Abstract
Eosinophilic disorders include a wide array of conditions in which eosinophils play a primary pathophysiologic role. While historically treated with corticosteroids and immunosuppressants, knowledge of eosinophil biology has led to the development of several biologics targeting eosinophils. In this review, we discuss the current US Food and Drug Administration (FDA) approved eosinophil-specific biologics targeting IL-5 (mepolizumab and reslizumab) and IL-5R (benralizumab) along with biologics under investigation targeting siglec-8 (lirentelimab). We discuss efficacy and safety data from trials of these medications in conditions including eosinophilic asthma, hypereosinophilic syndrome (HES), eosinophilic granulomatosis with polyangiitis (EGPA), chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis (EoE), and eosinophilic gastrointestinal disease (EGID). Additionally, we discuss case reports utilizing these medications in conditions including drug reaction with eosinophilia and systemic symptoms (DRESS), allergic bronchopulmonary aspergillosis (ABPA), and eosinophilic pneumonia, among others. While eosinophilic targeting biologic therapy has been successful in eosinophilic asthma, HES, EGPA, and CRSwNP leading to FDA approval for these conditions, trials treating EoE and EGID have been disappointing to date. Given the increasing number of trials utilizing these biologics, it will be imperative for the allergist-immunologist to stay up to date on the latest treatment options to provide the most optimal care for eosinophilic disorders.
Keywords: Eosinophil, Biologics, Interleukin 5, Treatment, Siglec-8
Introduction
Eosinophils are bone-marrow derived, multifunctional leukocytes that initiate and propagate diverse inflammatory pathways, modulate innate and adaptive immune processes, and serve as major effector cells producing tissue injury and dysfunction through release of toxic granule proteins and lipid mediators.1 Eosinophilic disorders encompass a wide variety of conditions in which eosinophils have a primary pathophysiologic role. They may affect any body compartment and organ, including the upper and lower airways, skin, connective tissues, gastrointestinal tract, and the hematopoietic, immune, cardiovascular, and nervous systems.2 Common eosinophilic disorders include eosinophilic asthma, hypereosinophilic syndrome (HES), chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic granulomatosis with polyangiitis (EGPA), eosinophilic esophagitis (EoE), and eosinophilic gastrointestinal disease (EGID), among others.2 These have been historically treated with corticosteroids and immunosuppressants, which are often, but not always, effective at reducing eosinophil counts and associated eosinophilic inflammation. However, these medications have long-term side effects that often limit dosing and efficacy.3 Capitalizing on knowledge regarding eosinophil cell biology has led to a remarkable increase in the development of eosinophil-specific therapies.
Eosinophil biology and therapeutic strategies
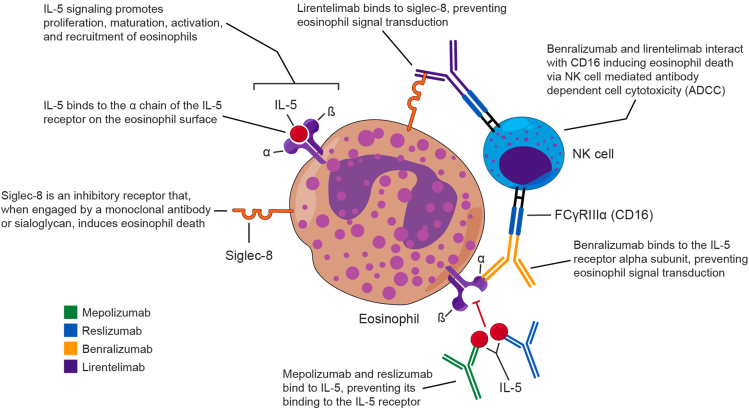
Interleukin-5 (IL-5) is essential to the eosinophil life cycle, stimulating the proliferation, differentiation, and maturation of eosinophil precursors.1,4 Additionally, IL-5 plays a role in the exit of eosinophils from the bone marrow into the blood stream, serves as a chemotactic factor, and prolongs eosinophil survival in tissue.4 The IL-5 receptor (IL-5R) is selectively expressed on eosinophils, basophils, and mast cells.3,5 Thus, therapies targeting IL-5 or its receptor represent a reasonably specific way to target eosinophils in eosinophilic disorders.
Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an inhibitory cell surface receptor expressed selectively on mature eosinophils and mast cells (and basophils to a lesser degree), which, when engaged, causes cells to undergo natural killer (NK) cell mediated, antibody dependent cellular cytotoxicity (ADCC).6, 7, 8 The restricted expression of Siglec-8 on eosinophils and subsequent cell death that occurs upon its engagement makes therapeutics targeting Siglec-8 an attractive prospect.
