Abstract
Recently, developing countries have focused on using innovative feed in poultry nutrition. The plant Moringa oleifera is native to India but grows worldwide in tropical and subtropical climates. Moringa is planted on a large scale as it can tolerate severe dry and cold conditions. All parts of this plant can be used for commercial or nutritional purposes, and it has a favorable nutritional profile. Beneficial phytochemicals, minerals, and vitamins are abundant in the leaves. The leaf extracts can be used to treat malnutrition; they also possess anticancer, antioxidant, antidiabetic, antibacterial, and anti-inflammatory properties. Further, moringa contains antinutritional substances, such as trypsin inhibitors, phytates, tannins, oxalates, cyanide, and saponins, which have a harmful effect on mineral and protein metabolism. Previous research suggested that including moringa in chicken diets boosts their growth and productivity. Therefore, this review focuses on the characterization and application of M. oleifera in poultry nutrition and its potential toxicity. Furthermore, we discuss the nutritional content, phytochemicals, and antioxidants of M. oleifera leaf meal and its applicability in poultry rations.
Key words: antioxidants, antibiotic alternatives, antimicrobials, Moringa oleifera, organic poultry
INTRODUCTION
Poultry products are rich sources of various proteins and minerals (Abd El-Hack et al., 2022a,b). However, several environmental hazards, such as heat stress and infectious agents (viral, bacterial, parasitic, and fungal pathogens), threaten the poultry industry worldwide, causing severe economic losses (El-Shall et al., 2022; Salem et al., 2022a,b). Currently, using natural antibiotic alternatives to improve poultry production and avoid the residual impact of antibiotic use in poultry products is considered a global initiative (Abd El-Hack et al., 2016a; Rehman et al., 2020).
Probiotics, prebiotics, synbiotics, amino acids, herbal extracts, exogenous enzymes, essential oils, green synthesized nanoparticles, and phytogenic products are the most common natural feed additives used to improve the production of poultry and livestock (Abou-Kassem et al., 2021, 2022; Alagawany et al., 2021; Arif et al., 2022). Various phytogenic poultry feed additives, such as aromatic herbs or fragrance oils, have also been explored in the recent decades (Ahossi et al., 2016; Abd Elkader et al., 2021; Reda et al., 2021). Indeed, their premium varieties are validated at different inclusion levels to identify nutritional supplements that are natural, inexpensive, and safe and provide high financial returns (Cross et al., 2007; Abd El-Hack et al., 2021a,b,d, Abd El-Hack et al., 2022d).
Moringa oleifera is a member of the family Moringaceae that originated in several countries, including southern Asia, and has now spread to the tropical and subtropical regions of the world (Fahey, 2005). Moringa has been used by the ancient Greeks and Romans (Duke, 2001). M. oleifera was introduced to Kenya by Indians during the construction of the Kenya–Uganda railway, and it is currently grown in Kenyan rangelands, including the lower Eastern, Baringo, and the Coast regions (Maundu and Tegnas, 2005). Moringa is a perennial shrub that grows well in a wide variety of soils, especially in sandy loams and slightly alkaline clay soils because of their good drainage ability (Abdul, 2007). It thrives best at altitudes of 0 to 1,800 m above sea level, with an annual rainfall of 500 to 1,500 mm (Sánchez et al., 2006; Nouman et al., 2014). Therefore, it is suitable for tropical and subtropical regions that are characterized by hot, humid, and dry climate and provides ample nutritional value even under marginal growth conditions (Nouman et al., 2014).
M. oleifera has high biomass yields of 4.2 to 8.3 metric tons/hectare (ha). Biomass and leaf nutritional composition are influenced by seasons; planting density; soil factors, such as fertilizer use, irrigation, and harvesting frequencies (Sánchez et al., 2006; Radovich, 2011). Similar to fodder shrubs, considerable time is required for the establishment of M. oleifera for strong root growth before the first defoliation. This period is approximately 1 yr when M. oleifera grows up to 1 to 1.5 m in height (Sánchez et al., 2006). Makkar and Becker (1997) documented that the appropriate plant spacing for the optimal performance of M. oleifera ranges from 1 m × 1 m (10,000 plants per ha) to 2.50 cm × 2.50 cm (16,000,000 plants per ha).
The total yield of fresh (FM) and dry matter (DM), growth rate, and height of M. oleifera during the first and second years increase significantly as the cutting interval is increased from 45 to 75 d (Sánchez et al., 2006). The same authors reported that during the first year of growth, DM, neutral detergent fiber (NDF), and ash contents were highest and the in vitro digestibility was lowest at the longest harvesting frequency, whereas crude protein (CP) and acid detergent fiber (ADF) contents were not affected by the harvesting frequency. For intensive biomass production, it is recommended that M. oleifera should be densely planted, with 50 to 75 plants per square meter, and harvested every 75 d (Sánchez et al., 2006).
The concentration of fragrant plant parts in the feed increased from 100 to 30,000 mg/kg. In layer diets, anise seed powder was used at a concentration of 250 to 1,500 mg/kg and rosemary powder were used at 400 to 500 mg/kg (Botsoglou et al., 2005). In addition, Cross et al. (2007) used marjoram, rosemary, and yarrow powder in broiler diets at 10,000 mg/kg. Furthermore, Govaris et al. (2007) added rosemary powder to turkey diets at 5,000 to 10,000 mg/kg. Christaki et al. (2011) supplemented the laying Japanese quail diet with oregano at 10,000 to 30,000 mg/kg. These diets resulted in significant benefits to chicken health and productivity.
Small inclusions of volatile oils in the feed showed a similar level of influence as other plant parts at higher concentrations. Some examples include herbal essential oils, such as thyme, marjoram, rosemary, and yarrow oil, at a concentration of 1,000 mg/kg (Cross et al., 2007) and oregano essential oils at 50 to 300 mg/kg (Botsoglou et al., 2002; Giannenas et al., 2003; Govaris et al., 2005). Ciftci et al. (2005) used aniseed oil at 100 to 400 mg/kg, Lopez-Bote et al. (1998) used rosemary extracts and sage at 500 mg/kg, and Amerah et al. (2011) used thymol and cinnamaldehyde at 100 mg/kg.
Feed quality can be improved by incorporating phytogenic substances (Abdelnour et al., 2020a,b; Ashour et al., 2020). First, these substances can inhibit the growth of mycotoxigenic fungi (Soliman and Badea, 2002). Second, the antibacterial and antioxidant properties of phytogenic substances, such as carvacrol, rosmarinic acid, and thymol, are critical factors for improving the overall feed quality (Burtis and Bucar, 2000). The antioxidant quality may be essential when the feed contains a higher amount of polyunsaturated fatty acids than saturated fatty acids. It has been suggested that the antimycotic properties of phytogenic extracts/essential oils are responsible for the prevention of mycotoxin formation in stored wheat grains. These substances could act as antioxidant, antispasmodic, immunomodulatory, antimicrobial, anti-inflammatory, and anticancer agents. They can also promote the production of intestinal flora and enhance digestion, absorption, and nutrient metabolic activity (Abd El-Hack et al., 2016b, 2018; Arif et al., 2019; Ashour et al., 2020). Abd El-Hack and Alagawany (2015) and Abd El-Hack et al. (2016a) reported that anise, thyme, and cinnamon prevent the development of toxigenic fungi, such as Fusarium moniliforme, Aspergillus parasiticus, Aspergillus flavus, and Aspergillus ochraceus.
This review article provides additional information on the nature, nutritional benefits, and prospective use of M. oleifera as a chicken feed additive.
Description of M. oleifera
M. oleifera is also known as drumstick tree, ben oil tree, benzoyl tree, and horseradish tree. The leaves, pods, seeds, flowers, fruits, and roots of the moringa tree are edible (Orwa et al., 2009). The family Moringaceae comprises 13 species (Table 1).
Table 1.
Species of the family Moringaceae.
| Species name | Properties |
|---|---|
| Moringa hildebrandtii | Medicinal |
| Moringa drouhardii | Medicinal |
| Moringa stenopetala | Edible delicious leafs |
| Moringa ovalifolia “aka ghost tree” | Medicinal |
| Moringa peregrina | Edible |
| Moringa oleifera | Edible delicious leaves |
| Moringa concanensis | Edible leaves |
| Moringa rivae | Medicinal |
| Moringa ruspoliana | Medicinal |
| Moringa arborea | Medicinal |
| Moringa borziana | Medicinal |
| Moringa pygmaea | Medicinal |
| Moringa longituba | Medicinal |
Several studies have been conducted on the nutritional composition of M. oleifera in different geographical locations. The leaves of M. oleifera contain Calcium (Ca) 99.1mg, Phosphorous (P) 70.8, Magnesium (Mg) 35.1, Iron (Fe) 1.3, Zinc (Zn) 0.85, Sodium (Na) 70, Manganese (Mn) 0.119, and Potassium (K) 471mg in DM (Abbas et al., 2018). Because of its rich nutritional composition, M. oleifera is used in human and animal feeds and for medicinal purposes (Richter et al., 2003; Sánchez et al., 2006). M. oleifera leaves contain high levels of soluble carbohydrates (10%) and β-carotenes (2.33 × 102 μg/L) (Mustapha and Babura, 2009).
