Abstract
Atrial fibrillation (AF) accounts for one-quarter of the global ischemic stroke burden. Population growth and an increasing prevalence of stroke risk factors underscores the critical need to recognize and address the worldwide crisis in underutilization of antithrombotic therapy for patients with AF. Failure to address key patient, clinician, and societal gaps in AF care will result in a worldwide increase in stroke-related morbidity and mortality while overwhelming global healthcare systems. The failure to adhere to evidence-based guideline recommendations for stroke prevention in AF reflects a critical gap in implementation of best clinical practice among providers and healthcare systems. Globally, these include inadequate provider education, limited public awareness, underdiagnosis, and underutilization of treatments, including antithrombotic therapy. In specific regions, efforts are further complicated by availability of specific medications, variation in drug metabolism across racial and ethnic populations, socioreligious considerations, and lack of universally available electronic health records. Efforts are needed at both global and regional levels to address key barriers to evidence-based care for patients with AF. Investing in clinical tools and teams that improve stroke prevention for patients with AF will likely improve population health.
Keywords: Atrial Fibrillation, Stroke, International Barriers
1. Introduction
The most common sustained cardiac dysrhythmia worldwide, atrial fibrillation (AF), accounts for 25% of the global burden of ischemic stroke [1], [2]. Growth of the global population of patients with AF and an increasing prevalence of stroke risk factors underscores the critical need to recognize and address the worldwide crisis in underutilization of antithrombotic therapy. Failure to address the crisis in failure to prevent stroke in AF is likely to result in a worldwide increase in stroke-related morbidity and mortality and overwhelm global healthcare systems.
In this Cardiovascular Perspective, we present international insights into the crisis of failure to prevent stroke in AF, universal barriers to evidence-based stroke prevention, and regional challenges in antithrombotic therapy from a Global Anticoagulation Roundtable representing Asia, Africa and the Middle East, and Latin America.
2. Methodology of the global anticoagulation Roundtable
This Global Anticoagulation Roundtable was held as a virtual conference on October 16, 2020 in response to travel restrictions in place due to the global COVID-19 pandemic. The main objective of the Global Anticoagulation Roundtable was to identify global and regional barriers to stroke prevention in AF and promote pathways for Quality Improvement at the clinician and healthcare system levels by harnessing the knowledge and expertise of participants from key areas of interest: Asia, Africa and the Middle East, and Latin America. The roundtable included 3–4 representatives from each region (Appendix).
3. Universality of failure to prevent stroke in AF
Failure to prescribe antithrombotic therapy for patients with AF and increased risk of stroke is a pervasive public health concern in the U.S. and worldwide [3]. The global failure to adhere to evidence-based guideline recommendations for stroke prevention in AF reflects a critical gap in implementation of best practice among providers and healthcare systems. Underutilization of anticoagulation for stroke prevention in at-risk patients with AF has been well-documented in many Asian, European, Middle Eastern, and South American countries in addition to North America [3], [4], [5], [6], [7], [8]. Barriers to appropriate stroke prevention exist at the patient-, physician-, and system-levels. The patient- and physician-level barriers are most often common across regions of the world while system-level barrier have a greater degree of variation.
3.1. Common barriers to stroke prevention in AF
Common barriers to evidence-based stroke prevention in AF include inadequate provider education, limited public awareness of AF as a risk factor for stroke, underdiagnosis of AF, limited resources for antithrombotic therapy, reduced access to direct oral anticoagulants (DOACs), underdosing of anticoagulation, and reliance of antiplatelet therapy for thromboprophylaxis, to name a few. Such obstacles were identified in this Global Anticoagulation Roundtable as universal across the regions of interest.
A major impediment to optimal stroke prevention in AF is the lack of community recognition of AF as cause of ischemic stroke. A campaign to increase patient and provider awareness and detection of AF through primary care practices in Japan was shown to increase detection, especially in elderly patients [9]. An international randomized controlled trial of a multifaceted educational intervention to increase AF awareness and improve stroke prevention efforts in patients with AF increased prescription of anticoagulation from 68% to 80% and reduced stroke by 52% [10].
