karaki et al., 2006; Chambers et al., 2015; Psichas et al., 2015). For example, mice lacking FFAR2/FFAR3 have reduced SCFA-triggered PYY and GLP-1 secretion (Samuel et al., 2008; Tolhurst et al., 2012). In the current study, intra-cecal infusion with propionate also upregulated the mRNA and protein expression of FFAR2/FFAR3 in the colonic mucosa. Thus, these results suggest that propionate stimulates PYY and GLP-1 secretion via FFAR2/FFAR3 activation in pigs. However, there are some conflicting results reported by other researchers. For instance, Voortman and his colleagues reported that the release of PYY and GLP-1 was unchanged in the pig colon after exposure to individual SCFA with 5 mmol/L (Voortman et al., 2012). In addition, a previous study showed that SCFA, except propionate, stimulate PYY and GLP-1 secretion from the isolated rat colon through activating the voltage-gated Ca2+ channels instead of FFAR2/FFAR3 (Christiansen et al., 2018).
Appetite regulation involves a complex signaling network. The hypothalamus is the most important neuronal site responsible for appetite control. The arcuate nucleus of the hypothalamus contains subpopulations of neurons that produce orexinergic neuropeptides AgRP/NPY and anorexinergic neuropeptides POMC/CART in mammals (Mitchell and Begg, 2021). In this study, although propionate administration has no influence on NPY and POMC expression in the hypothalamus, the mRNA expression levels of AgRP and CART were upregulated. These results could be caused by the increased levels of PYY and GLP-1. A study reported that GI hormones, especially PYY and GLP-1, stimulate POMC/CART and AgRP/NPY expression via the periphery circulation system (Pizarroso et al., 2021). Interestingly, PYY and GLP-1 levels in plasma were increased after propionate infusion in the current study. Moreover, the brain and gastrointestinal tract can interact with another complex and bidirectional pathway, such as the vagus nerve (Pizarroso et al., 2021). Previous studies showed that PYY and GLP-1 could not inhibit appetite in mice after vagotomy (Abbott et al., 2005; Morton et al., 2006), confirming the vagus nerve pathway in regulating appetite. PYY and GLP-1 also inhibit gastrointestinal motility and slow gastric-emptying by acting on the vagal nerve (Pappas et al., 1986; Broberger et al., 1997; Näslund et al., 1998; Edholm et al., 2010); thus, enhancing satiety and suppressing appetite. A previous study has demonstrated that SCFA can cross the blood–brain barrier to reach the brain (Vijay and Morris, 2014). Frost et al. (2014) indicated that acetate directly increase POMC and reduce AgRP expression in the hypothalamus. Interestingly, we found that the different doses of propionate also downregulated AgRP expression in hypothalamic N38 cells, suggesting that propionate has a direct role in suppressing appetite. Collectively, the effect of propionate on pig appetite and feed intake has central and peripheral pathways.
5. Conclusion
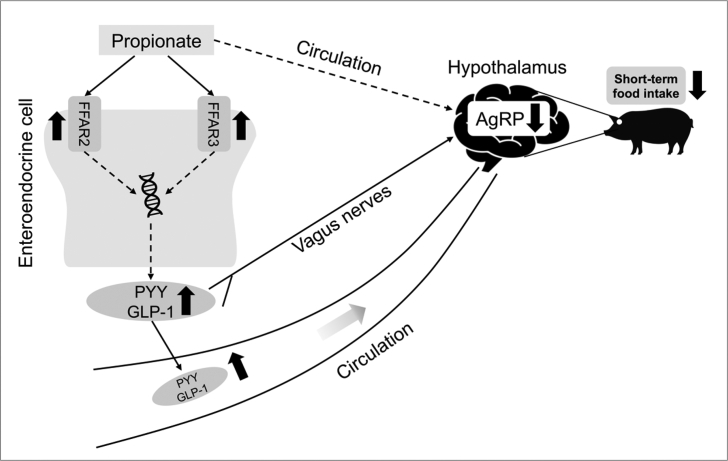
To summarize the effect of intra-cecal infusion of propionate on the secretion of GI hormones and feed intake in pigs, we proposed a potential scheme as shown in Fig. 7. This study demonstrated that cecal propionate may trigger FFAR2/FFAR3 to stimulate the secretion of satiety hormones from colonic tissue, which regulates expression levels of neuropeptides involved in appetite control via the gut–brain axis to reduce the short-term feed intake. These findings provide evidence for microbial-derived propionate regulating the physical functions of the host.
Fig. 7.
The structure of the potential mechanism of intra-cecal infusion of propionate suppressing acute appetite in pigs. The black up and down arrows indicate an increase and decrease after propionate treatment, respectively. The solid and dashed arrows indicate direct and indirect processes, respectively. FFAR2 = free fatty acid receptor 2; FFAR3 = free fatty acid receptor 3; PYY = peptide YY; GLP-1 = glucagon-like peptide 1; AgRP = agouti-related protein.
