Abstract
Branched-chain amino acid (BCAA) catabolism has been considered to have an emerging role in the pathogenesis of metabolic disturbances in obesity and type 2 diabetes (T2D). Several studies showed elevated plasma BCAA levels in humans with insulin resistance and patients with T2D, although the underlying reason is unknown. Dysfunctional BCAA catabolism could theoretically be an underlying factor. In vitro and animal work collectively show that modulation of the BCAA catabolic pathway alters key metabolic processes affecting glucose homeostasis, although an integrated understanding of tissue-specific BCAA catabolism remains largely unknown, especially in humans. Proof-of-concept studies in rodents -and to a lesser extent in humans – strongly suggest that enhancing BCAA catabolism improves glucose homeostasis in metabolic disorders, such as obesity and T2D. In this review, we discuss several hypothesized mechanistic links between BCAA catabolism and insulin resistance and overview current available tools to modulate BCAA catabolism in vivo. Furthermore, this review considers whether enhancing BCAA catabolism forms a potential future treatment strategy to promote metabolic health in insulin resistance and T2D.
Subject terms: Type 2 diabetes, Obesity
Introduction
Type 2 diabetes (T2D) is one of world’s most prevalent diseases, and is related to the epidemic of obesity [1]. Obesity can lead to the onset of T2D when pancreatic β-cells are no longer able to compensate higher insulin secretion for the reduced insulin sensitivity that often accompanies obesity [2]. Over the last decade, branched-chain amino acids (BCAA) catabolism has increasingly been considered to have an emerging role in the development of insulin resistance in people with obesity and T2D. In these individuals, BCAA levels are considerably elevated in plasma and tissues [3–9]. Furthermore, elevated BCAA levels in plasma strongly associate with insulin resistance in people with obesity and T2D [3, 4, 6–8, 10–13]. Although it is still unknown why these BCAA levels are elevated and why they associate with insulin resistance, a dysfunctional BCAA catabolism may be one of the underlying factors. This review aims to provide insight into the mechanisms behind elevated plasma BCAA levels in people with obesity and/or T2D and its role in the pathogenesis of insulin resistance. Furthermore, this review will overview pharmaceutical and alternative lifestyle intervention strategies in order to lower plasma BCAA levels and its effects on metabolic health.
Why investigate BCAA levels?
Leucine, isoleucine and valine are grouped together as BCAA because they share a structural feature with a branched-side chain and common initiation steps of catabolism [14].
In general, BCAA play several important metabolic and physiological roles, aside from being considered as substrates for synthesis of proteins. Reports show that BCAA act as signaling molecules regulating metabolism of glucose, lipid, and protein [15]. In addition, BCAA levels play a key role in interorgan metabolic crosstalk and, therefore, dysregulation of BCAA catabolism may play a significant role in several metabolic diseases [16].
Several studies showed that plasma BCAA levels in overweight and obese humans with insulin resistance [3–7] and patients with T2D [8, 9] were elevated compared to healthy individuals. Recently, in an observational study, we confirmed this finding and showed that plasma BCAA levels were elevated in patients with T2D compared to age- and BMI-matched controls without having T2D [13]. Some [17–19], but not all studies [20, 21] found elevated plasma BCAA levels to be associated with increased risk of T2D and suggest that BCAA levels in plasma may predict future diabetes [17].
It has repeatedly been reported that the accumulation of plasma BCAA levels strongly associate with insulin resistance in obesity and T2D [3, 4, 6–8, 10–13]. Similarly, a short-term intravenous infusion with amino acids in young, human volunteers induced temporary insulin resistance [22]. However, as a mixture of amino acids were infused, it cannot be deduced from this study whether the BCAA per se are responsible for the development of insulin resistance. So far, there are no reports investigating whether particularly a raise of BCAA plasma levels in humans induces insulin resistance. Therefore, the underlying mechanisms of elevated BCAA plasma levels on insulin-stimulated glucose uptake in humans remain largely unknown.
Why are plasma BCAA levels elevated with insulin resistance?
BCAA homeostasis and levels in plasma are defined by BCAA appearance and disappearance, affected by several processes. Processes contributing to BCAA appearance in the blood include protein breakdown in tissues (a process which is inhibited by insulin), food intake and gut microbial synthesis. The major processes involved in disappearance of BCAA are protein synthesis, excretion and BCAA catabolism [4, 23]. As a result, an interplay between these mechanisms defines the levels of BCAA in plasma, and therefore multifactorial causes could underlie the elevated BCAA plasma levels seen in people with insulin resistance and patients with T2D.
Effect of insulin on protein breakdown and BCAA catabolism
Insulin is known to be one of the most important regulators of carbohydrate, fat and protein metabolism. Protein metabolism, or more specifically, protein turnover, is defined by the balance between protein synthesis and protein breakdown [24]. During periods of steady state, the rate of protein synthesis equals the rate of protein breakdown. Both insulin as well as BCAA concentrations affects protein turnover in muscle [25], adipose tissue [26] and liver [27].
The effect of insulin on leucine flux has been investigated in humans with use of an intravenous infusion of insulin combined with [1-13C] or [1-14C]-leucine tracer [28–30]. An intravenous insulin infusion in people without diabetes provoked a decline in the leucine flux due to a reduction in protein breakdown, without an effect on protein synthesis [28–30]. The activation of protein kinase B (Akt) in response to insulin by the insulin receptor (IRS-1) induces phosphorylation of the Forkhead box class (FOXO) transcription, and indirectly activate mTOR, which seems to be responsible for the inhibited muscle protein breakdown via [31–35].
In humans with insulin resistance, the effect of insulin on reducing muscle protein breakdown is blunted causing increased muscle wasting [36], as is confirmed in rodent models [37–40]. BCAA are reported to activate the mTOR pathway [41] and stimulate protein synthesis in muscle of humans. However, the inhibitory effect of insulin on protein breakdown occurs independently of the levels of circulating plasma BCAA [42–44]. Normally, insulin’s inhibitory action on protein breakdown in muscle tissue [45–47] results in lower amino acid concentrations in plasma [42, 48, 49], with the most marked decline seen for BCAA [50–53]. The effect of insulin on BCAA plasma levels has been investigated for the first time in patients with type 1 diabetes [54, 55] and results showed that the withdrawal of insulin treatment was associated with a substantial increase in circulating BCAA concentrations, as confirmed by others [58, 59]. We recently confirmed the strong insulin-suppressive effect on BCAA levels in plasma during a euglycemic hyperinsulinemic clamp in healthy, insulin sensitive people with obesity, however, this insulin-suppressive effect was blunted in people with obesity, diagnosed with non-alcoholic fatty liver (NAFL) and/or T2D [56]. Also others found less efficient BCAA reduction upon insulin infusion in obese humans with insulin resistance [57–59]. The suggestion that increased BCAA levels could merely be a consequence of impaired insulin action is in accordance with the results from a recent mendelian randomization study [60], showing that insulin resistance drives higher plasma BCAA levels [60, 61]. In contrast, a large-scale human genetic study by Lotta et al. pointed towards a causal role of diminished BCAA catabolism underlying insulin resistance [62], which is described below.
Diet and microbiome
BCAA cannot be synthesized by humans and are therefore essential dietary components that must originate from ingested food [63]. In addition, gut microbiota is able to produce and degrade BCAA [64].
Major dietary sources of BCAA include milk, red meat, poultry, and high fat dairy products [65, 66]. BCAA make up almost 20% of dietary protein [63]. Since the Western diet is characterized by high fat and protein intake [3], one could assume that dietary intake of protein may contribute to changes in plasma BCAA levels. Indeed, evidence suggests that consumption of dietary protein increases the risk of diabetes and insulin resistance [3, 66, 67]. Newgard et al. [3] reported that individuals with obesity and insulin resistance consumed more protein compared to lean individuals. Since in the individuals with obesity and insulin resistance BCAA levels in plasma were increased, this data matches the assumption that higher protein intake leads to increase of BCAA in plasma [3]. However, in these studies only intake of total protein had been assessed, and not the BCAA consumption. In contrast, others found that BCAA levels were elevated in individuals with insulin resistance compared to healthy participants, despite equal rates of protein intake. Furthermore, a weak correlation was found between BCAA dietary intake and plasma BCAA levels [4, 19, 65]. McCormack et al. found that plasma BCAA levels, but not dietary BCAA intake, was associated with obesity and insulin resistance [19].
Besides direct dietary intake, BCAA can also be metabolized by the gut microbiome [68–71]. More specifically, a recent study by Pedersen et al. [70] showed that a gut microbiome having a higher potential for biosynthesis of BCAA and reduced number of inward bacterial transporters for these amino acids were associated with increased levels of BCAA in plasma [70]. Interestingly, increased potential for BCAA biosynthesis and reduced potential for bacterial BCAA uptake are both linked with insulin resistance [70]. Above all, it has been reported that circulating BCAA levels were increased in mice following transplantation of stool derived from individuals with insulin resistance [64]. This data indicates that microbiota indeed contributes to changes in BCAA plasma levels, in which altered gut microbiota could be another underlying cause of elevated BCAA levels in individuals with insulin resistance.
BCAA catabolism
BCAA catabolism in health
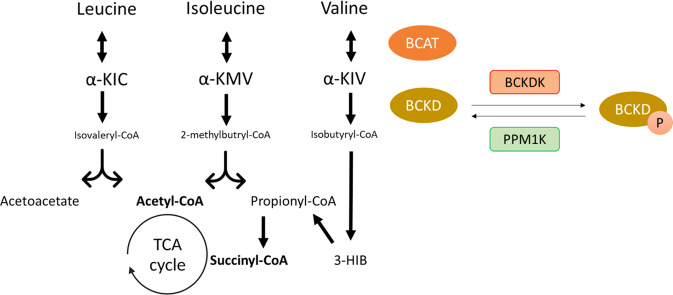
Catabolism of all three BCAA, leucine, isoleucine and valine, is located inside the mitochondria, in which the first two steps are common for all BCAA (Fig. 1) [72, 73]. The first reaction is the reversible transamination catalyzed by the branched-chain amino acid aminotransferases (BCAT) to form branched-chain α-keto acids (BCKA): α-ketoisocaproate (α-KIC), α-keto-B-methylvalerate (α-KMV), and α-ketoisovalerate (α-KIV), respectively formed out of leucine, isoleucine and valine [74]. The second step is the irreversible oxidative decarboxylation by the branched-chain α -keto acid dehydrogenase (BCKD) complex, the rate-limiting enzyme of this pathway [75]. BCKD comprising three catalytic components (E1, E2 and E3) is regulated by a phosphorylation-dephosphorylation catalyzing process, whereby a specific kinase (BCKDK) is responsible for inactivation and a phosphatase (PPM1K) for activation of this complex [76, 77], both regulated by nutrient status and BCAA levels itself [78–80]. It has been reported that phosphorylation occurs in the E1 component of the BCKD complex, whereas dephosphorylation reaction interacts with both the E1 and E2 domain [77, 81–83]. Ultimately, the CoA compounds formed by the BCKD-complex are further metabolized to acetyl-CoA and succinyl-CoA, which are incorporated into the tricarboxylic acid (TCA) cycle [84]. TCA cycle fueling also occurs via the alanine cycle (or termed Cahill cycle), which is tightly linked to BCAA catabolism. The alanine cycle involves series of reactions in which amino groups and carbons from skeletal muscle are transported to the liver [85]. In short, in skeletal muscle, the reaction of BCAA to BCKA yields glutamate which then combines with pyruvate to generate alanine [86]. Alanine is released by skeletal muscle and taken up by the liver [87, 88], where it forms an important source for gluconeogenesis [89]. The glucose produced by the liver is shuttled into the circulation, taken up by muscle cells [87], and consequently converted back to glutamate, entering the TCA cycle via α-ketoglutarate [86].
