The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see https://www.journals.elsevier.com/academic-pathology/news/pathology-competencies-for-medical-education-pcme.1
Primary objective
SP5.1: Objective SP5.1: Special Studies: Describe the roles of immunohistochemistry, flow cytometry, cytogenetics, and molecular diagnostics in the diagnosis and classification of lymphoma, and explain how, with examples, different techniques are most appropriate in diagnosis, staging, and management of the disease.
Competency 3: Diagnostic Medicine and Therapeutic Pathology; Topic: Surgical Pathology (SP); Learning Goal 5: Classification of Leukemia and Lymphomas.
Secondary objectives
Objective SP5.3: Differential Diagnosis: Discuss how a pathologist can use a diagnostic decision tree to make a diagnosis efficiently, minimize the time to report results to the oncologist, and optimize treatment decisions.
Competency 3: Diagnostic Medicine and Therapeutic Pathology; Topic: Surgical Pathology (SP); Learning Goal 5: Classification of Leukemia and Lymphomas.
Objective SP1.1: Obtaining the Specimen: Describe the procedures for obtaining a biopsy of a tissue lesion or mass in different sites, including superficial and deep soft tissues, solid organs, and tubular organs. Associate each procedure and specimen type to either cytology or surgical pathology and give examples of possible reasons and follow-up for false negative biopsies.
Competency 3: Diagnostic Medicine and Therapeutic Pathology; Topic: Surgical Pathology (SP); Learning Goal 1: Role in Diagnosis.1
Patient presentation
A 5-year-old boy is brought to his primary care manager by his parents who note increasing fatigue for the past month and complaints of pain in his legs for the past week. In addition, they report a history of a mild upper respiratory infection around a month ago which does not seem to have fully resolved. He does not have significant past medical history. There is a family history of hypertension and type 2 diabetes in his father. His parents deny the family history of cancer or hereditary conditions. They note that he attends pre-school and multiple other children have had recent upper respiratory infections. They deny recent travel history.
Diagnostic findings, part 1
On physical examination, the patient has a temperature of 100.9 °F, heart rate is 105 beats per minute, blood pressure is 110/70 mmHg, and respirations are 24 breaths per minute on room air. The pharynx is mildly erythematous. There is bilateral painless cervical lymphadenopathy measuring roughly 2cm in size by palpation. Heart sounds have a regular S1 and S2 without appreciable murmurs, rubs, or gallop. The lungs are clear to auscultation bilaterally. The liver is palpable 2 cm below the costal margin and the spleen tip is palpable. The thighs are tender to deep palpation bilaterally but there is a full range of motion in all extremities. The neurologic exam is unremarkable.
Questions/discussion points, part 1
Given the clinical history and physical examination, what would be included in an initial differential diagnosis?
Fever and lymphadenopathy in a child are most often secondary to infectious causes which could be viral or bacterial in nature.2 Common childhood infections might include influenza, strep throat, HIV infection, or mononucleosis, as well as parasitic infections including toxoplasmosis. Other items in a broad differential would include autoimmune conditions, malignancy, medications, and hereditary conditions.3 In addition, a more chronic infection must be considered with the upper respiratory infection that has not resolved. From these items, viral mononucleosis secondary to Epstein-Bar Virus could explain hepatosplenomegaly, lymphadenopathy, and fever. As a next step to narrow the differential diagnosis list, a complete blood count (CBC) with automated differential and a heterophile antibody test were ordered. If the heterophile antibody test is negative, it would help rule out Epstein-Bar Virus as a cause for this child's presentation. The CBC with automated differential may help to distinguish between infectious and malignant causes.
Diagnostic findings, part 2
The CBC is shown in Table 1. The heterophile antibody test was done and yielded a negative result.
Table 1.
CBC.
| Test | Patient | Reference Range and Units |
|---|---|---|
| WBC | 87.9 | 4.2–9.2 K/UL |
| HCT | 23.9 | 39.7–50.3% |
| HGB | 7.9 | 13.2–16.5 g/dl |
| RBC | 2.53 | 4.2–9.2 K/UL |
| MCV | 95 | 82.9–99.9 fl |
| MCH | 31.2 | 27.4–32.7 pg |
| MCHC | 33 | 31.3–35.0% |
| ROW | 18.1 | 11.6–14.7% |
| Platelet count | 91 | 166–407 K/UL |
Abbreviation: WBC, white blood cell; HCT, hematocrit; HGB, hemoglobin; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; ROW, red cell distribution width.
Questions/discussion points, part 2
Prior to looking at the differential cell count, discuss the detailed differential diagnoses based on the findings in the screening CBC. What makes up the white blood cell count on a CBC? How can the initial CBC help to narrow the differential list?
