Summary
Ailanthus Desf. (Simaroubaceae), now widespread in southern Asia to northern Australia, was widely distributed in the Northern Hemisphere during the Cenozoic, but has few fossil records at low latitudes. Here we report the fossil samaras of Ailanthus confucii Unger from South China and its occurrences indicate that this genus has been distributed in low latitude regions since the middle Eocene. According to the recent fossil records, Ailanthus is considered to have originated from the Indian subcontinent and dispersed rapidly to East Asia and western North America following the early Paleogene onset of the India-Eurasia collision. In the Eocene, Ailanthus became widespread across the Northern Hemisphere. Subsequent to global cooling, Ailanthus gradually disappeared in the mid-high latitudes and may have continued to spread southward from Asia to northern Australia following the Asia-Australia collision in the late Oligocene, thus forming its modern distribution pattern.
Subject areas: Biological sciences, Natural sciences, Paleobiology, Plant biology, Plant evolution
Graphical abstract

Highlights
-
•
Fossil Ailanthus confucii are described from the Eocene and Oligocene of South China
-
•
The genus Ailanthus has been distributed in South China since the middle Eocene
-
•
New fossil records still support the origin of this genus in the Indian subcontinent
-
•
Post-late Oligocene Asia-Australia collision promoted its modern distribution pattern
Biological sciences; Natural sciences; Paleobiology; Plant biology; Plant evolution
Introduction
The family Simaroubaceae sensu stricto is a mostly pantropical group of woody plants consisting of 22 genera and approximately 120 species (Devecchi et al., 2022; Pirani et al., 2021). Among them, genus Ailanthus Desf. has attracted much attention because of its significance in Chinese and western cultures, horticultural uses, and medicinal value (Hu, 1979; Sladonja et al., 2015). Ailanthus is traditionally placed among the basal clade of the Simaroubaceae family, a position supported by molecular phylogeny and biochemical data (Simao et al., 1991; Fernando et al., 1995; Chase et al., 1999; Clayton et al., 2009). This genus encompasses six extant species of deciduous or evergreen trees naturally distributed in Asia to northern Oceania, with the modern distribution center being in Southeast Asia, i.e. A.altissima (Mill.) Swingle, A.excelsa Roxb., A.fordii Noot., A.integrifolia Lam., A. vietnamensis Sam et Nooteboom, and A.triphysa (Dennst.) Alston (Nooteboom, 1962; Van Sam and Nooteboom, 2007; Song and Xu, 2014; Liu et al., 2019). Among them, A. altissima (Mill.) Swingle and A. fordii Noot. are endemic in China. The temperate species, A. altissima (chu-mu or tree of heaven), is now widely cultivated and also an invasive species in North America and Europe (Corbett and Manchester, 2004).
Ailanthus is widely recorded in Cenozoic deposits across the Northern Hemisphere (Corbett and Manchester, 2004; Su et al., 2013; Song et al., 2014; Liu et al., 2019; Yang et al., 2021). Samaroid mericarps of Ailanthus are easily recognized in the fossil record based on their morphological characters, which include a samara with a seed situated in the center, as well as the main ventral vein and style scar being on the same side of the seed (Nooteboom, 1962; Corbett and Manchester, 2004). By analyzing the samara morphology, Corbett and Manchester (2004) assigned all previously described Ailanthus samaras to three fossil species, A. confucii Unger, A.tardensis Hably, and A.gigas Unger. Later, Song et al. (2014) reassigned A. gigas to the species A. confucii, and Liu et al. (2019) recognized a new fossil species, A.maximus Liu, Su et Zhou. Currently, all these fossil species are known in China. A. tardensis was reported from the Oligocene of Ningming, Guangxi Province (Corbett and Manchester, 2004; Song et al., 2014), and A. maximus was described from the middle Eocene of Jianglang and upper Eocene of Dayu and Nima, central Tibetan Plateau (Liu et al., 2019; Su et al., 2020; Xiong et al., 2022). A. confucii is the most widely distributed species known from the lower-middle Eocene of Fushun, Liaoning Province (WGCPC, 1978; Corbett and Manchester, 2004), the lower Oligocene of Wenshan, Yunnan Province (Su et al., 2013; Tian et al., 2021), the Qaidam Basin, northeastern Qinghai-Tibetan Plateau, Qinghai Province (Yang et al., 2021), the Oligocene of Ningming, Guangxi Province (Song et al., 2014), and the Miocene of Linqi (WGCPC, 1978; Corbett and Manchester, 2004) and Shanwang, Shandong Province (Tanai and Suzuki, 1963; Sun et al., 1999; Corbett and Manchester, 2004). Despite abundant Ailanthus fossils at the middle latitudes of the Northern Hemisphere, there are still only a few fossil records from low-latitude areas. Thus, understanding the distribution of this genus at low latitudes in the geological past is critical for understanding its phytogeographic history and the formation of its modern distribution pattern.
Here, fossil Ailanthus samaras from the middle Eocene of the Changchang Basin, Hainan Island, and the lower Oligocene of the Maoming Basin, South China, are described and compared with related species, and the paleogeographic history of the genus is reviewed and revised. Moreover, by comparing the timing of geological events and occurrences of fossil records, we infer the formation process of the modern distribution pattern of the genus.
Results
Systematic paleontology
Family: Simaroubaceae de Candolle, 1811.