M. oleifera is also rich in tocopherols (γ and α), phenolic compounds, vitamin C, essential sulfur amino acids (methionine and cysteine), unsaturated fatty acids (oleic acids), and minerals (Ferreira et al., 2008; Mustapha and Babura, 2009). Furthermore, these leaves have a high total antioxidant capacity (260 mg/100 g) and high levels of total polyphenols (260 mg/100 g), quercetin (100 mg/100 g), kaempferol (34 mg/100 g), and β-carotene (34 mg/100 g) (Lako et al., 2007). M. oleifera seeds contain high levels of stable oleic acids followed by palmitic acids and behenic acids (Sánchez-Machado et al., 2015). The nutritional contents of each part of the M. oleifera tree are summarized in Figure 1. M. oleifera possesses a wide range of biological activities, including antioxidant, tissue protective (liver, kidneys, heart, testes, and lungs), analgesic, antiulcer, antihypertensive, radioprotective, and immunomodulatory properties in addition to being an important nutritional agent (Stohs and Hartman, 2015; Okumu et al., 2017).
Figure 1.
The nutritional contents of each part of Moringa oleifera tree.
M. oleifera is a deciduous tree that can quickly grow up to 10 to 12 m in height, and the diameter of its trunk is approximately 45 cm. The flowers are approximately 1.0 to 1.5 cm long and 2.0 cm wide. The flowering process begins within 6 months of transplantation. The fruit is a brown, 3-sided pendulous capsule with a diameter of 20 to 45 cm that contains dark brown globular seeds (1 cm in diameter). The seeds are dispersed by 3 thin whitish wings through water and wind (Olson and Carlquist, 2001). The tree requires an average annual rainfall of 250 to 3,000 mm and can be sustained at temperatures between 25°C and 40°C, which is ideal for tropical regions.
Nutritional Composition of M. oleifera Leaves
Moringa leaves are rich in iron, protein, carotenoids, and ascorbic acid (Table 2). In terms of highly digestible nutrients, the consumption of M. oleifera leaves is encouraged in several underdeveloped nations worldwide, where they may be consumed in fresh or roasted forms or preserved as a dry powder (Fahey, 2005). M. oleifera leaves could be used as a food additive to improve feed intake and animal profitability. Alternatively, they could substitute traditional harvests for more effective economic, ecofriendly, and safe production (Aregheore, 2002; Richter et al., 2003).
Table 2.
Mineral contents of dried Moringa oleifera leaves (source: Moyo et al. (2011).
| Mineral | Dry leaves |
|---|---|
| Calcium (Ca) (%) | 3.65 |
| Phosphorus (P) (%) | 0.30 |
| Magnesium (Mg) (%) | 0.50 |
| Potassium (K) (%) | 1.50 |
| Sodium (Na) (%) | 0.164 |
| Sulphur (S) (%) | 0.63 |
| Zinc (Zn) (mg/kg) | 31.03 |
| Copper (Cu) (mg/kg) | 8.25 |
| Iron (Fe) (mg/kg) | 490 |
| Manganese (Mn) (mg/kg) | 86.8 |
| Selenium(Se) (mg/kg) | 36.30 |
| Boron (B) (mg/kg) | 49.93 |
Rubanza et al. (2005) found improved feed absorption in animals fed with M. oleifera leaves, which was attributed to the plant's superior nutritional profile, particularly amino acids, CP, NDF, ADF, and ether extract. Toxins and other antinutritional substances are present in some parts of M. oleifera, rendering them unfit for human or animal consumption. Alkaloids, tannins, saponins, and inhibitors abound in the tree's bark (Becker and Makkar, 1999; Foidl et al., 2001). Therefore, it has been recommended that more attention should be paid to the leaf nutritional composition of moringa because they are used for rations, particularly in carp nutrition (Becker and Makkar, 1999). Antinutritional substances, such as tannins, saponins, and alkaloids, can obstruct digestive enzymes and reduce feed usage (Foidl et al., 2001).
Phytochemicals in M. oleifera Leaves
The phytochemical analyses of M. oleifera leaves revealed several unusual chemicals, including rhamnose, glucosinolates, and isothiocyanate (Fahey et al., 2001; Bennett et al., 2003). Leaves also contain benzyl glucosinolates, 4-(a-L-rhamnopyranosyloxy)-benzylisothiocyanate, and 4-(4’-Oacetyl-a-L-rhamnopyranosyloxy) benzylthiocyanate. These compounds exhibit anticancer, hypotensive, and antibacterial properties (Fahey et al., 2001). The chemical structures of M. oleifera isothiocyanates are illustrated in Figure 2. The flowers of M. oleifera contain high amounts of flavonoid pigments, including isoquercitrin, kaempferitrin, rhamnetin, quercetin, and kaempferol (Fahey et al., 2001; Bennett et al., 2003).
Figure 2.
The chemical structures of moringa isothiocyanates.
Foidl et al. (2001) and Pandey et al. (2012) found that the leaf extracts of M. oleifera in 80% ethanol contain cytokine-like hormones. In an experiment conducted by Al-Asmari et al. (2015), it was demonstrated that such extracts possess cancer-preventive properties, as measured by the differentiation activity against human myeloid leukemia (HL-60) cells. Grubben and Denton (2004) discovered the presence of moringinine and moringine, which are alkaloids with medicinal properties, in the root bark of M. oleifera.
Antioxidants in M. oleifera Leaves
Yameogo et al. (2011) reported that M. oleifera is the ultimate source of various natural antioxidants, including flavonoids such as quercetin and kaempferol. According to Siddhuraju and Becker (2003), M. oleifera solvent extracts from different agroclimatic regions have distinct antioxidant profiles. The dry weights of ascorbate (vitamin C), phenolics, α-tocopherol, and β-carotene are 70 to 100 mol/g, 74 to 210 mol/g, 0.7 to 1.1 µmol/g, and 1.1 to 2.8 mol/g, respectively. The leaves have much higher antioxidant levels than the fruits and vegetables. For example, strawberries have 190 mol/g of gallic acid (GA)/g and carrots have 1.8 mol/g of β-carotene. Soybeans have 1.8 mol/g of α-tocopherol and hot peppers have 110 mol/g of ascorbate (Abbas and Ahmed, 2012).
The leaves of M. oleifera contain essential flavonoids, such as quercetin and kaempferol, which are stronger antioxidants than vitamin C (Anwar and Bhanger, 2003; Siddhuraju and Becker, 2003). M. oleifera leaves also possess a significant amount of ascorbic acid (Siddhuraju and Becker, 2003). According to Verma et al. (2009) and Sreelatha and Padma (2009), these antioxidants protect animals from degenerative illnesses and infections through the significant binding of free radicals, thus preventing oxidative DNA damage.
Antimicrobial Activity of M. oleifera Leaf Meal
Infectious diseases are currently one of the main global public health concerns. Bacteria, fungi, parasites, and viruses, are the causative agents of infectious diseases in humans and animals (El-Saadony et al., 2021; Yaqoob et al., 2021; Abd El-Hack et al., 2022c; El-Saadony et al., 2022c) therefore, scientists and researchers focus on exploring alternative methods for combating antibiotic resistance in pathogenic microorganisms. This purpose can be achieved by adding plant extracts and some effective and safe natural substances to foods (El-Saadony et al., 2020, 2022a,b; Abd El-Hack et al., 2021a,b,c; Saad et al., 2021a,b).
Various parts of M. oleifera possess antimicrobial potentials, which are useful in the eradication of pathogenic microorganisms, biofilm menace, and water purification. The safety of M. oleifera usage has been demonstrated by its long-term use and ethnopharmacological properties. M. oleifera has not only been used for its antimicrobial activity but also for bioenhancement and as nanoparticles in drug delivery (Arora et al., 2013). At present, there is concern regarding the safety of water disinfectants as most of them are implicated in health-related anomalies (Righi et al., 2012). Several studies have reported that M. oleifera seed kernels possess water purifying properties. Eilert et al. (1981) discovered a compound, 4-α-rhamnosyloxybenzyl isothiocyanate, presently known as glucosidal mustard oil, and found that it coagulates solid matter into water and eliminates most of the suspended bacteria.
Madsen et al. (1987) revealed that crushed M. oleifera seed kernels release natural polyelectrolytes, which function as flocculating agents by binding to suspended particles in a colloidal suspension. These bound particles form large sedimenting particles, known as flocs, and microorganisms attach to these flocs. Madsen et al. (1987) suggested that treating water with M. oleifera seed kernels (press cake) removes 90 to 99% of the fecal coliform bacterial load. However, they noted that after several hours of storage, the residual bacteria re-grew within the storage container and an additional disinfection process was required.
In similar studies, the flocculants obtained from M. oleifera seeds were further characterized as basic polypeptides with molecular weights of 6 to 16 kDa, with an isoelectric pH of 10 to 11 (Jahn, 1988). In a study based on traditional water purification using M. oleifera seeds, another group of researchers discovered a steroidal glycoside, strophantidin, as a bioactive agent that was highly efficient in the clarification and sedimentation of inorganic and organic matter in raw water. It reduced up to 65% of the microbial load after 24 h compared with an 83% reduction by alum under similar conditions (Olayemi and Alabi, 1994).
The potential of the active ingredients in M. oleifera seeds has been explored. Mangale et al. (2012) demonstrated that the number of bacterial and fungal colonies on plates decreased with increasing concentrations of the extract. Other studies revealed the inhibitory effects of various M. oleifera seed extracts on Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Vibrio cholera, Aspergillus niger, and Candida albicans (Atieno et al., 2011). The antibiotic agent in M. oleifera seeds has been identified as pterygospermin (Aney et al., 2009). It is believed that these extracts have a synergistic potential to restore the effectiveness of β-lactam antibiotics against methicillin-resistant S. aureus (Karthy et al., 2009).