Underdiagnosis of AF has been identified as a challenge in reducing the global burden of stroke. Greater global integration of Health Information Technology and mobile monitoring strategies has the potential to aid providers in quantifying the denominator of patients at increased risk for stroke and to result in a more targeted approach to risk reduction. Currently, access to such Health Information Technology solutions is quite limited worldwide. And even in high resource settings where electronic health records are common, integration of clinical risk predictions tools and/or clinical decision support is often overlooked or logistically challenging.
For patients and clinicians across the globe, resource limitations often hinder the use of guideline-recommended therapies. This includes limited access to DOACs due to their high cost or lack of regulatory approval and drug availability. Similarly, use of anticoagulation management services are less common in many countries outside of North America and Europe. To address these concerns, collaboration between medical societies, governmental and leading healthcare institutions, and pharmaceutical companies should address these resource constraints. Specifically, the financial benefits of DOAC therapy for patients with AF must be calculated at a population level (both societal and healthcare-related) to realize the benefit of paying for newer (and often more expensive medications) for the benefit of fewer life-threatening intracranial hemorrhage events. Additionally, utilizing specialized nurses and/or pharmacists to assist with evidence-based anticoagulation care can improve overall quality of care for large populations of patients.
Underdosing of DOACs is common barrier to high-quality, evidence-based anticoagulation care for patients with AF in nearly all countries. In particular, patients receiving subtherapeutic DOAC doses for stroke prevention in AF experience a higher risk of all-cause mortality despite similar or slightly reduced rates of major bleeding [11]. Potential solutions to address this significant healthcare delivery gap include the use of computer decision support built into electronic health records, phone-based applications to assist with appropriate DOAC dose selection, broader use of anticoagulation clinic nurses and/or pharmacists to review DOAC dosing, and educational efforts targeting both patients and clinicians.
In many cases, patients with AF are given antiplatelet therapy instead of anticoagulants for stroke prevention [12], [13]. While there are numerous potential rationales (e.g., perceived lower risk of bleeding, lower cost, easier medication access), antiplatelet therapy is recommended by neither European nor North American cardiology societal guidelines [1], [2]. To address these concerns, robust implementation efforts leveraging both education- and technology-based solutions have demonstrated success in improving use of anticoagulation therapy, often with similar or lower rates of antiplatelet use.
Clinical emphasis in stroke prevention typically centers on appropriate use of anticoagulant therapy. Yet managing comorbid conditions can significantly impact AF-related stroke risk and morbidity. Effective management of obesity, obstructive sleep apnea, hypertension, and diabetes can have important impacts on reducing the burden of stroke. Finally, efforts to change lifestyle can also impact AF-related stroke risk, including smoking cessation and reduced alcohol consumption. These efforts are of unique importance in Asia, where tobacco use is highly prevalent.
3.2. Unique regional barriers to stroke prevention in Atrial fibrillation
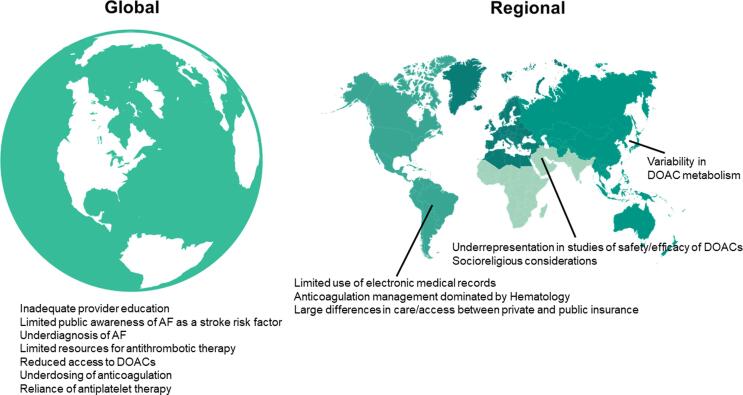
While some challenges to effective AF-related stroke prevention are present around the globe, others are unique to individual regions or countries (Fig. 1). In this way, many one-size-fits-all efforts to improve stroke prevention may not work if these unique barriers are not specifically addressed.