Genus: Ailanthus Desfontaines, 1788
Species: A. confucii Unger
The detailed synonomy of A. confucii is in Corbett and Manchester (2004, p. 677) and Table S1.
Specimens examined: CC916, MM3A-163a, b, MM3A-537a, b, MM3A-538, MMLS-321.
Localities and ages: Jiazi Town, Qiongshan City, Hainan Island, China, middle Eocene; Lishan Village, Maoming City, Guangdong, China, early Oligocene.
Repository: Museum of Biology, Sun Yat-sen University.
Description: Samaroid mericarps are elongated and elliptic, with a single centrally positioned seed surrounded by a narrowly elliptic wing that tapers apically and basally (Figures 1B, 1E, and 1F). The apex and the base of samaras are acute, sometimes with a twist of the wing lamina near the distal tip. The samaras are 49–55 mm long, and 9–11 mm wide (Figures 1B–1F). Seeds are nearly round to ovate with a blunt tip, about 5–7 mm in diameter (Figures 1A–1F). One side of the samara is emarginate at the middle level of the seed, where the stylar scar is situated, while the other side of the margin is flat (Figures 1A–1F). A short, thick vein connects the stylar scar and the seed (Figures 1A–1C, 1E, and 1F). A number of nearly parallel veins could be observed on the wing, with about 17–19 subparallel veins extending from the margin of the seed to the two ends, some of them interconnecting to form a stretched network (Figures 1A–1C, 1E, and 1F). A marginal ventral vein extends from the base of the samara to the seed (Figures 1A–B, 1E, and 1F).
Figure 1.
Samaras morphology of fossil and extant species
Samaras of Ailanthus confucii from Changchang (A) and Shangcun formations (B–C, E–F) and extant species (D, G–K), showing the stylar scar (S) and the ventral vein (V).
(A) CC916.
(B) MM3A-538.
(C) Line drawing of MM3A-538.
(D) A. altissima, IBSC0393098.
(E) MM3A-537a.
(F) MM3A-537b.
(G) A. excelsa, E00179790.
(H) A. triphysa, IBSC0393450.
(I) A. integrifolia, L0017722.
(J) A. fordii, PE01381352.
(K) A. vietnamensis from Van Sam and Nooteboom (2007). Scale bars are 3mm in A, 1cm in B–F, H and J, 2cm in G, I and K.
Discussion
Comparison with extant and fossil species
The fossil fruits from South China are the samaroid mericarps with a centrally located single seed and stylar scar with main ventral veins on one side of the samara. These characteristics are unique features of the genus Ailanthus, which make it easily distinguishable from the winged fruits of other plants (Su et al., 2013; Liu et al., 2019; Yang et al., 2021).
Ailanthus comprises six extant species, distributed in South and Southeast Asia as well as northern Australia (Nooteboom, 1962; Van Sam and Nooteboom, 2007; Su et al., 2013; Song and Xu, 2014). These extant species have some characteristics in common with the fossils we describe here, including an elongated and elliptic samara with a seed situated in the center, stylar scar that is emarginate at the middle part of the samara, a short and thick vein connecting the stylar scar and the seed, and one thickened ventral vein located on one side of the samara extending from the base of samara to the seed. However, the fossils we describe differ from all extant species (Table 1, table data mainly according to Hably, 2001; Corbett and Manchester, 2004; Van Sam and Nooteboom, 2007; Su et al., 2013; Song et al., 2014; Liu et al., 2019).
Table 1.
Morphological comparison of the Ailanthus confucii from Changchang and Shangcun formations with fossil and extant Ailanthus species
| Species | Length of samara (mm) | Ventral vein | Position of stylar scar |
|---|---|---|---|
| This study | 49–55 | Marginal | Middle of seed |
| Extant species | |||
| Ailanthusaltissima (Mill) Swingle | 40–50 | Marginal | Middle of seed |
| A. excelsa Roxburghii | 25 | Intramarginal | Apex of seed |
| A. fordii Nooteboom | 30–50 | Intramarginal | Distal to seed |
| A. integrifolia Lamarck | 110–220 | Marginal | Base of seed |
| A. triphysa (Dennstedt) Alston | 45–80 | Intramarginal | Middle of seed |
| A. vietnamensis Sam et Nooteboom | 70–100 | Intramarginal | Base of seed |
| Fossil species | |||
| A. confucii Unger | 16–57 | Marginal | Middle of seed |
| A. tardensis Hably | 35–41 | Intramarginal | Top of seed |
| A. maximus Liu, Su et Zhou | 60 | Intramarginal | Middle of seed |
The fossil samaras from South China can be clearly distinguished from those of extant Ailanthusexcelsa Roxburghii, A. integrifolia Lamarck, and A.vietnamensis Sam et Nooteboom in terms of samara length. The current fossils are 49–55 mm long (except for the incomplete specimen CC916), whereas samaras of A. excelsa are shorter (approximately 25mm in length), and samaras of A. integrifolia and A. vietnamensis are longer (approximately 110–220 mm and 70–100 mm in length, respectively). In addition, the main ventral vein of fossil samaras is strictly located on the margin of the samara, while the ventral veins of A. excelsa, A. fordii Nooteboom, A. triphysa (Dennstedt) Alston, and A. vietnamensis are located on intramarginal part of the samara (Figures 1G, 1H, 1J, and 1K).