Fig. 1. Schematic overview of BCAA catabolism.
BCAT branched-chain amino acid transaminase, BCKD branched-chain keto acid dehydrogenase, α-KIC α-ketoisocaproate, α-KMV α-keto-methylvalerate, α-KIV α-ketoisovalerate, 3-HIB 3-hydroxyisobutyrate, BCKDK BCKDK kinase, PPM1K BCKDK phosphatase. Adapted from Neinast et al. [73].
Tissue-specific BCAA metabolism has been investigated in rodent models. Neinast et al. investigated whole-body BCAA catabolism in mice using in vivo isotopic tracing and found that most tissues actively oxidize BCAA, with the largest contribution likely in skeletal muscle and liver [84]. Other rodent studies showed that BCAT activity, the enzyme responsible for the BCAA transamination step, is relatively low in hepatocytes [90]. Moreover, unlike other amino acids, BCAA circumvent first-pass metabolism in the liver [84], and are primarily transaminated to BCKA in extra-hepatic tissues since BCAT is mainly expressed in muscle, kidney and heart tissue in rodents [63, 91, 92]. Next, BCKA are released back into the circulation and undergo oxidation by the BCKD complex in the liver [76]. Accordingly, it has been assumed that the liver of rodents has the highest BCKD activity [93], however, BCKD is also expressed in white adipose tissue (WAT) although to a lesser extent [75, 76].
Information on tissue-specific BCAA oxidation in humans is, however, very limited. In one study, enzymatic activities of BCAT and BCKD were evaluated in several human-derived tissues and showed large differences compared to the results observed in rodent tissues [76]. Thus, Suryawan et al. [76] reported that both skeletal muscle and liver in humans are key tissues involved in BCAA catabolism and express BCAT and BCKD, with the highest expression in muscle, which was also found by others [85]. Furthermore, human heart [94–96] and adipose tissue [75, 81, 97–102] depend on BCAA oxidative capacity as well.
BCAA catabolism in obesity and T2D
Since the first two steps of BCAA catabolism are common for all three BCAA, a reduced BCAA catabolic flux in one of these steps forms a plausible explanation underlying the rise in plasma BCAA levels of obese insulin resistant individuals with and without T2D. Indeed, several studies points towards diminished or altered function of the key enzymes involved in BCAA catabolism [23, 75, 103–105]. This has been confirmed in rodent studies showing that increased levels of BCAA in plasma are the result of reduced expression of BCAT [75, 106] or lower BCKD complex activity, via either increased expression of BCKDK [75, 84, 107, 108] or suppression of PPM1K [80, 103, 109, 110]. Animal models of obesity and T2D as well show affected BCAA catabolism [75, 111, 112]: tissue-specific expression of BCAA-catabolic enzymes are shown to be dysregulated [23, 27, 79, 108, 113–121] especially in adipose tissue [75, 122] and liver [75, 113]. Moreover, decreased BCAA catabolism in WAT is assumed to be a contributor to increased plasma levels of BCAA as seen in obesity and insulin resistance [75, 81, 97–102, 104]. The capacity of WAT to modulate circulating BCAA levels has been confirmed by Herman et al. [98], who demonstrated that transplantation of normal WAT into transgenic mice with defective peripheral BCAA catabolism reduced circulating BCAA levels.
Although only limited knowledge derives from human studies, collecting evidence supports the hypothesis that dysfunctional BCAA catabolism could underlie a rise in BCAA plasma levels. For instance, in patients with maple syrup urine disease (MSUD), an inborn error of metabolism caused by loss-of-function mutation in components of the BCKD complex [123–126] or its regulatory phosphatase, PPM1K [127], BCAA levels in plasma are found to be elevated. Others confirmed that altered activity of BCAT or the BCKD complex, at least in muscle and liver, plays a role in plasma BCAA levels [25, 62, 93, 117, 128, 129]. Reduced expression levels of BCAT were found in skeletal muscle of insulin resistant patients with T2D, which could explain the observed elevated BCAA plasma levels [117]. Also expression of PPM1K in skeletal muscle of people with T2D failed to increase in contrast to healthy controls during in oral glucose challenge, which could indicate dysregulation of the BCAA pathway [62]. Indeed, gene expression studies revealed downregulation in multiple steps of the BCAA catabolic pathway in skeletal muscle of individuals with insulin resistance [25, 129] and patients with T2D [62]. In addition, individuals with obesity and/or T2D were shown to have a marked decrease in BCKD protein content in liver biopsies when compared to the non-obese control group [93]. In human liver cells, mutation or deletion of PPM1K resulted in elevated BCAA levels [128].
The BCAA catabolic pathway has also been shown to be downregulated in WAT of people with obesity [99]. The idea that BCKD in WAT contributes to changes in BCAA levels in humans is supported by the fact that BCAA levels in plasma significantly decreased after bariatric surgery [75, 130], while BCKD expression in WAT increased [75]. Together, these results demonstrate the capacity of WAT to modulate circulating BCAA levels. WAT is, however, suggested to be responsible for less than 5% of whole-body BCAA oxidation [84], meaning that the increase in plasma BCAA levels must have additional origins [131].
Others have suggested that reduced BCAA oxidation in adipose tissue and liver may induce BCAA overflow to skeletal muscle, driving its BCAA oxidation there [23, 84, 131–133]. Since skeletal muscle has a high capacity to oxidize BCAA, it could be postulated that muscle functions as the metabolic sink for impaired BCAA oxidation in adipose tissue and liver [132]. Interestingly, a recent study using a heavy isotope steady-state infusion of BCAA, showed a shift in BCAA oxidation from adipose tissue and liver toward skeletal muscle in obese, insulin resistant mice [84], consistent with the finding that BCKD enzyme activity in liver and adipose tissue is downregulated in animals with obese/insulin-resistant or diabetic states [75, 103, 111, 112, 134–138]. This was also confirmed by She et al. who found that BCKD activity was decreased in adipose tissue.
Recently, we reported that in vivo whole-body leucine oxidation rates were significantly lower in patients with T2D compared to control participants with similar age and BMI [13]. Previously, no differences were reported between FDR and matched controls [139] nor between obese and control participants [140]. As leucine, valine and isoleucine share the same oxidation route via the BCKD complex, one could assume that in vivo 1-13C leucine tracer kinetics represent the total BCAA pool [141–143]. Nevertheless, it would be of interest to measure the oxidation rates of the three individual BCAA (i.e., with 1-13C leucine, 1-13C isoleucine, and 1-13C isoleucine), which has never been investigated in humans. Furthermore, as BCAA and BCAA-derived catabolites has mostly been investigated in plasma, levels in human peripheral tissues would give more insight into tissue-specific BCAA catabolism. These considerations highlight the need for future research to investigate whether tissue-specific BCAA catabolic defects occur in individuals with obesity, insulin-resistance or T2D individuals.
How do plamsa BCAA levels link to insulin resistance?
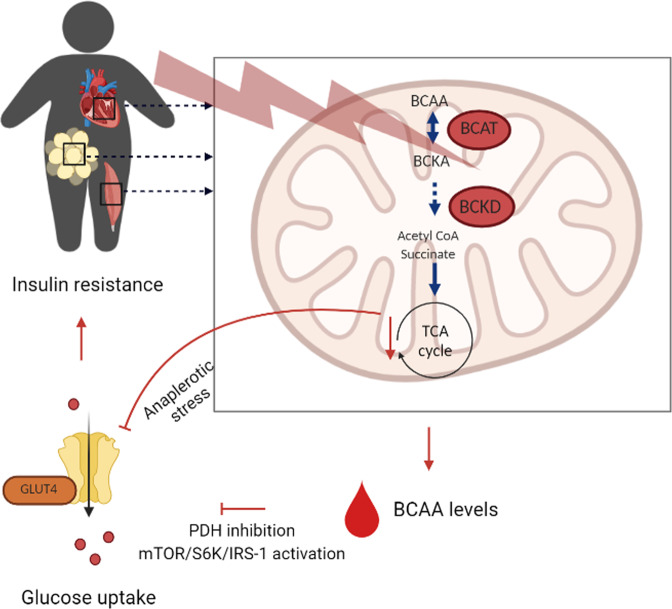
As already mentioned, several reports have been suggested that increased BCAA levels could merely be a consequence of impaired insulin [60, 61], however, evidence indicates that plasma BCAAs act as signaling molecules and contribute to the development of insulin resistance in humans [3, 5, 22, 25, 144–147]. Several mechanisms have been hypothesized explaining how plasma BCAA levels contribute to insulin resistance, which are overviewed in Fig. 2 and discussed in the following paragraphs.
Fig. 2. Schematic overview of mechanisms linking BCAA catabolism with insulin resistance.
BCAA branched-chain amino acids, mTOR mammalian target of rapamycin complex, S6K ribosomal S6 kinase, IRS-1 insulin receptor substrate-1, PDH pyruvate dehydrogenase complex, GLUT4 glucose transporter type 4.
Dysfunctional mitochondrial BCAA catabolism
We as well as others have repeatedly reported that people with insulin resistance and patients with T2D feature low muscle mitochondrial oxidative capacity [13, 148, 149]. The end products of BCAA catabolism inside the mitochondria, succinyl-CoA and acetyl-CoA, enter the TCA cycle and are important anaplerotic substrates fueling the TCA cycle. Defects in BCAA-catabolic enzymes may cause so-called anaplerotic stress and underlie low mitochondrial respiratory rates resulting in disturbed glucose and fat oxidation seen in this population [25, 62], which has been supported by in vitro studies [150–153]. In humans, it has been hypothesized that individuals with impaired or incomplete BCAA metabolism are susceptible to develop insulin resistance [23], in which anaplerotic stress originating from reduced BCAA-derived carbon flux to TCA cycle intermediates is an important underlying factor [23, 138, 154–158]. Additional studies investigating this concept, are however warranted.