Numerous studies have demonstrated the antimicrobial potential of M. oleifera leaves. For instance, a study on leaf extracts prepared in different solvents revealed that ether extract of M. oleifera leaves was the most effective component against the clinical and environmental isolates of Proteus mirabilis, a well-known causative agent of urinary tract infections (Arun and Rao, 2011). Several studies have been documented on the antibacterial activity of M. oleifera leaves against some bacterial species (Abalaka et al., 2011). Chuang et al. (2007) reported that the crude extracts and essential oils from M. oleifera leaves exhibit antifungal activity against Trichophyton rubrum, T. mentagrophytes, Epidermophyton floccosum, and Microsporum canis.
Patel et al. (2014) observed that both ethanolic and aqueous extracts of M. oleifera leaves were active against Saccharomyces cerevisiae and Candida tropicalis but not against C. albicans. Limited research is available on the antimicrobial activity of M. oleifera roots because of conservation-related concerns of this important plant. However, a few studies have shown the antimicrobial potential of the roots of this plant. For instance, in vitro studies on different extracts of the root bark of M. oleifera against S. aureus, E. coli, Salmonella gallinarum, P. aeruginosa, and others showed that ethyl acetate and acetone extracts exhibit maximum activity compared with other solvents (Anitha et al., 2011). Similarly, limited research has been conducted on the antimicrobial potential of the stem bark of M. oleifera, except a few preliminary reports. Studies on the antimicrobial activity of root extract of M. oleifera against some human pathogens have demonstrated the presence of active organic extracts with varying levels of activity (Bolin and Satyabrat, 2011).
Bioenhancers are molecules that do not exhibit any drug activity of their own but promote and augment the biological activity, bioavailability, or uptake of drugs in combination therapy, resulting in reduced drug-associated toxicity, cost, and duration of chemotherapy. The first bioenhancing property of M. oleifera pod extract was reported by Khanuja et al. (2005), where a niaziridin-rich fraction enhanced the bioactivity of commonly used antibiotics, such as rifampicin, tetracycline, and ampicillin, against gram-positive and gram-negative bacteria. It also facilitated the absorption of drugs, vitamins, and nutrients through the gastrointestinal membrane, which increased their bioavailability (Khanuja et al., 2005).
This observation was corroborated by a recent preclinical study on the influence of M. oleifera pods on the pharmacokinetic disposition of rifampicin using high-performance liquid chromatography–photodiode array. The active fraction isolated from air dried pods of the plant mixed with rifampicin and administered to the experimental animals enhanced systemic availability of the drug and suppressed the drug metabolizing cytochrome P-450 (Pal et al., 2010). Few reports are available on the antimicrobial activity of M. oleifera pods. Sayeed et al. (2012) studied the in vitro antimicrobial activity of methanolic extracts of M. oleifera fruits and reported that it was effective against some bacteria and fungi. Similar to roots and pods, the antimicrobial potential of the seed coat has not been sufficiently studied.
Alikwe et al. (2013) conducted a preliminary study on the antibacterial activity of the ethanolic leaf extracts of M. oleifera against P. aeruginosa, E. coli, Klebsiella sp., Salmonella typhimurium, and Proteus mirabilis. This plant part has shown antibiofilm potential against Staphylococcus epidermis as reported by Dayal et al. (2013). The increasing failure of conventional antibiotics because of side effects and the emergence of resistant microorganisms drive the search for better alternatives. Plants have proven to be a useful source of potential bioactive molecules; however, only a small percentage of these potential candidates have been explored. For instance, based on the scientifically supported ethnopharmacological claims, M. oleifera possesses antimicrobial potential. Most of the antimicrobial studies are preliminary; therefore, further studies to authenticate its candidature might be relevant. As recommended by Rios and Recio (2005), substantial knowledge could be obtained by the identification of the active fraction of extracts, mechanisms of action, and pharmacokinetic profile of extracts.
The presence of alkaloids, flavonoids (quercetin and kaempferol), saponins, and tannins in all extracts have been linked to various physiological actions in the human and animal bodies (Abdulkadir et al., 2015). Flavonoids have a hydroxyl group that confers antioxidant activity on M. oleifera and is thus used as a therapeutic agent (Pace-Asciak et al., 1995). Over the years, M. oleifera has been used as a traditional remedy for some diseases. It is rich in phytochemicals that exhibit effective antibacterial, antimycotic, antiviral, and potential anticancer activities. For instance, M. oleifera leaf extracts showed antiviral activity against foot and mouth disease (Younus et al., 2015). The antimicrobial properties of M. oleifera leaf extracts have also been reported against both gram-positive and gram-negative bacteria, such as S. aureus, E. coli and Salmonella (Van Weyenberg and Jacobs, 2013; Abdulkadir et al., 2015).
The antifungal properties of M. oleifera leaves have also been explored (Donli and Dauda, 2003). The extraction modes of the active ingredients play a role in the effectiveness of M. oleifera extracts against various pathogenic microorganisms. For instance, the antibacterial activity of the methanolic extracts of M. oleifera showed broader antimicrobial action compared with aqueous extracts (Donli and Dauda, 2003). M. oleifera has been known to exhibit both anti-inflammatory and anticancer properties (Cárceres et al., 1993; Bharali et al., 2003). Dried fruit powder possesses ameliorative potential against industrial fluorosis in cattle because altered hemato-biochemical parameters were restored after supplementation in fluorotic cows (Jena et al., 2016).
Antinutritional Factors in M. oleifera Leaves
M. oleifera leaves contain low amounts of polyphenols, such as tannins (1.4%) and total phenols (2.7%), which are less harmful to animals if consumed in appropriate amounts (Makkar and Becker, 1997). However, when consumed in large amounts, these factors may negatively affect the ability of an animal to utilize dietary nutrients and consequently deteriorate their health. The M. oleifera leaf meal also contains 5.6% of the saccharides raffinose and stachyose, which produce flatulence in monogastrics when consumed in excessive amounts (Gupta et al., 1989). Additionally, it contains nitrates (0.5 Mmol/100 g), oxalates (4.1%), saponins (1.2%), and phytates (3.1%). The high phytate concentrations in the leaves decrease the bioavailability of minerals in monogastrics (Reddy et al., 1982). Saponins from some plants induce an adverse effect on the growth of animals but those present in M. oleifera leaves are safe and do not exhibit hemolytic activity; therefore, humans can consume them without any adverse effects (Makkar and Becker, 1997).
Potential Toxicity of M. oleifera
To the best of our knowledge, only a few studies have established the highly adverse effects of M. oleifera as food or medication in humans. According to Berger et al. (1984), in a study to investigate the potential toxicity of seeds from M. stenopetala and M. oleifera, no harmful effects were noticed during the study evaluation period and no modifications were found in the histologic pictures of 28 organs in outbred Sprague–Dawley rats (Berger et al., 1984).
In a safety assessment analysis, Adedapo et al. (2009) revealed that administering M. oleifera leaf extract to rodents for 3 wk at a maximum dosage of 2,000 mg/kg was safe. They also reported that a dosage of 400 mg/kg resulted in a substantial increase in the packed cell volume, whereas a dosage of 800 mg/kg resulted in a significant decrease in the hemoglobin level and red blood cell counts. Asare et al. (2012) also presented comparable results, confirming that ingestion is safe at a dosage of <1,000 mg/kg body weight. However, 4-hydroxyphenylacetonitrile, 4-hydroxyphenyl-acetamide, and phenyl acetonitrile are biosynthetically and chemically similar substances extracted from roasted M. oleifera seeds, which may be highly poisonous (Villasenor et al., 1989).
According to Bhattacharya et al. (1978), M. oleifera bark extracts can induce abortion and cause violent uterine contractions, which may lead to death. Kavitha et al. (2012) revealed that M. oleifera seed powder dramatically affects the biochemical and hematological parameters of aquatic organisms, which can be fatal to these organisms. Fahey (2005) stated that the presence of isothiocyanate producing glycosides might explain the varied effects of M. oleifera extracts on the hematological parameters. Das and Mukherjee (2000) showed that the consumption of M. oleifera decreased the blood protein levels and they demonstrated that glycoside cyanides and isothiocyanate may trigger stress-mediated protein mobilization. Furthermore, Becker and Makkar (1999) demonstrated that dietary tannic acid, a tannin found in M. oleifera, might negatively influence the growth performance of carps (Cyprinus carpio L.) after 28 d of treatment. Therefore, future research should focus on the potential negative effects of using M. oleifera products in fish diets.
General Uses of M. oleifera
M. oleifera is a valuable plant as most of its parts are edible. The leaves of this plant can be used as food or forage during the wet and dry season (FAO, 2014). Humans consume M. oleifera pods, blossoms, roots, and leaves as a green vegetable substitute throughout Asia and Africa (Pandey et al., 2012). The consumption of leaves is suitable for vegetarians, especially for pregnant and nursing mothers as well as children, because they contain a high percentage of protein, vital minerals, and vitamins (A, B, and C) (FAO, 2014). M. oleifera seeds can be consumed in the raw or cooked form. These seeds contain 30 to 40% edible oil (ben oil) (Pandey et al., 2012).