The position of the stylar scar is an important identifying character of the Ailanthus samara, and this feature of current fossils is obviously different in several extant species. The stylar scar is at the same level as the middle part of the seed in the fossils described here, while the stylar scars of A. excelsa and A. fordii are at the top of the seed, and at the base of the seed in A. integrifolia and A. vietnamensis (Figures 1G–1I and 1K).
In general, the fossil species we describe is most similar to Ailanthusaltissima (Mill) Swingle. The ventral veins of both species are located on the margin of the samara and the stylar scars are at the same level as the middle part of the seed. Although the samaras of the studied fossil species and A. altissima overlap in length, those of the extant species differ in having obtuse bases and apices in contrast to acute bases and apices in the fossil species. Moreover, the fossil species differ in the samara length/width ratio (4.2–4.6 in A. confucii vs. 3.2–3.9 in A. altissima) and the seed diameter (5–8 mm in A. confucii vs. 3–5 mm in A. altissima). Therefore, the current fossils cannot be assigned to any extant species (Figures 1D and 1G–1K).
Two of three known fossil species of the genus, Ailanthustardensis and A. maximus, also differ from the studied fossil species. The current fossil species is obviously different from A. tardensis in the location of the main ventral vein, which runs along the margin in our fossils, whereas it is intramarginal in A. tardensis. The position of the stylar scar in fossil samaras from South China is at the same level as the middle part of the seed, whereas it is at the same level as the top of the seed in A. tardensis (Corbett and Manchester, 2004; Song et al., 2014). Fossil samaras reported here are also easily distinguished from A. maximus in terms of the length of the samara and position of the main ventral vein. Samaras of A. maximus are the largest Ailanthus samara fossils found so far (about 60 mm long), and their main ventral vein is located in the intramarginal part of the samara (Liu et al., 2019). The samaras of fossil species A. confucii are characterized by the stylar scar located at the same level as the middle part of the seed, the main ventral vein running along the margin, acute samara bases, and apices, and with an overall length varying from 16mm to 57mm. The morphological characteristics of the studied fossils are very similar to those of A. confucii, so we assign the fossil samaras from the Changchang and Maoming basins to this species.
Phytogeographical implication
Based on the then-available fossil records of Ailanthus and its modern distribution, Corbett and Manchester (2004) proposed two hypotheses for the biogeographical origins of the genus. Ailanthus may have originated and become widespread in the Northern Hemisphere, and later moved southward to the Southern Hemisphere. Alternatively, Ailanthus may have arisen in the Southern Hemisphere and subsequently migrated into the Northern Hemisphere. However, the hypothesis of the southern origin lacks support from the fossil record. The oldest known fossils of Ailanthus come from the lower-middle Eocene of Fushun, northern China (WGCPC, 1978) and the lower Eocene of the Green River Formation in Wyoming, USA (Grande, 1984). Throughout the Eocene, this genus spread across Asia, Europe, and North America (MacGinitie, 1969; Grande, 1984; Collinson, 1988; Manchester, 1990; Akhmetiev, 1993; Fields, 1996; Wilf, 2000). From the Oligocene to Miocene, Ailanthus became distributed widely on all continents in the Northern Hemisphere (Tanai and Suzuki, 1963; WGCPC, 1978; Zhilin, 1967; Mai, 1995; He and Tao, 1997; Meyer and Manchester, 1997; Manchester, 2001; Teodoridis et al., 2015; Martinetto and Macaluso, 2018; Liu et al., 2019; Yang et al., 2021). Hence, it seems from this evidence that the genus originated in western North America or eastern Asia during the early Eocene and subsequently diversified in Europe and East Asia (Figure 2).
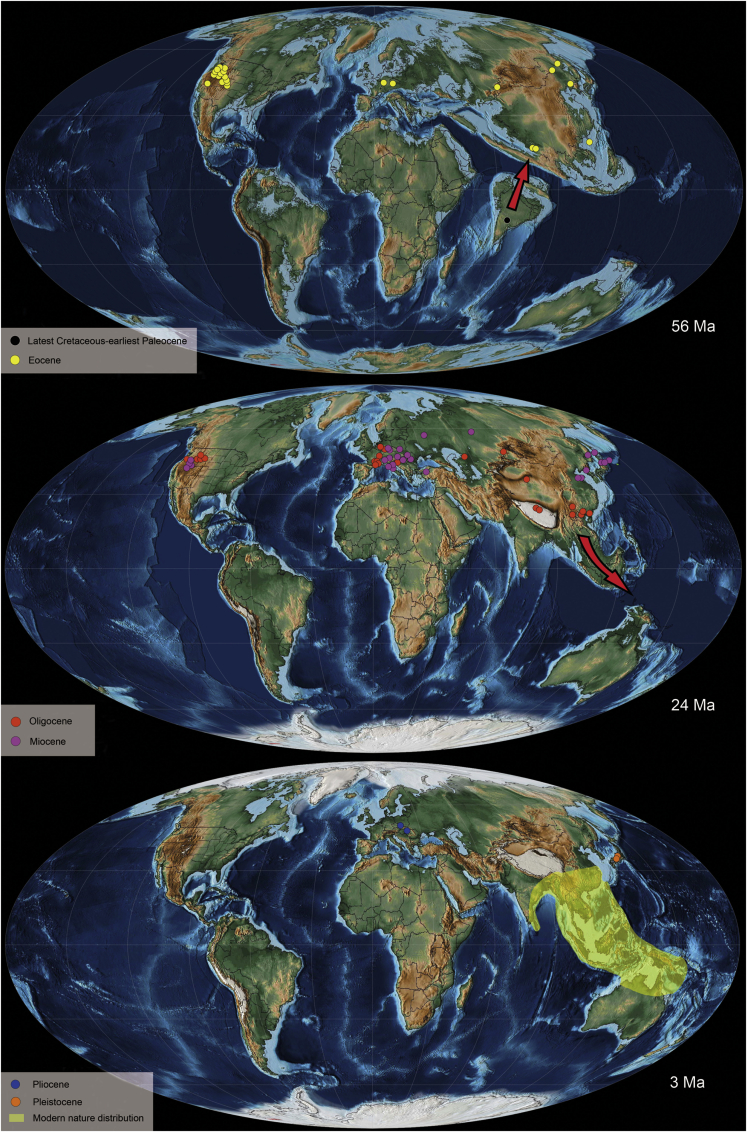
Figure 2.