Fig. 1.
Global and regional challenges to stroke prevention in atrial fibrillation (AF). DOAC – Direct Oral Anticoagulant.
In some Middle Eastern countries, generic versions of DOACs without published bioequivalence data are available and commonly prescribed. Concerns about the real-world efficacy and safety of DOACs, including generic formulations, in patients from the Middle East present an obstacle to implementation of evidence-based stroke prevention [14]. For example, in Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) only 4% of patients were enrolled from the Middle East and North Africa [15]. In addition, uncertainty and variability regarding dosing around fasting during the holy month of Ramadan can impact medication adherence and timing of medications prescribed twice daily (dabigatran and apixaban) or medications that must be taken with food (rivaroxaban) [16].
For patients of Asian ancestry, metabolism of DOAC medications may not mirror that of patients with Caucasian and/or Black ancestry [17]. Additionally, anticoagulant-related intracranial hemorrhage risk is higher in Asian patients as compared to patients of Caucasian race/ethnicity [18]. For these reasons, patients in many Asian countries are often treated with standard DOAC doses that are lower than what is used in the rest of the world. Adapting tools designed to improve use of anticoagulant therapy for stroke prevention in AF for Asian patients is critical to ensure their appropriate uptake and utilization.
In many Latin American countries, electronic health records are not broadly utilized. This limits the ability to apply population health approaches for anticoagulation management and adoption of DOAC therapy. It also limits the ability to implement clinical decision support tools that help clinicians select appropriate stroke prevention methods for patients with AF. In some contries, hematologists oversee nearly all anticoagulantion therapy (including for cardiac indications). This hampers the ability for cardiovascular specialists to start patients on DOACs or help paitents change from warfarin to DOAC therapy when clinically appropriate. Finally, marked differeces in cost for AF-related care and medications exist between patients who rely on public health systems and patients who can afford private health care. To address these barriers, nation-wide efforts are needed to increase investment in electronic health records and broaden access to proven, cost-effective medications. Additionally, cardiovascular societies should lead efforts to disseminate clinican education reguarding anticoagulant therapy and empower cardiovascular specialists to take the lead on initiating and managing anticoagulant medications.
4. Conclusions
To address the global burden of AF-related stroke, a combination of global and regional/national efforts are needed to address specific barriers to evidence-based use of anticoagulation and other stroke prevention methods. Inclusive clinical trials and real world evidence studies are needed to understand variability in practice, obstacles to optimal stroke prevention, and outcomes across diverse populations. Leveraging technology is likely to be a widely effective approach, as long as the technology is adapted to local barriers and needs. While many countries struggle with limited financial and clinical resources, the COVID-19 pandemic has exacerbated those struggles. Still, investing in clinical tools and teams that improve stroke prevention for patients with AF will improve the health of broad populations.
Financial Disclosures
This manuscript is based on a roundtable organized by the American College of Cardiology. Funding for the roundtable and support of manuscript publication was provided by Pfizer directly to the American College of Cardiology. The sponsor was not involved in the creation or writing process of this manuscript.
Dr. Barnes has received consulting fees from Pfizer/Bristol-Myers Squibb, Janssen, Acelis Connected Health, Abbott Vascular, and Boston Scientific and serves on the board of directors for the Anticoagulation Forum.
Dr. Piazza has received research grant support from EKOS, a BTG International Group company, Bayer, the Bristol Myers Squibb/Pfizer Alliance, Alexion, and Janssen and consulting fees from Amgen, Pfizer, Boston Scientific Corporation, Agile, Syntactx, and Prairie Education and Research Cooperative.
Dr. Chao has no financial disclosures to share.
Dr. Njeim has no financial disclosures to share.
Dr. Poh has no financial disclosures to share.