The CBC demonstrates normocytic anemia (low hemoglobin, hematocrit, and a normal mean corpuscular volume), thrombocytopenia (low platelets), and leukocytosis (increased white blood cells (WBCs). The WBC count is made up of granulocytes (neutrophils, eosinophils, and basophils) and non-granulocytes (lymphocytes and monocytes). Any of these cell types could be elevated to cause the leukocytosis seen on this patient's CBC. As stated, the current differential is broad and includes infection, autoimmune conditions, malignancy, medications, and hereditary conditions.3 Bacterial infection could cause either neutrophilia or lymphocytosis depending on the type of bacteria involved. Infections from bacteria such as group A Streptococcus or Staphylococcus could present with neutrophilia. On the other hand, bacterial infections from Bordetella pertussis and viral infections common to the pediatric population such as Epstein-Barr virus, Coxsackie viruses, Varicella, Influenza, and Enteroviruses could cause a lymphocytosis. Although, lymphocytosis secondary to bacterial or viral infection is unlikely to cause the WBC count to be elevated to the degree seen in this example. Autoimmune conditions, including juvenile idiopathic arthritis, juvenile dermatomyositis, and lupus, can occur in a pediatric population. These autoimmune conditions may be associated with leukocytosis and anemia (secondary to anemia of chronic disease or hemolytic anemia).4 Certain medications, such as corticosteroids, could cause neutrophilia. Malignancy, including acute and chronic leukemias, are also a cause of leukocytosis in the pediatric population. Malignancy could also cause normocytic anemia and thrombocytopenia secondary to bone marrow involvement.
What clinical features can help you narrow your differential and help decide what is most likely?
The clinical picture of progressive symptoms including painless lymphadenopathy, hepatosplenomegaly, and bony tenderness coupled with the laboratory findings of leukocytosis, anemia, and thrombocytopenia raises the concern for malignancy. Painful lymphadenopathy is suggestive of inflammation and commonly occurs secondary to infection. On the other hand, painless lymphadenopathy is more suggestive of a malignancy. Hepatosplenomegaly is not a specific finding of malignancy, but it can frequently occur secondary to infiltration of the organ by metastatic cells or extramedullary hematopoiesis. Malignancy can cause bony tenderness and pain in a variety of different mechanisms including bone marrow infiltration by malignant cells causing subsequent bone marrow engorgement and bone resorption secondary to paraneoplastic effects. Combined, these findings put malignancy higher on the differential and it must be ruled out.5
Malignant disorders on the differential include acute lymphoblastic leukemia (ALL), Burkitt Lymphoma, Acute Myeloid Leukemia (AML), chronic myeloid leukemia (CML), and acute undifferentiated leukemia (AUL). Of these, ALL should be at the top of the differential given the patient's age. ALL is the most common childhood malignancy and is most typically further classified into one of the following two categories: B-cell lineage or T-cell lineage. B-Cell ALL is the most common variant of ALL seen in children while T-cell ALL is commonly seen in adolescent males with superior vena cava symptoms.6
Diagnostic findings, part 3
The automated analyzer flagged the high WBC count in this patient's peripheral blood with a message “blast/abnormal lymphocyte” which triggered a manual differential and review by a pathologist. A manual differential and morphologic assessment of the peripheral blood smear was performed and is shown in Table 2 and the patient's peripheral blood smear is shown in Fig. 1. Please evaluate these findings.
Table 2.
WBC manual differential.
| Test | Patient | Reference Range and Units |
|---|---|---|
| Neutrophil % | 2 | 40–75% |
| Blasts % | 89 | 0% |
| Lymphocyte % | 9 | 12–47% |
| Monocyte % | 0 | 4.0–10.0% |
| Eosinophil% | 0 | 0.0–3.0% |
| Basophil % | 0 | 0.0–1.0% |
Fig. 1.
Peripheral Blood. (A) Peripheral blood smear at 400x. Large immature cells are present. RBCs are normochromic in appearance with anisocytosis appreciable. (B) Peripheral blood smear at 1000x. Immature cells are appreciated with characteristic partially condensed chromatin, high nuclear to cytoplasmic ratio, and scant non-granular cytoplasm. There are normal red blood cells in the background. RBC, red blood cells.
Questions/discussion points, part 3
How will a manual differential help to further narrow the differential diagnosis?
A manual differential will allow for morphologic assessment to be performed which will give further insight into the increased WBC count. Findings of immature WBCs in the blood would increase the concern for malignancy. More specifically, lymphoblasts would raise the concern for ALL, while myeloblasts or blast equivalents (promyelocytes and promonocytes) would raise the concern for AML. Abnormal increases in the percentage of mature myeloid lineage cells relative to lymphocytes increase the concern for CML. CML characteristically has a range of immature myeloid elements in the peripheral blood. Reactive leukocytosis secondary to infection could present with a relative increase in neutrophils or lymphocytes along with an increase in the total WBC count. Although, in reactive leukocytosis, the WBC count will typically not be elevated to the degree seen in some leukemias.
What does the manual differential show?
The manual differential showed a decreased percentage of neutrophils, monocytes, eosinophils, and basophils which made the diagnosis of CML unlikely. The manual differential also showed an abnormal increase in blast cells which raised the concern for acute leukemia.
Morphologically, how does ALL appear on peripheral blood smear?