Based on this knowledge, several biologic medications have been developed that target IL-5, IL-5R, and Siglec-8 (Fig. 1). Key information regarding each of these medications is presented in Table 1. Mepolizumab and reslizumab are fully humanized, IgG1 and IgG4, respectively, antibodies that target IL-5 with high affinity and specificity.9,10 Benralizumab is a fully humanized, afucosylated IgG1 antibody that targets the IL-5Rα chain, which prevents the binding of IL-5.11 Unique to benralizumab, compared to other anti-IL-5 therapies, is its ability to induce ADCC with resultant eosinophil death and tissue depletion.12 Lirentelimab is a humanized, nonfucosylated IgG1 antibody that targets Siglec-8 and depletes eosinophils in the blood via NK cell mediated ADCC and in the tissue via apoptosis.13,14
Fig. 1.
Antibody targeting in eosinophil-associated diseases
Table 1.
Characteristics of selected eosinophil selective biologics.
| Mepolizumab | Reslizumab | Benralizumab | Lirentelimab | |
|---|---|---|---|---|
| Target | IL-5 | IL-5 | IL-5Rα | Siglec-8 |
| FDA approved Indications |
|
|
|
|
| Dose |
|
|
|
|
| Ongoing Clinical Trials |
|
|
|
|
Abbreviations: IL-5, Interleukin 5; IL-5Rα, IL-5 receptor alpha subunit; Siglec-8, sialic acid-binding immunoglobulin-like lectin 8; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; SC, subcutaneous; IV, intravenous; CRSwNP, chronic rhinosinusitis with nasal polyposis; EoE, eosinophilic esophagitis; CSU, chronic spontaneous urticaria; COPD, chronic obstructive pulmonary disease; EGID, eosinophilic gastrointestinal disease; AD, atopic dermatitis; ABPA, allergic bronchopulmonary aspergillosis
This review will address relevant trials investigating use of biologics targeting IL-5 and Siglec-8 in eosinophilic disorders. Table 2, Table 3, Table 4 summarize details of the trials being discussed.
Table 2.
Relevant clinical trials in asthma.
| Author | Year | Study design | Study population | Drug | Dose | Primary outcome | Secondary outcome |
|---|---|---|---|---|---|---|---|
| Pavord19 (DREAM) | 2012 | Multicenter RDBPC |
|
Mepolizumab | 75, 250, or 750 IV every 4 weeks for 52 weeks |
|
|
| Bel17 (SIRIUS) | 2014 | Multicenter RDBPC |
|
Mepolizumab | 100 mg SC every 4 weeks for 20 weeks |
|
|
| Ortega18 (MENSA) | 2014 | Phase 3 multicenter RDBPC |
|
Mepolizumab | 75 IV or 100 mg SC every 4 weeks for 32 weeks |
|
|
| Castro25,a | 2015 |
|
|
Reslizumab | 3 mg/kg IV every 4 weeks for 52 weeks |
|
|
| Corren26 | 2016 | Phase 3 multicenter RDBPC |
|
Reslizumab | 3 mg/kg IV every 4 weeks for 16 weeks |
|
|
| Bjermer27 | 2016 | Phase 3 multicenter RDBPC |
|
Reslizumab |
|
|
|
| Bleecker28 (SIROCCO) | 2016 | Phase 3 multicenter RDBPC |
|
Benralizumab |
|
|
|
| Fitzgerald29 (CALIMA) | 2016 | Phase 3 multicenter RDBPC |
|
Benralizumab | Same as SIROCCO, except 52 weeks total |
|
|
| NAIR30 (ZONDA) | 2017 |
|
Benralizumab | Same as SIROCCO/CALIMA for 28 weeks |
|
|
Abbreviations: RDBPC, randomized double-blind placebo-controlled; SABA, short acting beta agonist; AHR, airway hyperresponsiveness; AEC, absolute eosinophil count; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow; BAL, bronchoalveolar lavage; MBP, major basic protein; AQLQ, Asthma Quality of Life Questionnaire; SEA, severe eosinophilic asthma; ICS, inhaled corticosteroid; ACQ, Asthma Control Questionnaire; OCS, oral corticosteroid; FeNO, Fraction of exhaled nitric oxide; ppb, parts per billion; SC, subcutaneous; SGRQ, St. George respiratory questionnaire; ASUI, asthma symptom utility index; FEF25-75, forced expiratory flow at 25–75% of forced vital capacity, FVC, forced vital capacity; RDB, randomized double-blind; LABA, long acting beta agonist; AQLQ(S)+12, Asthma quality of life questionnaire, standardized, age 12+; ER, emergency room; IV, intravenous.
Two separate trials published as one paper
Table 3.
Relevant clinical trials in HES, EGPA, and CRSwNP.