Author contributions
Yanan Zhang: Methodology, Investigation, Data curation, Writing – original draft. Xuan Li: Methodology, Investigation. Guowen Huang: Investigation. Haifeng Wang: Investigation, Resources. Huizi Chen: Data curation. Yong Su: Validation. Kaifan Yu: Conceptualization, Supervision, Methodology, Writing – review & editing, Funding acquisition. Weiyun Zhu: Conceptualization, Writing – review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This research was supported by grants from the National Natural Science Foundation of China (31972528 and 31501962), and the Jiangsu Agricultural Science and Technology Innovation Fund (CX(19)3012).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abbott C.R., Monteiro M., Small C.J., Sajedi A., Smith K.L., Parkinson J.R., Ghatei M.A., Bloom S.R. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044(1):127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Acuna-Goycolea C., van den Pol A.N. Peptide YY3-36 inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis. J Neurosci. 2005;25(45):10510–10519. doi: 10.1523/JNEUROSCI.2552-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C., Landry M., Wong H., Walsh J.N., Hökfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66(6):393–408. doi: 10.1159/000127265. [DOI] [PubMed] [Google Scholar]
- Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Dewever C., Delzenne N.M. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92(3):521–526. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E., MacDougall K., Preston T., Tedford C., Finlayson G.S., Blundell J.E., Bell J.D., Thomas E.L., Mt-Isa S., Ashby D., Gibson G.R., Kolida S., Dhillo W.S., Bloom S.R., Morley W., Clegg S., Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C.B., Gabe M.B.N., Svendsen B., Dragsted L.O., Rosenkilde M.M., Holst J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- Cummings J.H., Beatty E.R., Kingman S.M., Bingham S.A., Englyst H.N. Digestion and physiological properties of resistant starch in the human large bowel. Br J Nutr. 1996;75(5):733–747. doi: 10.1079/bjn19960177. [DOI] [PubMed] [Google Scholar]
- Darzi J., Frost G.S., Robertson M.D. Effects of a novel propionate-rich sourdough bread on appetite and food intake. Eur J Clin Nutr. 2012;66(7):789–794. doi: 10.1038/ejcn.2012.1. [DOI] [PubMed] [Google Scholar]
- Darzi J., Frost G.S., Swann J.R., Costabile A., Robertson M.D. L-rhamnose as a source of colonic propionate inhibits insulin secretion but does not influence measures of appetite or food intake. Appetite. 2016;98:142–149. doi: 10.1016/j.appet.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Edholm T., Degerblad M., Grybäck P., Hilsted L., Holst J.J., Jacobsson H., Efendic S., Schmidt P.T., Hellström P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neuro Gastroenterol Motil. 2010;22(11):1191–1200. doi: 10.1111/j.1365-2982.2010.01554.x. [DOI] [PubMed] [Google Scholar]
- Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L., Anastasovska J., Ghourab S., Hankir M., Zhang S., Carling D., Swann J.R., Gibson G., Viardot A., Morrison D., Louise Thomas E., Bell J.D. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M., Colmers W.F., Cone R.D. PYY3-36 inhibits the action potential firing activity of POMC neurons of arcuate nucleus through postsynaptic Y2 receptors. Cell Metabol. 2005;2(3):191–199. doi: 10.1016/j.cmet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Goswami C., Iwasaki Y., Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem. 2018;57:130–135. doi: 10.1016/j.jnutbio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Haenen D., Zhang J., Souza da Silva C., Bosch G., van der Meer I.M., van Arkel J., van den Borne J.J., Pérez Gutiérrez O., Smidt H., Kemp B., Müller M., Hooiveld G.J. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr. 2013;143(3):274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- Han H., Yi B., Zhong R., Wang M., Zhang S., Ma J., Yin Y., Yin J., Chen L., Zhang H. From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators. Microbiome. 2021;9(1):162. doi: 10.1186/s40168-021-01093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki S., Mitsui R., Hayashi H., Kato I., Sugiya H., Iwanaga T., Furness J.B., Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324(3):353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- Kasubuchi M., Hasegawa S., Hiramatsu T., Ichimura A., Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.C., Steyn L.V., Kitzmann J.P., Anderson M.J., Mueller K.R., Hart N.J., Lynch R.M., Papas K.K., Limesand S.W. Function and expression of sulfonylurea, adrenergic, and glucagon-like peptide 1 receptors in isolated porcine islets. Xenotransplantation. 2014;21(4):385–391. doi: 10.1111/xen.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda S., Date Y., Murakami N., Shimbara T., Hanada T., Toshinai K., Niijima A., Furuya M., Inomata N., Osuye K., Nakazato M. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146(5):2369–2375. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Krautkramer K.A., Fan J., Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19(2):77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- Larsen P.J., Tang-Christensen M., Jessop D.S. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138(10):4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- Li G., Su H., Zhou Z., Yao W. Identification of the porcine G protein-coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., Marsh D.J. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Zhang B., Yu C., Li J., Zhang L., Sun H., Gao F., Zhou G. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C.S., Begg D.P. The regulation of food intake by insulin in the central nervous system. J Neuroendocrinol. 2021;33(4) doi: 10.1111/jne.12952. [DOI] [PubMed] [Google Scholar]