ALL lymphoblasts on peripheral blood smear are immature appearing cells that are typically intermediate in size, but significantly larger than the red blood cells, see Fig. 1. They have a high nuclear to cytoplasmic ratio and have scant cytoplasm that is agranular and lightly basophilic. Lymphoblasts may vary in size from small blasts with scant cytoplasm and condensed appearing chromatin to cells that are intermediate in size with fine chromatin, a varying number of nucleoli, and moderate to abundant pale blue cytoplasm.7 Mature lymphocytes on the other hand are characteristically smaller than lymphoblasts with mature condensed chromatin, round to slightly irregular nuclear contours and scant basophilic cytoplasm. Some lymphocytes, called large granular lymphocytes, are slightly larger appearing and have more cytoplasm that is lightly basophilic with azurophilic granules. The nucleus of the large granular lymphocytes maybe be indented with condensed chromatin.8
The patient's blood smear shows findings consistent with the morphology of ALL. The patient's pediatrician was notified by the pathologist. After discussion with the patient's parents, the child was scheduled to undergo an urgent bone marrow aspiration and biopsy. The bone marrow aspiration and biopsy were performed at the posterior superior iliac crest which is the preferred site for both children and adults in the United States.9,10 Bone marrow aspirate was performed along with a core biopsy from the patient's left posterior iliac crest.
Diagnostic findings, part 4
Please review Fig. 2, Fig. 3 and interpret the bone marrow findings.
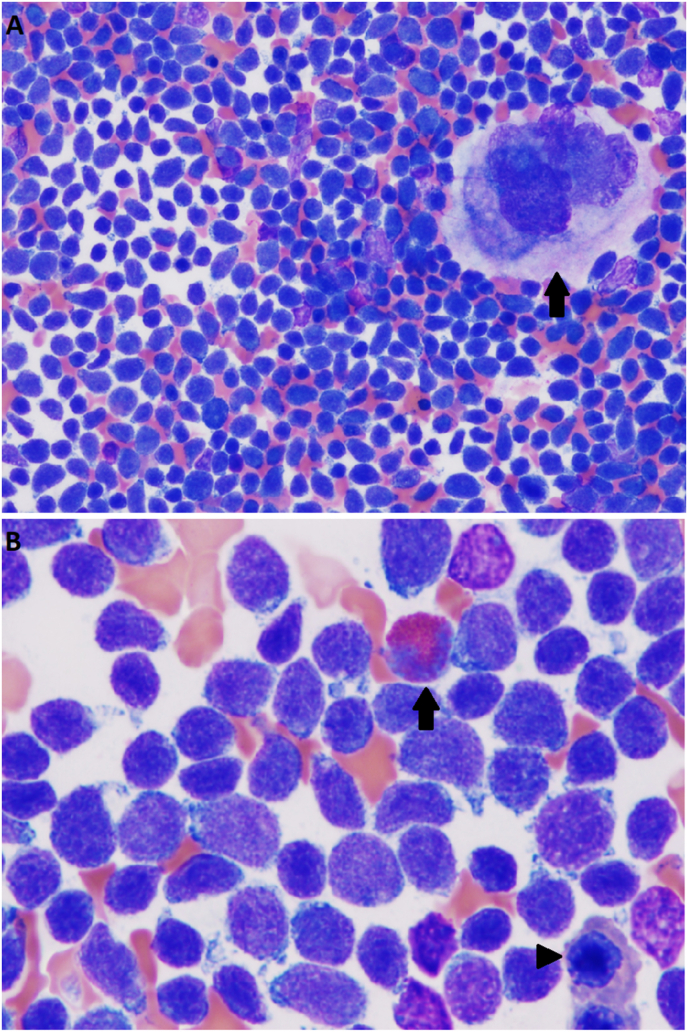
Fig. 2.
Bone marrow aspirate. (A) A smear of bone marrow aspirate at 400x. Immature appearing cells are present and make up the majority of the cell count with occasional erythroid precursors and granulocyte precursors appreciated. A megakaryocyte can be appreciated in the upper right portion of the image (arrow). (B) Smear of bone marrow aspirate at 1000x. Cells with characteristic partially condensed chromatin and scant lightly basophilic cytoplasm make up the majority of the cells. Granulocyte precursors are seen, such as the eosinophilic band on the upper part of the slide (arrow). Erythroid precursors and erythrocytes are present (arrowhead).
Fig. 3.
Bone marrow core biopsy. (A) Bone marrow core biopsy 40x. Trabecular bone is appreciated. Bone marrow is hypercellular in appearance. Trilineage hematopoiesis is markedly reduced and difficult to appreciate due to the monomorphic population of immature cells. (B) Bone marrow core biopsy 400x. Monomorphic immature cells make up the majority of the cells appreciated. Cells of erythroid (arrow head) and myeloid lineage (arrow) are appreciable but at decreased percentages from what would be expected, similar to what was seen in the bone marrow aspirate.
Questions/discussion points, part 4
What does the bone marrow biopsy show?