| Author | Year | Study design | Condition | Study population | Drug | Dose | Primary outcome | Secondary outcome |
|---|---|---|---|---|---|---|---|---|
| Rothenberg39 | 2008 | Multicenter RDBPC | HES |
|
Mepolizumab | 750 IV every 4 weeks for 32 weeks |
|
|
| Roufosse41 | 2020 | Phase 3 multicenter RDBPC | HES | 108 patients age 12+ with PDGFRA negative HES with ≥2 flares in previous year with AEC >1000/μl | Mepolizumab |
|
|
|
| Kuang42 | 2019 |
|
HES | 20 patients with symptomatic PDGFRA negative HES and AEC >1000-μl | Benralizumab |
|
|
|
| Wechsler45 | 2017 | Phase 3 multicenter RDBPC | EGPA |
|
Mepolizumab |
|
|
|
| Han49 (SYNAPSE) | 2021 | Phase 3 multicenter RDBPC | CRSwNP |
|
Mepolizumab |
|
|
|
| Bachert50 (OSTRO) | 2021 | Phase 3 multicenter RDBPC | CRSwNP |
|
Benralizumab |
|
|
|
Abbreviations: HES, hypereosinophilic syndrome; PDGFRA, platelet derived growth factor A; AEC, absolute eosinophil count; FEV1, forced expiratory volume in 1 s; RDBPC, randomized double-blind placebo controlled; CUP, compassionate use protocol; SC, subcutaneous; BFI, Big Five Inventory; EGPA, eosinophilic granulomatosis with polyangiitis; CBC, complete blood count; ESR, erythrocyte sedimentation rate; CRP, c-reactive protein; FeNO, fraction of exhaled nitric oxide; ACQ, Asthma Control Questionnaire; BVAS, Birmingham Vasculitis Activity; CRSwNP, chronic rhinosinusitis with nasal polyposis; PIF, peak inspiratory flow; AEC, absolute eosinophil count; VAS, visual analog scale; SNOT-22, Sino-Nasal Outcome Test 22; PFT, pulmonary function test; SC, subcutaneous; DSS, Difficulty with Sense of Smell; IV, intravenous Score; SC, subcutaneous; AEC, absolute eosinophil count; RDBPC, randomized, double-blind, placebo-controlled; SEA, severe eosinophilic asthma; ACT, Asthma Control Test; AQLQ, Asthma Quality of Life Questionnaire; SNOT-22, Sino-Nasal Outcome Test 22
Table 4.
Relevant clinical trials in EoE and EGID.
| Author | Year | Study design | Condition | Study population | Drug | Dose | Primary outcome | Secondary outcome |
|---|---|---|---|---|---|---|---|---|
| Assa'ad53 | 2011 | Multicenter RDB | EoE |
|
Mepolizumab |
|
|
|
| Spergel54 | 2012 | Multicenter RDBPC | EoE |
|
Reslizumab |
|
|
|
| Dellon60 (ENIGMA) | 2020 | Phase 2 multicenter RDBPC | EGID |
|
Lirentelimab |
|
|
|
Abbreviations: EoE, Eosinophilic esophagitis; EGID, eosinophilic gastrointestinal disease AEC, Absolute eosinophil count; RDBPC, randomized, double-blind, placebo-controlled; HPF, high-power field; RDB, Randomized, double-blind; CHQ, children's health questionnaire; CUP, compassionate use protocol
Asthma
Asthma is a heterogeneous condition characterized by multiple endotypes, each with its own nuances in pathophysiology and treatment.15 Eosinophilic asthma represents the largest endotype, accounting for 50–60% of all cases.16 All 3 currently available anti-IL-5 biologics are approved by the US Food and Drug Administration (FDA) for use in severe eosinophilic asthma.
Mepolizumab
Efficacy
Three trials established the efficacy of mepolizumab in asthma: DREAM, SIRIUS, and MENSA.17, 18, 19 DREAM randomized 621 patients ages 12–74 with severe eosinophilic asthma (defined as requiring 800 μg of inhaled fluticasone or its equivalent with at least 2 exacerbations in the prior year along with any of the following: sputum eosinophilia >3%, absolute eosinophil count (AEC) > 300/μl, or fraction of exhaled nitric oxide (FeNO) > 50 ppb) to receive placebo or 75, 250, or 750 mg intravenous (IV) mepolizumab monthly for 13 months.19 All mepolizumab groups met the primary endpoint of reduction in rate of clinically significant exacerbations (48%, 39%, and 52% reductions in the 75, 250, and 750 mg groups, respectively) with a significant increase in the time to first exacerbation.19 SIRIUS randomized 135 patients ages 16–74 with severe eosinophilic asthma (defined as requiring both high dose inhaled corticosteroid (ICS) and oral corticosteroid (OCS) with an AEC >300/μl in the year before screening or >150/μl during the 8 week optimization phase) to receive 100 mg subcutaneous (SC) mepolizumab monthly for 20 weeks or placebo.17 Primary outcomes included the degree of daily OCS dose reduction, lack of asthma control during weeks 20–24, and study withdrawal.17 There was a 50% median reduction in OCS dose in the mepolizumab group compared to 0% in the placebo group and a significantly lower number in the mepolizumab group who had no dose reduction, lack of asthma control, or withdrew from the study.17 Annualized exacerbation rate, a secondary endpoint, was reduced 32% in the mepolizumab group relative to the placebo group.17 MENSA randomized 576 patients ages 12–82 with severe eosinophilic asthma to receive 75 mg IV or 100 mg SC mepolizumab or placebo monthly for 32 weeks.18 The primary outcome (annualized exacerbation rate) was significantly reduced by 47% in the IV and 53% in the SC groups compared to placebo.18 Additionally, both groups showed statistically significant improvement in St. George Respiratory Questionnaire (SGRQ) and Asthma Control Questionnaire (ACQ-5) scores.18 None of these trials demonstrated statistically significant improvement in measures of airflow obstruction.17, 18, 19 A subsequent combined post hoc analysis of MENSA and DREAM published in 2016 showed that the magnitude of exacerbation rate reduction was associated with the degree of peripheral eosinophilia, with a 52% reduction in patients with an AEC >150/μl compared to a 70% reduction in patients with an AEC >500/μl.20 Importantly, there was decreased efficacy of mepolizumab in patients with an AEC <150/μl.20 Open-label extensions of MENSA and SIRIUS (COSMOS and COSMEX) showed that clinical response was durable with patients maintaining their exacerbation rate and OCS dose reductions throughout the extension.21,22
