Abstract
Introduction
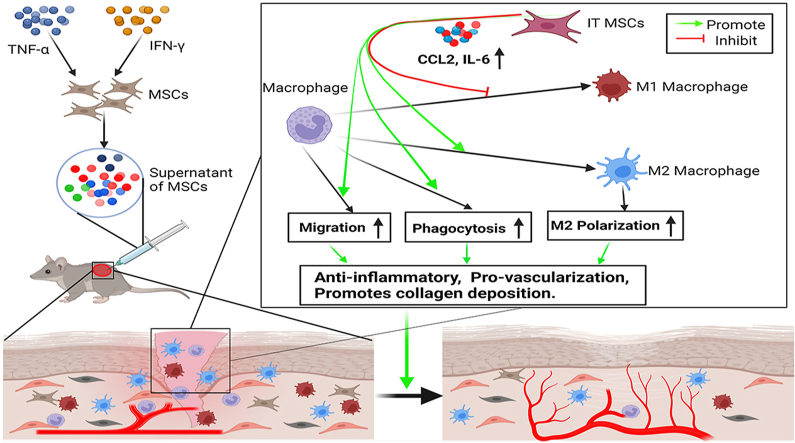
Numerous studies have shown that mesenchymal stem cells (MSCs) promote cutaneous wound healing via paracrine signaling. Our previous study found that the secretome of MSCs was significantly amplified by treatment with IFN-γ and TNF-α (IT). It has been known that macrophages are involved in the initiation and termination of inflammation, secretion of growth factors, phagocytosis, cell proliferation and collagen deposition in wound, which is the key factor during wound healing. In the present study, we used a unique supernatant of MSCs from human umbilical cord-derived MSCs (UC-MSCs) pretreated with IT, designated S-IT MSCs, to explore whether S-IT MSCs have a better effect on improving wound healing by improving the biological function of macrophages than the control supernatant of MSCs (S-MSCs).
Methods
In the present study, we used a unique supernatant of MSCs pretreated with IT subcutaneously injected into a mice total skin excision. We evaluated the effect of S-IT MSCs on wound healing and the quality of wound repair via promoting macrophages migration and M2 polarization in vivo. In addition, the effect of S-IT MSCs on macrophages migration, converting toward M2 phenotype and phagocytosis were also investigated in vitro.
Results
Indeed, S-IT MSCs were found to be more potent in promoting macrophage migration, M2 polarization, phagocytosis, and promoting wound closure than S-MSCs during the wound repair. High levels of CCL2 and IL-6 were found in S-IT MSCs, which indicated that the optimization of macrophage function by S-IT MSCs may be achieved through their high expression of CCL2 and IL-6.
Conclusions
Our results suggest that the beneficial paracrine effect of MSCs on wound healing can be amplified by pretreatment with IT, which may represent a new strategy for optimizing the therapeutic effect of MSCs on wound healing.
Keywords: Mesenchymal stem cell, IFN-γ and TNF-α, Macrophages, Migration, M2 polarization, Wound healing
Graphical abstract
Highlights
-
•
We found that S-IT MSCs were better at promoting macrophage migration and activation than S-MSCs in vitro.
-
•
Next, we reconfirmed that S-IT MSCs were more effective than S-MSCs in accelerating wound closure by promoting macrophage migration and activation.
-
•
High levels of CCL2 and IL-6 in S-IT MSCs may play a key role in improving macrophage function.
1. Introduction
Wound healing is a complex process and overlapping continuous process that depends on the presence of various types of cells, growth factors, cytokines and extracellular matrix elements. Studies have shown that MSCs can significantly accelerate the healing of acute wounds or diabetes ulcers, radiation ulcers and other chronic wounds in animals or humans [1,2]. In addition, the supernatant of MSCs has been showed to accelerate wound healing [3]. However, MSCs or MSCs supernatant did not show excellent effects in all cases [[4], [5], [6]], indicating that the beneficial effect of MSCs on promoting wound repair needs further improvement.
Our previous study found that the paracrine action of MSCs is plastic; that is, under the stimulation of a certain intensity of inflammatory factors, MSCs themselves will mass-produce cytokines, which are capable of regulating the immune microenvironment and stimulating the repair effect of macrophages, endothelial cells, fibroblasts and tissue precursor cells in wounds, such as interleukin-6 (IL-6), CCL2, tumorigenesis factor-induced Dutch gene-6 (TSG6), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) [[7], [8], [9]]. This suggests that stimulation of MSCs by acute inflammatory factors confers on MSCs an enhanced ability to promote wound repair.