Dysfunctional mitochondrial BCAA catabolism may explain the accumulation of a number of BCAA-catabolic metabolites in plasma in insulin-resistant people with obesity or T2D, including BCAA-derived acylcarnitines (C3 and C5), 3-hydroxyisobutyrate (3-HIB) and 2-hydroxbutyric acid (2-HB) and 2-ketobutyric acid (2-KB) [3, 10, 25, 81, 133, 137, 159, 160], which can have toxic effects on cellular function. It has been shown that acylcarnitines can cause mitochondrial dysfunction [3, 23, 47, 133, 161–164]. Furthermore, several studies link defective BCAA catabolism and consequently accumulation of toxic metabolites to increased lipotoxicity [109, 127, 128, 165, 166] and insulin resistance [3, 23, 47, 133, 161–164]. 3-hydroxyisobutyrate (3-HIB), a catabolic intermediate of valine, can exit the mitochondrion via the covalent binding to CoA [146]. Several reports have indicated an elevation of 3-HIB in plasma of people with insulin resistance [146, 167]. In addition, comprehensive metabolic profiling found that 2-HB and 2-KB, both catabolites of methionine/threonine metabolism, are elevated in individuals with reduced insulin sensitivity [168]. Moreover, in individuals with impaired glucose tolerance, plasma levels of 2-HB associate with hyperglycemia and insulin sensitivity and are an early marker for insulin resistance and risk for future T2D [169–171]. Interestingly, since 2-HB can be produced from and converted back into 2-KB, and 2-KB is an BCKD substrate, the increase in these metabolites may reflect impaired BCAA catabolism [172].
To summarize, dysfunctional mitochondrial BCAA catabolism in several tissues may cause anaplerotic stress thereby dysregulating glucose and fat oxidation (Fig. 2). Accumulation of either toxic BCAA-intermediates may exacerbate mitochondrial dysfunction, linked to impaired glucose homeostasis and insulin resistance.
Elevated BCAA levels hamper insulin signaling pathways
mTOR/S6K pathway
Both insulin and BCAA are known to stimulate the activity of mammalian target of rapamycin (mTOR), although the mechanisms for their action is not completely understood [173]. In normal conditions, insulin mediates phosphorylation of IRS-1, which in turn activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway [174]. Akt regulates glucose transport via the phosphorylation of Akt substrate of 160 kDa (AS160) to trigger GLUT4 translocation from intracellular site to the surface of the cell [23, 175–177]. In addition, Akt is able to activate mTOR via phosphorylation of tuberous sclerosis complex 1/2 (TSC 1/2) leading to degradation of Ras homolog enriched in brain (Rheb) [174], which alleviates the inhibition of mTOR [175]. To summarize, insulin is able to activate mTOR via the PI3K-Akt signaling pathway [178].
It has been suggested that increased BCAA levels in plasma or tissue also activate the mTOR pathway, although independently of TSC regulation [179, 180]. Elevated BCAA levels could lead to persistent activation of mTOR followed by serine phosphorylation of IRS-1 via S6 kinase (p70S6K). Phosphorylation of IRS-1 prevents further Akt-signaling leading to diminished glucose transport and consequently insulin resistance [181, 182]. Therefore, chronic accumulation of plasma BCAA levels could impede with the insulin signaling via activation of the mTOR/p70S6K pathway [181–184] with leucine as most potent mTOR activator [180].
BCAA-induced activation of the mTOR/p70S6K pathway has been shown by multiple rodent studies [3, 133, 146, 181, 182, 185, 186] and cell culture experiments [187–189]. In addition, in vivo and in vitro BCAA deprivation in mice reduced the activation of the mTOR pathway and increased pAkt in liver and muscle, resulting in improved insulin sensitivity [190–192]. Interestingly, Newgard et al. reported that dietary BCAA-induced mTOR activation only occurred in the presence of a high fat load [3, 104]. Moreover, mTOR-stimulated pAkt activation in muscle with the consequent development of insulin resistance, solely occurred when BCAA were supplemented in combination with a high-fat diet, and not upon BCAA supplementation combined with chow [3, 104]. Overall, collecting data in preclinical models support the notion that elevated BCAA availability - especially under high fat conditions - plays a key role in the development of insulin resistance, mediated by downregulation of PI3K-Akt signaling pathway and hyperactivation of the mTOR/p70S6K pathway.
Evidence for a role of BCAA in mTOR signaling and insulin resistance in humans is scarce. A short-term infusion of a mixture of amino acids, including BCAA, activated mTOR paralleled by reduced peripheral insulin sensitivity in humans [181, 184]. In addition, Weickert et al. [193] showed that a 6-week high-protein diet enriched with leucine and isoleucine, induced insulin resistance with increased p70S6K levels observed in adipose tissue [193]. Although these results show that BCAA-induced mTOR activation play a role in the development of insulin resistance in humans, normalized BCAA plasma levels which occurred after gastric bypass surgery, did not result in reduced mTOR activation [159], although insulin resistance improved substantially in these patients. The excessive weight loss in the latter study therefore seems to be the driving factor underlying improved insulin sensitivity, and not the change in BCAA plasma levels per se.
Inhibition of PDH
Pyruvate dehydrogenase complex (PDH) is the rate-limiting enzyme involved in glucose oxidation [194], linking glycolysis to the TCA cycle by transferring pyruvate into acetyl-coenzyme A (CoA) [94]. A common manifestation in obese individuals with insulin resistance is the inability to shift from fatty acid oxidation in the fasted state to glucose oxidation in the fed state, also called metabolic inflexibility [195]. This fatty acid-induced suppression of glucose oxidation as well glucose disposal can be explained by the model of Randle et al. [196]: by-products of fatty acid oxidation, such as acetyl-CoA, NADH and ATP, act as potent allosteric inhibitors of glycolysis and PDH [197]. Several studies in animals reported that accumulation of BCAA and its derived metabolites can also directly inhibit PDH activity, at least in liver [153, 198] and heart [94, 152, 199], resulting in a marked decrease in glucose uptake and oxidation. Moreover, animal studies show that dysfunctional BCAA oxidation result in accumulation of BCAA in cardiac tissue and forms a hallmark in cardiovascular disease [95, 200]. A mouse model with impaired BCAA oxidation revealed that the chronic accumulation of BCAA in heart tissue suppressed glucose metabolism [94]. More specifically, high levels of BCAA selectively disrupted mitochondrial pyruvate (end product of glucose oxidation) utilization through inhibition of PDH activity. It has long been established that PDH activity is a key determinant for insulin resistance of the heart [201, 202], in which BCAA may play a pivotal role. This link has not been investigated in humans, however, one study demonstrated that BCAA concentrations accumulate in failing heart tissue as a resultant of a coordinated decrease in BCAA oxidative genes [95], and was associated with impaired cardiac insulin signaling. However, whether BCAA-inhibited PDH activity played a role, was not investigated. In addition, one study showed that supplementing BCAA during exercise as well as during the recovery period resulted in increased plasma glucose levels due to reduced glucose uptake in the leg in the recovery period [203]. The authors suggest that the oxidation of supplemented BCAA resulted in increased BCAA-oxidative derived acetyl-CoA concentrations thereby inhibiting PDH activity, however, the elevated BCAA levels could as well be responsible for reduced pyruvate utilization.
Although there is evidence that elevated BCAA levels hamper insulin signaling pathways, it remains still unclear whether elevated BCAA levels are a cause or rather a consequence of insulin resistance. Future research, specifically cohort studies, could provide more information about causality between BCAA levels and insulin resistance.
Effective strategies to lower BCAA levels
Pharmaceutical strategies
BT2
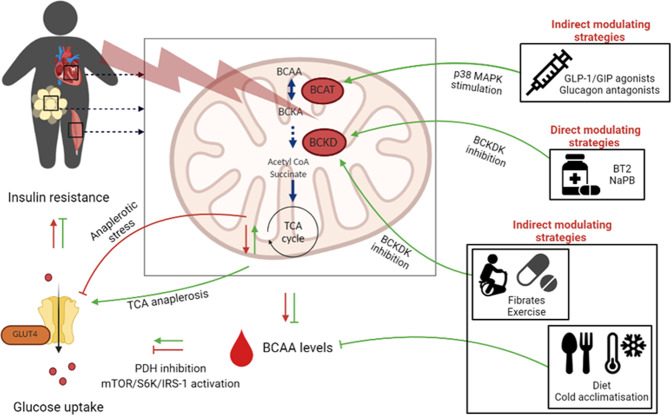
A compound called 3,6-dichlorobenzo(b)thiopene-2-carboxylic acid (BT2) is a small-molecule inhibitor of BCKDK and accelerates the BCAA catabolic pathway via increased activation of the BCKD complex (Fig. 3) [95, 204]. Its working mechanism has been confirmed in obese and diabetes mice models, who report accelerated BCAA catabolism in skeletal muscle [84, 200], liver, heart and adipose tissue [105, 107]. In these models, the administration of BT2 resulted in lower plasma BCAA levels, improved insulin sensitivity and hyperinsulinemia, and reduced hepatic fat levels [105, 107]. Together, these results demonstrate that BT2 is effective to restore BCAA catabolic activity in various tissues alleviating the BCAA catabolic defect, and thus improving insulin sensitivity, irrespective of the site.
Fig. 3. Schematic overview of pharmaceutical and alternative strategies and their hypothesized way of action to boost BCAA oxidation and lower BCAA levels.
BCAA branched-chain amino acids, mTOR mammalian target of rapamycin complex, S6K ribosomal S6 kinase, IRS-1 insulin receptor substrate-1, PDH pyruvate dehydrogenase complex, GLUT4 glucose transporter type 4, BT2 3,6-dichlorobenzo(b)thiopene-2-carboxylic acid, NaPB sodium phenylbutyrate, GLP-1 GCR-like peptide-1, GIP glucose-dependent insulinotropic polypeptide.
Furthermore, several studies administered BT2 in mice with heart failure [110, 205, 206], and collectively show that dysfunctional BCAA catabolism plays a pivotal role in the development of cardiac dysfunction. Results show that BT2-induced accelerated cardiac BCAA catabolism in failing hearts decreased cardiac BCAA levels, with beneficial effects on heart tissue remodeling, improved cardiac insulin sensitivity and function [110, 200, 205, 206]. The mechanisms underlying the cardiometabolic protective effects observed in these studies remain to be elucidated, however, results point out that restoring dysfunctional BCAA catabolism optimizes substrate use and attenuates mitochondrial function [110, 205, 206]. Interestingly, some studies show that the beneficial effects of BT2 on improved glucose metabolism were exerted by reduced mTOR activity and/or via a reduction in the formation of BCAA-derived toxic metabolites [110, 205]. To conclude, BT2 is a pharmacological agent which directly modulate BCAA catabolism via activating BCKD activity. As BT2 is not suitable for human use, so far, effects of pharmacologically modulating BCAA catabolism on the human heart and other tissues, as well on glucose homeostasis has not been investigated in humans.