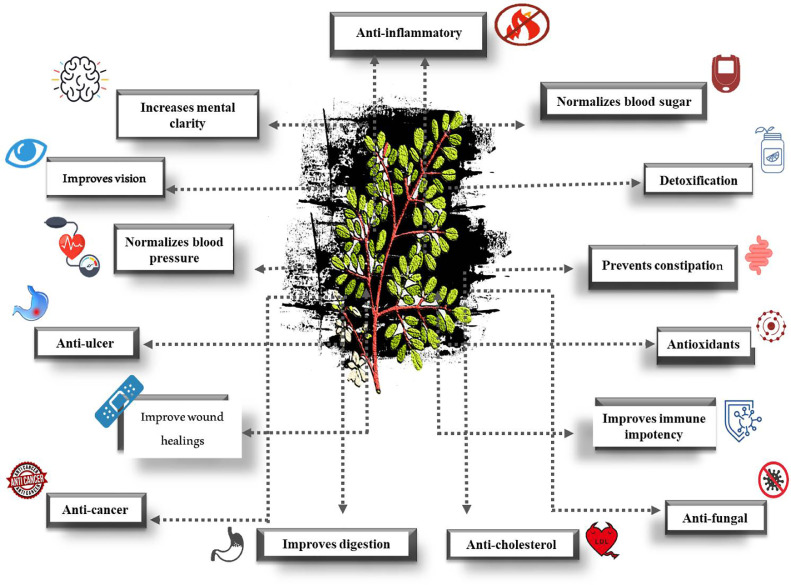
The high concentration of sterols, oleic acid, and tocopherols helps prevent rancidity (FAO, 2014). It possesses several medicinal properties, as shown in Figure 3. It has several therapeutic benefits, which include anti-inflammatory, anti-asthma, heart-protective, antioxidant, antiviral, and anticancer activities. M. oleifera seeds are rich in terygospermin with fungicidal properties as well as antibiotic properties against P. aeruginosa, Bacillus subtilis, S. aureus, and Fusarium solani (Price, 2007; Jabeen et al., 2008).
Figure 3.
Medicinal properties of Moringa oleifera tree.
M. oleifera leaves, which are rich in iron, can cure anemic individuals. Humans also use the bark and roots of this plant to treat heart diseases (Orwa et al., 2009). According to Ogbe and John (2012), feeding M. oleifera leaves with other concentrates might boost the efficiency of the concentrate. Similarly, Price (2007) determined that soybean meal provided with M. oleifera leaves had a significant impact on poultry growth performance, with improved bird feed conversion ratio. M. oleifera seeds can be used in water purification and biodiesel generation (Orwa et al., 2009). According to Aruna and Srilatha (2012), M. oleifera seed powder has antimicrobial properties that can disinfect and purify water in aquaculture ponds. Additionally, Egbuikwem and Sangodoyin (2013) determined the optimal amount of M. oleifera seed extract that was effective against E. coli in stream water; moreover, they found that the seed extract had a contaminant extraction efficiency of >90% for stream, well, and pond water specimens.
Rashid et al. (2008) reported that M. oleifera seeds have excellent properties necessary for biodiesel production. The oil cakes contain 1% flocculant proteins, which preserve the minerals and organic compounds during the purification of drinking water, making them suitable for water treatment. Further, they can be used for efficient fiber deposition in the beer and juice industries. Although M. oleifera seeds are not as efficient as alum in removing turbidity, they have been suggested as a potential alternative to some traditional chemically manufactured coagulants, such as alum, which may increase the risk of cancer. This is because these seeds are natural, ecofriendly, biodegradable, and safe (Preston et al., 2010; Aruna and Srilatha, 2012; Egbuikwem and Sangodoyin, 2013).
According to Foidl et al. (2001) and Pandey et al. (2012), the oil obtained from M. oleifera seeds preserves aroma easily and can be used in the perfume industry. It is also less prone to rancidity, thus making it superior to other oils. The bark of M. oleifera can be used for dyeing; however, the wood is not suitable for use in heavy construction (Pandey et al., 2012). Sprinkling M. oleifera leaf extract on the leaves of peanut, sugarcane, soybean, and coffee improved the plant yield by 20 to 35% (Foidl et al., 2001). Owing to its antiviral effect, M. oleifera leaf extract can treat some viral diseases, especially the Newcastle disease virus (Chollom et al., 2012).
M. oleifera Leaf Inclusion in Poultry Diets
The nutritional properties of M. oleifera leaf meal are summarized in Table 3. The leaves of M. oleifera are free of heavy metals, such as mercury, cadmium, and arsenic. They also have adequate amounts of vitamins, particularly A, B, and C; thus, including M. oleifera leaves in chicken diets is safe and can improve output performance in poultry production (Donkor et al., 2013). However, feeding M. oleifera leaves or leaf powder to poultry may have poor palatability (Price, 2007). Figure 4 highlights the effects of including M. oleifera leaves in poultry diets.
Table 3.
The nutritional qualities of Moringa oleifera leaves’ meal.
| Nutritive value | Dry leaves | References |
|---|---|---|
| Crude protein (CP %) | 25.1 to 30.29 | Moyo et al., 2011; Foidl et al., 2001 |
| Neutral detergent fibers (NDF%) | 11.40 to 21.9 | Foidl et al., 2001; Richter et al., 2003; Moyo et al., 2011 |
| Acid detergent fibers (ADF%) | 8.49 to 11.4 | Foidl et al., 2001; Richter et al., 2003; Moyo et al., 2011 |
| Gross energy (MJ/kg DM) | 18.7 | Foidl et al., 2001 |
| Ether extract (EE%) | 5.4 | Foidl et al., 2001 |
| Lysine (Lys%) | 1.1 to 1.64 | Richter et al., 2003; Moyo et al., 2011 |
| Histidine (His%) | 0.6 to 0.72 | Richter et al., 2003; Moyo et al., 2011 |
| Threonine (Thr%) | 0.8 to 1.36 | Richter et al., 2003; Moyo et al., 2011 |
| Arginine (Arg%) | 1.2 to 1.78 | Richter et al., 2003; Moyo et al., 2011 |
| Methionine (Met%) | 0.30 | Moyo et al., 2011 |
| Total phenolics (%) | 2.02 to 2.74 | Richter et al., 2003; Moyo et al., 2011 |
| Tannins (%) | 0.53 | Richter et al., 2003 |
| Condensed tannins (mg/g) | 3.12 | Moyo et al., 2011 |
Figure 4.
The impacts of applying Moringa oleifera leaves in poultry diets.
Several enzymes, including phytase, may be introduced to a diet containing M. oleifera leaves (Gaia, 2005; Fuglie, 2009). A sufficient amount of dietary M. oleifera leaves may positively influence the growth, carcass traits, and production performance of poultry. Ayssiwede et al. (2011) suggested that digestibility and antibacterial capabilities against gastrointestinal pathogens contribute to improved feed efficiency. M. oleifera forage can be safely added up to 10% to a cassava-based diet without lowering feed consumption. According to Olugbemi et al. (2010), M. oleifera has hypocholesterolemic properties and can be added to chicken rations to significantly reduce the cholesterol levels in eggs. Abou-Elezz et al. (2011) showed that M. oleifera leaf meals (approximately 10%) might improve yolk color without significant negative impacts on the egg-laying rate. According to previous research, 10% M. oleifera leaf meal is recommended as a long-term feed additive for layer diets. Compared with the control, food supplementation with 2.5% M. oleifera leaf meal improved the quality of the internal eggs, as determined by Ebenebe et al. (2013).
The incorporation of different amounts of M. oleifera leaf meal (0, 5, 10, and 15%) to the layer diet resulted in lower egg production and egg-laying rate. In contrast, egg weight continued to increase quadratically with increasing doses of M. oleifera leaf meal (Olugbemi et al., 2010). Overall, 5% M. oleifera leaf meals were helpful to birds. However, 15 and 20% leaf meals showed negative effects according to Kakengi et al. (2007) and Abou-Elezz et al. (2011).
Other researchers reported that a high dose of M. oleifera can reduce the egg-laying rate. Replacing sunflower seed powder with 20% M. oleifera leaf powder in the layer diet significantly reduced the egg yield and total egg weight (Kakengi et al., 2007). Likewise, Mutayoba et al. (2003) stated that 20% M. oleifera leaf meal intake affected the production of large eggs and egg-laying rate even with food consumed. In contrast, a consumption rate of 5% supplementation had no negative impact. Etalem et al. (2013) claimed that M. oleifera leaf meal could replace soybean meal in broiler chicken rations, but the increased levels of dietary leaf meal decreased the growth rate in those chickens. The negative effect of high dose of leaf meal on nutrition may be due to inadequate protein digestion (Olugbemi et al., 2010).
Kakengi et al. (2007) reported that a significant amount of DM and forage intake was observed when laying hens were fed a diet supplemented with 10% and 20% M. oleifera leaf meals. This result was consistent with that of a previous study by Abou-Elezz et al. (2011), which revealed that the consumption of DM showed a squared trend with higher levels of M. oleifera leaf powder (0–15%). In contrast, high levels of M. oleifera leaf powder reduced the chicken feed system intake (Kakengi et al., 2007). When a small amount of M. oleifera leaf meal was added to the feed, there were no negative impacts on feed utilization, and it may enhance the feed conversion rate. Approximately 10% dietary addition of M. oleifera leaf meal did not affect feed consumption, feed conversion efficiency, or body weight (Juniar et al., 2010). Broilers fed a diet supplemented with 5% M. oleifera leaf meal for 7 wk gained more weight, consumed more total feed, and converted feed more efficiently (Safa and Tazi, 2014). M. oleifera leaf meal can substitute groundnut cake in the grower rabbit diets, with an additional rate of up to 60% (Adeniji and Lawal, 2012).