An additional non-randomized trial investigated SC mepolizumab use in 36 children ages 6–11 with severe eosinophilic asthma at a dose of either 40 mg (if weight <40 kg) or 100 mg.23 Both groups showed trends toward exacerbation rate reduction and improvement in symptom scores.23
Safety
There was no significant difference in adverse events between placebo or treatment groups in MENSA, SIRIUS, or DREAM.17, 18, 19 Long-term safety of mepolizumab was established by COSMOS and COSMEX.21,22 Nasopharyngitis was the most commonly reported adverse effect, occurring in 30–42% of patients.21,22 Serious adverse effects occurred in 14–28% of patients with worsening or exacerbation of asthma being most common (6–10%).21,22 Systemic and local injection site reactions occurred in 2% and 4% of patients, respectively, but there were no reports of anaphylaxis or fatal adverse events.21,22 Similarly, the aforementioned pediatric trial and a subsequent open label extension showed no treatment related severe or fatal adverse effects, with minor adverse effects of headache and nasopharyngitis being among the most common.23,24
Clinical indications and dosing
These trials led to the 2015 FDA approval of mepolizumab for add-on therapy in patients age 12 or older with severe asthma at a dose of 100 mg SC monthly. In 2019, mepolizumab received FDA approval for the same indication in patients age 6–11 at a dose of 40 mg SC monthly.
Reslizumab
Efficacy
Four trials established the efficacy of reslizumab in the treatment of asthma. Castro and colleagues performed 2 separate trials randomizing a total of 953 patients ages 12–75 with uncontrolled eosinophilic asthma (defined as use of medium dose ICS with an OCS requiring exacerbation in the last month, an ACQ-7 of >1.5, and an AEC >400/μl) to receive 3 mg/kg IV reslizumab or placebo monthly for 1 year.25 The primary outcome of annual exacerbation rate was significantly reduced in both studies with rate ratios of 0.5 and 0.41.25 The probability of not having an exacerbation was significantly higher in the reslizumab groups (61%, 73%) compared to placebo (44%, 52%).25 Additionally, the time to first exacerbation was significantly increased in both studies, and there were significant improvements in FEV1 and a variety of symptom scores that were seen as soon as week 16 and sustained through week 52.25 A third trial randomized 492 patients ages 18–65 with uncontrolled asthma to receive 3 mg/kg IV reslizumab monthly for 4 months, but a key difference was their enrollment of patients regardless of AEC.26 The primary endpoint was change in FEV1 with secondary endpoints including change in FVC, SABA use, and ACQ-7.26 In the total population, none of these were significantly changed between groups, but when stratified by those with an AEC >400/μl, there was a statistically significant increase in FEV1 (mean increase 270 ml) in the reslizumab group with non-significant improvements in FVC, ACQ-7, and SABA use.26 A fourth trial randomized 315 patients with poorly controlled eosinophilic asthma (same criteria as the Castro trials with the additional requirement of documented airway reversibility) to receive either 0.3 mg/kg or 3 mg/kg IV reslizumab or placebo monthly for 4 months.27 The primary endpoint of change in pre-bronchodilator FEV1 was met in both groups, with an increase of 115 ml and 160 ml in the low and high dose groups, respectively.27 Key secondary outcomes included changes in FVC, FEF25-75, multiple patient reported surveys, and SABA use.27 The high dose reslizumab group showed a significant increase in FVC (130 ml) and a non-significant increase in FEF25-75 (233 ml).27 Additionally, ACQ, ASUI, and SABA use were significantly improved in both groups.27
Safety
In these trials, there were 3 episodes of anaphylaxis deemed related to reslizumab.25, 26, 27 Otherwise, there was no significant difference in severe adverse events between the placebo and treatment groups.25, 26, 27 The most common adverse events included asthma worsening, headache, nasopharyngitis, and upper respiratory tract infections, which occurred in 8–22% of patients in the treatment groups.25, 26, 27
Clinical indications and dosing
These trials led to the 2016 FDA approval of reslizumab for add-on therapy in patients age 18 or older with severe asthma at a dose of 3 mg/kg IV monthly.