The bone marrow biopsies are shown in Fig. 2, Fig. 3, respectively. On the bone marrow aspirate, immature appearing cells are present and make up almost all of the cell count with occasional erythroid precursors and granulocyte precursors appreciated. A megakaryocyte can be appreciated in the upper right corner of Fig. 2A. Note how large this cell is by comparison. On higher power, cells with characteristic partially condensed chromatin and scant lightly basophilic cytoplasm make up the majority of the cells in Fig. 2B. Granulocyte precursors are seen, such as the eosinophilic band on the upper part of the slide. Erythroid precursors and erythrocytes are present. Fig. 3A shows a bone marrow core biopsy at 40x. Trabecular bone is appreciated. Bone marrow is hypercellular in appearance with essentially 100% cellularity. Trilineage hematopoiesis is markedly reduced and difficult to appreciate due to the monomorphic population of immature cells. In Fig. 3B, monomorphic immature cells make up the majority of the cells appreciated. Cells of erythroid and myeloid lineage are appreciable but at decreased percentages from what would be expected, similar to what was seen in the bone marrow aspirate. In summary, the bone marrow aspirate and core biopsy both show a predominance of immature cells. It is difficult to further characterize the malignancy without immunophenotypic analysis.
What is flow cytometry? What markers are suggestive of ALL on flow cytometry or immunohistochemistry? How do we differentiate between B-ALL and T-ALL?
Flow cytometry is a useful tool to aid in the immunophenotyping of leukemias. In flow cytometry, cells are first fixed, commonly using paraformaldehyde. The cells are then resuspended in a buffered media and passed in a single file through a beam of light generated by a laser or lasers, each of which has a unique wavelength. This process can generate information about cell size and complexity based on how the light scatters upon contact with each cell. This process can also be used to detect cell membrane, cytoplasmic, or nuclear proteins using antibodies with fluorochromes to probe the protein of interest. Flow cytometry can be used to analyze thousands of cells per second in both peripheral blood and bone marrow aspirate. This is useful because B and T cells have cell markers that vary from one another; moreover, immature lymphocytes express different markers than mature lymphocytes. Terminal deoxynucleotidyl transferase (TdT) is a common marker of immaturity which is often seen in B-ALL and T-ALL. CD10 is a marker commonly seen in ALL, as well as Follicular Lymphoma, Burkitt Lymphoma, and Diffuse Large B Cell Lymphoma. Common B cell markers include CD19, CD22, CD79a, and PAX5 while T cell markers commonly include CD1a, CD3, CD4, CD5, CD7, and CD8. For both lineages, these markers may be variably expressed depending on the stage at which the lymphoblasts growth was arrested. Blasts cannot always be reliably identified as lymphoid or myeloid in lineage using B and T cell markers so myeloid cell markers are also typically obtained. Common myeloid markers include the following: myeloperoxidase (MPO), HLA-DR, CD13, CD14, CD16, CD56, and CD64.
Flow cytometric analysis of bone marrow aspirate is typically thought of as the gold standard for the diagnosis of ALL. However, flow cytometric analysis of peripheral blood is less invasive and can still yield a result that is both sensitive and specific. Recent studies indicate that flow cytometry of peripheral blood also shows high specificity and sensitivity, 99.7% and 98.5% respectively, for the lineage classification of pediatric leukemia.11
What is immunohistochemistry? What stains are commonly used in cases which are suspect for B-ALL?
Immunohistochemistry is a process by which tissue specimens can be selectively stained for specific antigens. This is a multistep process. First, tissue samples are incubated with an antibody that is specific to an antigen of interest. Next, the tissue will be incubated with a second antibody conjugated to a chromogenic reporter which is specific to the fragment crystallizable region (Fc) of the initial antibody. Finally, substrate can be added which interacts with the chromogenic reporter to generate a pigmented precipitate. The antigen staining can then be visualized using regular light microscopy.12
Flow cytometry is often the primary method of diagnosing ALL, and immunohistochemistry may not be performed in cases that are straightforward to diagnosis with flow cytometry. However, immunohistochemistry can still provide unique insight and help to confirm the diagnosis of ALL. Immunohistochemistry is particularly useful for cases in which a bone marrow aspirate cannot be obtained. Tissue specimens obtained from bone marrow biopsy can be stained to probe for antigens of interest. Common antigens which are stained for in suspect cases of ALL include MPO, TdT, CD3, CD10, CD19, CD34, and CD79a.13 All of these antigens and the specific cells they are located on are further discussed in the flow cytometry section.
Diagnostic findings, part 5
This patient had the flow cytometric analysis of his bone marrow aspirate and the results are shown in Fig. 4. In a different case of ALL in which flow cytometry could not be performed due to inadequate aspirate, immunohistochemistry was performed on a bone marrow biopsy and the results are shown in Fig. 5. Please evaluate these findings.
