Abstract
The sternum is a stabilizing element in the axial skeleton of most tetrapods, closely linked with the function of the pectoral girdle of the appendicular skeleton. Modern mammals have a distinctive sternum characterized by multiple ossified segments, the origins of which are poorly understood. Although the evolution of the pectoral girdle has been extensively studied in early members of the mammalian total group (Synapsida), only limited data exist for the sternum. Ancestrally, synapsids exhibit a single sternal element and previously the earliest report of a segmental sternum in non-mammalian synapsids was in the Middle Triassic cynodont Diademodon tetragonus. Here, we describe the well-preserved sternum of a gorgonopsian, a group of sabre-toothed synapsids from the Permian. It represents an ossified, multipartite element resembling the mammalian condition. This discovery pulls back the origin of the distinctive “mammalian” sternum to the base of Theriodontia, significantly extending the temporal range of this morphology. Through a review of sternal morphology across Synapsida, we reconstruct the evolutionary history of this structure. Furthermore, we explore its role in the evolution of mammalian posture, gait, and ventilation through progressive regionalization of the postcranium as well as the posteriorization of musculature associated with mammalian breathing.
Subject terms: Evolution, Palaeontology
Introduction
In the skeleton of tetrapods, the sternum acts as a ventral stabilizing element. It usually comprises a bony rod or plate in cartilaginous contact with the distal ends of the ribs, serving to reinforce the rib cage. It is also functionally associated with the pectoral girdle (part of the appendicular skeleton), which in Permian synapsids consists of the paired scapulae, procoracoids, coracoids (sometimes called metacoracoids1), cleithra, clavicles, and the unpaired interclavicle2. By anatomical convention, the sternum is not included in the pectoral complex, instead being classified as part of the axial skeleton, even though the ontogeny of these elements is interconnected1. Because of its functional importance, the pectoral girdle has been extensively studied in many fossil tetrapods. An animal’s stance and musculature can be inferred from the morphology of the pectoral girdle e.g.3–6, providing insights into its function and lifestyle. This is especially important in extinct clades, where one is generally not able to infer behavior by reference to extant representatives of the group. Despite its relevance to the function of the pectoral girdle in extant taxa, the sternum has received comparably little attention in fossil tetrapods. This is due in large part to its rarity of preservation; it is frequently lost in disarticulated specimens, and if it was cartilaginous in life (as in many extant reptiles and amphibians), it would be unlikely to fossilize.
In extant mammals the sternum is a well-ossified and segmental abaxial structure consisting of a large anterior element (the manubrium), a series of posterior segments (the sternebrae), and a usually pointed terminal (xiphoid) element, although in some cases these segments can fuse during ontogeny (e.g. in Homo). The sternal segments are also a prevalent and phylogenetically conserved pattern for all major Mesozoic clades of crown Mammalia e.g.7–11, with a single exception of Zhangheotherium quinquecuspedens11. The majority of extant reptiles also possess a segmental sternum, generally divided into (from anterior to posterior) pre-, meso-, and xiphisternum12. However, the homology of the sternal divisions of extant reptiles with those of mammals is questionable. Reptilian sterna are mostly cartilaginous in structure2 (with notable exceptions in Aves, in which the sternum forms an ossified keel-like structure for the attachment of flight muscles, and some lizards, e.g. Iguana12) and some reptiles have no sternum at all (i.e. snakes13). Hence, the fossil record provides only limited information on sternal evolution in reptiles, as the sternal cartilage rarely fossilizes.
However, the rich fossil record of late Paleozoic and Mesozoic taxa on the mammalian stem suggests that the modern mammalian sternum did not evolve from an ancestral morphology similar to that of extant reptiles1. No sternum is known in the earliest diverging synapsids (“pelycosaurs”), but the sternum is preserved in several non-mammalian therapsid groups and typically forms a single, large, rounded plate (Fig. 1). Previously, the earliest record of a mammal-like ossified segmental sternum was in the Middle Triassic Diademodon tetragonus14, a member of Cynodontia, the synapsid subclade that includes mammals. Based on this specimen, it had been assumed that the “mammalian-type” sternum was an apomorphy of cynodonts, one of the many mammalian characters that first appeared in this clade during their rapid radiation following the end-Permian mass extinction15.
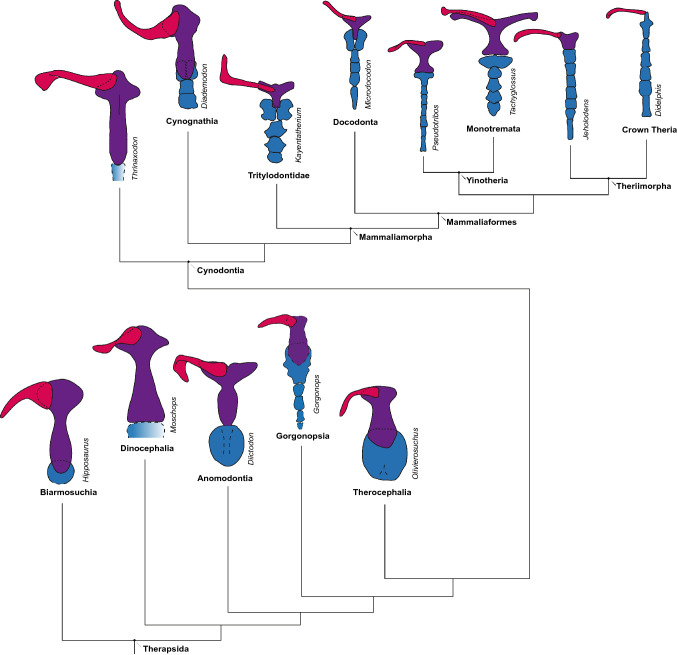
Figure 1.
Schematic representation after Luo et al.10 of the evolution of the pectoral girdle elements interclavicle (purple), clavicle (pink) and sternum (blue) in the major therapsids clades Biarmosuchia (Hipposaurus26), Dinocephalia (Moschops29), Anomodontia (Diictodon20), Gorgonopsia (SAM-PK-K10591, Gorgonops torvus), Therocephalia (Olivierosuchus21) and the cynodont Thrinaxodon71, as well as Cynognathia (Diademodon14), Tritylodontidae (Kayentatherium), Docodonta (Microdocodon9), Yinotheria (Pseudotribos10 and the monotreme Tachyglossus (drawing after specimen ZMB-35995 in the Museum für Naturkunde, Berlin) and Theriimorpha (Jeholodens10 and the crown therian Didelphis10). All views from ventral. Simplified phylogeny after Hopson and Barghusen24 and Sidor and Hopson25.