Benralizumab
Efficacy
Three trials established the efficacy of benralizumab in asthma: SIROCCO, CALIMA, and ZONDA. SIROCCO randomized 1205 patients ages 12–75 with asthma who had ≥2 exacerbations in the past year while on high dose ICS plus LABA to receive placebo or 30 mg SC benralizumab at intervals of either every 4 weeks for 48 weeks or every 4 weeks for the first 3 doses then every 8 weeks through week 48.28 The primary endpoint was annual exacerbation rate ratio with key secondary endpoints including time to first exacerbation and pre-bronchodilator FEV1 and total asthma symptom scores at week 48 with results stratified by an AEC >300/μl.28 In those with an AEC >300/μl, both dosing groups had significant reductions in exacerbation rate, improvements in FEV1, and increased time to first exacerbation.28 Additionally, the 8 week dosing group had a significant decrease in total asthma symptom score.28 In those with an AEC <300/μl, the 4 week dosing group had a significantly decreased exacerbation rate and the 8 week group had significant improvement in asthma symptom scores, but no other endpoints achieved statistical significance.28 CALIMA utilized the exact dosing scheme, outcomes, and patient population characteristics (with the exception of allowing patients on medium dose ICS) as SIROCCO.29 In patients with an AEC >300/μl, there was a significant reduction in exacerbation rate (36% and 28% in every 4 week and every 8 week group, respectively), increased time to first exacerbation, increase in FEV1, and decreased total symptom score.29 A significant reduction in exacerbation rate was also seen in those with an AEC <300/μl, but no secondary outcomes achieved statistical significance.29 ZONDA randomized 220 patients with asthma requiring medium to high dose ICS plus LABA, OCS for at least the past 6 months, and an AEC >150/μl to benralizumab or placebo at the same dosing scheme as SIROCCO and CALIMA, but for a duration of 28 weeks.30 The primary outcome was the percent change in OCS dose at 28 weeks with key secondary outcomes including annual exacerbation rates, time to first exacerbation, percentage of patients with an OCS dose ≤5 mg, pre-bronchodilator FEV1, and total symptom scores.30 Both groups experienced a 75% reduction in OCS dose compared to 25% in the placebo group, and 61% and 59% of patients in the 4 week and 8 week dosing group, respectively, achieved a final OCS dose of ≤5 mg compared to 33% in the placebo group.30 There was a 55% decrease in exacerbation rate in the 4 week group and a 70% decrease in the 8 week group.30 No significant changes were seen in FEV1 in any group.30 Two open label extensions of these trials, BORA and MELTEMI, showed durable reductions in rates of exacerbation in those with an AEC >300/μl and sustained improvements in FEV1.31, 32, 33 Additionally, in a recent single arm study (PONENTE) 490 of 598 OCS dependent severe asthmatics (81.9%) were able to achieve an OCS dose of ≤5 mg following benralizumab treatment irrespective of baseline eosinophil counts or baseline OCS dosage, with 75% of patients doing so without experiencing asthma exacerbations during the OCS taper.34
Safety
In SIRROCO, CALIMA, and ZONDA, there were no significant differences in severe adverse effects between treatment and placebo groups.28, 29, 30 Nasopharyngitis was the most common adverse effect occurring in 12–21% of patients.28, 29, 30 Worsening asthma was the most common serious adverse effect occurring in 5–13% of patients.28, 29, 30 Drug-related hypersensitivity reactions were uncommon, occurring in 0–2% of patients in the treatment groups.28, 29, 30 Long-term safety was confirmed by BORA and MELTEMI, which showed similar findings to the original trials in terms of safety and tolerability with patients on treatment for up to 5 years.31, 32, 33
Clinical indications and dose
These trials led to the 2017 FDA approval of benralizumab for add-on therapy in patients age 12 or older with severe asthma at a dose of 30 mg SC every 4 weeks for 3 doses with subsequent increase in dosing interval to every 8 weeks.