NaPB
Sodium phenylbutyrate (NaPB) is a commonly used medication for the treatment of patients with urea cycle disorders [207]. NaPB is an aromatic fatty acid that is converted in vivo by β-oxidation into phenylacetate followed by conjugation with glutamine to form phenylacetylglutamine, which is excreted in the urine [208]. Via this mechanism NaPB act as an ammonia scavenger in patients with urea cycle disorders [209]. Interestingly, it has been demonstrated in mice [210] and human cells [208] that NaPB, as BT2, also directly enhance BCAA catabolism through stimulation of the BCKD complex by preventing the phosphorylation of BCKDK (Fig. 3). Holecek et al. [211] showed that in vitro and in vivo administration of NaPB resulted in augmented BCAA catabolism resulting in reduced BCAA levels in plasma and muscle [211]. In another in vitro study in mice, NaPB treatment resulted in lower BCAA concentrations paralleled by improved insulin-stimulated glucose uptake [189, 212] via an improved insulin signaling in skeletal muscle cells [189]. This result was confirmed in a diabetic mouse model showing substantial improved glucose metabolism upon NaPB treatment [213]. These data postulate that NaPB-induced lowering of BCAA levels alleviate the inhibition of insulin signaling leading to an improved glucose uptake, in which skeletal muscle plays an important role.
Although limited research has been performed in humans, some studies show that NaPB lowers BCAA levels in patients with urea cycle disorders, patients with MSUD and healthy subjects [207, 208, 214–217]. In a study with male people with overweight or obesity, NaPB administration was effective in partially improving lipid-induced insulin resistance, although circulating plasma BCAA levels were not measured [218]. As previously done in mouse skeletal muscle cells [189], it would be of interest to study effects of NaPB administration on insulin signaling and glucose uptake in primary human muscle cells, to acquire missing physiological insights on the metabolic consequences of modulating BCAA catabolism in humans.
Fibrates
Fibrate is a class of drugs widely used to treat dyslipidaemia by reducing cholesterol and triglyceride levels, decreasing the risk for the development of cardiovascular diseases [219, 220]. Fibrate mechanism of action includes activation of peroxisome proliferator-activated receptor alpha (PPARα), a transcriptional factor of genes involved in fatty acid oxidation [219, 220]. The major adverse effect of the clinical use of fibrates is the development of myopathy [221–223], however, the pathogenesis of fibrate-induced myopathy is still unclear.
In rodents, several studies showed that fibrate treatment decreased BCAA and BCKA plasma levels [224–226] as well in skeletal muscle and liver tissue [227]. Fibrates inhibit gene expression of the BCKDK in the liver (Fig. 3) [225, 226, 228–231], an effect which was not found in skeletal muscle [228]. This could imply that fibrates enhance BCAA catabolism specifically in the liver.
Interestingly, it has been shown that fibrate treatment improved insulin sensitivity in patients with T2D, although the underlying mechanisms were not investigated [232–234]. Fibrate treatment decreased the activation of the mTOR/p70S6K pathway in rats [226], as well lowered BCAA plasma levels in humans [235]. Whether the fibrate-induced improvement in insulin sensitivity is attributable to improved BCAA catabolism, lower BCAA levels and/or decreased activation of the mTOR-pathway, cannot be deduced from these studies.
Novel T2D therapies targeting incretin and glucagon receptors
In recent years, new therapies targeting receptors including GCR-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and glucagon have been developed. Tirzepatide, a dual GIP and GLP-1 agonist and potential new glucose-lowering medication for patients with T2D, has been shown to improve hyperglycemia [236]. Obese insulin resistant mouse models feature improved glycaemic control in the presence of reduced BCAA and BCKA plasma levels upon Trizepatide treatment [237]. The observed effects were accompanied by an increased expression of BCAT via the p38-MAPK pathway particularly in BAT (Fig. 3) [237]. Interestingly, in humans, Tirzepatide treatment reduced BCAA, BCKA and other BCAA-derived metabolites in plasma, including 3-HIB and 2-HB, previously shown to associate with insulin resistance and T2D [238]. Together, tirzepatide may alter expression of genes regulating BCAA catabolism explaining these results [238]. Also, antagonizing the glucagon receptors has shown to be effective in improving insulin sensitivity in models of diabetes and obesity [239]. In failing heart, inhibition of the glucagon receptor improved insulin-stimulated glucose oxidation and enhanced cardiac function, which were attributable to an improved BCAA catabolism via the p38-MAPK pathway [240]. Although these findings suggests that T2D treatment targeting receptors as GLP-1, GIP and glucagon may activate BCAA catabolism, future studies will be required to investigate if and how activated BCAA catabolism helps to improve glycaemic control upon this treatment in individuals with insulin resistance and T2D.
Alternative strategies
Physical activity and exercise
Generally, it has been assumed that amino acids do not contribute substantially to energy supply during endurance exercise training [241]. In contrast, others suggest that this assumption may underestimate the role of proteins and that endurance exercise may result in promotion of amino acid catabolism in general, and especially the oxidation of BCAA [242]. To provide energy, endurance training promotes the transamination of BCAA to BCKA [75], which are further metabolized into acyl-coenzymes which can enter the TCA cycle [84]. Indeed, it is well established that endurance exercise training in rodents [243] and combined endurance and resistance training in humans with overweight [244] decreased plasma BCAA levels and toxic intermediates of BCAA catabolism, such as acylcarnitines. Consistent with this finding, a recognized effect of endurance exercise training is an accelerated BCAA catabolism represented by an increased BCKD activity [245]. More specifically, it has been found that BCKD is activated due to decreased phosphorylation by BCKD kinase (Fig. 3) [246–250]. Several exercise intervention studies in rats found that BCKD complex was activated in skeletal muscle [78, 251], as well as in liver [248, 249]. The mechanisms responsible for activating these enzymes are not fully understood. One report demonstrated that inactivity potently downregulated expression of BCAA metabolic genes in mice and vice versa that expression of BCAA metabolic enzymes were upregulated in response to endurance exercise training [25]. Contrarily, others suggest that the relative short exercise training sessions, as performed in the beforementioned studies, could not underlie altered gene expression or phosphorylation status of the kinase and that other mechanisms are possibly involved [248, 252].
Recently, we found that levels of BCAA were lower in more active individuals compared to less active individuals [56], which is in line with another observational study showing an association between high physical activity level and low plasma BCAA levels [253]. Nevertheless, 12-week combined endurance and resistance-exercise training in people with obesity did not result in decreased plasma BCAA levels [56]. Although prolonged intense exercise has been shown to increase the activity of the BCKD complex in skeletal muscle of trained, healthy individuals [254], this effect might be blunted in people with insulin resistance. Controversy does exist on the effect of exercise on BCAA catabolism. Howarth et al. [255] showed that a single bout of endurance exercise increased BCKD kinase content in human skeletal muscle, which was associated with a training-induced decrease in BCKD activity, although Poortmans et al. did not find a change in plasma BCAA levels [256]. The inconsistent responses of the different studies could be explained by different work load, duration of physical activity and exercise training, and individuals’ training status. In addition, changes in plasma BCAA levels upon exercise are not a good reflection of BCAA catabolism since exercise influence protein turnover, and therefore also BCAA levels. Exercise training studies combined with stable isotope would elucidate the impact of exercise on BCAA catabolism. The question, however, remains if improved BCAA catabolism is involved in the improvement in metabolic health after physical activity and exercise.
Dietary restriction of BCAA
As mentioned before, diet may contribute to the elevation of BCAA as observed in humans, and therefore diet intervention could potentially help to improve BCAA metabolism. Indeed, it has been shown that restricting dietary BCAA restores metabolic health, including lower adiposity and improved insulin sensitivity in obese rodents [257–259]. The positive metabolic effects were independent of alterations in BCKD activity [260] suggesting that low protein diets restrict plasma BCAA levels thereby alleviating its inhibitory effect on glucose uptake.
In humans, BCAA dietary restriction studies are limited since feasibility is a challenge: interpretation can be limited in case nitrogen and caloric content is different between intervention arms, and therefore any reported effects cannot be asserted as solely due to BCAA restriction. It has been shown, that BCAA levels decreased after a weight loss program, but was not related to changes in BCAA intake [10].
One study reported only modest changes in fasting BCAA levels, associated with an increase in insulin sensitivity upon short-term dietary restriction in healthy individuals [261]. Patients with T2D are characterized by higher plasma BCAA levels compared to healthy controls and therefore probably may benefit more from a BCAA restricted diet. Indeed, short-term dietary reduction of BCAA was effective in decreasing BCAA levels coinciding with improved postprandial insulin sensitivity and gut microbiome composition in patients with T2D [262]. Although, reports showed in vivo and in vitro that lowering BCAA levels alleviates the inhibition of the insulin signaling pathway by decreasing mTOR/S6K1 signaling resulting in increased insulin sensitivity [191, 262], when and how BCAA restriction influences metabolic health, particularly glucose homeostasis, remains unclear. Long-term studies in humans are needed to evaluate the safety and the metabolic efficiency in individuals with obesity and insulin resistance.
Cold acclimatization
Several rodent reports noted that cold exposure significantly decreases plasma BCAA levels, possibly by an increased BCAA uptake and oxidation merely located in BAT [263–266]. Consistent with their findings, it was recently reported that BCAA are actively utilized in BAT mitochondria for UCP1-mediated thermogenesis upon cold exposure in mice [266]. In turn, impaired capacity to take up BCAA and defective BCAA catabolism in BAT results in impaired BCAA clearance and thermogenesis leading to impairments in lipid and glucose metabolism [266, 267]. Thus, besides glucose and fatty acids, BCAA are likely to be important energy substrates in BAT during cold exposure, however, the relationship of BCAA metabolism to thermogenesis is still unclear.