- Mortensen P.B., Clausen M.R. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl. 1996;31:132–148. doi: 10.3109/00365529609094568. [DOI] [PubMed] [Google Scholar]
- Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Murphy K.G., Bloom S.R. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444(7121):854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Näslund E., Gutniak M., Skogar S., Rössner S., Hellström P.M. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68(3):525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- Óvilo C., González-Bulnes A., Benítez R., Ayuso M., Barbero A., Pérez-Solana M.L., Barragán C., Astiz S., Fernández A., López-Bote C. Prenatal programming in an obese swine model: sex-related effects of maternal energy restriction on morphology, metabolism and hypothalamic gene expression. Br J Nutr. 2014;111(4):735–746. doi: 10.1017/S0007114513002948. [DOI] [PubMed] [Google Scholar]
- Pappas T.N., Debas H.T., Chang A.M., Taylor I.L. Peptide YY release by fatty acids is sufficient to inhibit gastric emptying in dogs. Gastroenterology. 1986;91(6):1386–1389. doi: 10.1016/0016-5085(86)90191-5. [DOI] [PubMed] [Google Scholar]
- Pizarroso N.A., Fuciños P., Gonçalves C., Pastrana L., Amado I.R. A review on the role of food-derived bioactive molecules and the microbiota-gut-brain axis in satiety regulation. Nutrients. 2021;13(2):632. doi: 10.3390/nu13020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psichas A., Sleeth M.L., Murphy K.G., Brooks L., Bewick G.A., Hanyaloglu A.C., Ghatei M.A., Bloom S.R., Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes. 2015;39(3):424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., Gordon J.I. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hee B., Wells J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Vijay N., Morris M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharmaceut Des. 2014;20(10):1487–1498. doi: 10.2174/13816128113199990462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voortman T., Hendriks H.F., Witkamp R.F., Wortelboer H.M. Effects of long- and short-chain fatty acids on the release of gastrointestinal hormones using an ex vivo porcine intestinal tissue model. J Agric Food Chem. 2012;60(36):9035–9042. doi: 10.1021/jf2045697. [DOI] [PubMed] [Google Scholar]
- Wanders A.J., Mars M., Borgonjen-van den Berg K.J., de Graaf C., Feskens E.J. Satiety and energy intake after single and repeated exposure to gel-forming dietary fiber: post-ingestive effects. Int J Obes. 2014;38(6):794–800. doi: 10.1038/ijo.2013.176. [DOI] [PubMed] [Google Scholar]
- Wang C., Kang C., Xian Y., Zhang M., Chen X., Pei M., Zhu W., Hang S. Sensing of L-arginine by gut-expressed calcium sensing receptor stimulates gut satiety hormones cholecystokinin and glucose-dependent insulinotropic peptide secretion in pig model. J Food Sci. 2018;83(9):2394–2401. doi: 10.1111/1750-3841.14297. [DOI] [PubMed] [Google Scholar]
- Wang L., Chen X., Li X., Shu G., Yan H., Wang X. Evaluation of adrenocorticotropin regulated glucocorticoid synthesis pathway in adrenal of different breeds of pigs. Livest Sci. 2014;169:185–191. [Google Scholar]
- Yu K., Zhang Y., Chen H., Zhu W. Hepatic metabolomic and transcriptomic responses induced by cecal infusion of sodium propionate in a fistula pig model. J Agric Food Chem. 2019;67(47):13073–13081. doi: 10.1021/acs.jafc.9b05070. [DOI] [PubMed] [Google Scholar]
- Yu M., Mu C., Yang Y., Zhang C., Su Y., Huang Z., Yu K., Zhu W. Increases in circulating amino acids with in-feed antibiotics correlated with gene expression of intestinal amino acid transporters in piglets. Amino Acids. 2017;49(9):1587–1599. doi: 10.1007/s00726-017-2451-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu K., Chen H., Su Y., Zhu W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb Biotechnol. 2018;11(5):859–868. doi: 10.1111/1751-7915.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Xian Y., Wang C., Ding L., Meng X., Zhu W., Hang S. Calcium-sensing receptor-mediated L-tryptophan-induced secretion of cholecystokinin and glucose-dependent insulinotropic peptide in swine duodenum. J Vet Sci. 2018;19(2):179–187. doi: 10.4142/jvs.2018.19.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Nutrient requirements of swine. 11th ed. Washington, DC: National Academy Press; 2012. [Google Scholar]