Summary
While these 3 anti-IL-5 biologics are FDA approved and are effective in reducing exacerbation rates, no trial has directly compared any of them. An indirect treatment comparison assessed the relative efficacy of each through analysis of several pre-existing trials.35 Outcomes included exacerbation rate, ACQ score, and change in pre-bronchodilator FEV1.35 Comparisons were stratified by AEC cutoffs that had been established in the prior clinical trials (>150/μl, >300/μl, >400/μl for mepolizumab, benralizumab, and reslizumab, respectively).35 Mepolizumab significantly reduced the rate of exacerbations and improved ACQ scores compared to both benralizumab and reslizumab in patients with an AEC >400/μl and compared to benralizumab in patients with an AEC >150/μl and >300/μl.35 Benralizumab was associated with a significantly greater improvement in FEV1 compared to reslizumab in patients with an AEC >400/μl, but there was otherwise no significant difference at any AEC threshold between mepolizumab and benralizumab or reslizumab.35
Hypereosinophilic syndrome
HES comprises a rare group of systemic disorders characterized by peripheral and/or tissue eosinophilia, the clinical evaluation and treatment of which has been extensively reviewed elsewhere.36, 37, 38, 39
Mepolizumab
Efficacy
The first large trial demonstrating efficacy of mepolizumab in HES randomized 85 patients with PDGFRA negative HES (defined as an AEC >1500/μl for at least 6 months with organ involvement and no identifiable secondary cause) to receive 750 mg IV mepolizumab or placebo monthly for 8 months after a run-in period where controller medications were adjusted to where clinical stability was achieved using prednisone monotherapy at 20–60 mg.40 In the mepolizumab group, 84% of patients achieved the primary end point of prednisone dose reduction to ≤10 mg for at least 8 consecutive weeks.40 Key secondary end points included achievement of an AEC <600/μl for at least 8 consecutive weeks, time to treatment failure, mean prednisone dose at week 36, achievement of a prednisone dose ≤7.5 mg, achievement of a prednisone dose ≤10 mg by week 20 for at 8+ weeks, and receipt of no prednisone for at least 1 day, all of which favored the mepolizumab group.40 An open label extension demonstrated durability of mepolizumab's corticosteroid sparing effect with 83% of patients able to maintain a prednisone dose ≤10 mg for ≥12 weeks during the extension.41
After years of compassionate use, questions arose surrounding optimal dosing of mepolizumab for HES. This led to a trial randomizing 108 patients age 12+ with PDGFRA negative HES with an AEC ≥1000/μl and 2+ flares in the preceding 12 months to receive either 300 mg SC mepolizumab monthly or placebo for 32 weeks, in addition to pre-existing HES therapy.42 The primary outcome, the proportion of patients who experienced a disease flare in 32 weeks, was met with 28% of patients in the mepolizumab group experiencing a flare compared to 56% in the placebo group.42 Secondary outcomes included the time to first flare, proportion of patients with a flare during weeks 20–32, annualized flare rate, and change in fatigue severity. There was a significantly decreased proportion of patients in the mepolizumab group with a flare during weeks 20–32, decreased annualized rate of flare (0.50 vs. 1.46), and improvement in fatigue scores.42
Safety
Long-term safety of mepolizumab in HES was established in the open label extension where 78 patients received a mean of 25 mepolizumab doses over 251 weeks with 20 patients experiencing adverse effects attributed to mepolizumab, the most common of which were fatigue (n = 6) and nausea (n = 3).41 One fatal (angioimmunoblastic T cell lymphoma) and 3 non-fatal (motor neuron disease, transverse myelitis, increased T lymphocyte count) serious adverse events were attributed to mepolizumab.41
In the post compassionate use trial, there was a similar percentage of adverse events in the placebo and mepolizumab groups with no reported severe adverse events attributed to mepolizumab.42 Additionally, there were no reports of malignancy or anaphylaxis in the mepolizumab group.42 The most common adverse events included bronchitis (15%), upper respiratory tract infection (15%), headache (13%), and nasopharyngitis (13%).42
Clinical indications and dosing
These trials led to the 2020 FDA approval of mepolizumab for patients age 12 or older with HES with no identifiable secondary cause at a dose of 300 mg SC monthly.
Benralizumab
Efficacy
A phase 2 trial investigated the efficacy of benralizumab at a dose of 30 mg SC in 20 patients with symptomatic PDGFRA negative HES with an AEC ≥1000/μl.43 The study was randomized, double-blind, and placebo-controlled for the initial 12 weeks, open-label for the next 12 weeks, and transitioned to an open label extension for the next 24 weeks for those who had a clinical or laboratory response in the initial 24 weeks.43 The primary endpoint, the percentage of patients with a 50% reduction in AEC at 12 weeks was met in 9/10 patients in the benralizumab group compared to 3/10 in the placebo group.43 Key secondary endpoints included the frequency and severity of adverse events and the proportion of patients with reductions in concomitant therapy at 48 weeks.43 At week 48, 9 of 14 patients still receiving benralizumab were able to taper other HES therapy, and 7 of those 9 were on benralizumab alone.43 A phase 3 trial investigating benralizumab efficacy in HES is ongoing (NATRON, NCT04191304).
Safety
There was a similar number of adverse events reported in the benralizumab and treatment groups with lymphocytopenia (n = 6) and headache (n = 5) being most common.43 There were no serious adverse events reported in the benralizumab group.43
Eosinophilic granulomatosis with polyangiitis
Eosinophilic granulomatosis with polyangiitis (EGPA) is a systemic disorder characterized by peripheral eosinophilia, severe eosinophilic asthma, sinus disease, and small vessel vasculitis with demonstrated increases in airway levels of IL-5.44,45
Mepolizumab
Efficacy
The efficacy of mepolizumab in EGPA was established in a trial that randomized 136 patients age 18+ with relapsing or refractory EGPA requiring a stable prednisone dose of 7.5–50 mg to receive 300 mg SC mepolizumab or placebo every 4 weeks for 52 weeks.46 The 2 primary endpoints were the accrued weeks of remission (defined as a Birmingham Vasculitis Activity Score (BVAS) of 0 and prednisone dose ≤4 mg) over 52 weeks and the proportion of patients in remission at weeks 36 and 48.46 Secondary endpoints included the time to first relapse, average OCS dose during weeks 48–52, and the proportion of patients with remission within the first 24 weeks.46 There was a significantly greater accrued time in remission with 28% of participants in the mepolizumab group experiencing 24+ weeks of remission compared to 3% in the placebo group with an increased time to first exacerbation in the mepolizumab group.46 Additionally, there were significantly more patients in the mepolizumab group in remission at weeks 36 and 48 compared to placebo (32% vs. 3%).46 Prednisone doses were significantly lower in the mepolizumab group with 44% achieving a dose ≤4 mg and 18% discontinuing prednisone compared to 7% and 3%, respectively, in the placebo group.46
Safety
The overall rate of adverse effects was similar in the mepolizumab and treatment groups.46 Similar to trials in asthma and HES, the most common adverse effects reported in the mepolizumab group were headache (32%), upper respiratory infection (21%), nasopharyngitis (18%), and local injection site reaction (15%).46 There were only 3 serious adverse events considered to be related to mepolizumab, and there were no reports of anaphylaxis.46
Clinical indications and dosing
This trial led to the 2017 FDA approval of mepolizumab for add-on therapy in patients age 18 or older with EGPA at a dose of 300 mg SC monthly.