Paleogeographical maps showing the fossil records and modern distribution of Ailanthus
The red arrows indicate the hypothetical routes of Ailanthus dispersal from the Indian subcontinent and the formation of its modern distribution. The paleogeography map is a Mollweide view and shows the landforms of 56 Ma, 24 Ma, and 3 Ma, respectively.
Recent re-identification of Ailanthoxylon wood from the uppermost Cretaceous-lowermost Paleocene of the Deccan Intertrappean Beds, India, revealed that the combination of characteristics exhibited in this wood is unique to Ailanthus, and these Ailanthus-type wood fossils are the oldest known occurrences of Simaroubaceae (Prasad et al., 2007; Wheeler et al., 2017). Phylogenetic analysis of the family based on molecular data revealed that Ailanthus is a monophyletic group that differentiated during the Late Cretaceous (Clayton et al., 2009), a result consistent with this wood fossil occurrence.
With the re-identification of the Indian Deccan wood fossils (Wheeler et al., 2017) and the subsequent discovery of Ailanthus samaras from the supposedly uppermost Paleocene-lower Eocene of Jianglang, central Tibetan Plateau (Liu et al., 2019), the existing fossil records seemed to undermine support for a Northern Hemisphere origin of this genus. Liu et al. (2019) supposed that Ailanthus most likely had a Gondwanan origin, and dispersed to central Tibet following the early Paleogene onset of the India-Eurasia collision and that during the early Eocene Ailanthus may have spread from Tibetan Plateau to Northeast Asia, and then to North America via the Bering Land Bridge. It was further supposed that in the middle Eocene this genus dispersed to Europe via central Asia and became widespread in the Northern Hemisphere after the Eocene (Liu et al., 2019).
Following further work in the central Tibetan Plateau, the Jianglang and Dayu assemblages are now confirmed to be Lutetian-Bartonian (middle Eocene) and late Eocene, respectively, based on U-Pb dating of detrital zircons and primary ash beds (Su et al., 2020; Xiong et al., 2022). According to these existing, but re-dated fossil records, it is possible that Ailanthus may still be of Gondwanan origin and moved between Northeast China and North America through the Bering Land Bridge during the early Eocene, before becoming widely distributed in East Asia and western North America during the middle Eocene. In the late Eocene, a possible spread to Europe was through the North Atlantic Bridge or Central Asia, but its specific migration pathway still needs to be confirmed by new fossil discoveries. Newly discovered occurrences of Ailanthus samaroid mericarps in the Changchang Formation (Hainan Island), the Shangcun Formation of Guangdong (this study), and earlier fossil occurrences from the Xiaolongtan Formation of Yunnan (Su et al., 2013) and the Ningming Formation of Guangxi (Song et al., 2014), indicate that Ailanthus had arrived in South China at least by the middle Eocene, and became widely distributed there during the early Oligocene (Figure 2). Overall, understanding the distribution of this genus at low-latitudes of southern regions of Asia in the geological past is crucial for inferring the biogeographic origin of Ailanthus and its dispersal routes as well as further verification of one of the existing hypotheses of the genus origin (Corbett and Manchester, 2004; Liu et al., 2019).
How did the modern natural distribution pattern of the genus Ailanthus form? During the Eocene, Ailanthus was present in Asia, North America, and Europe and so was an almost cosmopolitan samara species represented by A. confucii and the Asian species A. maximus (Table S1). In the Oligocene, diversity increased with two additional fossil species: the samara species A. tardensis and the leaflet species A.ailanthifolia (Web.) Weyland (Table S1). A. tardensis appeared in the Oligocene of South China and Europe, probably because of the shrinking of the Turgai Strait during the Oligocene affording increased floristic exchanges between the two continents (Song et al., 2014; Liu et al., 2019). However, owing to progressive global cooling, the distribution range of Ailanthus gradually decreased in the higher latitudes and the genus became extinct in North America and Europe after the middle Miocene and Pliocene, respectively (Table S1). In Asia, this genus continued to flourish and diversify in middle and low latitudes during the Pleistocene (Table S1). Simultaneously, the continuous island chain formed by the accelerated collision of the Asian plate and Australian plates after the late Oligocene promoted floristic exchange between the two continents (Hall, 2009; Sniderman and Jordan, 2011; Wu et al., 2019a), which would also have provided an opportunity for Ailanthus to continue to spread from southern China to Southeast Asia and Australasia, and so form the modern distribution pattern (Figure 2).