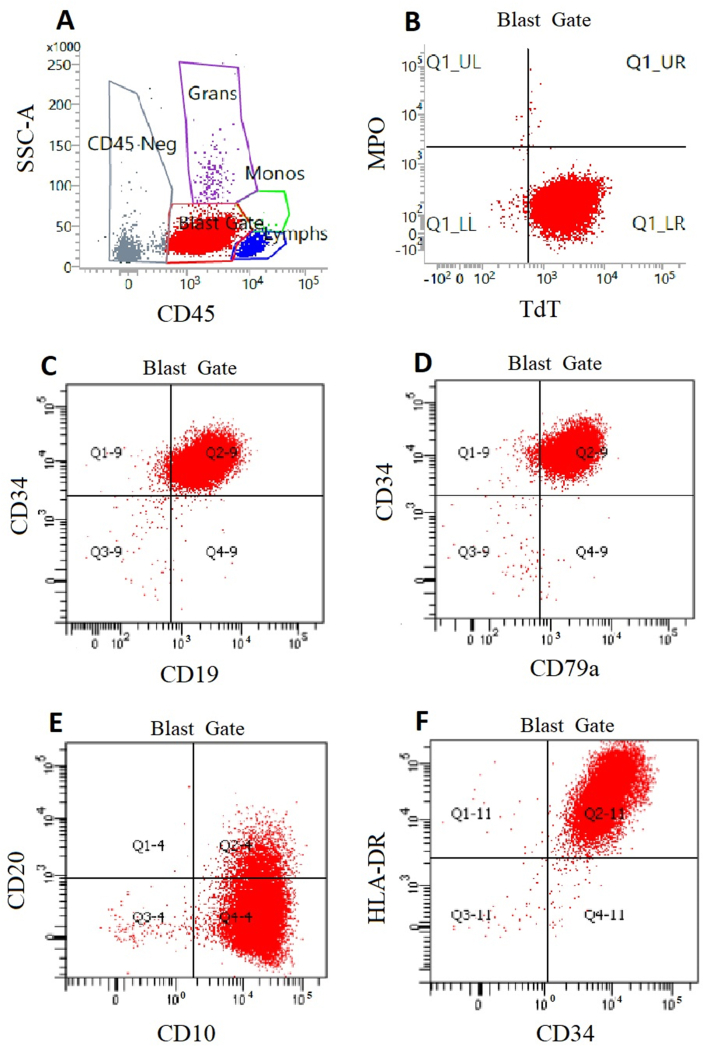
Fig. 4.
Flow cytometry analysis of patient's bone marrow aspirate. (A) CD45 vs SSC. Blast gate is assigned to the population of cells with positive CD45 and minimal side scatter. (B) Negative staining for MPO and positive staining for TdT. (C) Positive staining for CD34 and CD19. (D) Positive staining for CD34 and CD79a. (E) Positive staining for CD10 partially positive (dim intensity) staining for CD20. (F) Positive staining for HLA-DR and CD34. MPO, myeloperoxidase; SSC, side scatter; TdT, terminal deoxynucleotidyl transferase.
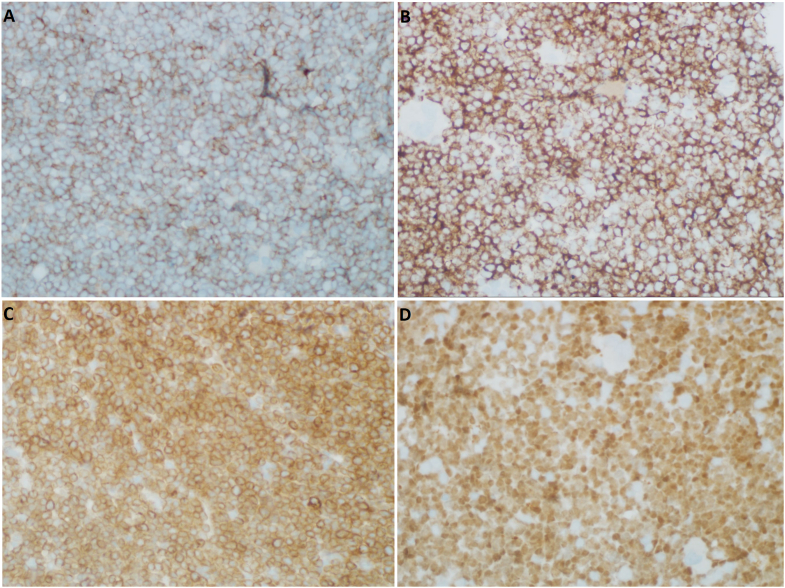
Fig. 5.
Immunohistochemical staining of the core biopsy taken from the patient's bone marrow. (A) Staining for CD19 at 400x shows a positive membranous staining pattern. (B) Staining for CD34 at 400x shows positive membranous/cytoplasmic staining. (C) Staining for CD79a at 400x shows positive membranous staining. (D) Staining for TdT at 400x shows positive nuclear staining. TdT, terminal deoxynucleotidyl transferase.
Questions/discussion points, part 5
What do the flow cytometry results show?