Benralizumab
A phase 2 trial evaluating the efficacy of benralizumab is ongoing (BITE, NCT03010436). Additionally, a randomized trial directly comparing the efficacy of benralizumab compared to mepolizumab is ongoing (MANDARA, NCT04157348), which would be the first randomized trial to perform a head to head comparison of 2 anti-IL-5 biologics in any eosinophilic disease.
Chronic rhinosinusitis with nasal polyposis
It is known that nasal polyps in patients with CRSwNP have increased levels of IL-5 and that eosinophilic inflammation is associated with polyp recurrence after surgical therapy, hence the role of anti-IL-5 therapy in this condition.47, 48, 49
Mepolizumab
Efficacy
A phase 3 trial, SYNAPSE, established the efficacy of mepolizumab in CRSwNP. SYNAPSE randomized 407 adult patients from 11 countries with severe bilateral nasal polyposis (defined as a nasal obstruction visual analog scale (VAS) score of >5) with a history of at least 1 nasal surgery in the past 10 years who were currently eligible for repeat surgery to receive 100 mg SC mepolizumab or placebo monthly for 52 weeks following a 4 week run in period where all patients were treated with intranasal mometasone.50 Both primary outcomes were achieved, which were significant decreases in the endoscopic nasal polyp score (adjusted median decrease of 0.73) and nasal obstruction VAS score (adjusted median decrease of 3.14) in the mepolizumab group.50 Secondary end points included time to first nasal surgery, proportion of patients requiring systemic steroids, and changes from baseline in mean overall VAS score, Sinonasal Outcome Test 22 scores (SNOT-22), mean composite VAS score, and mean VAS score for loss of smell, all of which showed statistically significant changes favoring mepolizumab.50 A second phase 3 trial (MERIT, NCT04607005) is ongoing.
Safety
In SYNAPSE, adverse events occurred in 15% of patients in the mepolizumab group compared to 9% in the placebo group with nasopharyngitis (25%) and headache (18%) being most common.50 There were no serious adverse events that were attributed to mepolizumab and no cases of anaphylaxis.50
Clinical indications and dosing
This trial led to the 2021 FDA approval of mepolizumab for patients age 18 or older with CRSwNP at a dose of 100 mg SC monthly.
Benralizumab
Efficacy
A phase 3 trial, OSTRO, established the efficacy of benralizumab in CRSwNP. OSTRO randomized 413 adult patients with bilateral nasal polyposis with a total nasal polyp score ≥5 and a history of either systemic steroid use or prior nasal surgery to receive 30 mg SC benralizumab (every 4 weeks for the first 3 doses and every 8 weeks thereafter) or placebo for 56 weeks.51 Key exclusion criteria included a history of nasal or sinus surgery in the 3 months prior to enrollment or an asthma exacerbation in the 4 weeks prior to enrollment.51 Both co-primary endpoints were achieved, which were significant improvements in total mean endoscopic nasal polyp score (mean reduction of 0.57) and biweekly nasal obstruction score (mean reduction of 0.27), both measured at week 40.51 There was no statistically significant improvement in key secondary efficacy endpoints including SNOT-22 at week 40, time to first nasal polyp surgery or systemic steroid use, or Lund-Mackay score at end of treatment.51 An additional phase 3 trial (ORCHID, NCT 04157335) is ongoing.
Safety
The most common adverse events were nasopharyngitis (17.4%) and asthma (9.2%).51 Serious adverse events occurred at rates of 8.4% in the placebo group and 11.1% in the benralizumab group, although none of these were deemed related to benralizumab.51 Only 2 serious adverse events occurred in more than 1 patient (pericarditis and gastritis each occurred in 2 patients in the benralizumab group).51 There were no reports of anaphylaxis, and local injection site reactions occurred at similar rates (1.9% benralizumab, 2.0% placebo).51
Reslizumab
There are no large published trials of reslizumab use in CRSwNP, but a phase 3 trial investigating its use is ongoing (NCT02799446).