It has been shown that after skin injury, monocytes accumulate at the wound surface and differentiate into macrophages, which subsequently exert phagocytosis, promote angiogenesis and re-epithelialization [10,11]. Therefore, timely chemotaxis of monocytes to the wounded tissue is a prerequisite for accelerated wound healing. In addition, classically activated M1 macrophages, labeled by specific proteins such as CD86, iNOS and TNF-α, mainly show proinflammatory properties [12]. Alternatively activated anti-inflammatory M2 macrophages, labeled by specific proteins such as CD206, CD163, Arg-1 and IL-10, exert anti-inflammatory effects, promoting vascularization and collagen deposition to prevent delayed healing [13,14]. Therefore, promoting macrophage migration, phagocytosis and M2 polarization is important to regulate the immune microenvironment of wounds and accelerate wound healing.
In this study, we explored effects of human umbilical cord-derived MSCs with respect to their ability to promote macrophage migration, M2 polarization, phagocytosis and wound healing, which depend on their preconditioning with IT. We hypothesize that pretreatment with IT could be a better strategy for the future application of MSCs to wound healing in the clinic.
2. Materials and methods
2.1. Human UC-MSCs
The human UC-MSCs used in this experiments were taken from human umbilical cords, the method is previously described [7]. And the Medical Ethics Committee of Affiliated Hospital of Jiangnan University approved all experimental procedures. Ethics Approval No.:LS2021046. Briefly, after parental consent, healthy full-term delivery umbilical cords were obtained and transferred to a biosafety cabinet with sterile PBS within 4 h. Under aseptic conditions, Waldron's Jelly was isolated from the umbilical cord, then cut into small pieces and transferred to 10-cm dishes, and Dulbecco's modified Eagle's medium (DMEM, Gibco) containing 15% FBS (Gibco), 10 ng/ml bFGF (PeproTech), and 100 mg/ml penicillin/streptomycin (Gibco) was used to cover the tissue pieces, then cultured in a humid 37 °C, 5% CO2 incubator. Medium replenishment was performed every 3 days, and nonadherent cells and tissues were removed after 14 days.
2.2. Macrophages
Macrophages were extracted from C57BL/6J mice (age 4–6 weeks) bone marrow, method as previously described [15]. Briefly, the isolated femur and tibia bones were transferred to a biosafety cabinet after 5 min in 75% alcohol and then washed twice in sterile PBS. Bone marrow was isolated from bones using a 1 ml syringe with DMEM/F12 (Gibco) containing 10% FBS (Gibco), penicillin/streptomycin (100 μg/ml) (Gibco), and 20 ng/mL M-CSF (PeproTech), then cultured in a humid 37 °C, 5% CO2 Incubator for 7 days. Expression of the macrophage marker, F4/80 (PE/Cyanine7-labeled) antibodies (BioLegend), was verified by flow cytometry. Only macrophages with a purity greater than 95% were used for subsequent experiments.
2.3. Preparation of conditioned medium
MSCs were cultured in 10 cm diameter dishes, and they were stimulated with IT (20 ng/ml, PeproTech) for 24h when they reached 90% confluence and then were washed three times with PBS, and 3 min each time to remove the effect of IT, then DMEM was added and continue to incubate cells for 12h, ultimately, the supernatant was collected after removing cell debris by centrifugation at 350g for 5 min. And then it was condensed by a 3 kDa ultrafiltration membrane at 3234×g for 45 min, which condensed it to a tenth of its original volume and then it was stored at −80 °C.
2.4. Scratch wound assay
The scratch wound assay was conducted as previously described [16]. Briefly, when the macrophages formed a 100% confluent monolayer, a scratch was made in each culture using a disposable pipette tip (200 μl). Then, the cells were treated with DMEM, S-MSCs or S-IT MSCs. The wound area was photographed using a Motic AE 2000 inverted microscope (Motic Corporation, China) immediately and 12 h, 24 h and 48 h later. The wound area was then measured using ImageJ software.