Here, we present a mammal-like sternum in a late Permian gorgonopsian therapsid, which is phylogenetically stemward and geologically much earlier than the cynodont Diademodon. This specimen, referable to the species Gorgonops torvus, helps illuminate the transition between a primitive unipartite sternum and a derived multipartite (segmental) sternum along the mammalian stem-lineage. Placing the new specimen in the broader context of synapsid sternal evolution reveals a complex history of variation that provides insights into the functional diversity of early synapsids, and links it to the evolution of the mammalian thorax which was essential for the development for aspiration during breathing.
Results
The gorgonopsian specimen SAM-PK-K10591 (here referred to Gorgonops torvus, a mid-sized gorgonopsian recovered as an early-diverging member of the group in the most recent phylogenetic analysis16), constitutes a nearly complete skeleton. Of exceptional significance is the preservation in partial articulation of the pectoral girdle and associated sternum (Fig. 2). The sternum (visible dorsally) consists of a large, plate-like, roughly oval element and three smaller, flat elements. In other groups of therapsids with multipartite sterna, i.e., non-mammalian cynodonts, the former element is often called the ‘manubrium’ in anatomical literature14,17,18, as this is the term for the anteriormost and largest element of the sternum in Mammalia. But given the uncertainty regarding the homology between these elements15 we refer to this structure in quotes. In Gorgonops torvus, the ‘manubrium’ element bears two triangular articular facets for ribs on each side and shares a third articulation area with the first sternebra. The three smaller, discrete penta- or hexagonal elements, the sternebrae, are situated posterior to the ‘manubrium’. Just as the first sternebra contributes to the third rib attachment facet with the ‘manubrium’, additional ribs would have articulated lateral to the contacts between subsequent adjacent sternebrae via cartilage. This configuration of the sternum is similar to that of modern Mammalia, but contrasts with that of contemporary non-mammalian synapsids such as anomodonts e.g.19,20 and therocephalians e.g.21,22. In these groups, the ossified sternum commonly consists of a single large, rounded element and is generally considered unipartite.
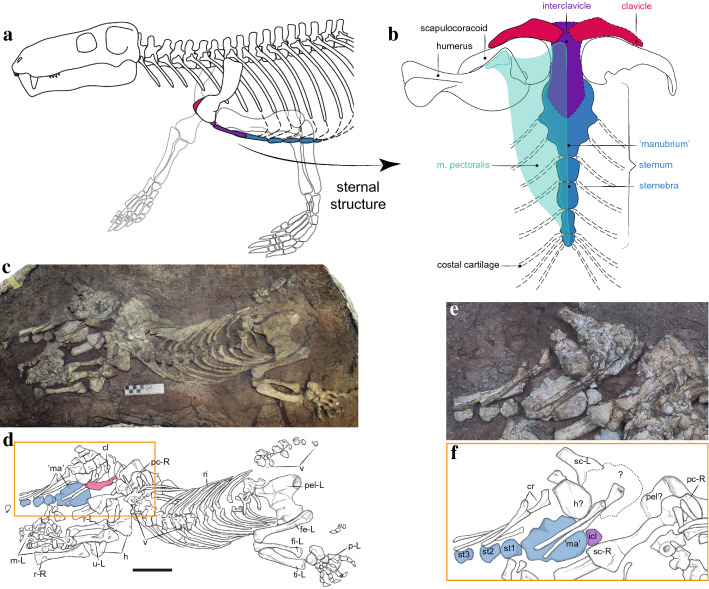
Figure 2.
(a) Partial skeletal reconstruction of Gorgonops torvus in lateral aspect and (b) schematic reconstruction of the pectoral girdle, sternum, interclavicle and humerus with hypothetical position of m. pectoralis in ventral aspect. (c) Photograph and (d) interpretative drawing of Gorgonops torvus (SAM-PK-K10591), highlighting the pectoral region in an orange rectangle as an (e) photograph and (f) interpretative drawing (e and f after further preparation to reveal the interclavicle). For explanation of the taphonomic disturbance of the specimen, see Materials and methods section. Abbreviations: cl, clavicle; cr, cervical ribs; cv, cervical vertebrae; fe-L, left femur; fi-L, left fibula; h, partial humeri; icl, interclavicle; m-L, left manus; ’ma’, ‘manubrium’; p-L, left pes; pc-R, right pectoral girdle; pel?, partial pelvic girdle?; pel-L, left pelvic girdle; r-L, left radius; r-R, right radius; ri, ribs; sc-L; left scapula; sc-R, right scapula; st1-3, sternebra 1–3; ti-L, left tibia; u-L, left ulna; v, vertebrae.
Although damaged, part of the interclavicle is preserved as a plate-like element at the anterior edge of the sternum. Its posterior border is smaller than the anterior border of the ‘manubrium’ but is in close contact with it. The overlap of ‘manubrium’ and interclavicle is not preserved, but can be reconstructed based on other well-preserved gorgonopsian specimens (e.g. NHCC LB350, SAM-PK-9344, USNM 412381—see Supplementary Fig. S1). The right clavicle is a robust element preserved in dorsal view, which is slightly displaced from life position but shows a facet for articulation with the interclavicle, permitting reconstruction of the connection between these bones.