Also in humans, Yoneshiro et al. [266] observed that cold exposure for 2 h preferentially decreased BCAA plasma levels in participants with high BAT activity, suggesting a potential link between BAT and BCAA metabolism. Surprisingly, muscle mass showed no correlation with cold-induced changes in BCAA levels although skeletal muscle is a major organ that utilizes BCAA [266]. Feasibility
To summarize, catabolism and levels of BCAA can be modulated by several pharmaceutical and alternative strategies, although their mechanisms are not completely known in humans. Further research would be needed to study feasibility and optimization for alternative strategies. As a side note, BT2 and NaPB are the only interventions able to directly target the BCAA catabolic defect to improve glucose homeostasis. Other pharmaceutical and alternative interventions, known to improve metabolic health, have also shown to influence BCAA catabolism and levels, however, it has not yet been investigated whether this improved metabolic health is attributable to change in BCAA catabolism and levels.
Conclusion
Dysregulation of BCAA catabolism is closely related to obesity- and T2D related metabolic disturbances since BCAA levels plays a key role in interorgan metabolic crosstalk. Findings from animal and human studies provided evidence that dysfunctional BCAA catabolism in several tissues could be a plausible explanation for the elevated plasma BCAA levels seen in obesity and T2D, however, huge knowledge gaps exist in tissue-specific BCAA catabolism in humans. Insulin resistance can occur via dysfunctional BCAA catabolism or BCAA levels acting as signaling molecules hampering the insulin signaling pathways. Therefore, exploring intervention strategies to increase BCAA oxidation and/or lower BCAA levels is important to investigate whether this could be a new potential strategy in the treatment of metabolic diseases, including obesity and T2D.
Author contributions
FV and EP were responsible for writing the manuscript. EP and PS were responsible for designing the review protocol, and provided feedback on the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
EP was granted with a senior fellowship from the Dutch Diabetes Foundation (Grant no. 2017.82.010), a “VENI” Research Grant for innovative research from the Netherlands Organization for Scientific Research (91613132), and an EFSD/Lilly grant from the European Foundation for the Study of Diabetes (EFSD).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH Global Report on Diabetes. France: WHO; 2016.
- 2.Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:476279. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26.. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–67. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- 6.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–83. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perng W, Gillman MW, Fleisch AF, Michalek RD, Watkins SM, Isganaitis E, et al. Metabolomic profiles and childhood. Obes Obes. 2014;22:2570–8. doi: 10.1002/oby.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer ND, Stevens RD, Antinozzi PA, Anderson A, Bergman RN, Wagenknecht LE, et al. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab. 2015;100:E463–8. doi: 10.1210/jc.2014-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J Clin Endocrinol Metab. 2013;98:E1060–5. doi: 10.1210/jc.2012-4132. [DOI] [PubMed] [Google Scholar]
- 10.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55:321–30. doi: 10.1007/s00125-011-2356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE, et al. Metabolite profiles of diabetes incidence and intervention response in the diabetes prevention program. Diabetes. 2016;65:1424–33.. doi: 10.2337/db15-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wurtz P, Soininen P, Kangas AJ, Ronnemaa T, Lehtimaki T, Kahonen M, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–55. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanweert F, de Ligt M, Hoeks J, Hesselink MKC, Schrauwen P, Phielix E. Elevated plasma branched-chain amino acid levels correlate with type 2 diabetes-related metabolic disturbances. J Clin Endocrinol Metab. 2021;106:e1827–e36.. doi: 10.1210/clinem/dgaa751. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura J, Masaki T, Arakawa M, Seike M, Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J Nutr. 2010;140:496–500. doi: 10.3945/jn.109.108977. [DOI] [PubMed] [Google Scholar]
- 15.Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19:954.. doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gancheva S, Jelenik T, Alvarez-Hernandez E, Roden M. Interorgan metabolic crosstalk in human insulin resistance. Physiol Rev. 2018;98:1371–415.. doi: 10.1152/physrev.00015.2017. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–48.. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamley S, Kloosterman D, Duthie T, Dalla Man C, Visentin R, Mason SA, et al. Mechanisms of hyperinsulinaemia in apparently healthy non-obese young adults: role of insulin secretion, clearance and action and associations with plasma amino acids. Diabetologia. 2019;62:2310–24.. doi: 10.1007/s00125-019-04990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merino J, Leong A, Liu CT, Porneala B, Walford GA, von Grotthuss M, et al. Metabolomics insights into early type 2 diabetes pathogenesis and detection in individuals with normal fasting glucose. Diabetologia. 2018;61:1315–24.. doi: 10.1007/s00125-018-4599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–84. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 23.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eagle H, Piez KA, Fleischman R, Oyama VI. Protein turnover in mammaliar cell cultures. J Biol Chem. 1959;234:592–7. doi: 10.1016/S0021-9258(18)70251-2. [DOI] [PubMed] [Google Scholar]
- 25.Lerin C, Goldfine AB, Boes T, Liu M, Kasif S, Dreyfuss JM, et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol Metab. 2016;5:926–36.. doi: 10.1016/j.molmet.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng S, Wiklund P, Autio R, Borra R, Ojanen X, Xu L, et al. Adipose tissue dysfunction and altered systemic amino acid metabolism are associated with non-alcoholic fatty liver disease. PLoS One. 2015;10:e0138889. doi: 10.1371/journal.pone.0138889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lake AD, Novak P, Shipkova P, Aranibar N, Robertson DG, Reily MD, et al. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids. 2015;47:603–15. doi: 10.1007/s00726-014-1894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meek SE, Persson M, Ford GC, Nair KS. Differential regulation of amino acid exchange and protein dynamics across splanchnic and skeletal muscle beds by insulin in healthy human subjects. Diabetes. 1998;47:1824–35. doi: 10.2337/diabetes.47.12.1824. [DOI] [PubMed] [Google Scholar]
- 29.Flakoll PJ, Kulaylat M, Frexes-Steed M, Hourani H, Brown LL, Hill JO, et al. Amino acids augment insulin’s suppression of whole body proteolysis. Am J Physiol. 1989;257:E839–47. doi: 10.1152/ajpendo.1989.257.6.E839. [DOI] [PubMed] [Google Scholar]
- 30.Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, et al. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985;76:2306–11. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267–78. doi: 10.1007/82_2010_78. [DOI] [PubMed] [Google Scholar]
- 32.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 2015;6:6670. doi: 10.1038/ncomms7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez AM, Candau RB, Bernardi H. FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci. 2014;71:1657–71.. doi: 10.1007/s00018-013-1513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neill BT, Bhardwaj G, Penniman CM, Krumpoch MT, Suarez Beltran PA, Klaus K, et al. FoxO transcription factors are critical regulators of diabetes-related muscle atrophy. Diabetes. 2019;68:556–70.. doi: 10.2337/db18-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59:44–55. doi: 10.1007/s00125-015-3751-0. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–8. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 38.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 39.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–45.. doi: 10.1097/01.ASN.0000127211.86206.E1. [DOI] [PubMed] [Google Scholar]
- 41.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everman S, Meyer C, Tran L, Hoffman N, Carroll CC, Dedmon WL, et al. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans. Am J Physiol Endocrinol Metab. 2016;311:E671–E7.. doi: 10.1152/ajpendo.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffer LJ, Taveroff A, Robitaille L, Hamadeh MJ, Mamer OA. Effects of leucine on whole body leucine, valine, and threonine metabolism in humans. Am J Physiol. 1997;272:E1037–42. doi: 10.1152/ajpendo.1997.272.6.E1037. [DOI] [PubMed] [Google Scholar]
- 44.Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263:E928–34. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- 45.Pozefsky T, Felig P, Tobin JD, Soeldner JS, Cahill GF., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969;48:2273–82. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–54. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adeva MM, Calvino J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids. 2012;43:171–81. doi: 10.1007/s00726-011-1088-7. [DOI] [PubMed] [Google Scholar]
- 48.Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein synthesis. J Clin Invest. 1987;80:1784–93. doi: 10.1172/JCI113272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanetti M, Barazzoni R, Kiwanuka E, Tessari P. Effects of branched-chain-enriched amino acids and insulin on forearm leucine kinetics. Clin Sci. 1999;97:437–48. doi: 10.1042/CS19990163. [DOI] [PubMed] [Google Scholar]
- 50.Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–55. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- 51.Aoki TT, Brennan MF, Muller WA, Soeldner JS, Alpert JS, Saltz SB, et al. Amino acid levels across normal forearm muscle and splanchnic bed after a protein meal. Am J Clin Nutr. 1976;29:340–50. doi: 10.1093/ajcn/29.4.340. [DOI] [PubMed] [Google Scholar]
- 52.Elia M, Livesey G. Effects of ingested steak and infused leucine on forelimb metabolism in man and the fate of the carbon skeletons and amino groups of branched-chain amino acids. Clin Sci. 1983;64:517–26. doi: 10.1042/cs0640517. [DOI] [PubMed] [Google Scholar]
- 53.Hagenfeldt L, Eriksson LS, Wahren J. Amino acids in liver disease. Proc Nutr Soc. 1983;42:497–506. doi: 10.1079/PNS19830056. [DOI] [PubMed] [Google Scholar]
- 54.Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983;25:400–3. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- 55.Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest. 1995;95:2926–37.. doi: 10.1172/JCI118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanweert F, Boone SC, Brouwers B, Mook-Kanamori DO, de Mutsert R, Rosendaal FR, et al. The effect of physical activity level and exercise training on the association between plasma branched-chain amino acids and intrahepatic lipid content in participants with obesity. Int J Obes. 2021;45:1510–20. doi: 10.1038/s41366-021-00815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forlani G, Vannini P, Marchesini G, Zoli M, Ciavarella A, Pisi E. Insulin-dependent metabolism of branched-chain amino acids in obesity. Metabolism. 1984;33:147–50. doi: 10.1016/0026-0495(84)90127-6. [DOI] [PubMed] [Google Scholar]