2.5. Transwell migration assay
For the Transwell assay, 3 × 104 cells/well were suspended in medium and seeded into the upper chambers of Transwell 24-well plates (Corning, USA) with 8 μm pore filters. Then, medium with or without S-MSCs and S-IT MSCs was added to the lower chamber. After 12 h, the migrated cells on the lower surface were stained with 0.5% crystal violet for 10 min. The extent of migration was observed under an optical microscope (Motic Corporation, China).
2.6. Flow cytometry (FCM)
After culturing with the supernatant from each group of stem cells for 24 h, the macrophages were collected and the density was adjusted to 1 × 106/ml, after which the following monoclonal fluorescent antibodies were added: CD206-APC (Rat IgG, Monoclonal, BioLegend Cat. No. 141708), CD86-PE (Rat IgG, Monoclonal, BioLegend Cat. No. 105008), Arg-1-PE (Syrian Hamster IgG, Monoclonal, BioLegend Cat. No. 138408) and iNOS-FITC (Rat IgG, Monoclonal, BioLegend Cat. No. 696802). For identification of the MSCs, a suspension of P3 generation UC-MSCs was collected, after the following monoclonal fluorescent antibodies were added: CD31-ECD, CD34-ECD, CD45-ECD, HLA-DR-ECD, CD29-ECD, CD90-ECD, CD73-ECD and CD105-ECD (Mouse IgG, Monoclonal, BioLegend Cat. No. 303106, 343502, 304002, 307606, 921304, 328110, 344004, 323206). The expression of the various antigens was detected by flow cytometry.
2.7. Phagocytosis of macrophages
Macrophages were inoculated in 96-well plates at a density of 1 × 105 cells/well, and cells were then treated according to the above experimental grouping after adhered. After 24 h of stimulation, the supernatant of each group was removed, and 100 μl/well of dextran MW 4000 solution (1 mg/ml) labeled with FITC was added. Cells continue to be incubated in the cell incubator for 30 min. Then, cells were washed three times with 200 μl/well PBS. Finally, cells were fixed with 4% paraformaldehyde and were observed using an inverted fluorescence microscope.
2.8. Wound healing model and treatment
Six-to eight-week-old C57BL/6J female mice were purchased from Shanghai SLAC Laboratory (Shanghai, China) and housed in the Medical Laboratory Animal Center of Jiangnan University. The Animal Ethics Committee of Jiangnan University authorized all experimental procedures in this study. Animal Ethics Approval No: JN. No20210430c0641130[113]. A mouse skin excision wound healing model was established according to the previously described method [17]. Briefly, 7 days after adaptive feeding, fourteen C57BL/6J female mice weighing 20–25 g were anesthetized with pentobarbital sodium 40 mg/kg intraperitoneal injection, and a combination of lidocaine for local anesthetic analgesia was used, then two circular holes of 6 mm in diameter were formed in the back of the mice. The mice were randomly divided into three groups and treated on alternate days with DMEM, S-MSCs, and S-IT MSCs (20 μl per treatment) by injecting evenly into four points of the wound bed. Then cover the wound with 3M film (1624W, 3M) followed by a layer of medical tape to protect the wound from dryness and infection. Take photographs of the wounds every other day after the injury. ImageJ software was used to count the areas of the wound.
2.9. Hematoxylin-eosin staining (H&E) and immunofluorescence (IF) analysis
On the third and tenth day after the first supernatant treatment, the mice were euthanized via cervical dislocation, and samples were harvested for tissue H&E staining and IF. Briefly, the tissues were fixed with 4% paraformaldehyde for 48 h and then covered with paraffin before sectioning and histological analysis. Blocks were cut into 4 μm thick sections and stained with H&E (YESEN). For IF assay, Briefly, Samples from the wound bed on day 3 were first dewaxed and rehydrated and then repaired antigen via boiling in a 100 °C citrate buffer water bath for 25 min. The tissue sections were blocked with immunohistochemical blocking solution (Beyotime, China) for 90 min. The primary antibodies used in this experiment were incubated at 4 °C overnight, as follows: F4/80 (Rat IgG, monoclonal, Abcam Cat. No.: ab6640), CD86 (Rabbit IgG, Polyclonal, SAB Cat. No.: 32223-2) and CD163 (Mouse IgG, monoclonal, GeneTex Cat. No.: ED2). Then incubated with the following secondary antibodies for 90 min at room temperature: Alexa 594-conjugated goat anti-Rat IgG (ab150160, Abcam), Alexa 488-conjugated goat anti-rabbit IgG (ab150077, Abcam) and Alexa 594-conjugated goat anti-mouse IgG (ab150116, Abcam). The nuclei were stained with DAPI (YESEN, China). Images were acquired using a laser-scanning confocal microscope (Carl Zeiss LSM880, Germany).