Limitations of the study
The majority of paleobotanical research are subject to some limitations related to the objects of study. The first limitation is the preservation quality of plant macrofossils. The second limitation concerns the representativeness of data. Both of these factors may influence more accurate taxonomic identification of plant fossils, the assessment of morphological variability of different plant organs, the possibility of whole-plant reconstruction, and, eventually, paleoclimatic and paleogeographic reconstructions.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Ailanthus confucii fossil specimens | Museum of Biology, Sun Yat-sen University, Guangzhou | CC916; MM3A-163a, b; MM3A-537a, b; MM3A-538; MMLS-321 |
| A. altissima | South China Botanical Garden, Chinese Academy of Science | IBSC0393098 |
| A. excelsa | The Royal Botanic Garden Edinburgh | E00179790 |
| A. triphysa | South China Botanical Garden, Chinese Academy of Science | IBSC0393450 |
| A. integrifolia | Naturalis Biodiversity Centre, Leiden, Netherlands | L0017722 |
| A. fordii | The Botanical Garden, Institute of Botany, CAS | PE01381352 |
| Software and algorithms | ||
| Post-processing of images and colour markings were performed with Adobe Photoshop 2020 | Adobe Inc. | RRID:SCR_014199, URL: https://www.adobe.com/products/photoshop.html |
| DigiCamControl-Free Windows DSLR camera controlling solution | Duka, 2015 | http://digicamcontrol.com/ |
| Helicon Focus | Helicon Inc. | RRID:SCR_014462, URL: http://www.heliconsoft.com/heliconsoft-products/helicon-focus/ |
| PaleoDataPlotter for GPlates | Scotese, 2016 | http://www.earthbyte.org/paleomap-paleoatlas-for-gplates/ |
Resource availability
Lead contact
Further questions should be directed to the lead contact, Jianhua Jin (lssjjh@mail.sysu.edu.cn).
Materials availability
Specimens CC916, MM3A-163(a, b), MM3A-537(a, b), MM3A-538, and MMLS-321 are deposited in the Museum of Biology, Sun Yat-sen University, Guangzhou, China. The fossil records used in phytogeographic analyses is available from supplemental information.
Experimental model and subject details
Plants
All specimens used here were obtained as herbarium specimen from the source organizations listed in the key resources table.
Method details
Geological setting
The Ailanthus samara fossils described in this paper were collected from the Changchang Basin of Hainan Island and the Maoming Basin of Guangdong, South China (see Figure S1). Only a fragment of samaroid mericarp was obtained from the middle Eocene Changchang Formation in the Changchang Basin on northeast Hainan Island, near Jiazi Town, Qiongshan City (19°38′N, 110°27′E). The Changchang Formation consists of predominantly lacustrine gray and brown mudstones and siltstones interbedded with alluvial sandstones, along with coaly shales with thin coal seams formed in lacustrine environments (Jin et al., 2002). Well-preserved palynomorphs and plant megafossils including leaves, flowers, fruits, seeds, and wood are abundant in the lower part of this formation (Lei et al., 1992; Yao et al., 2009; Spicer et al., 2014, 2017; Hofmann et al., 2019). The age of the Changchang Formation is middle Eocene (Lutetian-Bartonian) based on the palynological data and plant assemblages (Spicer et al., 2014).
Another four samaroid mericarps of Ailanthus (parts and counterparts) were collected from the Shangcun Formation of the Maoming Basin, southwest Guangdong Province. Fossiliferous horizons were located in the Lishan opencast mine (21°50′N; 110°46′E), about 25 km northwest of Maoming City. Here the Shangcun Formation consists mainly of gray-green to gray-white mudstones, shales, argillaceous siltstones and dark brown oil shales intercalated with calcareous siltstones and lignites (BGMRGD, 1988). This formation contains gastropods, fish, and abundant well-preserved plant fossils representing the families Osmundaceae, Polypodiaceae, Pinaceae, Cupressaceae, Platanaceae, Lauraceae, Fagaceae, Malvaceae, Juglandaceae, Myricaceae, Simaroubaceae, and Palmae (Herman et al., 2017; Kodrul et al., 2018; Wu et al., 2019b; Xu et al., 2021). The age of the Shangcun Formation has been assigned to the early Oligocene based on palynological and magnetostratigraphic studies (Wang et al., 1994; Herman et al., 2017).
Specimens imaging and terminology
Plant megafossils and herbarium specimens were observed using a Zeiss Stereo Discovery V20 stereomicroscope (AxioCam HRc; Carl Zeiss, Göttingen, Germany) in the Museum of Biology, Sun Yat-sen University (Guangzhou, China) and photographed using Canon EOS 500D digital camera. Images were processed with DigiCamControl-Free Windows DSLR camera controlling solution (Duka, 2015) and Helicon Focus 6.6.1 (Helicon Soft Ltd., Kharkov, Ukraine). Figures were made using Photoshop 2020 (Adobe, San Jose, California, USA) and PALEOMAP PaleoAtlas for GPlates (Scotese, 2016). All the specimens are housed in the Museum of Biology, Sun Yat-sen University, China. Specimens of living species of Ailanthus were studied in the herbaria of South China Botanical Garden, Chinese Academy of Sciences (SCBC) and Sun Yat-sen University (SYS). The terminology of Ailanthus samaras follows Corbett and Manchester (2004).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 41872015, 32100172, 42111530024), State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (Grant No. 193118), the Russian Foundation for Basic Research (RFBR, Grant No. 21-54-53001 for NM), and the State program (No. 0135-2019-0045, Geological Institute, Russian Academy of Sci. for TK). The authors are thankful to Robert Spicer from Open University, UK for his helpful comments and language help. We also express sincere gratitude to graduate students majoring in botany at Sun Yat-sen University for their assistance in field work. We greatly thank Jia Liu from Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (XTBG) for his helpful information. We are grateful to the staff of Sun Yat-sen University Herbarium, and the Herbarium of the South China Botanical Garden for their permission to examine extant specimens. Our deepest gratitude goes to the editor and reviewers for their careful work and thoughtful suggestions.