- 58.Chevalier S, Burgess SC, Malloy CR, Gougeon R, Marliss EB, Morais JA. The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Diabetes. 2006;55:675–81.. doi: 10.2337/diabetes.55.03.06.db05-1117. [DOI] [PubMed] [Google Scholar]
- 59.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–6. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 60.Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jorgensen ME, et al. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia. 2017;60:873–8. doi: 10.1007/s00125-017-4222-6. [DOI] [PubMed] [Google Scholar]
- 61.Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation. Diabetes Care. 2017;40:1779–86.. doi: 10.2337/dc17-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 2016;13:e1002179. doi: 10.1371/journal.pmed.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 64.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jennings A, MacGregor A, Pallister T, Spector T, Cassidy A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: a twin study. Int J Cardiol. 2016;223:992–8. doi: 10.1016/j.ijcard.2016.08.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45:1482–92.. doi: 10.1093/ije/dyw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez AM, Noriega LG, Diaz M, Torres N, Tovar AR. Plasma branched-chain and aromatic amino acid concentration after ingestion of an urban or rural diet in rural Mexican women. BMC Obes. 2015;2:8. doi: 10.1186/s40608-015-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, et al. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:1336–7. doi: 10.1016/j.cell.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 69.Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31:283–93. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 70.Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81.. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 71.Kappel BA, Federici M. Gut microbiome and cardiometabolic risk. Rev Endocr Metab Disord. 2019;20:399–406. doi: 10.1007/s11154-019-09533-9. [DOI] [PubMed] [Google Scholar]
- 72.Holecek M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab. 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. 2019;81:139–64.. doi: 10.1146/annurev-physiol-020518-114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holecek M. Branched-chain amino acids and branched-chain keto acids in hyperammonemic states: metabolism and as supplements. Metabolites. 2020;10:324.. doi: 10.3390/metabo10080324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293:E1552–63. doi: 10.1152/ajpendo.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68:72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- 77.Wynn RM, Kato M, Machius M, Chuang JL, Li J, Tomchick DR, et al. Molecular mechanism for regulation of the human mitochondrial branched-chain alpha-ketoacid dehydrogenase complex by phosphorylation. Structure. 2004;12:2185–96. doi: 10.1016/j.str.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 78.Shimomura Y, Obayashi M, Murakami T, Harris RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care. 2001;4:419–23.. doi: 10.1097/00075197-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 79.Joshi M, Jeoung NH, Popov KM, Harris RA. Identification of a novel PP2C-type mitochondrial phosphatase. Biochem Biophys Res Commun. 2007;356:38–44. doi: 10.1016/j.bbrc.2007.02.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou M, Lu G, Gao C, Wang Y, Sun H. Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm) J Biol Chem. 2012;287:23397–406.. doi: 10.1074/jbc.M112.351031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–81. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chuang JL, Wynn RM, Chuang DT. The C-terminal hinge region of lipoic acid-bearing domain of E2b is essential for domain interaction with branched-chain alpha-keto acid dehydrogenase kinase. J Biol Chem. 2002;277:36905–8. doi: 10.1074/jbc.C200430200. [DOI] [PubMed] [Google Scholar]
- 83.Islam MM, Wallin R, Wynn RM, Conway M, Fujii H, Mobley JA, et al. A novel branched-chain amino acid metabolon. Protein-protein interactions in a supramolecular complex. J Biol Chem. 2007;282:11893–903. doi: 10.1074/jbc.M700198200. [DOI] [PubMed] [Google Scholar]
- 84.Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019;29:417–29 e4. doi: 10.1016/j.cmet.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976;57:987–99. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites. 2013;3:931–45.. doi: 10.3390/metabo3040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Felig P. The glucose-alanine cycle. Metabolism. 1973;22:179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- 88.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284:E855–62. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 89.Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, Bier DM, et al. Glutamine: a major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J Clin Invest. 1995;95:272–7. doi: 10.1172/JCI117651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J Biol Chem. 1992;267:15681–6. doi: 10.1016/S0021-9258(19)49589-6. [DOI] [PubMed] [Google Scholar]
- 91.Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr. 2005;135:1557S–64S. doi: 10.1093/jn/135.6.1557S. [DOI] [PubMed] [Google Scholar]
- 92.Ding C, Li Y, Guo F, Jiang Y, Ying W, Li D, et al. A cell-type-resolved liver proteome. Mol Cell Proteom. 2016;15:3190–202.. doi: 10.1074/mcp.M116.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, Zhou JY, et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20:898–909. doi: 10.1016/j.cmet.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li T, Zhang Z, Kolwicz SC, Jr, Abell L, Roe ND, Kim M, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 2017;25:374–85.. doi: 10.1016/j.cmet.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18:86. doi: 10.1186/s12933-019-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buse MG, Biggers JF, Friderici KH, Buse JF. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972;247:8085–96. doi: 10.1016/S0021-9258(20)81813-4. [DOI] [PubMed] [Google Scholar]
- 97.Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–87. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285:11348–56.. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pietilainen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keranen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5:e51. doi: 10.1371/journal.pmed.0050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leskinen T, Rinnankoski-Tuikka R, Rintala M, Seppanen-Laakso T, Pollanen E, Alen M, et al. Differences in muscle and adipose tissue gene expression and cardio-metabolic risk factors in the members of physical activity discordant twin pairs. PLoS One. 2010;5:e12609.. doi: 10.1371/journal.pone.0012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Klimcakova E, Roussel B, Marquez-Quinones A, Kovacova Z, Kovacikova M, Combes M, et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J Clin Endocrinol Metab. 2011;96:E73–82. doi: 10.1210/jc.2010-1575. [DOI] [PubMed] [Google Scholar]
- 102.Stancakova A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes. 2012;61:1895–902. doi: 10.2337/db11-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Doisaki M, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, et al. Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem Biophys Res Commun. 2010;393:303–7. doi: 10.1016/j.bbrc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011;2:445–56. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68:1730–46.. doi: 10.2337/db18-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–94. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 2018;27:1281. doi: 10.1016/j.cmet.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lian K, Du C, Liu Y, Zhu D, Yan W, Zhang H, et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes. 2015;64:49–59. doi: 10.2337/db14-0312. [DOI] [PubMed] [Google Scholar]
- 109.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–87. doi: 10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation. 2016;133:2038–49. doi: 10.1161/CIRCULATIONAHA.115.020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem Biophys Res Commun. 2008;373:94–8. doi: 10.1016/j.bbrc.2008.05.167. [DOI] [PubMed] [Google Scholar]
- 112.Bajotto G, Murakami T, Nagasaki M, Sato Y, Shimomura Y. Decreased enzyme activity and contents of hepatic branched-chain alpha-keto acid dehydrogenase complex subunits in a rat model for type 2 diabetes mellitus. Metabolism. 2009;58:1489–95. doi: 10.1016/j.metabol.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 113.Biswas D, Duffley L, Pulinilkunnil T. Role of branched-chain amino acid-catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. Faseb J. 2019;33:8711–31.. doi: 10.1096/fj.201802842RR. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, et al. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33:645–51.. doi: 10.2337/dc09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takeuchi Y, Yahagi N, Aita Y, Murayama Y, Sawada Y, Piao X, et al. KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting. Cell Rep. 2016;16:2373–86. doi: 10.1016/j.celrep.2016.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salinas-Rubio D, Tovar AR, Torre-Villalvazo I, Granados-Portillo O, Torres N, Pedraza-Chaverri J, et al. Interaction between leucine and palmitate catabolism in 3T3-L1 adipocytes and primary adipocytes from control and obese rats. J Nutr Biochem. 2018;59:29–36. doi: 10.1016/j.jnutbio.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 117.Hernandez-Alvarez MI, Diaz-Ramos A, Berdasco M, Cobb J, Planet E, Cooper D, et al. Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Sci Rep. 2017;7:13850. doi: 10.1038/s41598-017-14120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sperringer JE, Addington A, Hutson SM. Branched-chain amino acids and brain metabolism. Neurochem Res. 2017;42:1697–709.. doi: 10.1007/s11064-017-2261-5. [DOI] [PubMed] [Google Scholar]
- 119.Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, et al. Gene expression in human NAFLD. Am J Physiol Gastr L. 2008;294:G1281–G7.. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 120.Hirata Y, Nomura K, Senga Y, Okada Y, Kobayashi K, Okamoto S, et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight. 2019;4:e124952.. doi: 10.1172/jci.insight.124952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, et al. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2007;104:7074–9. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL, Thapar D, et al. Multi-tissue, selective PPARgamma modulation of insulin sensitivity and metabolic pathways in obese rats. Am J Physiol Endocrinol Metab. 2011;300:E164–74. doi: 10.1152/ajpendo.00219.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang B, Zhao Y, Harris RA, Crabb DW. Molecular defects in the E1 alpha subunit of the branched-chain alpha-ketoacid dehydrogenase complex that cause maple syrup urine disease. Mol Biol Med. 1991;8:39–47. [PubMed] [Google Scholar]
- 124.Strauss KA, Puffenberger EG, Carson VJ. Maple syrup urine disease. 2006 Jan 30 In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. GeneReviews((R)). Seattle (WA): University of Washington, Seattle; 1993.