Discussion
Sternal morphology in Synapsida
The earliest-diverging synapsids, the paraphyletic “pelycosaurs”, do not preserve an ossified sternum in any known taxa23. However, a large, ossified interclavicle is always present. The broad interclavicle tends to be mostly uniform in shape (“spoon-shaped” as per Romer and Price23, with a cruciate anterior part and an elongate posterior rod). The first appearance of an ossified sternum in Synapsida occurs within the diverse and long-lived subclade Therapsida. Although some uncertainty exists as to the relationships between the major therapsid clades, the earliest-diverging group is generally considered to be Biarmosuchia24,25. Few biarmosuchian postcrania are known, but the sternum is preserved in a few taxa (e.g. Hipposaurus26), where it is unipartite and probably incompletely ossified. In known examples the sternum is relatively small compared to the interclavicle and roughly circular in outline (see Fig. 1). No sternum is known in the Dinocephalia2,27. As several nearly complete dinocephalian skeletons are known e.g.28,29, it seems that the sternum, if present, must have been cartilaginous in this group, and the lack of discovered sterna is not simply due to incomplete preservation of the bony elements (likely also the case for “pelycosaurs”).
Anomodontia is the most diverse Permo-Triassic therapsid clade30, and also exhibits a diversity of sternal morphologies. Although an ossified sternum seems to be lacking in basal (non-dicynodont) anomodonts, as indicated by its absence in the well-preserved and fairly complete skeletons of Suminia31, Galechirus, and Galepus32, an ossified sternum is present in Dicynodontia30. In dicynodonts, it is always unipartite and generally a simple, plate-like element (e.g. in Diictodon20 and Eosimops33). However, the sternum is more complex in the burrowing dicynodont Cistecephalus (wide anteriorly, with a strongly tapering posterior edge and pronounced attachment sites for the ribs)19. In the largest known dicynodonts, the Late Triassic stahleckeriids, the sternum is extremely deep dorsoventrally, with a well-developed ventral keel6. The number of ribs attaching to the sternum varies in the clade, with one (e.g. Dinodontosaurus34), two (e.g. Aulacephalodon35), or three (e.g. Cistecephalus19) attachment sites per side.
Few well-described postcrania are known for Gorgonopsia. Previously-described gorgonopsian sterna consist of one element with up to three articulations for ribs on either side (i.e. in the holotypes of Lycaenops ornatus36, Aelurognathus tigriceps36, “Aelurognathus” microdon37, and Viatkogorgon ivakhnenkoi38). The discovery of an ossified and segmental abaxial sternal structure in Gorgonops torvus, however, raises the possibility that the apparently unipartite sterna of other species reflect incompleteness rather than the true absence of discrete sternebrae. With the exception of V. ivakhnenkoi, the aforementioned specimens were all collected and prepared in the early twentieth century, with damage to the more delicate parts of the anatomy. Also, although complete, well-preserved, and well-prepared, the skeleton of V. ivakhnenkoi is preserved on its side, and the base of the pectoral complex is poorly exposed, making the morphology of the sternum somewhat uncertain.
Similar to the condition in Gorgonopsia, few skeletons of Therocephalia are complete enough to determine whether a sternum was present. An ossified sternum appears to be absent in basal (non-eutherocephalian) therocephalians, as no trace of this element is present even in well-preserved, articulated skeletons of this grade (i.e. Glanosuchus39, Lycosuchus40). However, an ossified sternum is known in a number of eutherocephalian taxa (e.g. Regisaurus22 and Olivierosuchus21) and likely was present throughout that subclade41. In these taxa, the preserved portion of the sternum consists of a single element and is a remarkably large, plate-like structure dwarfing the interclavicle (Fig. 1).
Prior to the discovery of the gorgonopsian specimen described here, the earliest record of an ossified multipartite sternum was in the Middle Triassic cynodont Diademodon tetragonus14. No ossified sternal elements are known in any earlier cynodonts (including taxa known from numerous complete skeletons, such as Thrinaxodon), suggesting that the sternum was cartilaginous in those taxa. Therefore, no conclusions can be drawn about the sternal shape in the earliest cynodonts. However, a multipartite sternum is known in several later-occurring non-mammalian cynodonts (e.g. the Jurassic Kayentatherium wellesi17 and Bienotheroides wansienensis18), in which the anteriormost section of the sternum is paired. Although rare, all the non-mammalian cynodont sterna thus far described consist of multiple elements. The connection between all these elements is assumed to have been cartilaginous18.
A fully-ossified multipartite sternum is known in several extinct mammaliaform taxa (e.g. Sinoconodon42, Maiopatagium43, Microdocodon9) (Fig. 1) as well as all modern mammals44. Adult monotremes and non-crown group therians retain a distinct interclavicle, which acts as an anchor for the proximal attachment of the clavicle, and the first rib attaches to the largest anterior sternal element (the manubrium). Marsupials and placentals do not preserve an interclavicle as adults, as this element fuses with the manubrium during development. In these taxa, the clavicles and the first ribs both connect to the anteriormost sternal element on either side45.
The new multipartite sternum of a gorgonopsian presented here appears substantially earlier in geological time and is phylogenetically more stemward than any previous records of a “mammalian-type” sternum. The partial interclavicle shows some similarities to the interclavicles in other gorgonopsian specimens (see Supplementary Fig. S1) as well as those of Therocephalia (e.g. Olivierosuchus21), but the sternum of Gorgonops torvus is novel in its configuration.
The sternal variation within Synapsida discussed above allows us to distinguish between three morphologically differentiated groups:
-
A)
Synapsids inferred to have an unossified sternum, such as “pelycosaurs”, dinocephalians, and basal anomodonts, therocephalians, and cynodonts. The lack of an ossified sternum in the predominantly large-bodied Dinocephalia demonstrates that sternal ossification is not necessarily correlated with body size.
-
B)
Synapsids with usually large, unipartite (singular), and well-ossified sterna, for instance dicynodonts and eutherocephalians. Although it is possible that additional cartilaginous elements were present in life, the lack of a well-developed articular facet on the posterior margin of the sternum in these groups suggests that is unlikely.
-
C)
Synapsids with segmental and ossified sterna such as Gorgonops torvus, Diademodon tetragonus, Mesozoic mammaliaforms, and extant mammals. The condition in close relatives of Gorgonops and Diademodon is uncertain, due to limited fossil data.
The discovery of the sternal complex of Gorgonops torvus now presents two equally possible hypotheses for the earliest evolution of the mammalian sternum: 1) the “mammal-like” condition arose first in gorgonopsians (as represented by Gorgonops torvus) but then was lost in eutheriodonts (therocephalians and cynodonts, in which the sternum ancestrally seems to have been cartilaginous) or 2) the condition in Gorgonops torvus evolved convergently to that of cynodonts, originating from a unipartite ancestral state common to both gorgonopsians and eutheriodonts. Until further discoveries of fossil taxa with different sternal conditions provide more evidence, it is impossible to test either of these hypotheses thoroughly, but functional considerations may provide some insight as to which is more likely (see below).