2.10. Analysis of cytokine production by enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-6 and CCL2 in S-MSCs and S-IT MSCs were measured by ELISA according to the manufacturer's directions (70-EK106/2, 85-39-7399-65, MultiSciences). The absorbance (450 nm) for each supernatant was analyzed by a microplate reader (Cytation5, Bio Tek) and was interpolated with a standard curve.
2.11. Statistical analysis
All results are expressed as the mean ± SD. The experiments were independently repeated three times. Comparisons were performed by one-way analysis of variance followed by Tukey's multiple comparison post hoc test. Statistical analysis was performed using SPSS 22.0 software. P values of less than 0.05 were considered statistically significant.
3. Results
3.1. S-IT MSCs efficiently promote macrophage migration in vitro
It is a prerequisite for macrophages to exert their anti-inflammatory and accelerating wound repair function that migrate to the wound in a timely and targeted manner in the early stage of wound healing. Therefore, we conducted scratch wound assays and Transwell migration assays to detect the migration of macrophages after treatment with DMEM, S-MSCs, and S-IT MSCs. The results showed that the migration of macrophages was higher in the S-IT MSCs-treated groups than in the DMEM- or S-MSCs-treated groups (Fig. 1a–d), indicating enhanced migration of macrophages by S-IT MSCs in vitro. The MSCs used in this study were assayed by flow cytometry using a series of surface markers for characterize MSCs populations (Fig. S1a). Bone marrow differentiation-derived macrophages were characterized using F4/80 (Fig. S1b).
Fig. 1.
S-IT MSCs promoted macrophages migration in vitro. (a, b) Scratch assay of macrophages following treatment with DMEM, S-MSCs and S-IT MSCs for 2 days. Photographs were taken at 0, 12, 24 and 48 h after scratching. We calculated the migration rate using the following formula: (1-(current denuded zone area/initial denuded zone area)) × 100. (c, d) We determined the migration ability 12 h after treatment in the above groups using a Transwell cell migration assay. Cells migrating through the polycarbonate membrane were counted by detecting the average cell number in three randomly chosen fields using a light microscope. ∗p < 0.05, ∗∗p < 0.001, ∗∗∗p < 0.0001.
3.2. S-IT MSCs efficiently promote polarization toward the M2 phenotype in vitro
Macrophages profoundly influence the course of inflammation, proliferation and remodeling during wound healing through their phagocytosis and production of corresponding cytokines and mediators. M1-type macrophages are present in large numbers during the initial inflammatory response to trauma repair and produce large amounts of pro-inflammatory factors, whereas M2-type macrophages predominated during the resolution phase, secreting mainly anti-inflammatory and growth factors and having higher phagocytic activity to remove necrotic and damaged cells from the trauma surface [18]. Therefore, the M2 phenotype and phagocytosis of macrophages is crucial for wound repair.
Our FCM results revealed that the number of CD206-positive macrophages was significantly higher in the S-IT MSCs-treated group than in the S-MSCs- or DMEM-treated group, whereas the trend was reversed for the number of CD86-positive macrophages (Fig. 2a and b). It can be concluded from the above results that S-IT MSCs could promote polarization toward the M2 phenotype via their paracrine factors, which is superior to S-MSCs and DMEM.
Fig. 2.
S-IT MSCs efficiently promote polarization toward the M2 phenotype in vitro. (a) The percentage of CD206-positive, CD86-positive, Arg-1-positive and iNOS-positive cells in macrophages after coculture with DMEM, S-MSCs or S-IT MSCs by flow cytometry. (b) The corresponding bar chart. ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.0001.
3.3. S-IT MSCs enhanced the phagocytic ability of M2 macrophages in vitro
As is known that M2 macrophages predominate during the resolution phase, secreting mainly anti-inflammatory and growth factors, and they have high phagocytic activity, allowing them to remove necrotic and damaged cells from the trauma surface [5]. Next, we detected whether the supernatant from IT MSCs has a greater effect in improving the phagocytic activity of M2 macrophages. Our results showed that the S-MSCs and S-IT MSCs treatments all enhanced the phagocytosis of FITC-labeled dextran by macrophages compared to the DMEM treatment. Moreover, S-IT MSCs treatment is more pronounced (Fig. 3a and b). Thus, these findings indicated that the supernatant from IT MSCs enhanced phagocytosis of M2 macrophages and this effect was superior to that of the S-MSCs and DMEM supernatants.