Author contributions
X.W., N.M., T.K., and J.J. conceived and designed the project and photographed the material. J.J. organized field work and led the data acquisition. X.W., T.K., and N.M. prepared and imaged fossil and modern specimens. X.W., Y.W., and J.J. contributed to the initial article preparation. All authors discussed the results, read, and approved the final article.
Declaration of interests
The authors declare no conflict of interest.
Published: August 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104757.
Supporting citations
The following reference appears in the supplemental information: Xu et al., 2015.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
No novel code was used in this study.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Akhmetiev M.A. Phytostratigraphy of Paleogene and Miocene continental deposits of boreal Asia. Trans. Geol. Inst. Russ. Acad. Sci. 1993;475:1–142. in Russian. [Google Scholar]
- BGMRGD (Bureau of Geology and Mineral Resources of Guangdong Province) Geological Publishing House; 1988. Regional Geology of the Guangdong Province; p. 971. in Chinese with English abstract. [Google Scholar]
- Chase M.W., Morton C.M., Kallunki J.A. Evaluation of the six subfamilies of Rutaceae using evidence from rbcL and atpB sequence variation. Am. J. Bot. 1999;86:1191–1199. [PubMed] [Google Scholar]
- Clayton J.W., Soltis P.S., Soltis D.E. Recent long-distance dispersal overshadows ancient biogeographical patterns in a pantropical angiosperm family (Simaroubaceae, Sapindales) Syst. Biol. 2009;58:395–410. doi: 10.1093/sysbio/syp041. [DOI] [PubMed] [Google Scholar]
- Collinson M.E. The special significance of the middle Eocene fruit and seed flora from Messel, Germany. Cour. Forsch. Senckenberg. 1988;107:187–197. [Google Scholar]
- Corbett S.L., Manchester S.R. Phytogeography and fossil history of Ailanthus (Simaroubaceae) Int. J. Plant Sci. 2004;165:671–690. doi: 10.1086/386378. [DOI] [Google Scholar]
- Devecchi M.F., Thomas W.W., Pirani J.R. Flora of the reserva Ducke, Amazonas, Brazil: Simaroubaceae. Rodriguésia. 2022;73:e00182020. doi: 10.1590/2175-7860202273045. [DOI] [Google Scholar]
- Duka I. 2015. Digicam Control: Free Windows DSLR Camera Controlling Solution.http://digicamcontrol.com/ [Google Scholar]
- Fernando E.S., Gadek P.A., Quinn C.J. Simaroubaceae, an artificial construct: evidence from rbcL sequence variation. Am. J. Bot. 1995;82:92–103. [Google Scholar]
- Fields P.F. PhD Dissertation. Michigan State University; 1996. The Succor Creek Flora of the Middle Miocene Sucker Creek formation, Southwestern Idaho and Eastern Oregon: systematics and paleoecology. [Google Scholar]
- Grande L. Geological Survey of Wyoming; 1984. Paleontology of the Green River Formation, with a review of the fish fauna; p. 333. [Google Scholar]
- Hably L. Fruits and leaves of Ailanthus Desf. from the tertiary of Hungary. Acta Palaeobot. 2001;41:207–219. [Google Scholar]
- Hall R. Southeast Asia’s changing palaeogeography. Blumea. 2009;54:148–161. doi: 10.3767/000651909X475941. [DOI] [Google Scholar]
- He C.X., Tao J.R. A study on the Eocene flora in Yilan County, Heilongjiang. Acta Phytotaxon. Sin. 1997;35:249–256. in Chinese, with English abstract. [Google Scholar]
- Herman A.B., Spicer R.A., Aleksandrova G.N., Yang J., Kodrul T.M., Maslova N.P., Spicer T.E.V., Gang C., Jin J.H. Eocene-early Oligocene climate and vegetation change in southern China: evidence from the Maoming Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017;479:126–137. doi: 10.1016/j.palaeo.2017.04.023. [DOI] [Google Scholar]
- Hofmann C.C., Kodrul T.M., Liu X.Y., Jin J.H. Scanning electron microscopy investigations of middle to late Eocene pollen from the Changchang Basin (Hainan Island, South China) – Insights into the paleobiogeography and fossil history of Juglans, Fagus, Lagerstroemia, Mortoniodendron, Cornus, Nyssa, Symplocos and some Icacinaceae in SE Asia. Rev. Palaeobot. Palynol. 2019;265:41–61. [Google Scholar]
- Hu S.Y.A. Ailanthus. Arnoldia. 1979;39:29–50. [Google Scholar]
- Jin J.H., Liao W.B., W B.S., Peng S.L. Paleodiversification of the environment and plant community of Tertiary in Hainan Island. Acta Ecol. Sin. 2002;22:425–432. [Google Scholar]