In another study, Gadzirayi et al. (2012) stated that when M. oleifera leaf meal was included in the diet, the DM intake increased in broilers due to the increased bulk and metabolizable concentrations. M. oleifera leaf meal is a good source of yolk pigments and had no detrimental impacts on shell thickness or egg shape index (Kaijage et al., 2003). This may be due to the high carotene levels in M. oleifera leaf meal, which extended from 15.25 to 16.30 mg/100 g (Etalem et al., 2013). More egg albumens and less yolk index indicate decreased cholesterol levels, which is a positive trait among egg consumers (Kaijage et al., 2003).
Low levels of M. oleifera supplementation enhanced egg quality, whereas excessive levels resulted in lower output of eggs (Abou-Elezz et al., 2011). The combination of 5% sunflower seed meal and M. oleifera leaf meal in the diet had a significant impact on the egg weight (Kakengi et al., 2007). The lower egg mass at high concentrations of M. oleifera leaf meal was probably because of decreased energy requirement, protein preservation, and CF nutritional value, as determined using 10% M. oleifera leaf meal (Lu et al., 2016). M. oleifera showed hypocholesterolemic characteristics, indicating that it lowers the cholesterol content of eggs in layer diets (Olugbemi et al., 2010). M. oleifera is widely known for its antioxidant properties, high phytochemical content, consumer acceptability, manufacturing properties, poultry product stability, and storage life (Jung et al., 2010; Abbas and Ahmed, 2012). The most essential antioxidant in M. oleifera is flavonoids, particularly flavonols (Pandey et al., 2012). They have a higher antioxidant capacity than vitamin C and can extend the life span of chicken products (Pennington and Fisher, 2009). These findings indicatd that M. oleifera leaves are an excellent addition to the chicken diet; however, dietary amounts should be carefully monitored. Employing biosecurity measures, hygienic disposal of poultry waste along with a proper vaccination program, and the usage of natural feed additives can enhance poultry production worldwide and help to avoid the risk of infection with different avian pathogens (Abd El-Hack et al., 2022d; Attia et al., 2022; El-Naggar et al., 2022; Mahmoud et al., 2022).
M. oleifera as a Nonruminant Feed
M. oleifera has extensive potential as a livestock feed (Richter et al., 2003). The low levels of antinutritional factors and high levels of protein, lipid, and sulfur-containing amino acids make it an excellent source of protein for nonruminants (Richter et al., 2003; Ferreira et al., 2008). M. oleifera leaves also contain all essential amino acids, including sulfur-containing amino acids, in higher concentration than the recommended daily intake, making it a good source of protein for monogastrics (Ferreira et al., 2008).
In Ghana, improved weight gains were reported in rabbits fed with M. oleifera; therefore, it was recommended for use either as partial or total replacement for soybean meal without any adverse effects on the productive performance and blood indices of weaned rabbits (Nuhu, 2010). In broilers, it was established that M. oleifera can replace soybean at 25% of the total diet without negatively affecting growth performance, feed intake, and feed conversion efficiency (Gadzirayi et al., 2012).
Other Uses of M. oleifera
M. oleifera seeds contain naturally occurring proteins that are more effective coagulants than alum (Ndabigengesere et al., 1995). Furthermore, M. oleifera seed extracts significantly improved water quality, particularly the number of cryptosporidium oocysts and water turbidity (Petersen et al., 2016). The relative lack of toxic compounds in the seed and its ability to clarify and purify muddy water make it a suitable alternative to obtaining clean drinking water (Ndabigengesere et al., 1995; Anwar et al., 2007; Ferreira et al., 2008). M. oleifera seed oil has high oxidative stability, making it fit for the cosmetic manufacturing (Sánchez-Machado et al., 2015). Alternatively, the pods can be used as litter in poultry and other housed livestock. These uses ensure the sustainable utilization of natural resources and environmental conservation.
CONCLUSIONS
The valuable M. oleifera tree, also called the “drumstick” or horseradish tree, can be grown in severe dry and cold conditions. It has a wide range of nutrients. M. oleifera contains crude protein ranging from 71.2 to 391.7 g/kg in different parts of the plant. In previous studies, M. oleifera leaf meal has partially replaced sunflower seed cake and soybean meal as a protein source in chicken rations. Leaf meal has been associated with significant economic benefits, no adverse side effects, and even improved growth and product quality at appropriate dietary integration levels (5–10% in broiler rations and 10% in layer rations). Nevertheless, M. oleifera leaf meal (20% for broilers or layers and 80% for rabbits) may have specific harmful effects, possibly because of high levels of unique substances, such as tannins, which influence feed absorption or nutrient digestibility. The components and nutrients, including bioavailability and amino acid profile, of M. oleifera leaf meal must be studied in detail so that it can be frequently and widely used in poultry diets.
ACKNOWLEDGMENTS
Author contributions: All authors equally contributed to writing this review article. All authors reviewed and approved the final version of the manuscript. Prof. Khaled A. El-Tarabily thanks the library at Murdoch University, Australia, for the valuable online resources and comprehensive databases.
DISCLOSURES
The authors declare no conflict of interest.
References
- Abalaka M.E., Daniyan S.Y., Oyeleke S.B., Adeyemo S.O. The antibacterial evaluation of Moringa oleifera leaf extracts on selected bacterial pathogens. J. Microbiol. Res. 2011;1:1–4. [Google Scholar]
- Abbas R.K., Elsharbasy F.S., Fadlelmula A.A. Nutritional values of Moringa oleifera, total protein, amino acid, vitamins, minerals, carbohydrates, total fat and crude fiber, under the semi-arid conditions of Sudan. J. Microb. Biochem. Technol. 2018;10:56–58. [Google Scholar]
- Abbas T.E., Ahmed M.E. Use of Moringa oleifera seeds in broilers diet and its effects on the performance and carcass characteristics. Int. J. Appl. Poult. Res. 2012;1:1–4. [Google Scholar]
- Abd El-Hack M.E., Alagawany M. Performance, egg quality, blood profile, immune function, and antioxidant enzyme activities in laying hens fed diets with thyme powder. J. Anim. Feed Sci. 2015;24:127–133. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Elrys A.S., Desoky E.S.M., Tolba H.M.N., Elnahal A.S.M., Elnesr S.S., Swelum A.A. Effect of forage Moringa oleifera L. (moringa) on animal health and nutrition and its beneficial applications in soil, plants and water purification. Agriculture. 2018;8:145. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Karthik K., Dhama K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharmacol. 2016;12:232–248. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Kumaragurubaran K., Dhama K., Zorriehzahra J., Adel M. Beneficial impacts of thymol essential oil on health and production of animals, fish and poultry: a review. J. Essent. Oil Res. 2016;28:365–382. [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Gado A.R., Nader M.M., Saad A.M., El-Tahan A.M., Taha A.E., Salem H.M., El-Tarabily K.A. Hot red pepper powder as a safe alternative to antibiotics in organic poultry feed: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A.M., Al-Wajeeh A.S., Al-Shargi O.Y.A., Taha A.E., Mesalam N.M., Abdel-Moneim A.M.E. Prebiotics can restrict Salmonella populations in poultry: a review [e-pub ahead of print] Anim. Biotechnol. 2021:1–10. doi: 10.1080/10495398.2021.1883637. Accessed July 2022. doi: [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd Elkader A.M., Labib S., Taha T.F., Althobaiti F., Aldhahrani A., Salem H.M., Saad A., Ibrahim F.M. Phytogenic compounds from avocado (Persea americana L.) extracts; antioxidant activity, amylase inhibitory activity, therapeutic potential of type 2 diabetes. Saudi J. Biol. Sci. 2021;29:1428–1433. doi: 10.1016/j.sjbs.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-Shargi O.Y.A., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdulkadir I.S., Nasir I.A., Sofowora A., Yahaya F., Ahmad A.A., Hassan I.A. Phytochemical screening and antimicrobial activities of ethanolic extracts of Moringa oleifera Lam on isolates of some pathogens. J. App. Pharm. 2015;7:1–7. [Google Scholar]
- Abdul, D. A. S. 2007. Economic importance of Moringa oleifera in Tafa local government area of Niger State. NDE Project. Federal College of Forestry Mechanization. Kaduna, Nigeria.