Fig. 3.
S-IT MSCs efficiently enhanced the phagocytosis ability of M2 macrophages in vitro, and high levels of CCL2 and IL-6 were detected in S-IT MSCs. (a, b) FITC-labeled (green) dextran with a molecular weight of 4 kD was used to detect the effect of DMEM, S-MSCs and S-IT MSCs on macrophage phagocytosis. (c, d) The levels of CCL2 and IL-6 expression in MSCs and IT MSCs were detected by ELISA. Scale bar 20 μm ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.0001.
3.4. S-IT MSCs efficiently accelerated wound closure
It has been shown that MSCs can accelerate wound repair via their paracrine factors. To explore whether this effect of S-MSCs on wound healing can be strengthened by pretreated with IT, we examined the effects of DMEM, S-MSCs and S-IT MSCs on skin regeneration using a whole skin excisional wound mice model. Compared to the DMEM group, the wound-healing rate was accelerated in all groups of stem cell supernatant-treated wounds at days 3 and 7. And the reduction in the wound areas of the S-IT MSC-treated group was more pronounced starting at day 7 (Fig. 4a and b).
Fig. 4.
S-IT MSCs accelerate skin wound healing in mice. (a) Gross view of wounds treated with DMEM, S-MSCs or S-IT MSCs at days 0, 3, and 7 post administration. (b) The rate of wound closure in wounds receiving different treatments at the indicated times. (c) Histological images (H&E staining) of wound sections treated with DMEM, S-MSCs or S-IT MSCs at Day 3 post administration. (d) Corresponding bar graphs of granulation tissue thickness. Scale bar 100 μm ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.0001.
Timely coverage of the wound by granulation tissue is more conducive to the crawling of surrounding epithelial cells to the wound bed, which is the key to accelerated wound repair. Our H&E staining results showed that the granulation tissue formed in the wound bed treated with S-IT MSCs was thicker on the third day compared with DMEM and S-MSCs (Fig. 3c and d). These results indicate that S-IT MSCs not only accelerates wound closure but also promotes the formation of granulation tissue in the early stage of wound healing.
3.5. S-IT MSCs promote macrophage migration to the wound bed and increased the percentage of M2 phenotype in the local wounds of mice
To reconfirm whether the soluble factors in S-IT MSCs have a more significant effect on macrophage migration in vivo, we next subjected the wounded tissue on the third day to staining with an F4/80 antibody. The F4/80 (red) positivity rate in the S-IT MSCs group was significantly higher than that in the DMEM and S-MSCs groups (Fig. 5a and b), which reconfirmed that the chemotaxis ability of S-IT MSCs to macrophages was better than that of DMEM and S-MSCs.
Fig. 5.
S-IT MSCs promoted macrophage migration to the local wounds of mice. (a) Tissue sections of the wound bed at 3 days after treatment were immunostained with specific antibodies against F4/80 (red). Nuclei were stained with DAPI (blue). Scale bar 20 μm. (b) Frequency of F4/80-positive macrophages in the wound bed tissue. ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.0001.
To investigate the effects of DMEM, S-MSCs or S-IT MSCs on the phenotype of infiltrating macrophages on day 3 post treatment, wound tissues were immunostained for CD86 (green) and CD163 (red) antibodies, which are surface-specific marker proteins of M1 and M2 macrophages, respectively. As shown in Fig. 6, a large number of CD163-and CD86-positive macrophages were observed in the wound tissue of the mice. The number of CD163-positive macrophages in the wound tissue of S-IT MSCs-treated was significantly higher than that of DMEM- or S-MSCs-treated mice, whereas the number of CD86-positive cells in the wound tissue of S-IT MSCs-treated was significantly lower than that of DMEM- or S-MSCs-treated mice (Fig. 6a and b).
Fig. 6.
S-IT MSCs increased the percentage of M2 macrophages in the local wounds of mice. (a) Tissue sections of the wound bed at 3 days after treatment were immunostained with specific antibodies against CD163 (red) and CD86 (green). Nuclei were stained with DAPI (blue). Scale bar 20 μm. (b) The rate of CD163/CD86-positive macrophages in the wound bed tissue. ∗P < 0.05, ∗∗P < 0.001, ∗∗∗P < 0.0001.