- Kodrul T., Gordenko N., Sokolova A., Maslova N., Wu X.K., Jin J.H. A new Oligocene species of Cunninghamia R. Brown ex Richard et A. Richard (Cupressaceae) from the Maoming Basin, South China. Rev. Palaeobot. Palynol. 2018;258:234–247. doi: 10.1016/j.revpalbo.2018.09.003. [DOI] [Google Scholar]
- Lei Y.Z., Zhang Q.R., He W., Cao X.P. Geological Publishing House; 1992. Tertiary. Geology of Hainan Island; pp. 218–266. [Google Scholar]
- Liu J., Su T., Spicer R.A., Tang H., Deng W.Y.D., Wu F.X., Srivastava G., Spicer T., Van D.T., Deng T., Zhou Z.K. Biotic interchange through lowlands of Tibetan Plateau suture zones during Paleogene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019;524:33–40. doi: 10.1016/j.palaeo.2019.02.022. [DOI] [Google Scholar]
- MacGinitie H.D. Vol. 83. University of California Press; 1969. The Eocene Green River Flora of Northwestern Colorado and Northeastern Utah; pp. 1–140. [Google Scholar]
- Mai D.H. Gustav Fischer; 1995. Tertiäre Vegetationsgeschichte Europas; p. 691. [Google Scholar]
- Manchester S.R. In: Symposium proceedings, paleofloristic and paleoclimatic changes in the Cretaceous and Tertiary. Knobloch E., Kvaček Z., editors. Geological Survey; 1990. Eocene to Oligocene floristic changes recorded in the Clarno and John Day formations, Oregon, USA; pp. 183–187. [Google Scholar]
- Manchester S.R. In: Fossil flora and stratigraphy of the Florissant Formation, Colorado. Evanoff E., Gregory-Wodzicki K.M., Johnson K.R., editors. Vol. 4. Proceedings of the Denver Museum of Nature and Science; 2001. Update on the megafossil flora of Florissant, Colorado, USA; pp. 137–161. [Google Scholar]
- Martinetto E., Macaluso L. Quantitative application of the whole-plant concept to the Messinian-Piacenzian flora of Italy. Fossil Imprint. 2018;74:77–100. [Google Scholar]
- Meyer H.W., Manchester S.R. Vol. 141. University of California Press; 1997. The Oligocene Bridge Creek flora of the John Day Formation; pp. 1–195. [Google Scholar]
- Nooteboom H.P. Flora Malesiana (Ser I, 6) Noordhoff International Publishing; 1962. Ailanthus; pp. 215–220. [Google Scholar]
- Pirani J.R., Majure L.C., Devecchi M.F. An updated account of Simaroubaceae with emphasis on American taxa. Braz. J. Bot. 2021 doi: 10.1007/s40415-021-00731-x. [DOI] [Google Scholar]
- Prasad M., Kapgate D.K., Manadokar B.D. Fossil wood “Ailanthoxylon indicum” Prakash from the Deccan intertrappean beds of Shibla, Yeotmal district, Maharashtra, India. J. Appl. Biosci. 2007;33:141–144. [Google Scholar]
- Scotese C.R. 2016. PALEOMAP PaleoAtlas for GPlates and the PaleoData Plotter Program, PALEOMAP Project.http://www.earthbyte.org/paleomap-paleoatlas-for-gplates/ [DOI] [Google Scholar]
- Simao S.M., Barreiros E.L., Da Silva M.F.G.F., Gottlieb O.R. Chemogeographical evolution of quassinoids in Simaroubaceae. Phytochemistry. 1991;30:853–865. [Google Scholar]
- Sladonja B., Sušek M., Guillermic J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015;56:1009–1034. doi: 10.1007/s00267-015-0546-5. [DOI] [PubMed] [Google Scholar]
- Sniderman J.M., Jordan G.J. Extent and timing of floristic exchange between Australian and Asian rain forests. J. Biogeogr. 2011;38:1445–1455. doi: 10.1111/j.1365-2699.2011.02519.x. [DOI] [Google Scholar]
- Song Z.Q., Shi G.L., Chen Y.F., Wang Q. Winged fruits of Ailanthus (Simaroubaceae) from the Oligocene Ningming Formation of Guangxi, and there taxonomic and biogeographic implications. Acta Palaeontol. Sin. 2014;53:191–200. [Google Scholar]
- Song Z.Q., Xu D.X. The identity of Ailanthus guangxiensis (Simaroubaceae) and lectotypification of A. integrifolia Lamarck. Phytotaxa. 2014;173:177–180. doi: 10.11646/phytotaxa.173.2.10. [DOI] [Google Scholar]
- Spicer R.A., Herman A.B., Liao W., Spicer T.E.V., Kodrul T.M., Yang J., Jin J. Cool tropics in the middle Eocene: evidence from the Changchang flora, Hainan Island, China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014;412:1–16. doi: 10.1016/j.palaeo.2014.07.011. [DOI] [Google Scholar]
- Spicer R., Yang J., Herman A., Kodrul T., Aleksandrova G., Maslova N., Spicer T., Ding L., Xu Q., Shukla A., et al. Paleogene monsoons across India and South China: drivers of biotic change. Gondwana Res. 2017;49:350–363. doi: 10.1016/j.gr.2017.06.006. [DOI] [Google Scholar]
- Su T., Jacques F., Ma H.J., Zhou Z.K. Fossil fruits of Ailanthus confucii from the upper Miocene of Wenshan, Yunnan Province, southwestern China. Palaeoworld. 2013;22:153–158. doi: 10.1016/j.palwor.2013.07.002. [DOI] [Google Scholar]