- Abou-Elezz F.M.K., Sarmiento-Franco L., Santos-Ricalde R., Solorio-Sanchez F. Nutritional effects of dietary inclusion of Leucaena leucocephala and Moringa oleifera leaf meal on Rhode Island Red hens’ performance. Cuban J. Agric. Sci. 2011;45:163–169. [Google Scholar]
- Abou-Kassem D.E., El-Abasy M.M., Al-Harbi M.S., Abol-Ela S., Salem H.M., El-Tahan A.M., El-Saadony M.T., Abd El-Hack M.E., Ashour E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2022;29:1683–1693. doi: 10.1016/j.sjbs.2021.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Adedapo A., Mogbojuri O., Emikpe B. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J. Med. Plant. Res. 2009;3:586–591. [Google Scholar]
- Adeniji A., Lawal M. Effects of replacing groundnut cake with Moringa oleifera leaf meal in the diets of grower rabbits. Int. J. Mol. Vet. Res. 2012;2:8–13. [Google Scholar]
- Ahossi P.K., Dougnon J.T., Kiki P.S., Houessionon J.M. Effects of Tridax procumbens powder on zootechnical, biochemical parameters and carcass characteristics of Hubbard broiler chicken. J. Anim. Health Prod. 2016;4:15–21. [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., El-Naggar K., Taha A.E., Khafaga A.F., Madkour M., Salem H.M., Eltahan A., El-Saadony M.T., Abd El-Hack M.E. Betaine and related compounds: chemistry, metabolism, and role in mitigating heat stress in poultry. J. Therm. Biol. 2021;104 doi: 10.1016/j.jtherbio.2021.103168. [DOI] [PubMed] [Google Scholar]
- Al-Asmari A.K., Albalawi S.M., Athar M.T., Khan A.Q., Al-Shahrani H., Islam M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PloS One. 2015;10 doi: 10.1371/journal.pone.0135814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikwe P.C.N., Ohimain E.I., Zige D.V., Angaye T.N.C. Antibacterial activity of ethanol extract of the defatted seed and seed coat of Moringa oleifera. J. Pharm. Biol. Sci. 2013;8:38–41. [Google Scholar]
- Amerah A.M., Péron A., Zaefarian F., Ravindran V. Influence of whole wheat inclusion and a blend of essential oils on the performance, nutrient utilization, digestive tract development and ileal microbiota profile of broiler chickens. Br. Poult. Sci. 2011;52:124–132. doi: 10.1080/00071668.2010.548791. [DOI] [PubMed] [Google Scholar]
- Aney J.S., Rashmi T., Maushumi K., Kiran B. Pharmacological and pharmaceutical potential of Moringa oleifera: a review. J. Pharm. Res. 2009;2:1424–1426. [Google Scholar]
- Anitha J.R., Velliyur K.G., Sangilimuthu A.Y., Sudarsanam D. Antimicrobial activity of Moringa oleifera Lam. root extract. J. Pharm. Res. 2011;4:1426–1427. [Google Scholar]
- Anwar F., Bhanger M.I. Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J. Agric. Food Chem. 2003;51:6558–6563. doi: 10.1021/jf0209894. [DOI] [PubMed] [Google Scholar]
- Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: a food plant with multiple medicinal uses. Phyther. Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Aregheore E.M. Intake and digestibility of Moringa oleifera–batiki grass mixtures by growing goats. Small Rumin. Res. 2002;46:23–28. [Google Scholar]
- Arif M., Hayat Z., Abd El-Hack M.E., Saeed M., Imran H.M., Alowaimer A.N., Saadeldinm I.M., Taha A.E., Swelum A.A. Impacts of supplementing broiler diets with a powder mixture of black cumin, moringa and chicory seeds. S. Afr. J. Anim. Sci. 2019;49:564–572. [Google Scholar]
- Arif M., Rehman A., Naseer K., Abdel-Hafez S.H., Alminderej F.M., El Saadony M.T., Abd El-Hack M.E., Taha A.E., Elnesr S.S., Salem H.M., Alagawany M. Effect of Aloe vera and clove powder supplementation on growth performance, carcass and blood chemistry of Japanese quails. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D.S., Onsare J.G., Kuar H. Bioprospecting of moringa (Moringaceae): microbiological perspective. J. Pharmacogn. Phytochem. 2013;1:193–215. [Google Scholar]
- Arun T., Rao P.C.H. Phytochemical screening and antibacterial activity of Moringa oleifera Lam. against Proteus mirabilis from urinary tract infected patients. Int. J. Pharm.Tech. Res. 2011;3:2118–2123. [Google Scholar]
- Aruna M., Srilatha N. Water clarification using Moringa oleifera Lam. seed as a natural coagulant. Curr. Biot. 2012;5:472–486. [Google Scholar]
- Asare G.A., Gyan B., Bugyei K., Adjei S., Mahama R., Addo P., Out-Nyarko L., Wiredu E.K., Nyarko A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012;139:265–272. doi: 10.1016/j.jep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Ashour E.A., El-Kholy M.S., Alagawany M., Abd El-Hack M.E., Mohamed L.A., Taha A.E., El Sheikh A.I., Laudadio V., Tufarelli V. Effect of dietary supplementation with Moringa oleifera leaves and/or seeds powder on production, egg characteristics, hatchability and blood chemistry of laying Japanese quails. Sustainability. 2020;12:2463. [Google Scholar]
- Atieno W., Wagai S., Arama P., Ogur J. Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n- hexane seed extracts on bacteria implicated in water borne diseases. Afr. J. Microbiol. Res. 2011;5:153–157. [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2022;29:1644–1652. doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayssiwede S.B., Zanmenou J.C., Issa Y., Hane M.B., Dieng A., Chrysostome C.A.A.M., Houinato M.R., Hornick J.L., Missohou A. Nutrient composition of some unconventional and local feed resources available in Senegal and recoverable in indigenous chickens or animal feeding. Pak. J. Nutr. 2011;10:707–717. [Google Scholar]
- Becker K., Makkar H.P.S. Effects of dietary tannic acid and quebracho tannin on growth performance and metabolic rates of common carp (Cyprinus carpio L.) Aquaculture. 1999;175:327–335. [Google Scholar]
- Bennett R.N., Mellon F.A., Foidl N., Pratt J.H., Dupont M.S., Perkins L., Kroon P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003;51:3546–3553. doi: 10.1021/jf0211480. [DOI] [PubMed] [Google Scholar]
- Berger M.R., Habs M., Jahn S.A., Schmahl D. Toxicological assessment of seeds from Moringa oleifera and Moringa stenopetala, two highly efficient primary coagulants for domestic water treatment of tropical raw waters. East Afr. Med. J. 1984;61:712–717. [PubMed] [Google Scholar]
- Bharali R., Tabassum J., Azad M.R.H. Chemomodulatory effect of Moringa oleifera Lam. on hepatic carcinogen metabolizing enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pac. J. Cancer Prev. 2003;4:131–139. [PubMed] [Google Scholar]
- Bhattacharya J., Guha G., Bhattacharya B. Powder microscopy of bark-poison used for abortion: moringa pterygosperma gaertn. J. Indian Forensic Sci. 1978;17:47. [PubMed] [Google Scholar]
- Bolin C., Satyabrat G. Antibacterial activity of the methanolic extract of stem bark of Spondias pinnata, Moringa oleifera and Alstonia scholaris. Asian J. Tradit. Med. 2011;6:163–167. [Google Scholar]
- Botsoglou N., Florou-Paneri P., Botsoglou E., Dotas V., Giannenas I., Koidis A., Mitrakos P. The effect of feeding rosemary, oregano, saffron and a-tocopheryl acetate on hen performance and oxidative stability of eggs. S. Afr. J. Anim. Sci. 2005;35:143–151. [Google Scholar]
- Botsoglou N.A., Christaki E., Fletouris D.J., Florou-Paneri P., Spais A.B. Effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002;62:259–265. doi: 10.1016/s0309-1740(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Burtis M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cárceres A., Saravia A., Rizzio S., Zabala L., De Leon E., Nave F. Pharmacological properties of Moringa oleifera. 2: screening for antispasmodic, anti-inflammatory and diuretic activity. J. Ethnopharmacol. 1993;36:233–237. doi: 10.1016/0378-8741(92)90049-w. [DOI] [PubMed] [Google Scholar]
- Chollom S.C., Agada G.O.A., Gotep J.G., Mwankon S.E., Dus P.C., Bot Y.S., Nyango D.Y., Singnap C.L., Fyaktu E.J., Okwori A.E.J. Investigation of aqueous extract of Moringa oleifera lam seed for antiviral activity against Newcastle disease virus in ovo. J. Med. Plant. Res. 2012;6:3870–3875. [Google Scholar]
- Christaki E.V., Bonos E.M., Florou-Paneri P.C. Comparative evaluation of dietary oregano, anise and olive leaves in laying Japanese quails. Braz. J. Poult. Sci. 2011;13:97–101. [Google Scholar]
- Chuang P.H., Lee C.W., Chou J.Y., Murugan M., Shieh B.J., Chen H.M. Antifungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007;98:232–236. doi: 10.1016/j.biortech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ciftci M., Guler T., Dalkilic B., Ertas O.N. The effect of anise oil (Pimpinella anisum) on broiler performance. Inter. J. Poult. Sci. 2005;4:851–855. [Google Scholar]
- Cross D.E., Mcdevith R.M., Hillman K., Agamovic T. The effect of herbs and their associated essential oils on performance, digestibilities and gut microflora in chickens 7 to 28d of age. Br. Poult. Sci. 2007;4:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Das B.K., Mukherjee S.C. Sublethal effect of quinalphos on selected blood parameters of Labeo rohita (Ham.) Asian Fish. Sci. 2000;13:225–233. [Google Scholar]
- Dayal B., Yannamreddy R.V., Ragunath C., Ramasubbu N., Roy T., Amin R., Nirujogo S.P., Lea M.A. Pages 177–191 in Advances in Applied Nanotechnology for Agriculture. American Chemical Society Symposium Series; Washington, DC: 2013. Breaking up of biofilms with Moringa oleifera: insights into mechanisms. [Google Scholar]
- Donkor A.M., Glover R.L.K., Addae D., Kubi K.A. Estimating the nutritional value of the leaves of Moringa oleifera on poultry. Food Nutr. Sci. 2013;4:1077–1083. [Google Scholar]
- Donli P.O., Dauda H. Evaluation of aqueous moringa seed extract as a seed treatment biofungicide for groundnuts. Pest Manag. Sci. 2003;59:1060–1062. doi: 10.1002/ps.595. [DOI] [PubMed] [Google Scholar]
- Duke J.A. Pages 214–217 in Handbook of Nuts (Ed. Duke JA) CRC Press; Boca Raton, FL: 2001. Moringa oleifera Lam. (Moringaceae) [Google Scholar]
- Ebenebe C.I., Anigbogu C.C., Anizoba M.A., Ufele A.N. Effect of various levels of moringa leaf meal on the egg quality of Isa Brown breed of layers. Adv. Life Sci. Technol. 2013;14:1–6. [Google Scholar]
- Egbuikwem P.N., Sangodoyin A.Y. Coagulation efficacy of Moringa oleifera seed extract compared to alum for removal of turbidity and E. coli in three different water sources. E. I. J. S. T. 2013;2:13–20. [Google Scholar]
- Eilert U., Wolters B., Nahrstedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala. Planta Med. 1981;42:55–61. doi: 10.1055/s-2007-971546. [DOI] [PubMed] [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2022;10:55–61. [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Swelum A.A., Abo Ghanim M.M., Shukry M., Omar A.A., Taha A.E., Salem H.M., El-Tahan A.M., El-Tarabily K.A., Abd El-Hack M.E. Shrimp production, the most important diseases that threaten it, and the role of probiotics in confronting these diseases: a review. Res. Vet. Sci. 2022;144:126–140. doi: 10.1016/j.rvsc.2022.01.009. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Cerbo A.D., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2022 doi: 10.1080/87559129.2021.1944183. In Press. [DOI] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etalem T., Getachew A., Mengistu U., Tadelle D. Moringa oleifera leaf meal as an alternative protein feed ingredient in broiler ration. Int. J. Poult. Sci. 2013;12:289–297. [Google Scholar]
- Fahey J.W. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Trees Life J. 2005;1:1–15. [Google Scholar]
- Fahey J.W., Zakmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Photochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- Ferreira P.M.P., Farias D.F., de A. Oliveira J.T., de F. U. Carvalho A. Moringa oleifera: bioactive compounds and nutritional potential. Rev. Nutr. 2008;21:431–437. [Google Scholar]
- Foidl, N., H. P. S. Makkar, and K. Becker, 2001.The potential of Moringa oleifera for agricultural and industrial uses. What development potential for Moringa products? Accessed May 2021. https://www.feedipedia.org/node/19286.