In Fig. 4 plots, each dot represents an individual cell, and each axis represents a measurement of interest. Fig. 4A demonstrates side scatter (SSC) on the y-axis and CD45 on the x-axis. CD-45 is a marker of hematopoietic cells. SSC is increased if a cell has increased internal complexity. For example, granulocytes have cytoplasmic granules which increase their internal complexity and thus increase their SSC. In Fig. 4A, the red population of cells most likely makes up lymphoblasts — they are dimly positive for CD45 with minimal SSC. Using a process called gating, that focuses on a specific group of cells, the lymphoblast cell population can be analyzed independently in the subsequent graphs.
In the graphs with four quadrants, the lower left quadrant represents cells that are double negative for each marker on the x and y axis, and the top right quadrant represents cells that are positive for both markers. In Fig. 4B, cells stain negative for MPO, making them less likely myeloid in origin. They are weakly for TdT which is a marker of immature lymphocytes. Fig. 4C shows that the lymphoblast population stains positive for CD34 (a marker of hematopoietic stem cells) and positive for CD19 (a B-cell marker). Fig. 4D shows positive staining for CD34 and positive staining for CD79a (a B-cell marker). Fig. 4E shows positive staining for CD10 (common acute lymphoblastic leukemia antigen) with a subset staining for CD20 (a marker of B-cells). Finally, Fig. 4F shows cells that are positive for CD34 and HLA-DR (major histocompatibility complex class II surface receptor).
Overall, this patient's flow cytometric analysis demonstrates that the cells of interest are positive for CD10, CD79a, TdT, and CD19, with a subset positive for CD20, indicating B-lymphoblasts. The lymphoblastic cells are also positive for CD34. CD34 and CD20 tend to have a variable expression in B-ALL. The patient's cells are negative for MPO which is common for B-ALL. MPO detected in blasts is normally indicative of AML.14 Based on the combined finding from the peripheral blood smear, bone marrow biopsy, and flow cytometry, we can conclude that this patient's malignancy is B-ALL.
Does the immunohistochemical staining support the results seen on flow cytometry?
The TdT staining of the bone marrow biopsy specimens shows a strong nuclear positivity which is the typical staining pattern for cells expressing TdT. Strong cytoplasmic staining can be appreciated when staining for CD79a. Strong membranous staining is seen when staining for CD19 and CD34. Overall, the results from immunohistochemical staining support findings of flow cytometry and are suggestive of B-ALL.
What additional genetic or molecular analysis could be done to further characterize the cancer?
B-ALL may be further defined by genetic abnormalities. Specific genetic abnormalities may affect clinical presentation, prognosis, and treatment options. Genetic abnormalities play a critical role in assigning patients to therapy. Genetic abnormalities can include translocations and chromosomal aneuploidies. Common translocations include t(9; 22) (BCR-ABL1), t(v; 11p23.3) (KMT2A-rearranged), t(12; 21) (ETV6-RUNX1), and t(1; 19) (TCF3-PBX1). In the previous sentence, the t prior to the parentheses denotes translocation. The numbers within the first set of parentheses denote which chromosomes are involved in the translocation (note a v denotes that various chromosomes can participate in the translocation). The letters within the second set of parentheses demonstrate the gene fusion that result from the translocation. For example, t(9; 22) (BCR-ABL1) denotes a translocation between chromosome 9 and 22 which result in the formation of the BCR-ABL1 fusion gene. B-ALL can also be defined by chromosomal abnormalities such as hyperdiploidy (blasts with greater than 50 chromosomes) or hypodiploidy (blasts with less than 46 chromosomes). Other genetic abnormalities used to define B-ALL that are not associated with a specific translocation or chromosomal number abnormality include the BRC-ABL-like subtype and the iAMP21 subtype of B-ALL.15 Of the genetic abnormalities mentioned, ETV6/RUNX1 and hyperdiploidy are associated with a good prognosis while BCR/ABL1 and KMT2AR are associated with a poorer prognosis.