Functional evolution of the sternum
The sternum of extant mammals has several functions. Notably, it helps to reinforce the rib cage, with a more stable, enclosed rib cage offering better protection of the thoracic organs than one exposed abaxially46. Furthermore, an ossified (and hence stronger) sternum is functionally important for forelimb locomotor function, as the ventral surface of the thorax has major attachment sites for pectoral muscles47. These complementary functions of the sternum reflect its integral part in the entire system of the forelimb, the shoulder girdle, and the thorax. In synapsid evolution, there are two major morphologies of ossified sterna (Fig. 1): the single, plate-like sternum present in earlier-diverging synapsids (e.g. dicynodonts) and the relatively narrow, segmental sternum seen in cynodonts such as Diademodon, some tritylodontids and mammaliaforms. The shift between these osteological configurations would have been part of a broader suite of functional changes occurring in this section of the synapsid tree.
The origin of mammals is associated with major changes in skeletal morphology, and the stepwise assembly of these changes in Permo-Triassic synapsids has historically been cited as one of the best bodies of evidence for macroevolution in the fossil record48,49. The inferred functional associations (and evolutionary drivers) of these changes can be roughly broken down into three areas: 1. dental (increasing complexity, both from differentiation in the heterodont tooth series, and from elaboration of individual teeth, particularly the postcanines, with multicusped and expanded crowns capable of occlusion); 2. cranial (formation of a complete secondary palate, loss of the postorbital bar, simplification of the jaw elements, increase in brain size/complexity); and 3. postcranial (increased regionalization of the axial column, changes in limb morphology associated with posture, origin of the segmental sternum). Each of these changes has functional implications—more efficient food processing driven by changes to the inferred muscular complement and jaw orientation for the craniodental characters50, and more active locomotion associated with an erect gait for the postcranial characters51. Each of these had downstream effects on portions of the anatomy not immediately subject to selection. For example, the expansion of jaw musculature attachment on the dentary is thought to have contributed to the decrease in size of the post-dentary bones and their eventual detachment to form middle ear bones52.
We offer a similar interpretation for the evolution of a segmental sternum in Permo-Triassic therapsids. On its own, this feature would have had little to do with improved gait in mammals—the forelimbs themselves, the shoulder girdle, and the thoracic vertebral column all have more immediate influences on locomotion. However, the sternum bridges the girdle to the axial skeleton and it is therefore connected with shifts in locomotor evolution. And it is involved in two ways of particular note in the evolution of mammal-like morphologies and function: 1. increased regionalization of the axial skeleton and 2. increased posteriorization of thoracic elements. For the former, mammals are well known to have greater differentiation of the axial column into discrete regions than reptiles, although this transition is now thought to be more complex and to have occurred earlier in synapsid evolution than previously believed53. In the typical mammalian condition, the thorax is a highly discrete unit readily distinguished by vertebral morphology, and it also differs in range of motion from the cervical, lumbar, and caudal regions. By contrast, in many reptiles and even early synapsids, the distinction between the thoracic and lumbar regions is less evident, and the cervical-thoracic transition is also difficult to discern54. The origins of the mammal-like rib cage, a structure surrounding the thoracic organs (the heart, lungs and muscular diaphragm), are intimately associated with changes in gait that took synapsids from the lateral undulation of early amniotes to the primarily dorsoventral flexion of mammals47, in a divergent evolutionary path from the evolution of modern reptiles55. In the context of this paradigm shift in synapsid history, a massive, plate-like sternum broadly overlapping the interclavicle would have been a hindrance, a relic of the “pelycosaurian” condition with sprawling forelimbs in close association with the substrate. In the evolution of theriodonts (the group containing gorgonopsians, therocephalians, and cynodonts), even as early as gorgonopsians there is a shift towards more cursorial locomotion and more erect gaits, with a focus on dorsoventral rather than side-to-side motion51,55. To facilitate this style of locomotion, it was necessary to reduce the size of the pectoral girdle, thereby enhancing its mobility relative to the axial skeleton.
There are multiple ways to reduce the weight of bony elements, one being simply to not ossify them. This may have been the ancestral condition in eutheriodonts, given that the sternum seems to have been cartilaginous in the earliest therocephalians and cynodonts (although this would imply a reversal to the pre-theriodont condition in eutherocephalians). Another is to transform from a single solid plate to a series of connected elements, which can retain the protective function of the sternum without limiting mobility (similar transitions can be seen in the evolution of armor, with trends towards multipartite structures offering greater flexibility56). This latter approach appears to characterize sternal evolution in Gorgonopsia.
Greater flexibility of the thorax also has importance beyond permitting dorsoventral flexion during locomotion, as shown by Jones et al.53,55 in their studies of the axial skeletal evolution in Synapsida. Increased potential for axial twisting can also aid in behaviors such as grooming and fast locomotory maneuvers, but this requires vertebral specializations for torsion. In earlier non-mammalian synapsids (i.e. most non-cynodont taxa), the functional regions of the vertebral column are not as distinct as in later taxa such as advanced cynodonts (e.g. the Jurassic Kayentatherium55), and there is little evidence of selection for performance under torsion in the anterior vertebrae. However, a general phylogenetic trend towards more regionalization into pre- and post-diaphragmic areas of the vertebrate column can be observed even in more stemward portions of synapsid phylogeny55. A more flexible, segmental sternum, as seen in Gorgonops torvus, may represent a prerequisite for accommodating intervertebral torsion in the thorax.
Therefore, we hypothesize that the evolution of the ossified segmental sternum in Theriodontia is a part of the broad evolutionary shift towards more mammal-like locomotion, which may have facilitated the rise of this group as the dominant carnivores of the late Permian. Selection for a lighter, more flexible sternum in the context of changing posture, gait, and vertebral mobility can be inferred regardless of the homology of the segmental sternum in Gorgonops—either this morphology evolved convergently in gorgonopsians and eucynodonts, or it would represent an ancestral adoption retained in cynodont evolution (albeit cartilaginous in taxa other than eucynodonts).