- Food and Agricultural Organization (FAO). 2014. Moringa traditional crop of the month. Accessed May 2021. http://www.fao.org/traditional-crops/moringa/en/
- Fuglie L. New uses of moringa studied in Nicaragua. Moringa leaf concentrate. ECHO Dev. Notes 2009, 2009;68:1–8. [Google Scholar]
- Gadzirayi C.T., Masamha B., Mupangwa J.F., Washaya S. Performance of broiler chickens fed on mature Moringa oleifera leaf meal as a protein supplement to soybean meal. Int. J. Poult. Sci. 2012;11:5–10. [Google Scholar]
- Gaia, S. 2005. Wonder tree 100 facts moringa fact 04 exceptional animal feed moringa as livestock feed & pet food. Moringa Mission Trust. Accessed Oct. 2013. http://gaiathelivingplanet.blogspot.com/2005/06/wondertree-100-facts-moringa-fact-04.html.
- Giannenas I., Floroupaneri P., Papazahariadou M., Christaki E., Botsoglou N., Spais A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003;57:99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Govaris A., Botsoglou E., Florou-Paneri P., Moulas A., Papageorgiou G. Dietary supplementation of oregano essential oil and α–tocopheryl acetate on microbial growth and lipid oxidation of turkey breast fillets during storage. Int. J. Poult. Sci. 2005;4:969–975. [Google Scholar]
- Govaris A., Florou-Paneri P., Botsoglou E., Giannenas I., Ambrosiadis I., Botsoglou N. The inhibitory potential of feed supplementation with rosemary and/or α-tocopheryl acetate on microbial growth and lipid oxidation of turkey breast during refrigerated storage. LWT-Food Sci. Technol. 2007;40:331–337. [Google Scholar]
- Grubben G.J.H., Denton O.A. PROTA Foundation; Wageningen, Netherlands: 2004. Plant Resources of Tropical Africa 2. Vegetables. [Google Scholar]
- Gupta K., Barat G.K., Wagle D.S., Chawla H.K.L. Nutrient contents and antinutritional factors in conventional and non-conventional leafy vegetables. Food Chem. 1989;31:105–116. [Google Scholar]
- Jabeen R., Shahid M., Jamil A., Ashraf M. Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera. Pak. J. Bot. 2008;40:1349–1358. [Google Scholar]
- Jahn S.A.A. Using Moringa seeds as coagulants in developing countries. J. Am. Water Works Assoc. 1988;80:43–50. [Google Scholar]
- Jena C.K., Gupta A.R., Patra R.C. Protective effect of Moringa oleifera on haematological and biochemical parameters of cattle from industrial fluoride polluted area. J. Anim. Res. 2016;6:91–97. [Google Scholar]
- Jung S., Choe J.H., Kim B., Yun H., Kruk Z.A., Jo C. Effect of a dietary mixture of garlic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010;86:520–526. doi: 10.1016/j.meatsci.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Juniar I., Widodo E., Sjofjan O. Effect of Moringa oleifera leaf meal in the feed on broiler production performance. Indones. J. Anim. Sci. 2010;18:238–242. [Google Scholar]
- Kaijage J.T., Sarwatt S.V., Mutayoba S.K. Numerical proceedings papers 126-129. 2003. Moringa oleifera leaf meal can improve quality characteristics and consumer preference of marketable eggs.http://www.costech.or.tz accessed Apr. 2009. [Google Scholar]
- Kakengi A.M.V., Kaijage J.T., Sarwatt S.V., Mutayoba S.K., Shem M.N., Fujihara T. Effect of Moringa oleifera leaf meal as a substitute for sunflower seed meal on performance of laying hens in Tanzania. Livest. Res. Rural De. 2007;19:120. [Google Scholar]
- Karthy E.S., Ranjitha P., Mohankumar A. Antimicrobial potential of plant seed extracts against multidrug resistant methicillin resistant Staphylococcus aureus (MDR – MRSA) Int. J. Biol. 2009;1:34–40. [Google Scholar]
- Kavitha C., Ramesh M., Kumaran S.S., Lakshmi S.A. Toxicity of Moringa oleifera seed extract on some hematological and biochemical profiles in a freshwater fish Cyprinus carpio. Exp. Toxicol. Pathol. 2012;64:681–687. doi: 10.1016/j.etp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Khanuja, S. P. S., J. S. Arya, R. S. K. Tiruppadiripuliyur, D. Saikia, H. Kaur, M. Singh, S. C. Gupta, A. K. Shasany, M. P. Darokar, S. K. Srivastava, M. M. Gupta, S. C. Verma, and A. Pal. 2005. Nitrile glycoside useful as a bioenhancer of drugs and nutrients, process of its isolation from Moringa oleifera. US Patent No. 6,858,588. Accesssed Apr. 2022. https://patents.google.com/patent/US6858588B2/en.