- 125.Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, et al. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333–45. doi: 10.1016/j.ymgme.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Homanics GE, Skvorak K, Ferguson C, Watkins S, Paul HS. Production and characterization of murine models of classic and intermediate maple syrup urine disease. BMC Med Genet. 2006;7:33. doi: 10.1186/1471-2350-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oyarzabal A, Martinez-Pardo M, Merinero B, Navarrete R, Desviat LR, Ugarte M, et al. A novel regulatory defect in the branched-chain alpha-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat. 2013;34:355–62. doi: 10.1002/humu.22242. [DOI] [PubMed] [Google Scholar]
- 128.Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–96.. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lefort N, Glancy B, Bowen B, Willis WT, Bailowitz Z, De Filippis EA, et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes. 2010;59:2444–52. doi: 10.2337/db10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arany Z, Neinast M. Branched chain amino acids in metabolic disease. Curr Diab Rep. 2018;18:76. doi: 10.1007/s11892-018-1048-7. [DOI] [PubMed] [Google Scholar]
- 132.Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–14. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steele RD, Weber H, Patterson JI. Characterization of alpha-ketobutyrate metabolism in rat tissues: effects of dietary protein and fasting. J Nutr. 1984;114:701–10. doi: 10.1093/jn/114.4.701. [DOI] [PubMed] [Google Scholar]
- 135.Gillim SE, Paxton R, Cook GA, Harris RA. Activity state of the branched chain alpha-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats. Biochem Biophys Res Commun. 1983;111:74–81. doi: 10.1016/S0006-291X(83)80119-3. [DOI] [PubMed] [Google Scholar]
- 136.Randle PJ. alpha-Ketoacid dehydrogenase complexes and respiratory fuel utilisation in diabetes. Diabetologia. 1985;28:479–84.. doi: 10.1007/BF00281981. [DOI] [PubMed] [Google Scholar]
- 137.She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, et al. Leucine and protein metabolism in obese Zucker rats. PLoS One. 2013;8:e59443. doi: 10.1371/journal.pone.0059443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olson KC, Chen G, Xu Y, Hajnal A, Lynch CJ. Alloisoleucine differentiates the branched-chain aminoacidemia of Zucker and dietary obese rats. Obesity. 2014;22:1212–5. doi: 10.1002/oby.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burgos SA, Chandurkar V, Tsoukas MA, Chevalier S, Morais JA, Lamarche M, et al. Insulin resistance of protein anabolism accompanies that of glucose metabolism in lean, glucose-tolerant offspring of persons with type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4:e000312. doi: 10.1136/bmjdrc-2016-000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luzi L, Castellino P, DeFronzo RA. Insulin and hyperaminoacidemia regulate by a different mechanism leucine turnover and oxidation in obesity. Am J Physiol. 1996;270:E273–81. doi: 10.1152/ajpendo.1996.270.2.E273. [DOI] [PubMed] [Google Scholar]
- 141.Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, AbouAssi H, et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58:2324–35.. doi: 10.1007/s00125-015-3705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matthews DE. Observations of branched-chain amino acid administration in humans. J Nutr. 2005;135:1580S–4S. doi: 10.1093/jn/135.6.1580S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tan HC, Hsu JW, Khoo CM, Tai ES, Yu S, Chacko S, et al. Alterations in branched-chain amino acid kinetics in nonobese but insulin-resistant Asian men. Am J Clin Nutr. 2018;108:1220–8. doi: 10.1093/ajcn/nqy208. [DOI] [PubMed] [Google Scholar]
- 144.Harris LLS, Smith GI, Patterson BW, Ramaswamy RS, Okunade AL, Kelly SC, et al. Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Diabetes. 2017;66:1871–8. doi: 10.2337/db16-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krebs M, Brehm A, Krssak M, Anderwald C, Bernroider E, Nowotny P, et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia. 2003;46:917–25.. doi: 10.1007/s00125-003-1129-1. [DOI] [PubMed] [Google Scholar]
- 146.Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–6. doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, Cao J, Zhang S, Lee AJ, Sun G, Larsen CN, et al. Genetic changes found in a distinct clade of Enterovirus D68 associated with paralysis during the 2014 outbreak. Virus Evol. 2016;2:vew015. doi: 10.1093/ve/vew015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714–21. doi: 10.1007/s00125-010-1764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Phielix E, Jelenik T, Nowotny P, Szendroedi J, Roden M. Reduction of non-esterified fatty acids improves insulin sensitivity and lowers oxidative stress, but fails to restore oxidative capacity in type 2 diabetes: a randomised clinical trial. Diabetologia. 2014;57:572–81. doi: 10.1007/s00125-013-3127-2. [DOI] [PubMed] [Google Scholar]
- 150.Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, et al. alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab Brain Dis. 2005;20:155–67.. doi: 10.1007/s11011-005-4152-8. [DOI] [PubMed] [Google Scholar]
- 151.Funchal C, Latini A, Jacques-Silva MC, Dos Santos AQ, Buzin L, Gottfried C, et al. Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain alpha-keto acids accumulating in maple syrup urine disease. Neurochem Int. 2006;49:640–50. doi: 10.1016/j.neuint.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 152.Jackson RH, Singer TP. Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids. J Biol Chem. 1983;258:1857–65.. doi: 10.1016/S0021-9258(18)33067-9. [DOI] [PubMed] [Google Scholar]
- 153.Walajtys-Rode E, Williamson JR. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. III. Interactions with pyruvate dehydrogenase. J Biol Chem. 1980;255:413–8. doi: 10.1016/S0021-9258(19)86189-6. [DOI] [PubMed] [Google Scholar]
- 154.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–9. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- 156.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 157.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81.. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes. 2013;62:2757–61.. doi: 10.2337/db13-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010;5:e15234. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, et al. Acylcarnitines: potential implications for skeletal muscle insulin resistance. Faseb J. 2015;29:336–45. doi: 10.1096/fj.14-255901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–18. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 163.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 164.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pr Res Clin Endocrinol Metab. 2005;19:471–82. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 165.Lu G, Sun H, Korge P, Koehler CM, Weiss JN, Wang Y. Functional characterization of a mitochondrial Ser/Thr protein phosphatase in cell death regulation. Methods Enzymol. 2009;457:255–73.. doi: 10.1016/S0076-6879(09)05014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikainen LP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44:269–76. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nilsen MS, Jersin RA, Ulvik A, Madsen A, McCann A, Svensson PA, et al. 3-hydroxyisobutyrate, a strong marker of insulin resistance in type 2 diabetes and obesity that modulates white and brown adipocyte metabolism. Diabetes. 2020;69:1903–16. doi: 10.2337/db19-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trico D, Prinsen H, Giannini C, de Graaf R, Juchem C, Li F, et al. Elevated alpha-hydroxybutyrate and branched-chain amino acid levels predict deterioration of glycemic control in adolescents. J Clin Endocrinol Metab. 2017;102:2473–81.. doi: 10.1210/jc.2017-00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. alpha-hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care. 2016;39:988–95. doi: 10.2337/dc15-2752. [DOI] [PubMed] [Google Scholar]
- 170.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–7. doi: 10.2337/db12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Plummer DT, Elliott BA, Cooke KB, Wilkinson JH. Organ specificity and lactate-dehydrogenase activity. 1. The relative activities with pyruvate and 2-oxobutyrate of electrophoretically separated fractions. Biochem J. 1963;87:416–22. doi: 10.1042/bj0870416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nepstad I, Hatfield KJ, Gronningsaeter IS, Aasebo E, Hernandez-Valladares M, Hagen KM, et al. Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct Target Ther. 2019;4:20. doi: 10.1038/s41392-019-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8:405.. doi: 10.3390/nu8070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–9. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006;17:72–8. doi: 10.1016/j.tem.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 177.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295:E29–37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 179.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–51. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 181.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 182.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 183.Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–7. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- 184.Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056–61.. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine-an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jeganathan S, Abdullahi A, Zargar S, Maeda N, Riddell MC, Adegoke OA. Amino acid-induced impairment of insulin sensitivity in healthy and obese rats is reversible. Physiol Rep. 2014;2:e12067.. doi: 10.14814/phy2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101:1519–29.. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhenyukh O, Civantos E, Ruiz-Ortega M, Sanchez MS, Vazquez C, Peiro C, et al. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med. 2017;104:165–77.. doi: 10.1016/j.freeradbiomed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 189.Crossland H, Smith K, Idris I, Phillips BE, Atherton PJ, Wilkinson DJ. Exploring mechanistic links between extracellular branched-chain amino acids and muscle insulin resistance: an in vitro approach. Am J Physiol Cell Physiol. 2020;319:C1151–C7.. doi: 10.1152/ajpcell.00377.2020. [DOI] [PubMed] [Google Scholar]
- 190.Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism. 2014;63:841–50. doi: 10.1016/j.metabol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 191.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–56. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rivera ME, Rivera CN, Vaughan RA. Branched-chain amino acids at supraphysiological but not physiological levels reduce myotube insulin sensitivity. Diabetes Metab Res Rev. 2021;38:e3490.. doi: 10.1002/dmrr.3490. [DOI] [PubMed] [Google Scholar]
- 193.Weickert MO, Roden M, Isken F, Hoffmann D, Nowotny P, Osterhoff M, et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr. 2011;94:459–71. doi: 10.3945/ajcn.110.004374. [DOI] [PubMed] [Google Scholar]
- 194.Lee IK. The role of pyruvate dehydrogenase kinase in diabetes and obesity. Diabetes Metab J. 2014;38:181–6. doi: 10.4093/dmj.2014.38.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–83.. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 196.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. doi: 10.1016/S0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 197.Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15:764–77.. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Williamson JR, Walajtys-Rode E, Coll KE. Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. I. Regulation of branched chain alpha-ketoacid metabolism. J Biol Chem. 1979;254:11511–20.. doi: 10.1016/S0021-9258(19)86514-6. [DOI] [PubMed] [Google Scholar]
- 199.Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93:652–65. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lian K, Guo X, Wang Q, Liu Y, Wang RT, Gao C, et al. PP2Cm overexpression alleviates MI/R injury mediated by a BCAA catabolism defect and oxidative stress in diabetic mice. Eur J Pharm. 2020;866:172796. doi: 10.1016/j.ejphar.2019.172796. [DOI] [PubMed] [Google Scholar]
- 201.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation. 1995;91:2071–9. doi: 10.1161/01.CIR.91.7.2071. [DOI] [PubMed] [Google Scholar]
- 202.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–57. doi: 10.1016/S0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 203.Blomstrand E, Saltin B. BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am J Physiol Endocrinol Metab. 2001;281:E365–74. doi: 10.1152/ajpendo.2001.281.2.E365. [DOI] [PubMed] [Google Scholar]
- 204.Tso SC, Gui WJ, Wu CY, Chuang JL, Qi X, Skvora KJ, et al. Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain alpha-ketoacid dehydrogenase kinase. J Biol Chem. 2014;289:20583–93.. doi: 10.1074/jbc.M114.569251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;311:H1160–H9.. doi: 10.1152/ajpheart.00114.2016. [DOI] [PubMed] [Google Scholar]