However, posture and gait were not the only major changes in thoracic anatomy occurring in Permo-Triassic therapsids. The transition to a mammal-like thoracic morphology is also tied to the way for therapsids to break Carrier’s constraint: the respiratory limitation driven by dual use of the axial musculature during lateral flexion and costal breathing during rapid locomotion47. Dorsoventral flexion in mammals, and a more rigid thorax centered more anteriorly along the vertebral column, fundamentally altered synapsid ventilation, permitting both lungs to be expanded or compressed simultaneously, a metabolically more efficient method advantageous for active locomotion. For this to work, however, it is necessary that the dorsal and ventral limits (i.e. the vertebral column and sternum) of the bony enclosures of the lungs (i.e. the rib cage) are both strong and pliable, conferring functional advantage over a single stiff interclavicle-sternal plate in managing volume of the thoracic cavity57. A multipartite sternum with cartilaginous tissue between the ‘manubrium’ and the sternebrae is consistent with this requirement. However, while this on its own would have helped to reduce the impact of Carrier’s constraint, actually breaking the constraint required an additional innovation: the diaphragm, a muscular sheet at the base of the thoracic cavity capable of pumping air through the lungs independently of locomotion.
Amongst the basic requirements for a diaphragm is that it must functionally be positioned caudad to the sternum, because by contracting during respiration, it creates negative pressure in the chest that is stabilized by the robust yet flexible complex of ribs, costal cartilages, and the segmental sternum. The origins of the diaphragm are obscure, however; it has been proposed to be unique to mammals or to have originated in some of the earliest “pelycosaurs” (e.g. caseids)58. Recent research taking data from developmental studies suggests that the diaphragm originated from ancestral pharygneal muscles of the cervico-thoracic region by posteriorization of elements associated with it, i.e. the forelimb bud during development and the brachial plexus nerve59. Accordingly, if the diaphragm did indeed originate from cervico-thoracic pharyngeal muscles, then the two requisite changes associated with the diaphragm may have been well underway in gorgonopsians: a) the posteriorization, evidenced by the likely presence of seven cervical vertebrae60 and the herein described elongate segmental sternum. And b) the elongate configuration itself of the sternum of Gorgonops, providing the needed caudad-positioned attachment for the diaphragm. This indicates that a mammalian-style diaphragm should already have been present in this taxon (and possibly, by inference, in theriodonts generally) to support the changes in ventilatory function.
Ontogenetic development of the sternum is well studied in extant mammals, with a particularly robust literature in the realms of human medicine and mouse embryology, demonstrating that formation of the characteristic segmental sternum is mediated by interactions with the developing ribs15,61. Specifically, the rib tips inhibit skeletal maturation, resulting in ossification of the intermediary regions but maintenance of cartilaginous connections between them62. As such, we must consider whether the segmental sternum would even have been selected for at all, or merely is an inherent consequence of developmental formation of a thoracic rib cage between the axial skeleton and sternum. Here, the fossil record is instructive. The plate-like sternum of dicynodonts has a variable number of rib attachments (see above), but a number of taxa clearly show multiple ribs attached to the single sternal element19. Therefore, it is apparently not an inherent developmental feature of Synapsida that rib attachments inhibit sternal growth and cause segments of the sternebrae to form. Rather, we propose that this system evolved through co-opting developmental mechanisms during a period of selection towards lighter and more jointed thoracic structures. Unfortunately, the cartilaginous nature of these elements in many synapsid groups (notably early cynodonts) makes it difficult to establish a precise understanding of the shift between dicynodont- and therocephalian-like structures and those of mammals. However, discoveries like that of the new Gorgonops specimen provide strong support for an early origin of the functional suite of derived mammalian locomotion and ventilation in the Permian antecedents of the clade.
Materials and methods
The gorgonopsian specimen SAM-PK-K10591 was discovered, excavated and mechanically prepared by Georgina Farrell (see Fig. 2c), and more recently given additional preparation by Nyaniso Nofingxana (see Fig. 2e) at the Iziko South African Museum in Cape Town. The skeleton is almost complete and maintained in situ on the slab to retain preservational context. SAM-PK-K10591 was found on the farm Wilgersbosch Kloof 449 in Oukloof Pass (Lat -32.187473 Long. 21.820334), some 120 km south east of Fraserburg in the Northern Cape Province, South Africa. The locality is in a low sandstone-capped cliff exposure of Tropidostoma-Gorgonops Subzone63,64 mudrocks, indicating an early late Permian (Wuchiapingian) age. A partial cranium was found as several loose pieces in close association with the semi-articulated postcranial skeleton, allowing for confident identification of the specimen as Gorgonops torvus (i.e., the presence of four postcanines, a prominent precanine ‘step’ to the maxillary alveolary margin, and proportions of the snout are diagnostic—for measurements of the skull, see Supplemementary Table S1).
The thinly bedded mudrocks that host the specimen contain isolated smooth surfaced calcareous nodules and claystone-lined root casts indicative of an immature palaeosol in a distal floodplain paleoenvironment65. The bones are patchily peri-mineralized with a thin, 5 mm-thick encrustation of the same micritic nodular material. Such bacterially mediated precipitation of carbonate suggests that most of the skeleton was buried before the soft tissue had completely decomposed66,67. The presence of fine longitudinal cracks in the limb bones represents Behrensmeyer’s68 weathering stage 1 and is an indication that the bones were exposed to the elements for 0.5 to 2.5 years before final burial.
The unusual distribution of elements of the SAM PK-K10591 skeleton is worthy of further description, as it indicates what happened in the post-mortem/pre-burial phase. When discovered, only the anterior part of the skeleton was partly exposed, the posterior half being fully enclosed in mudrock. Thus, the five articulated dorsal vertebrae that have been removed from the carcass (see Fig. 2c and d), without displacing the articulating ribs, must have occurred before burial. This atypical occurrence, combined with an unnatural 180-degree rotation of the neck (and likely the skull as well, although this cannot be confirmed) along with its articulated cervical ribs (see Fig. 2) and complete sternum, is interpreted as most likely the work of scavenging tetrapods. Efforts by co-occurring carnivores (likely other therapsids) to dismember the carcass may have been hampered by tough desiccated skin and connective tissue, which not only resisted their attempts, but also kept the ribs and sternum in articulation. Similar taphonomic modification is observed in modern mid-sized carcasses that have been scavenged by non-bone-cracking predator/scavengers such as jackals and coyotes, e.g.69,70.