Genetic analysis can be used to further characterize the malignancy. Next-generation sequencing (NGS) employs parallel sequencing technologies and can be used to sequence large amounts of either DNA or RNA. NGS works by generating small reads of DNA or RNA which are then aligned against one another. Comparison to a reference DNA or RNA can determine alterations in the sequence of interest. NGS can be used for a variety of applications including whole-genome sequencing, exome sequencing, and transcriptome sequencing. NGS can be performed on live cells and cells that are formalin-fixed paraffin-embedded (FFPE). There are numerous reports about the use of NGS in B-ALL to determine gene mutations as well as fusion genes.15
Polymerase chain reaction (PCR) and reverse transcription-PCR (RT-PCR) are sensitive tools that can provide useful information such as translocations and gene fusions. PCR employs primers that flank a site of interest and a heat-stable polymerase to amplify a portion of DNA. Generally, the first step of PCR involves raising the temperature to denature the DNA. Next, the temperature is lowered to allow the primers of interest to anneal to their respective DNA sequence. The temperature is then raised to an optimal temperature to allow the polymerase to bind to the primers and begin synthesizing the DNA in the region of interest. This process is repeated many times to create cyclic amplification of the target region. PCR can be performed on DNA extracted from live cells or FFPE tissue. Besides the detection of fusion genes and translocation, this tool has proved particularly useful in the monitoring of minimal residual disease (MRD). MRD describes the evidence of leukemic cells that are present in the body after therapeutic treatment but not appreciable by morphology alone. The detection of MRD requires tests with a high sensitivity. PCR and flow cytometry can determine MRD. MRD is useful in monitoring response to treatment and identifying patients who may need more intensive therapy.16
Fluorescence in situ hybridization (FISH) involves the binding of labeled DNA fluorescent probes to metaphase or interphase chromosomes. There are locus-specific probes which can be used to reveal translocations, inversions, copy number variations, and gene deletions. Centromere-specific probes exist which are complementary to sequences of a specific chromosome's centromere. These probes can be used to identify chromosomal aneuploidies such as monosomy or trisomy which are often associated with malignant cells. Whole chromosomes can be bound in FISH analysis using chromosome-specific libraries. These libraries can be useful to determine rearrangements between chromosomes. FISH is typically performed on FFPE tissue. Finally, chromosomal banding can be done on cells arrested in metaphase. This process requires live B-ALL cells obtained from the patient which are stimulated to grow in vitro. The cell growth would be stopped in metaphase. Chromosomal banding is then performed using special stains to identify each individual chromosome and determine structural abnormalities or aneuploidies.17
Flow cytometric analysis has demonstrated that this malignancy is consistent with B-ALL. Why is further characterization important?
Further characterization is important because it impacts prognosis and may also lead to targeted therapy. In this patient, FISH analysis was performed. A red fluorescent probe specific for ABL on 9q34 and a green fluorescent probe specific for BCR of chromosome 22q11 were used. The fluorescent markers were present in close proximity to one another on the same chromosome, such that the red and green blend to create a yellow hue. This result was suggestive of the presence of the BCR-ABL 1 fusion gene, also known as the Philadelphia chromosome. Further analysis using RT-PCR suggested that the patient's tumor cells contained RNA consistent with both the BCR exon b3/ABL exon a2 fusion product (b3a2) and the BCR exon b2/ABL exon a2 fusion product (b2a2). The RT-PCR and FISH findings confirmed that the Philadelphia chromosome is present. This is relevant because the BRC-ABL 1 gene fusion presents the opportunity for target therapy with a tyrosine kinase inhibitor. Imatinib is one such BRC-ABL specific tyrosine kinase inhibitor.18 Targeted therapy with Imatinib coupled with standard chemotherapy regimens has shown a significant improvement in long-term outcomes compared to chemotherapy alone in pediatric patients.19 Outcomes with Imatinib and intensive chemotherapy were not statistically different from pediatric patients who underwent a bone marrow transplant.20
Follow-up
Following the diagnosis of B-ALL, the patient underwent an induction round of chemotherapy followed by multiple cycles of intensive chemotherapy and imatinib immunotherapy. MRD testing of the bone marrow was performed by flow cytometry at multiple time points after the start of therapy. MDR was initially positive after induction therapy but negative after multiple rounds of chemotherapy combined with imatanib immunotherapy. This patient is currently 5 years post-therapy without relapse.
Teaching points
-
•
The classic morphology of ALL on peripheral blood smear is large lymphoblasts which appear as immature cells with a high nuclear to cytoplasmic ratio, scant cytoplasm that is agranular and lightly basophilic, and chromatin that varies from fine to moderately condensed. A differential diagnosis is generated based on clinical and laboratory data which are interpreted and used to develop the most likely cause of the patient's ailment and used to guide additional testing to confirm the true diagnosis and timely treatment.
-
•
For leukemias, it is necessary to obtain a biopsy from the bone marrow because it allows for microscopic analysis to determine if there is proper bone marrow cellularity and trilineage hematopoietic differentiation. The bone marrow biopsy also allows for immunohistochemical analysis of the tissue.
-
•
Flow cytometry is a special study where cells are passed single-file through lasers of varying wavelengths to determine the presence of specific cellular markers including TdT, CD10, CD 19, CD20, CD34, CD79a, and MPO (as demonstrated above). Flow cytometry is required to confirm the diagnosis of B-ALL.
-
•
Immunohistochemistry is the process by which specific cellular markers can be selectively visualized on tissue. Tissues are commonly evaluated for the following cellular markers in cases of suspect B-ALL: MPO, TdT, CD3, CD10, CD20, and CD79a.
-
•
Cytogenetics and molecular diagnostics are tools which help in the classification of disease through the determination of specific mutations, translocations, and chromosomal abnormalities. Genetic characterization of malignancies is important for both prognosis and treatment. In B-ALL the most common defining mutations are t(9; 22) (BCR-ABL1), t(v; 11p23.3) (KMT2A-rearranged), t(12; 21) (ETV6-RUNX1), t(5; 14) (EGH/IL3), and t(1; 19) (TCF3-PBX1). When denotating a translocation, the numbers within the first set of parentheses represent which chromosomes are involved in the translocation while the letters within the second set of parentheses demonstrate the gene fusion that results from the translocation.