Dr. Zimerman has received consulting fees from Libbs, Pfizer, Daiichi-Sankyo, and Bayer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Geoffrey D. Barnes, Email: gbarnes@umich.edu.
for the Global Anticoagulation Roundtable Working Group:
Geoffrey D. Barnes, Tze-Fan Chao, Mario Njeim, Kian Keong Poh, Leandro Zimerman, and Gregory Piazza
Appendix.
Participating Members in the Global Anticoagulation Roundtable
Program Chairs
Geoffrey Barnes, MD, MSc, FACC
Gregory Piazza, MD, MS, FACC
Members
Africa and the Middle East
Mohamed Aljaabaari, MD, FACC
Ahmed Shawky Elserafy, MD
Mario Njeim, MD
Latin America
Nester Lopez-Cabanillas, MD
Manuel Odin de los Rios Ibarra, MD, FACC
Leandro Zimerman, MD, FACC
Asia
Pantep Angchaisuksiri, MD, PhD
Tze-Fan Chao, MD, PhD
Kian Keong Poh, MBBChir, FACC
Hung-Fat Tse, MD, PhD, FACC.
References
- 1.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., Fauchier L., Filippatos G., Kalman J.M., La Meir M., Lane D.A., Lebeau J.P., Lettino M., Lip G., Pinto F.J., Thomas G.N. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Jr, Ellinor P.T., Ezekowitz M.D., Field M.E., Furie K.L., Heidenreich P.A., Murray K.T., Shea J.B., Tracy C.M., Yancy C.W. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg B.A., Gao H., Shrader P., Pieper K., Thomas L., Camm A.J., Ezekowitz M.D., Fonarow G.C., Gersh B.J., Goldhaber S., Haas S., Hacke W., Kowey P.R., Ansell J., Mahaffey K.W., Naccarelli G., Reiffel J.A., Turpie A., Verheugt F., Piccini J.P. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am. Heart J. 2017;194:132–140. doi: 10.1016/j.ahj.2017.08.011. ORBIT-AF Investigators. [DOI] [PubMed] [Google Scholar]
- 4.Bassand J.P., Accetta G., Al Mahmeed W., Corbalan R., Eikelboom J., Fitzmaurice D.A., Fox K., Gao H., Goldhaber S.Z., Goto S., Haas S., Kayani G., Pieper K., Turpie A., van Eickels M., Verheugt F., Kakkar A.K. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation. PLoS ONE. 2018;13(1) doi: 10.1371/journal.pone.0191592. GARFIELD-AF Investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koretsune Y., Etoh T., Katsuda Y., Suetsugu T., Kumeda K., Sakuma I., Eshima K., Shibuya M., Ando S.I., Yokota N., Goto S., Pieper K.S., Allu J., Kakkar A.K. Risk profile and 1-year outcome of newly diagnosed atrial fibrillation in Japan - Insights from GARFIELD-AF. Circulation J.: Official J. Japanese Circulation Soc. 2018;83(1):67–74. doi: 10.1253/circj.CJ-18-0655. GARFIELD-AF Investigators. [DOI] [PubMed] [Google Scholar]
- 6.Chao T.F., Chiang C.E., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Tuan T.C., Liao J.N., Chung F.P., Chen T.J., Lip G., Chen S.A. Evolving Changes of the Use of Oral Anticoagulants and Outcomes in Patients With Newly Diagnosed Atrial Fibrillation in Taiwan. Circulation. 2018;138(14):1485–1487. doi: 10.1161/CIRCULATIONAHA.118.036046. [DOI] [PubMed] [Google Scholar]
- 7.Hersi A., Abdul-Moneim M., Almous'ad A., Al-Samadi F., AlFagih A., Sweidan R. Saudi Atrial Fibrillation Survey: national, observational, cross-sectional survey evaluating atrial fibrillation management and the cardiovascular risk profile of patients with atrial fibrillation. Angiology. 2015;66(3):244–248. doi: 10.1177/0003319714529180. [DOI] [PubMed] [Google Scholar]