These findings demonstrated that S-IT MSCs can promote macrophages migration and increased the proportion of M2 macrophages more significantly in mouse trauma tissue, which contributing to the regulation of the inflammatory response and enhancing the healing of wounds.
3.6. IT MSCs optimized the quality of the regenerated skin by promoting macrophages polarization toward M2
At day 10, the wound was completely covered by newly formed skin, the thickness of newly formed skin was evaluated by H&E staining. The results showed that the epithelial structure and thickness of the S-IT MSCs treated group was more similar to normal skin compared to the S-MSCs and DMEM treated groups (Fig. 7a and b). This indicated that S-IT MSCs not only promoted wound healing, but also improved the quality of wound healing by promoting macrophage polarization toward M2 and shortening the inflammatory phase.
Fig. 7.
Posterior wound-healing assessment after 10 days. (a) Representative images of H&E. (b) The thickness of newly formed skin after wound healing.
3.7. S-IT MSCs improved the function of macrophages and accelerated wound closure via high expression of CCL2 and IL-6
CCL2 has been shown to be a major chemotactic factor for macrophages and has recently been shown to promote the polarization of macrophages toward the M2 phenotype [[19], [20], [21]]. Similarly, high levels of IL-6 have been shown to promote the polarization of macrophages toward the M2 phenotype by activating STAT3 and STAT6, thus exerting anti-inflammatory and pro-repair effects [[22], [23], [24]].
In this study, we found that the expression of CCL2 and IL-6 was enhanced significantly after mesenchymal stem cells were pretreated with IT, and their secretion levels of CCL2 and IL-6 were thousands or even tens of thousands of times higher than those of stem cells not stimulated with IT (Fig. 3c and d). Therefore, these results indicate that the optimization of macrophage function by S-IT MSCs may be achieved through their high expression of CCL2 and IL-6.
4. Discussion
The skin is the body's first line of defense against environmental exposure and it provides basic functions. Therefore, skin tissue integrity must be restored quickly to prevent infection and reduce fluid loss after injury [25]. A growing number of studies show that MSCs can accelerate wound healing [5,26]. There is, however, growing evidence that less than 1% of stem cells survive for more than a week after transplantation [27,28]. Some studies have shown that MSC-derived prostaglandin E2 (PGE2), IL-6 and miR-223 accelerate wound healing by promoting macrophage polarization toward the M2 phenotype [18,29,30]. Consequently, we explored whether the effect of S-IT MSCs was superior to that of S-MSCs on recruiting macrophages into the wound bed and polarizing macrophages to the M2 phenotype, ultimately accelerating the wound healing process. In the present study, we confirmed that S-MSCs can promote wound healing, which is consistent with previous reports. Furthermore, we found that S-IT MSCs could more significantly enhance wound healing than S-MSCs in mice (Fig. 4a–d).
Numerous studies have reported that CCL2 is a major chemotactic factor for macrophages and it has recently been shown to promote the polarization of macrophages toward the M2 phenotype [[19], [20], [21]]. In addition, M2 activation has been shown to involve various transcription factors. STAT3 and STAT6 are key proteins in M2 activation [31,32]. The knockdown of STAT3 and STAT6 in mouse and human macrophages has been reported to prevent the switch to the M2 phenotype [32,33]. Moreover, high levels of IL-6 have been shown to promote the polarization of macrophages toward the M2 phenotype by activating STAT3 and STAT6, thus exerting anti-inflammatory and pro-repair effects [[22], [23], [24]]. It has also been found that CCL2 and IL-6 have synergistic effects in promoting macrophages polarization toward M2 to promote wound repair [34].
Indeed, our in vitro and in vivo studies found that the migration of macrophages can effectively occur via chemotaxis in the S-IT MSC-treated group. Furthermore, the promotion of phagocytosis and the M2 phenotype of macrophages was also enhanced by S-IT MSCs. Meanwhile, the secretion of CCL2 and IL-6 by MSCs was amplified after pretreatment with IT, which indicated that the promotion of macrophage migration and the M2 phenotype may be mediated by S-IT MSCs via high levels of CCL2 and IL-6.