- Su T., Spicer R.A., Wu F.X., Farnsworth A., Huang J., Del Rio C., Deng T., Ding L., Deng W.Y.D., Huang Y.J., et al. A middle Eocene lowland humid subtropical “Shangri-La” ecosystem in central Tibet. Proc. Natl. Acad. Sci. USA. 2020;117:32989–32995. doi: 10.1073/pnas.2012647117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Tao J.R., Wang X.Z., Li J.Y. Shandong Science and Technology Publishing House; 1999. Plant fossils from Shanwang; p. 167. in Chinese. [Google Scholar]
- Tanai T., Suzuki N. On the genus Ailanthus from the Tertiary of Japan. Trans. Proc. Palaeontol. Soc. Jpn. 1963;52:135–144. [Google Scholar]
- Teodoridis V., Kvaček Z., Sami M., Utescher T., Martinetto E. Palaeoenvironmental analysis of the Messinian macrofossil floras of Tossignano and Monte Tondo (Vena del Gesso basin, Romagna Apennines, northern Italy) Acta Musei Natl. Pragae. 2015;71:249–292. doi: 10.14446/AMNP.2015.249. [DOI] [Google Scholar]
- Tian Y., Spicer R.A., Huang J., Zhou Z.K., Su T., Widdowson M., Jia L.B., Li S.F., Wu W.J., Xue L., et al. New early Oligocene zircon U-Pb dates for the ‘Miocene’ Wenshan basin, Yunnan, China: biodiversity and paleoenvironment. Earth Planet Sci. Lett. 2021;565:116929. doi: 10.1016/j.epsl.2021.116929. [DOI] [Google Scholar]
- Van Sam H., Nooteboom H.P. Ailanthus vietnamensis (Simaroubaceae): a new species from Vietnam. Blumea J. Plant Taxon. Plant Geogr. 2007;52:555–558. doi: 10.3767/000651907X608918. [DOI] [Google Scholar]
- Wang J., Li H., Zhu Z., Seguin M.K., Yang J., Zhang G. Magnetostratigraphy of Tertiary rocks from Maoming Basin, Guangdong Province, China. Chin. J. Geochem. 1994;13:165–175. doi: 10.1007/BF02838516. [DOI] [Google Scholar]
- WGCPC (Writing Group of Cenozoic Plants of China) Vol. 3. Science Press; 1978. Cenozoic Plants from China, Fossil Plants of China; p. 232. in Chinese. [Google Scholar]
- Wheeler E.A., Srivastava R., Manchester S.R., Baas P. Surprisingly modern Latest Cretaceous–earliest Paleocene woods of India. IAWA J. 2017;38:456–542. doi: 10.1163/22941932-20170174. [DOI] [Google Scholar]
- Wilf P. Late Paleocene–early Eocene climate changes in southwestern Wyoming: paleobotanical analysis. Bull. Geol. Soc. Am. 2000;112:292–307. [Google Scholar]
- Wu X.K., Liu X.Y., Kodrul T.M., Quan C., Jin J.H. Dacrycarpus pattern shedding new light on the early floristic exchange between Asia and Australia. Natl. Sci. Rev. 2019;6:1086–1090. doi: 10.1093/nsr/nwz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Jin J.H., Li N., He H.M., Chen T., Liu X.Y. Early Oligocene Calocedrus (Cupressaceae) from the Maoming Basin, South China, and its paleogeographic and paleoclimatic implications. J. Systemat. Evol. 2019;57:142–152. doi: 10.1111/jse.12424. [DOI] [Google Scholar]
- Xiong Z., Liu X., Ding L., Farnsworth A., Spicer R.A., Xu Q., Valdes P., He S.L., Zeng D., Wang C., et al. The rise and demise of the Paleogene central Tibetan valley. Sci. Adv. 2022;8:eabj0944. doi: 10.1126/sciadv.abj0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Qiu J., Zhou Z., Jin J. Eocene Podocarpium (Leguminosae) from South China and its biogeographic implications. Front. Plant Sci. 2015;6:938. doi: 10.3389/fpls.2015.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.L., Kodrul T.M., Wu Y., Maslova N.P., Jin J.H. Early Oligocene fruits and leaves of Burretiodendron (Malvaceae s.l.) from South China. J. Systemat. Evol. 2021;59:1100–1110. doi: 10.1111/jse.12577. [DOI] [Google Scholar]
- Yang T., Jia J.W., Chen H.Y., Zhang Y.X., Wang Y., Wang H.J., Bao L., Zhang L., Li W.J., Xie S.P., Yan D.F. Oligocene Ailanthus from northwestern Qaidam Basin, northern Tibetan Plateau, China and its implications. Geol. J. 2021;56:616–627. doi: 10.1002/gj.3904. [DOI] [Google Scholar]
- Yao Y.F., Bera S., Ferguson D.K., Mosbrugger V., Paudayal K.N., Jin J.H. Reconstruction of paleovegetation and paleoclimate in the early and middle Eocene, Hainan Island, China. Clim. Change. 2009;92:169–189. doi: 10.1007/s10584-008-9457-2. [DOI] [Google Scholar]
- Zhilin S.G. Ulmaceae and Simaroubaceae of the late Oligocene flora of Kinyak (northwestern Karakalpak A.S.S.R.) Bot. Zh. 1967;52:481–488. in Russian. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
No novel code was used in this study.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.