Supplementary Information
Acknowledgements
We are grateful to Georgina Farrell, who found and prepared the specimen as well as Nyaniso Nofingxana for further preparation. Furthermore, we want to thank Zaituna Skosan for enabling access to the collections of the Iziko Museums of South Africa and Christian Sidor for providing the image of NHCC LB350. We thank Christian Sidor and Katrina Jones for their constructive comments on earlier drafts of this manuscript that have helped improved this manuscript, although any errors and imperfection remaining are the authors’ own. This research was supported by an Elsa-Neumann-Scholarship of the State Berlin to E.-M.B. and a National Research Foundation/African Origins Platform entitled “Palaeoecology of Western Gondwana” grant to R.M.H.S. We acknowledge support by the Open Access Publication Fund of Humboldt-Universität zu Berlin.
Author contributions
E.-M.B., C.F.K. and J.F. conceived the study. E.-M.B. prepared the figures. All authors analyzed the data and contributed to writing the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17492-6.
References
- 1.Vickaryous MK, Hall BK. Homology of the reptilian coracoid and a reappraisal of the evolution and development of the amniote pectoral apparatus. J. Anat. 2006;208:263–285. doi: 10.1111/j.1469-7580.2006.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romer AS. Osteology of the Reptiles. University of Chicago Press; 1956. [Google Scholar]
- 3.Ray S. Functional and evolutionary aspects of the postcranial anatomy of dicynodonts (Synapsida, Therapsida) Palaeontology. 2006;49:1263–1286. doi: 10.1111/j.1475-4983.2006.00597.x. [DOI] [Google Scholar]
- 4.Fahn-Lai P, Biewener AA, Pierce SE. Broad similarities in shoulder muscle architecture and organization across two amniotes: Implications for reconstructing non-mammalian synapsids. PeerJ. 2020;8:e8556. doi: 10.7717/peerj.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai PH, Biewener AA, Pierce SE. Three-dimensional mobility and muscle attachments in the pectoral limb of the Triassic cynodont Massetognathus pascuali (Romer, 1967) J. Anat. 2018;232:383–406. doi: 10.1111/joa.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulej T, Niedźwiedzki G. An elephant-sized Late Triassic synapsid with erect limbs. Science. 2019;363:78–80. doi: 10.1126/science.aal4853. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann S, Hu Y, Krause DW. Postcranial morphology of Adalatherium hui (Mammalia, Gondwanatheria) from the Late Cretaceous of Madagascar. J. Vert. Paleontol. 2020;40:133–212. doi: 10.1080/02724634.2020.1799818. [DOI] [Google Scholar]
- 8.Ji Q, Luo Z-X, Ji S-A. A Chinese triconodont mammal and mosaic evolution of the mammalian skeleton. Nature. 1999;398:326–330. doi: 10.1038/19221. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C-F, Bhullar B-AS, Neander AI, Martin T, Luo Z-X. New Jurassic mammaliaform sheds light on early evolution of mammal-like hyoid bones. Science. 2019;365:276–279. doi: 10.1126/science.aau9345. [DOI] [PubMed] [Google Scholar]
- 10.Luo Z-X, Ji Q, Yuan C-X. Convergent dental adaptations in pseudo-tribosphenic and tribosphenic mammals. Nature. 2007;450:93. doi: 10.1038/nature06221. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Luo Z-X. Postcranial skeleton of the Cretaceous mammal Akidolestes cifellii and its locomotor adaptations. J. Mamm. Evol. 2013;20:159–189. doi: 10.1007/s10914-012-9199-9. [DOI] [Google Scholar]
- 12.Russell, A. & Bauer, A. in Biology of the Reptilia. Volume 21. Morphology I. The Skull and Appendicular Locomotor Apparatus of Lepidosauria (eds Gans, C., Gaunt, A. S. & Adler, K.) 1–466 (Society for the Study of Amphibians and Reptiles, 2008).
- 13.Stinner JN. Functional anatomy of the lung of the snake Pituophis melanoleucus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1982;243:R251–R257. doi: 10.1152/ajpregu.1982.243.3.R251. [DOI] [PubMed] [Google Scholar]
- 14.Gaetano LC, Mocke H, Abdala F. The postcranial anatomy of Diademodon tetragonus (Cynodontia, Cynognathia) J. Vert. Paleontol. 2018;38:e1451872. doi: 10.1080/02724634.2018.1451872. [DOI] [Google Scholar]
- 15.Buchholtz E, Yozgyur Z, Feldman A, Weaver A, Gaudin T. The therian sternum at the lateral somitic frontier: Evolution of a composite structure. J. Zool. 2020 doi: 10.1111/jzo.12809. [DOI] [Google Scholar]
- 16.Bendel E-M, Kammerer CF, Kardjilov N, Fernandez V, Fröbisch J. Cranial anatomy of the gorgonopsian Cynariops robustus based on CT-reconstruction. PLoS ONE. 2018;13:e0207367. doi: 10.1371/journal.pone.0207367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sues, H. D. & Jenkins Jr, F. A. 5 The postcranial skeleton of Kayentatherium wellesi from the lower Jurassic Kayenta formation of Arizona and the phylogenetic significance of postcranial features. Amniote Paleobiol. Perspect. Evol. Mamm. Birds Reptil.114 (2006).
- 18.Sun, A. & Li, Y. The postcranial skeleton of the late tritylodont Bienotheroides. Vertebr. PalAsiat.23 (1985).
- 19.Cluver, M. The skeleton of the mammal-like reptile Cistecephalus with evidence for a fossorial mode of life. 5 (1978).