In summary, we found that the effects of S-IT MSCs on the migration, phagocytosis and M2 phenotype of macrophages were superior to those of S-MSCs. These findings suggest that S-IT MSCs can be used for skin regeneration treatments and are a key factor that stimulates migration, phagocytosis and the M2 phenotype of macrophages during wound healing.
Authors’ contributions
Chenyang Liu, Ling Diao, Guozhong Lu designed research and Chenyang Liu wrote the paper. Chenyang Liu, Yichi Lu, Pan Du, Fengbo Yang, Peng Guo, Xinyao Yin performed experiments. Xiaoyu Tang analyzed data. All coauthors have discussed the results and reviewed the manuscript.
Declaration of competing interest
The authors have declared that no conflict of interest exists.
Acknowledgements
This study was supported by the Leading talent of TCM in Jiangsu Province (014000204/2018-00073 25), the National Key Research and Development Program of China (2016YFE0204400) and the Social Development Program of Jiangsu Province (BE2018626).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2022.06.009.
Contributor Information
Ling Diao, Email: ling.diao@yahoo.com.
Guozhong Lu, Email: luguozhong@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
fig.s1.tif.
References
- 1.Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y., Chen L., Scott P.G., Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cell. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 3.Kim W.S., Park B.S., Sung J.H., Yang J., Park S.B., Kwak S.J., et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Heo S.C., Jeon E.S., Lee I.H., Kim H.S., Kim M.B., Kim J.H. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Su W., Shi S., Wilder-Smith P., Xiang A., Wong A., et al. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cell. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura Y., Ishikawa H., Kawai K., Tabata Y., Suzuki S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials. 2013;34:9393–9400. doi: 10.1016/j.biomaterials.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 8.Meirelles L.S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Crisostomo P.R., Wang Y., Markel T.A., Wang M., Lahm T., Meldrum D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 10.Gillitzer R., Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 11.Baum C.L., Arpey C.J. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 12.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barros M.H., Hauck F., Dreyer J.H., Kempkes B., Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards J.P., Zhang X., Frauwirth K.A., Mosser D.M. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H., Wang S.Y., Kwak G., Yang Y., Kwon I.C., Kim S.H. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci (Weinh) 2019;6 doi: 10.1002/advs.201900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J., Nam D., Park K.S. Substance P enhances cellular migration and inhibits senescence in human dermal fibroblasts under hyperglycemic conditions. Biochem Biophys Res Commun. 2020;522:917–923. doi: 10.1016/j.bbrc.2019.11.172. [DOI] [PubMed] [Google Scholar]
- 17.Chen L., Xu Y., Zhao J., Zhang Z., Yang R., Xie J., et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qun Z., Wen R. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cell. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood S., Jayaraman V., Huelsmann E.J., Bonish B., Burgad D., Sivaramakrishnan G., et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida Y., Kuninaka Y., Nosaka M., Furuta M., Kimura A., Taruya A., et al. CCL2-Mediated reversal of impaired skin wound healing in diabetic mice by normalization of neovascularization and collagen accumulation. J Invest Dermatol. 2019;139:2517–2527. doi: 10.1016/j.jid.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Whelan D.S., Caplice N.M., Clover A.J.P. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci Rep. 2020;10:2642. doi: 10.1038/s41598-020-59174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philipp D., Suhr L., Wahlers T., Choi Y.H., Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. doi: 10.1186/s13287-018-1039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Z., Hao H., Tong C., Cheng Y., Liu J., Pang Y., et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cell. 2016;34:627–639. doi: 10.1002/stem.2238. [DOI] [PubMed] [Google Scholar]
- 24.Yin Z., Ma T., Lin Y., Lu X., Zhang C., Chen S., et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J Cell Biochem. 2018;119(11):9419–9432. doi: 10.1002/jcb.27259. [DOI] [PubMed] [Google Scholar]
- 25.Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 26.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong F., Caplan A.I. Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transplant. 2012;17:670–674. doi: 10.1097/MOT.0b013e328359a617. [DOI] [PubMed] [Google Scholar]
- 28.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S., Chen L., Zhang G., Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11:39. doi: 10.1186/s13287-020-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X., Dong Z., Cao Y., Wang H., Liu S., Liao L., et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cell Int. 2019;2019 doi: 10.1155/2019/7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Mandal P., Pratt B.T., Barnes M., McMullen M.R., Nagy L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKinnon A.C., Farnworth S.L., Hodkinson P.S., Henderson N.C., Atkinson K.M., Leffler H., et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]