- Lako J., Trenerry V.C., Wahlqvist M., Wattanapenpaiboon N., Sotheeswaran S., Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. [Google Scholar]
- Lopez-Bote C.J., Gray J.I., Gomaa E.A., Flegal C.J. Effect of dietary administration of oil extracts from rosemary and sage on lipid oxidation in broiler meat. Br. Poult. Sci. 1998;39:235–240. doi: 10.1080/00071669889187. [DOI] [PubMed] [Google Scholar]
- Lu W., Wang J., Zhang H.J., Wu S.G., Qi G.H. Evaluation of Moringa oleifera leaf in laying hens: effects on laying performance, egg quality, plasma biochemistry and organ histopathological indices. Ital. J. Anim. Sci. 2016;15:658–665. [Google Scholar]
- Madsen M., Schlundt J., Omer E.F. Effect of water coagulation by seeds of Moringa oleifera on bacterial concentrations. J. Trop. Med. Hyg. 1987;90:101–109. [PubMed] [Google Scholar]
- Mahmoud I., Hassan M., Aboelenin S.M., Soliman M.M., Attia H.F., Metwally K.A., Salem H.M., El-Tahan A.M., El-Saadony M.T., Khalaphallah R. Biogas manufacture from co-digestion of untreated primary sludge with raw chicken manure under anaerobic mesophilic environmental conditions. Saudi J. Biol. Sci. 2022;29:2969–2977. doi: 10.1016/j.sjbs.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar H.P.S., Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. 1997;128:311–322. [Google Scholar]
- Mangale S.M., Chonde S.G., Jadhav A.S., Raut P.D. Study of Moringa oleifera (Drumstick) seed as natural absorbent and antimicrobial agent for river water treatment. J. Nat. Prod. Plant Resour. 2012;2:89–100. [Google Scholar]
- Maundu P., Tengnäs B. World Agroforestry Centre; Nairobi, Kenya: 2005. Useful Trees and Shrubs for Kenya. [Google Scholar]
- Moyo B., Masika P.J., Hugo A., Muchenje V. Nutritional characterization of moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011;10:12925–12933. [Google Scholar]
- Mustapha Y., Babura S.R. Determination of carbohydrate and β-carotene content of some vegetables consumed in Kano metropolis, Nigeria. Bayero J. Pure Appl. Sci. 2009;2:119–121. [Google Scholar]
- Mutayoba S.K., Mutayoba B.M., Okot P. The performance of growing pullets fed diets with varying energy and Leucaena leaf meal levels. Livest. Res. Rural Dev. 2003;15:350–357. [Google Scholar]
- Ndabigengesere A., Narasiah K.S., Talbot B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995;29:703–710. [Google Scholar]
- Nouman W., Basra S.M.A., Siddiqui M.T., Yasmeen A., Gull T., Alcayde M.A.C. Potential of Moringa oleifera L. as livestock fodder crop: a review. Turk. J. Agric. For. 2014;38:1–14. [Google Scholar]
- Nuhu F. 2010. Page 122 in Effect of moringa leaf meal (MOLM) on nutrient digestibility, growth, carcass and blood indices of weaner rabbits. MSc, Faculty of Agriculture and Natural Resources, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
- Ogbe A.O., John P.A. Proximate study, mineral and anti-nutrient composition of Moringa oleifera leave harvested from Lafia, Nigeria: potential benefits in poultry nutrition and health. J. Microbiol. Biotechnol. Food Sci. 2012;3:296–308. [Google Scholar]
- Okumu M.O., Ochola F.O., Mbaria J.M., Kanja L.W., Gakuya D.W., Kinyua A.W., Okumu P.O., Kiama S.G. Mitigative effects of Moringa oleifera against liver injury induced by artesunate-amodiaquine antimalarial combination in wistar rats. Clin. Phytosci. 2017;3:18. [Google Scholar]
- Olayemi A.B., Alabi R.O. Studies on traditional water purification using Moringa oleifera seeds. Afr. Study Monogr. 1994;15:135–142. [Google Scholar]
- Olson M.E., Carlquist S. Stem and root anatomical correlations with life form diversity, ecology, and systematics in moringa (Moringaceae) Biol. J. Linn. Soc. 2001;135:315–348. [Google Scholar]
- Olugbemi T.S., Mutayoba S.K., Lekule F.P. Moringa oleifera leaf meal as a hypocholesterolemic agent in laying hen diets. Livest. Res. Rural Dev. 2010;22:84. [Google Scholar]
- Orwa C., Mutua A., Kindt R., Simons A., Jamnadass R.H. World Agroforestry Centre; Nairobi, Kenya: 2009. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. [Google Scholar]
- Pace-Asciak C.R., Hahn S., Diamandis E.P., Soleas G., Goldberg D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin. Chim. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- Pal A., Bawankule D.U., Darokar M.P., Gupta S.C., Arya J.S., Shanker K., Gupta M.M., Yadav N.P., Singh Khanuja S.P. Influence of Moringa oleifera on pharmacokinetic disposition of rifampicin using HPLC- PDA method: a pre-clinical study. Biomed. Chromatogr. 2010;25:641–645. doi: 10.1002/bmc.1494. [DOI] [PubMed] [Google Scholar]
- Pandey A., Pandey R.D., Tripathi P., Gupta P.P., Haider J., Bhatt S., Singh A.V. Moringa Oleifera Lam. (Sahijan) - a plant with a plethora of diverse therapeutic benefits: an updated retrospection. Med. Aromat. Plants. 2012;1:101. [Google Scholar]
- Patel P., Pater N., Patel D., Desai S., Meshram D. Phytochemical analysis and antifungal activity of Moringa oleifera. Int. J. Pharm. Pharm. Sci. 2014;6:144–147. [Google Scholar]
- Pennington J.A.T., Fisher R.A. Classification of fruits and vegetables. J. Food Compos. Anal. 2009;22S:S23–S31. [Google Scholar]
- Petersen H.H., Petersen T.B., Enemark H.L., Olsen A., Dalsgaard A. Removal of Cryptosporidium parvum oocysts in low quality water using Moringa oleifera seed extract as coagulant. Food Waterborne Parasitol. 2016;3:1–8. [Google Scholar]
- Preston K., Lantagne D., Kotlarz N., Jellison K. Turbidity and chlorine demand reduction using alum and moringa flocculation before household chlorination in developing countries. J. Water Health. 2010;8:60–70. doi: 10.2166/wh.2009.210. [DOI] [PubMed] [Google Scholar]
- Price M.L. The moringa tree. ECHO Tech. Note. 2007:1–19. Published 1985; Revised 2000, 2002, 2007 by ECHO Staff. [Google Scholar]
- Radovich T. In: Specialty Crops for Pacific Island Agroforestry. Elevitch C.R., editor. Permanent Agriculture Resources (PAR); Holualoa, Hawai‘i: 2011. Farm and Forestry Production and Marketing Profile for Moringa (Moringa oleifera)http://agroforestry.net/scps [Google Scholar]
- Rashid U., Anwar F., Moser B.R., Knothe G. Moringa oleifera oil: a possible source of biodiesel. Bioresour Technol. 2008;99:8175–8179. doi: 10.1016/j.biortech.2008.03.066. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy N.R., Sathe S.K., Salunkhe D.K. Phytates in legumes and cereals. Adv. Food Res. 1982;28:1–92. doi: 10.1016/s0065-2628(08)60110-x. [DOI] [PubMed] [Google Scholar]
- Rehman A., Arif M., Sajjad N., Al-Ghadi M.Q., Alagawany M., Abd El-Hack M.E., Alhimaidi A.R., Elnesr S.S., Almutairi B.O., Amran R.A., Hussein E.O.S., Swelum A.A. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 2020;99:6946–6953. doi: 10.1016/j.psj.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter N., Perumal S., Becker K. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.) Aquaculture. 2003;217:599–611. [Google Scholar]
- Righi E., Bechtold P., Tortorici D., Lauriola P., Calzolari E., Astolfi G., Nieuwenhuijsen M.J., Fantuzzi G., Aggazzotti G. Trihalomethanes, chlorite, chlorate in drinking water and risk of congenital anomalies: a population- based case-control study in Northern Italy. Environ. Res. 2012;116:66–73. doi: 10.1016/j.envres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Ríos J.L, Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Rubanza C.D.K., Shem M.N., Otsyina R., Bakengesa. T. Ichinohe S.S., Fujihara T. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Technol. 2005;119:129–142. [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;56:3255–3268. [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safa M.A., Tazi E. Effect of feeding different levels of Moringa oleifera leaf meal on the performance and carcass quality of broiler chicks. Int. J. Sci. Res. 2014;3:147–151. [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Swelum A.A., Attia M.M. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Salem M.A., Soliman M.M., Althobaiti S.A., Khafaga A.K., El-Tahan A.M., El-Saadony M.T., Attia M.M. Parasitological and histopathological examination of Cocktail love birds infected with Eimeria aratinga (Apicomplexa: Eimeriidae) Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez N.R., Ledin S., Ledin I. Biomass production and chemical composition of Moringa oleifera under different management regimes in Nicaragua. Agrofor. Syst. 2006;66:231–242. [Google Scholar]
- Sánchez-Machado D.I., López-Cervantes J., Núñez-Gastélum J.A., de la MoraLópez G.S., López-Hernández J., Paseiro-Losada P. Effect of the refining process on Moringa oleifera seed oil quality. Food Chem. 2015;187:53–57. doi: 10.1016/j.foodchem.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Sayeed M.A., Hossain M.S., Chowdhury M.E.H., Haque M. In vitro antimicrobial activity of methanolic extract of Moringa oleifera Lam. fruits. J. Pharmacogn. Phytochem. 2012;1:94–98. [Google Scholar]
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.) J. Agric. Food Chem. 2003;15:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Soliman K.M., Badea R.I. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chemi. Toxicol. 2002;40:1669–1675. doi: 10.1016/s0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Sreelatha S., Padma P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009;64:303–311. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- Stohs S.J., Hartman M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015;29:796–804. doi: 10.1002/ptr.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Weyenberg S.J.B., Jacobs M.A.J.M. Polypoid lymphoid hyperplasia of the terminal ileum. Video J. Encycl. GI Endosc. 2013;1:268. [Google Scholar]
- Verma A.R., Vijayakumar M., Mathela C.S., Rao C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chemi. Toxicol. 2009;9:2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Villasenor I.M., Finch P., Lim-Sylianco C.Y., Dayrit F. Structure of a mutagen from roasted seeds of Moringa oleifera. Carcinogenesis. 1989;10:1085–1087. doi: 10.1093/carcin/10.6.1085. [DOI] [PubMed] [Google Scholar]
- Yameogo C.W., Bengaly M.D., Savadogo A., Nikiema P.A., Traore S.A. Determination of chemical composition and nutritional values Moringa oleifera leaves. Pak. J. Nutr. 2011;10:264–268. [Google Scholar]
- Yaqoob M.U., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A.F., Batiha G.E., Yehia N., Elnesr S.S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younus I., Siddiq A., Assad T., Badar S., Jameel S., Ashraf M. Screening antiviral activity of Moringa oliefera L. leaves against foot and mouth disease virus. Glob. Vet. 2015;15:409–413. [Google Scholar]