- 8.Jerjes-Sanchez C., Corbalan R., Barretto A., Luciardi H.L., Allu J., Illingworth L., Pieper K.S., Kayani G. Stroke prevention in patients from Latin American countries with non-valvular atrial fibrillation: Insights from the GARFIELD-AF registry. Clin. Cardiol. 2019;42(5):553–560. doi: 10.1002/clc.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki A., Okamura T., Sasaki M., Matsuoka H., Ikeda Y., Takahashi A., Akiyama S., Ono F., Yoshihara N. Acceleration of opportunistic atrial fibrillation screening for elderly patients in routine primary care. PLoS ONE. 2020;15(12) doi: 10.1371/journal.pone.0244240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinereanu D., Lopes R.D., Bahit M.C., Xavier D., Jiang J., Al-Khalidi H.R., He W., Xian Y., Ciobanu A.O., Kamath D.Y., Fox K.A., Rao M.P., Pokorney S.D., Berwanger O., Tajer C., de Barros E., Silva P., Roettig M.L., Huo Y., Granger C.B. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet (London, England) 2017;390(10104):1737–1746. doi: 10.1016/S0140-6736(17)32165-7. IMPACT-AF investigators. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg B.A., Shrader P., Thomas L., Ansell J., Fonarow G.C., Gersh B.J., Kowey P.R., Mahaffey K.W., Naccarelli G., Reiffel J., Singer D.E., Peterson E.D., Piccini J.P. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J. Am. College Cardiol. 2016;68(24):2597–2604. doi: 10.1016/j.jacc.2016.09.966. ORBIT-AF Investigators and Patients. [DOI] [PubMed] [Google Scholar]
- 12.Hsu J.C., Maddox T.M., Kennedy K., Katz D.F., Marzec L.N., Lubitz S.A., Gehi A.K., Turakhia M.P., Marcus G.M. Aspirin instead of oral anticoagulant prescription in atrial fibrillation patients at risk for stroke. J. Am. Coll. Cardiol. 2016;67(25):2913–2923. doi: 10.1016/j.jacc.2016.03.581. [DOI] [PubMed] [Google Scholar]
- 13.Pritchett R.V., Bem D., Turner G.M., Thomas G.N., Clarke J.L., Fellows R., Lane D.A., Jolly K. Improving the prescription of oral anticoagulants in atrial fibrillation: a systematic review. Thromb. Haemost. 2019;119(2):294–307. doi: 10.1055/s-0038-1676835. [DOI] [PubMed] [Google Scholar]
- 14.Hersi A.S., Alhebaishi Y.S., Hamoui O., Hassan T., Khalifa Hamad A., Magdy M., Sabbour H., Shaheen S. Practical perspectives on the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation: a view from the Middle East and North Africa. J. Saudi Heart Assoc. 2018;30(2):122–139. doi: 10.1016/j.jsha.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huisman M.V., Rothman K.J., Paquette M., Teutsch C., Diener H.C., Dubner S.J., Halperin J.L., Ma C.S., Zint K., Elsaesser A., Bartels D.B., Lip G.Y., Investigators G.-L.-O.-R.-I.-A.-A.-F. The Changing Landscape for Stroke Prevention in AF: Findings From the GLORIA-AF Registry Phase 2. J. Am. Coll. Cardiol. 2017;69(7):777–785. doi: 10.1016/j.jacc.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 16.Aslam M., Assad A. Drug regimens and fasting during Ramadan: a survey in Kuwait. Public health. 1986;100(1):49–53. doi: 10.1016/s0033-3506(86)80086-5. [DOI] [PubMed] [Google Scholar]
- 17.Chao T.F., Chen S.A., Ruff C.T., Hamershock R.A., Mercuri M.F., Antman E.M., Braunwald E., Giugliano R.P. Clinical outcomes, edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur. Heart J. 2019;40(19):1518–1527. doi: 10.1093/eurheartj/ehy807. [DOI] [PubMed] [Google Scholar]
- 18.Shen A.Y., Yao J.F., Brar S.S., Jorgensen M.B., Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2007;50(4):309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]