- 20.Ray S, Chinsamy A. Functional aspects of the postcranial anatomy of the Permian dicynodont Diictodon and their ecological implications. Palaeontology. 2003;46:151–183. doi: 10.1111/1475-4983.00292. [DOI] [Google Scholar]
- 21.Botha-Brink J, Modesto SP. A new skeleton of the therocephalian synapsid Olivierosuchus parringtoni from the Lower Triassic South African Karoo Basin. Palaeontology. 2011;54:591–606. doi: 10.1111/j.1475-4983.2011.01048.x. [DOI] [Google Scholar]
- 22.Kemp T. The skeleton of a baurioid therocephalian therapsid from the Lower Triassic (Lystrosaurus Zone) of South Africa. J. Vert. Paleontol. 1986;6:215–232. doi: 10.1080/02724634.1986.10011617. [DOI] [Google Scholar]
- 23.Romer AS, Price LI. Review of the Pelycosauria. Geological Society of America; 1940. [Google Scholar]
- 24.Hopson J, Barghusen H. An Analysis of Therapsid Relationships. Smithsonian Institution Press; 1986. pp. 83–106. [Google Scholar]
- 25.Sidor CA, Hopson JA. Ghost lineages and “mammalness”: Assessing the temporal pattern of character acquisition in the Synapsida. Paleobiology. 1998;24:254–273. doi: 10.1666/0094-8373(1998)024[0254:GLAATT]2.3.CO;2. [DOI] [Google Scholar]
- 26.Boonstra LD. The girdles and limbs of the Gorgonopsia of the Tapinocephalus Zone. Ann. S. Afr. Mus. 1965;48:237–249. [Google Scholar]
- 27.Broom R. On a nearly complete therocephalian skeleton. Ann. Transvaal Mus. 1938;19:257–261. [Google Scholar]
- 28.Rubidge BS, Govender R, Romano M. The postcranial skeleton of the basal tapinocephalid dinocephalian Tapinocaninus pamelae (Synapsida: Therapsida) from the South African Karoo Supergroup. J. Syst. Palaeontol. 2019;17(20):1767–1789. doi: 10.1080/14772019.2018.1559244. [DOI] [Google Scholar]
- 29.Gregory WK, Broom R. The Skeleton of Moschops Capensis Broom: A Dinocephalian Reptile from the Permian of South Africa. USA: Order of the Trustees, The American Museum of Natural History; 1926. [Google Scholar]
- 30.Botha-Brink J, Angielczyk KD. Do extraordinarily high growth rates in Permo-Triassic dicynodonts (Therapsida, Anomodontia) explain their success before and after the end-Permian extinction? Zool. J. Linn. Soc. 2010;160:341–365. doi: 10.1111/j.1096-3642.2009.00601.x. [DOI] [Google Scholar]
- 31.Fröbisch J, Reisz RR. The postcranial anatomy of Suminia getmanovi (Synapsida: Anomodontia), the earliest known arboreal tetrapod. Zool. J. Linn. Soc. 2011;162:661–698. doi: 10.1111/j.1096-3642.2010.00685.x. [DOI] [Google Scholar]
- 32.Brinkman D. The Structure and Relationships of the Dromasaurs (Reptilia: Therapsida) Bulletin of the Museum of Comparative Zoology; 1981. [Google Scholar]
- 33.Angielczyk KD, Rubidge BS. Skeletal morphology, phylogenetic relationships and stratigraphic range of Eosimops newtoni Broom, 1921, a pylaecephalid dicynodont (Therapsida, Anomodontia) from the Middle Permian of South Africa. J. Syst. Palaeontol. 2013;11:191–231. doi: 10.1080/14772019.2011.623723. [DOI] [Google Scholar]
- 34.Lucas SG, Harris SK. Taxonomic and biochronological significance of specimens of the Triassic dicynodont Dinodontosaurus Romer 1943 in the Tübingen collection. Paläontol. Z. 1996;70:603–622. doi: 10.1007/BF02988096. [DOI] [Google Scholar]
- 35.Govender R. Description of the postcranial anatomy of Aulacephalodon baini and its possible relationship with 'Aulacephalodon peavoti’. S. Afr. J. Sci. 2008;104:479–486. doi: 10.1590/S0038-23532008000600023. [DOI] [Google Scholar]
- 36.Broom R. On the structure of the mammal-like reptiles of the Sub-Order Gorgonopsia. Philos. Trans. R Soc. Lond. Ser. B Biol. Sci. 1930;218:345–371. [Google Scholar]
- 37.Boonstra L. Additions to our knowledge of the South African Gorgonopsia, preserved in the British Museum (Natural History) Ann. S. Afr. Mus. 1934;31:175–213. [Google Scholar]
- 38.Tatarinov L. A postcranial skeleton of the gorgonopian Viatkogorgon ivachnenkoi (Reptilia, Theriodontia) from the Upper Permian Kotelnich locality, Kirov Region. Paleontol. J. 2004;38:437–447. [Google Scholar]
- 39.Fourie H, Rubidge BS. The postcranial skeleton of the basal therocephalian Glanosuchus macrops (Scylacosauridae) and comparison of morphological and phylogenetic trends amongst the Theriodontia. Palaeontol. Afr. 2009;44:27–40. [Google Scholar]
- 40.Boonstra LD. The girdles and limbs of the pristerognathid Therocephalia. Ann. S. Afr. Mus. 1964;48:121–165. [Google Scholar]
- 41.Sigurdsen T, Huttenlocker AK, Modesto SP, Rowe TB, Damiani R. Reassessment of the morphology and paleobiology of the therocephalian Tetracynodon darti (Therapsida), and the phylogenetic relationships of Baurioidea. J. Vert. Paleontol. 2012;32:1113–1134. doi: 10.1080/02724634.2012.688693. [DOI] [Google Scholar]
- 42.Luo ZX. Origin of the mammalian shoulder. In: Dial KP, Shubin N, Brainerd EL, editors. Great Transformations: Major Events in the History of Vertebrate Life. Chicago, Illinois: The University of Chicago Press; 2015. pp. 167–187. [Google Scholar]
- 43.Meng Q-J, et al. New gliding mammaliaforms from the Jurassic. Nature. 2017;548:291–296. doi: 10.1038/nature23476. [DOI] [PubMed] [Google Scholar]
- 44.Lessertisseur, J. & Saban, R. in Traité de zoologie: Anatomie, systématique, biologie. Vol. Tome XVI Mammifères, Fascicule I, Téguments et squelette. (ed Grassé, P.-P.) 585–708 (Masson et cie editeurs libraires de l’academie de medecine, 1967).