- 206.Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc. 2019;8:e011625. doi: 10.1161/JAHA.118.011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Burrage LC, Jain M, Gandolfo L, Lee BH, Nagamani SC. Members of the Urea Cycle Disorders Consortium. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol Genet Metab. 2014;113:131–5. doi: 10.1016/j.ymgme.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, Marini JC, et al. Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet. 2011;20:631–40. doi: 10.1093/hmg/ddq507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res. 1991;29:147–50. doi: 10.1203/00006450-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 210.Tso SC, Qi X, Gui WJ, Chuang JL, Morlock LK, Wallace AL, et al. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain alpha-ketoacid dehydrogenase kinase. Proc Natl Acad Sci USA. 2013;110:9728–33.. doi: 10.1073/pnas.1303220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Holecek M, Vodenicarovova M, Siman P. Acute effects of phenylbutyrate on glutamine, branched-chain amino acid and protein metabolism in skeletal muscles of rats. Int J Exp Pathol. 2017;98:127–33.. doi: 10.1111/iep.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hu H, Li L, Wang C, He H, Mao K, Ma X, et al. 4-Phenylbutyric acid increases GLUT4 gene expression through suppression of HDAC5 but not endoplasmic reticulum stress. Cell Physiol Biochem. 2014;33:1899–910. doi: 10.1159/000362967. [DOI] [PubMed] [Google Scholar]
- 213.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40.. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Darmaun D, Welch S, Rini A, Sager BK, Altomare A, Haymond MW. Phenylbutyrate-induced glutamine depletion in humans: effect on leucine metabolism. Am J Physiol. 1998;274:E801–7. doi: 10.1152/ajpendo.1998.274.5.E801. [DOI] [PubMed] [Google Scholar]
- 215.Scaglia F, Carter S, O’Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81:S79–85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 216.Seminara J, Tuchman M, Krivitzky L, Krischer J, Lee HS, Lemons C, et al. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100:S97–105. doi: 10.1016/j.ymgme.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60:918–24.. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, et al. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–63. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- 220.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension. 2012;60:1517–23.. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hodel C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicol Lett. 2002;128:159–68. doi: 10.1016/S0378-4274(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 222.Tahmaz M, Kumbasar B, Ergen K, Ure U, Karatemiz G, Kazancioglu R. Acute renal failure secondary to fenofibrate monotherapy-induced rhabdomyolysis. Ren Fail. 2007;29:927–30. doi: 10.1080/08860220701573640. [DOI] [PubMed] [Google Scholar]
- 223.Wang D, Wang Y. Fenofibrate monotherapy-induced rhabdomyolysis in a patient with hypothyroidism: a rare case report and literature review. Medicine. 2018;97:e0318. doi: 10.1097/MD.0000000000010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Paul HS, Adibi SA. Paradoxical effects of clofibrate on liver and muscle metabolism in rats. Induction of myotonia and alteration of fatty acid and glucose oxidation. J Clin Invest. 1979;64:405–12. doi: 10.1172/JCI109476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kadota Y, Kazama S, Bajotto G, Kitaura Y, Shimomura Y. Clofibrate-induced reduction of plasma branched-chain amino acid concentrations impairs glucose tolerance in rats. JPEN J Parenter Enter Nutr. 2012;36:337–43. doi: 10.1177/0148607111414578. [DOI] [PubMed] [Google Scholar]
- 226.Ishiguro H, Katano Y, Nakano I, Ishigami M, Hayashi K, Honda T, et al. Clofibrate treatment promotes branched-chain amino acid catabolism and decreases the phosphorylation state of mTOR, eIF4E-BP1, and S6K1 in rat liver. Life Sci. 2006;79:737–43. doi: 10.1016/j.lfs.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 227.Holecek M, Vodenicarovova M. Effects of low and high doses of fenofibrate on protein, amino acid, and energy metabolism in rat. Int J Exp Pathol. 2020;101:171–82.. doi: 10.1111/iep.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Paul HS, Liu WQ, Adibi SA. Alteration in gene expression of branched-chain keto acid dehydrogenase kinase but not in gene expression of its substrate in the liver of clofibrate-treated rats. Biochem J. 1996;317:411–7. doi: 10.1042/bj3170411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kobayashi R, Murakami T, Obayashi M, Nakai N, Jaskiewicz J, Fujiwara Y, et al. Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Arch Biochem Biophys. 2002;407:231–40.. doi: 10.1016/S0003-9861(02)00472-1. [DOI] [PubMed] [Google Scholar]
- 230.Knapik-Czajka M, Gozdzialska A, Jaskiewicz J. Adverse effect of fenofibrate on branched-chain alpha-ketoacid dehydrogenase complex in rat’s liver. Toxicology. 2009;266:1–5. doi: 10.1016/j.tox.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 231.Zhao Y, Jaskiewicz J, Harris RA. Effects of clofibric acid on the activity and activity state of the hepatic branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1992;285:167–72. doi: 10.1042/bj2850167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kobayashi M, Shigeta Y, Hirata Y, Omori Y, Sakamoto N, Nambu S, et al. Improvement of glucose tolerance in NIDDM by clofibrate. Randomized double-blind study. Diabetes Care. 1988;11:495–9. doi: 10.2337/diacare.11.6.495. [DOI] [PubMed] [Google Scholar]
- 233.Murakami K, Nambu S, Koh H, Kobayashi M, Shigeta Y. Clofibrate enhances the affinity of insulin receptors in non-insulin dependent diabetes mellitus. Br J Clin Pharm. 1984;17:89–91. doi: 10.1111/j.1365-2125.1984.tb05005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ferrari C, Frezzati S, Romussi M, Bertazzoni A, Testori GP, Antonini S, et al. Effects of short-term clofibrate administration on glucose tolerance and insulin secretion in patients with chemical diabetes or hypertriglyceridemia. Metabolism. 1977;26:129–39.. doi: 10.1016/0026-0495(77)90048-8. [DOI] [PubMed] [Google Scholar]
- 235.Wolfe BM, Kane JP, Havel RJ, Brewster HP. Mechanism of the hypolipemic effect of clofibrate in postabsorptive man. J Clin Invest. 1973;52:2146–59. doi: 10.1172/JCI107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392:2180–93.. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 237.Samms RJ, Christe ME, Collins KA, Pirro V, Droz BA, Holland AK, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J Clin Invest. 2021;131:e146353.. doi: 10.1172/JCI146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pirro V, Roth KD, Lin Y, Willency JA, Milligan PL, Wilson JM, et al. Effects of tirzepatide, a dual GIP and GLP-1 RA, on lipid and metabolite profiles in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2022;107:363–78. doi: 10.1210/clinem/dgab722. [DOI] [PubMed] [Google Scholar]
- 239.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, et al. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc Diabetol. 2019;18:1. doi: 10.1186/s12933-019-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Consolazio CF, Johnson HL, Nelson RA, Dramise JG, Skala JH. Protein metabolism during intensive physical training in the young adult. Am J Clin Nutr. 1975;28:29–35. doi: 10.1093/ajcn/28.1.29. [DOI] [PubMed] [Google Scholar]
- 242.Rennie MJ. Influence of exercise on protein and amino acid metabolism. Compr Physiol. 2011;25:995–1035. [Google Scholar]
- 243.Xiao W, Chen P, Liu X, Zhao L. The impaired function of macrophages induced by strenuous exercise could not be ameliorated by BCAA supplementation. Nutrients. 2015;7:8645–56.. doi: 10.3390/nu7105425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, AbouAssi H, et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58:2324–35.. doi: 10.1007/s00125-015-3705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fujii H, Shimomura Y, Murakami T, Nakai N, Sato T, Suzuki M, et al. Branched-chain alpha-keto acid dehydrogenase kinase content in rat skeletal muscle is decreased by endurance training. Biochem Mol Biol Int. 1998;44:1211–6. doi: 10.1080/15216549800202302. [DOI] [PubMed] [Google Scholar]
- 246.Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr. 2004;134:1583S–7S. doi: 10.1093/jn/134.6.1583S. [DOI] [PubMed] [Google Scholar]
- 247.Shimomura Y, Fujii H, Suzuki M, Murakami T, Fujitsuka N, Nakai N. Branched-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle: regulation of the activity and gene expression by nutrition and physical exercise. J Nutr. 1995;125:1762S–5S. doi: 10.1093/jn/125.suppl_6.1762S. [DOI] [PubMed] [Google Scholar]
- 248.Xu M, Nagasaki M, Obayashi M, Sato Y, Tamura T, Shimomura Y. Mechanism of activation of branched-chain alpha-keto acid dehydrogenase complex by exercise. Biochem Bioph Res Co. 2001;287:752–6. doi: 10.1006/bbrc.2001.5647. [DOI] [PubMed] [Google Scholar]
- 249.Kobayashi R, Shimomura Y, Murakami T, Nakai N, Otsuka M, Arakawa N, et al. Hepatic branched-chain alpha-keto acid dehydrogenase complex in female rats: activation by exercise and starvation. J Nutr Sci Vitaminol. 1999;45:303–9. doi: 10.3177/jnsv.45.303. [DOI] [PubMed] [Google Scholar]
- 250.Murakami T, Matsuo M, Shimizu A, Shimomura Y. Dissociation of branched-chain alpha-keto acid dehydrogenase kinase (BDK) from branched-chain alpha-keto acid dehydrogenase complex (BCKDC) by BDK inhibitors. J Nutr Sci Vitaminol. 2005;51:48–50. doi: 10.3177/jnsv.51.48. [DOI] [PubMed] [Google Scholar]
- 251.Kasperek GJ, Dohm GL, Snider RD. Activation of branched-chain keto acid dehydrogenase by exercise. Am J Physiol. 1985;248:R166–71. doi: 10.1152/ajpregu.1985.248.2.R166. [DOI] [PubMed] [Google Scholar]
- 252.Shimomura Y, Fujii H, Suzuki M, Fujitsuka N, Naoi M, Sugiyama S, et al. Branched-chain 2-oxo acid dehydrogenase complex activation by tetanic contractions in rat skeletal muscle. Biochim Biophys Acta. 1993;1157:290–6. doi: 10.1016/0304-4165(93)90112-L. [DOI] [PubMed] [Google Scholar]
- 253.Kujala UM, Peltonen M, Laine MK, Kaprio J, Heinonen OJ, Sundvall J, et al. Branched-chain amino acid levels are related with surrogates of disturbed lipid metabolism among older men. Front Med. 2016;3:57. doi: 10.3389/fmed.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wagenmakers AJ, Brookes JH, Coakley JH, Reilly T, Edwards RH. Exercise-induced activation of the branched-chain 2-oxo acid dehydrogenase in human muscle. Eur J Appl Physiol Occup Physiol. 1989;59:159–67. doi: 10.1007/BF02386181. [DOI] [PubMed] [Google Scholar]
- 255.Howarth KR, Burgomaster KA, Phillips SM, Gibala MJ. Exercise training increases branched-chain oxoacid dehydrogenase kinase content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1335–41. doi: 10.1152/ajpregu.00115.2007. [DOI] [PubMed] [Google Scholar]
- 256.Poortmans JR, Siest G, Galteau MM, Houot O. Distribution of plasma amino acids in humans during submaximal prolonged exercise. Eur J Appl Physiol Occup Physiol. 1974;32:143–7. doi: 10.1007/BF00421572. [DOI] [PubMed] [Google Scholar]
- 257.Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596:623–45.. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab. 2016;5:538–51.. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520–30.. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, et al. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2020;318:E216–E23.. doi: 10.1152/ajpendo.00334.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cavallaro NL, Garry J, Shi X, Gerszten RE, Anderson EJ, Walford GA. A pilot, short-term dietary manipulation of branched chain amino acids has modest influence on fasting levels of branched chain amino acids. Food Nutr Res. 2016;60:28592. doi: 10.3402/fnr.v60.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Karusheva Y, Koessler T, Strassburger K, Markgraf D, Mastrototaro L, Jelenik T, et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr. 2019;110:1098–107.. doi: 10.1093/ajcn/nqz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–53. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 264.Chondronikola M, Volpi E, Borsheim E, Chao T, Porter C, Annamalai P, et al. Brown adipose tissue is linked to a distinct thermoregulatory response to mild cold in people. Front Physiol. 2016;7:129. doi: 10.3389/fphys.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lopez-Soriano FJ, Fernandez-Lopez JA, Mampel T, Villarroya F, Iglesias R, Alemany M. Amino acid and glucose uptake by rat brown adipose tissue. Effect of cold-exposure and acclimation. Biochem J. 1988;252:843–9. doi: 10.1042/bj2520843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614–9. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cannavino J, Shao M, An YA, Bezprozvannaya S, Chen S, Kim J, et al. Regulation of cold-induced thermogenesis by the RNA binding protein FAM195A. Proc Natl Acad Sci USA. 2021;118:e2104650118.. doi: 10.1073/pnas.2104650118. [DOI] [PMC free article] [PubMed] [Google Scholar]