-
•
B-ALL is a disease driven by genetic aberrations making cytogenetics and molecular diagnostics useful in the management of disease through both developing potential targeted therapy and monitoring of residual disease.
Funding/support
The article processing fee for this article was funded by an Open Access Award given by the Society of ‘67, which supports the mission of the Association of Pathology Chairs to produce the next generation of outstanding investigators and educational scholars in the field of pathology. This award helps to promote the publication of high-quality original scholarship in Academic Pathology by authors at an early stage of academic development.
Disclaimer
The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Knollmann-Ritschel B.E.C., Regula D.P., Borowitz M.J., Conran R., Prystowsky M.B. Pathology competencies for medical education and educational cases. Acad Pathol. 2017;4 doi: 10.1177/2374289517715040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deosthali A., Donches K., DelVecchio M., Aronoff S. Etiologies of pediatric cervical lymphadenopathy: a systematic review of 2687 subjects. Global Pediatr Health. 2019;6 doi: 10.1177/2333794x19865440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley L.K., Rupert J. Evaluation of patients with leukocytosis. Am Fam Physician. 2015;92(11):1003–1011. [PubMed] [Google Scholar]
- 4.Behrens E.M., Beukelman T., Gallo L., et al. Evaluation of the presentation of systemic onset juvenile rheumatoid arthritis: data from the Pennsylvania Systemic Onset Juvenile Arthritis Registry (PASOJAR) J Rheumatol. 2008;35:343–348. Accessed December 5, 2021. www.jrheum.org/content/jrheum/35/2/343.full.pdf. Accessed. [PubMed] [Google Scholar]
- 5.Redaelli A., Laskin B.L., Stephens J.M., Botteman M.F., Pashos C.L. A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL) Eur J Cancer Care. 2005;14(1):53–62. doi: 10.1111/j.1365-2354.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 6.Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics. CA: A Cancer J Clin. 2014;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 7.Chiaretti S., Zini G., Bassan R. Diagnosis and subclassification of acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2014;6(1) doi: 10.4084/MJHID.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mescher A.L. In: Junqueira's Basic Histology: Text and Atlas. fourteenth ed. Mescher A.L., editor. McGraw-Hill Education; New York, NY: 2016. Chapter 12: blood. [Google Scholar]
- 9.Riley R.S., Hogan T.F., Pavot D.R., et al. A pathologist's perspective on bone marrow aspiration and biopsy: I. performing a bone marrow examination. J Clin Lab Anal. 2004;18(2) doi: 10.1002/jcla.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abla O., Friedman J., Doyle J. Performing bone marrow aspiration and biopsy in children: recommended guidelines. Paediatr Child Health. 2008;13(6):499–501. doi: 10.1093/pch/13.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam G., Punnett A., Stephens D., Sung L., Abdelhaleem M., Hitzler J. Value of flow cytometric analysis of peripheral blood samples in children diagnosed with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65(1) doi: 10.1002/pbc.26738. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Vara J.A. Principles and methods of immunohistochemistry. Methods Mol Biol. 2017;1641:115–128. doi: 10.1007/978-1-4939-7172-5_5. [DOI] [PubMed] [Google Scholar]
- 13.Al Gwaiz L.A., Bassioni W. Immunophenotyping of acute lymphoblastic leukemia using immunohistochemistry in bone marrow biopsy specimens. Histol Histopathol. 2008;23(10):1223–1228. doi: 10.14670/HH-23.1223. [DOI] [PubMed] [Google Scholar]
- 14.Borowitz M.J., Chan J.K.C., Downing J.R., et al. In: fourth ed. Swerdlow S.H., Campo E., Harris N.L., et al., editors. vol. 2. International Agency for Research on Cancer; Lyon, Fance: 2017. Chapter 11: precursor lymphoid neoplasms. (WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues). [Google Scholar]
- 15.Coccaro N., Anelli L., Zagaria A., Specchia G., Albano F. Next-generation sequencing in acute lymphoblastic leukemia. Int J Mol Sci. 2019;20(12) doi: 10.3390/ijms20122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse A., Nour A., Kim H.N., et al. Minimal residual disease detection in acute lymphoblastic leukemia. Int J Mol Sci. 2020;21(3) doi: 10.3390/ijms21031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayani J., Squire J.A. Fluorescence in situ hybridization (fish) Curr Protoc Cell Biol Chapter. 2004;22 doi: 10.1002/0471143030.cb2204s23. Unit 22 4. [DOI] [PubMed] [Google Scholar]
- 18.Terwilliger T., Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biondi A., Schrappe M., de Lorenzo P., et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9) doi: 10.1016/S1470-2045(12)70377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz K.R., Carroll A., Heerema N.A., et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: children's Oncology Group Study AALL0031. Leukemia. 2014;28(7) doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]