- 45.Klima M. Early Development of the Shoulder Girdle and Sternum in Marsupials (Mammalia: Metatheria) USA: Springer-Verlag; 1987. [DOI] [PubMed] [Google Scholar]
- 46.Linzey DW. Vertebrate Biology: Systematics, Taxonomy, Natural History, and Conservation. USA: JHU Press; 2020. [Google Scholar]
- 47.Carrier DR. The evolution of locomotor stamina in tetrapods: Circumventing a mechanical constraint. Paleobiology. 1987;13:326–341. doi: 10.1017/S0094837300008903. [DOI] [Google Scholar]
- 48.Crompton A, Jenkins FA., Jr Mammals from reptiles: A review of mammalian origins. Annu. Rev. Earth Planet. Sci. 1973;1:131–155. doi: 10.1146/annurev.ea.01.050173.001023. [DOI] [Google Scholar]
- 49.Hopson JA. The mammal-like reptiles: A study of transitional fossils. Am. Biol. Teach. 1987;49:16–26. doi: 10.2307/4448410. [DOI] [Google Scholar]
- 50.DeMar R, Barghusen HR. Mechanis and the evolution of the synapsid jaw. Evolution. 1972 doi: 10.1111/j.1558-5646.1972.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 51.Blob RW. Evolution of hindlimb posture in nonmammalian therapsids: Biomechanical tests of paleontological hypotheses. Paleobiology. 2001;27:14–38. doi: 10.1666/0094-8373(2001)027<0014:EOHPIN>2.0.CO;2. [DOI] [Google Scholar]
- 52.Lautenschlager S, Gill PG, Luo Z-X, Fagan MJ, Rayfield EJ. The role of miniaturization in the evolution of the mammalian jaw and middle ear. Nature. 2018;561:533–537. doi: 10.1038/s41586-018-0521-4. [DOI] [PubMed] [Google Scholar]
- 53.Jones KE, Gonzalez S, Angielczyk KD, Pierce SE. Regionalization of the axial skeleton predates functional adaptation in the forerunners of mammals. Nat. Ecol. Evol. 2020;4:470–478. doi: 10.1038/s41559-020-1094-9. [DOI] [PubMed] [Google Scholar]
- 54.Müller J, et al. Homeotic effects, somitogenesis and the evolution of vertebral numbers in recent and fossil amniotes. PNAS. 2010;107:2118–2123. doi: 10.1073/pnas.0912622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones KE, Dickson BV, Angielczyk KD, Pierce SE. Adaptive landscapes challenge the “lateral-to-sagittal” paradigm for mammalian vertebral evolution. Curr. Biol. 2021;31:1883–1892. doi: 10.1016/j.cub.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Yang W, Chen IH, Mckittrick J, Meyers MA. Flexible dermal armor in nature. J. Morphol. 2012;64:475–485. [Google Scholar]
- 57.Gillespie J. Mechanisms that determine functional residual capacity in different mammalian species. Am. Rev. Respir. Dis. 1983;128:S74–S77. doi: 10.1164/arrd.1983.128.2P2.S74. [DOI] [PubMed] [Google Scholar]
- 58.Lambertz M, Shelton CD, Spindler F, Perry SF. A caseian point for the evolution of a diaphragm homologue among the earliest synapsids. Ann. N. Y. Acad. Sci. 2016;1385:3–20. doi: 10.1111/nyas.13264. [DOI] [PubMed] [Google Scholar]
- 59.Hirasawa T, Kuratani S. A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J. Anat. 2013;222:504–517. doi: 10.1111/joa.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sigogneau-Russell D. Theriodontia I. Phthinosuchia, Biarmosuchia, Eotitanosuchia, Gorgonopsia. Fischer; 1989. [Google Scholar]
- 61.Ashley G. The morphological and pathological significance of synostosis at the manubrio-sternal joint. Thorax. 1954;9:159. doi: 10.1136/thx.9.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen JM. Studies on the morphogenesis of the mouse sternum: I. Normal embryonic development. J. Anat. 1952;86:373. [PMC free article] [PubMed] [Google Scholar]
- 63.Botha J, Angielczyk K. An integrative approach to distinguishing the Late Permian dicynodont species Oudenodon bainii and Tropidostoma microtrema (Therapsida: Anomodontia) Palaeontology. 2007;50:1175–1209. doi: 10.1111/j.1475-4983.2007.00697.x. [DOI] [Google Scholar]
- 64.Kammerer CF, Smith RM. An early geikiid dicynodont from the Tropidostoma Assemblage Zone (late Permian) of South Africa. PeerJ. 2017;5:e2913. doi: 10.7717/peerj.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith RM. Alluvial paleosols and pedofacies sequences in the Permian Lower Beaufort of the southwestern Karoo Basin, South Africa. J. Sediment. Res. 1990;60:258–276. doi: 10.1306/212F9142-2B24-11D7-8648000102C1865D. [DOI] [Google Scholar]
- 66.de Sousa DV, Eltink E, Oliveira RAP, Félix JF, de Moura Guimarães L. Diagenetic processes in Quaternary fossil bones from tropical limestone caves. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyman RL, Lyman C. Vertebrate Taphonomy. Cambridge University Press; 1994. [Google Scholar]
- 68.Behrensmeyer AK. Taphonomic and ecologic information from bone weathering. Paleobiology. 1978;4:150–162. doi: 10.1017/S0094837300005820. [DOI] [Google Scholar]
- 69.Nattrass, N., Conradie, B., Drouilly, M. & O’Riain, M. J. Understanding the black-backed jackal. Centre for Social Science Research University of Cape Town. Working Paper No. 399 10.13140/RG.2.2.14650.70085 (2017).
- 70.Weigelt J. Recent Vertebrate Carcasses and Their Paleobiological Implications. University of Chicago Press; 1989. p. 188. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez V, et al. Synchrotron reveals Early Triassic odd couple: Injured amphibian and aestivating therapsid share burrow. PLoS ONE. 2013;8:e64978. doi: 10.1371/journal.pone.0064978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.