Eosinophilic esophagitis
EoE has traditionally been treated with a mixture of elimination diets, proton pump inhibitors, and corticosteroids (systemic and swallowed topical) with a goal of reducing both symptoms and eliminating esophageal eosinophilia.52,53 While biologics targeting eosinophils are a plausible method of treating EoE, clinical trials to date have been disappointing.
Mepolizumab
Efficacy
The largest trial investigating the efficacy of mepolizumab in EoE randomized 59 children ages 2–17 with EoE with a peak esophageal eosinophil count >20/hpf refractory to medical therapy to receive 0.55, 2.5, or 10 mg/kg IV mepolizumab monthly for 3 months.54 The primary endpoint, the proportion of patients with an esophageal eosinophil count of <5/hpf was met in only 8.8% of patients.54 Secondary outcomes included changes in peak and mean esophageal eosinophil counts, improvement in histopathologic and endoscopic findings, and the frequency and severity of symptoms.54 Peak and mean esophageal eosinophil counts <20/hpf were observed in 31.6% and 89.5% of patients, respectively.54 While there were decreases in reports of pain, regurgitation, and vomiting, these findings were limited by wide confidence intervals.54 An additional phase 3 trial is ongoing (NCT03656380).
Safety
Only one serious adverse event related to mepolizumab was reported (chest pain), and there were no hypersensitivity reactions.54
Reslizumab
Efficacy
A multi-center trial assessing the effect of reslizumab in EoE randomized 227 patients ages 5–18 with moderately severe EoE with a peak esophageal eosinophil count >24/hpf to receive 1, 2, or 3 mg/kg IV reslizumab or placebo monthly for 4 months. 55 Primary outcomes included the percent change in peak esophageal eosinophil count and change in the physician's EoE global assessment score.55 Secondary outcomes included changes in the patient's predominant symptoms and the children's health questionnaire (CHQ).55 While there was a significant reduction in the peak esophageal eosinophil count in all the reslizumab groups (40–50% reduction compared to 5% increase in the placebo group), no group was able to achieve a peak esophageal eosinophil count of <5/hpf, and there was no significant improvement in the physician's EoE global assessment, patient's predominant symptoms, or CHQ scores.55
Safety
A 9-year open label extension demonstrated long-term safety of reslizumab with no serious adverse events reported.56
Benralizumab and lirentelimab
There are ongoing phase 3 trials investigating the efficacy of SC benralizumab (MESSINA, NCT04543409), and IV lirentelimab (KRYPTOS, NCT04322708). Preliminary results from KRYPTOS showed histologic improvement in the treatment group, but did not show significant symptom improvement.57
Eosinophilic gastrointestinal disease
Eosinophilic gastrointestinal disease distal to the esophagus (gastritis, duodenitis, colitis) can present with a wide range of symptoms and is treated similarly to EoE but has had less targeted therapeutic investigation.58, 59, 60
Lirentelimab
Efficacy
A phase 2 trial investigating the efficacy of lirentelimab in EGID randomized 65 patients ages 18–80 with eosinophilic gastritis or duodenitis not adequately controlled with pharmacologic therapy or dietary modification to receive 4 monthly infusions of IV lirentelimab at high dose (0.3, 1, 3, 3 mg/kg) or low dose (0.3, 1, 1, 1 mg/kg) or placebo.61 The primary outcome was the percent change in mean peak gastric or duodenal eosinophil count with secondary outcomes including treatment response (defined as those with >30% reduction in total symptom score AND >75% reduction in gastrointestinal eosinophil count) and the percent change in total symptom score.61 The primary outcome was achieved with 79% and 86% reductions in mean peak gastrointestinal eosinophil count in the low and high dose groups, respectively, compared to a 9% increase in the placebo group.61 Treatment response was seen at a significantly higher rate in both dosing groups (59%, 67%, and 5% in the low dose, high dose, and placebo groups, respectively).61 Total symptom scores were reduced in both dosing groups compared to placebo (55%, 42%, and 22% in the high dose, low dose, and placebo groups, respectively).61
ENIGMA 2 (NCT04322604) is an ongoing phase 3 trial investigating the efficacy of lirentelimab in EGID. Preliminary results showed histologic improvement in the treatment group, but (similarly to KRYPTOS for EoE) did not result in significant symptom improvement.57
Safety
Infusion related reactions were common, occurring in 60% of patients who received lirentelimab.61 The majority of these reactions were mild to moderate, consisting of flushing, warmth, headache, nausea, or dizziness.61 Otherwise, the rates of other adverse events were similar between the lirentelimab and placebo groups.61 Serious adverse events occurred in 4 patients in the lirentelimab group (hypoxia, abdominal pain, dehydration, and a grade 4 infusion reaction) and 3 patients in the placebo group (dehydration, anemia, and altered mental status).61
Benralizumab
A randomized trial investigating efficacy of benralizumab in EGID is ongoing (BEGS, NCT03473977).
Other
There have been case reports of successful anti-IL-5 therapy in a variety of other conditions. Positive effects have been reported in chronic spontaneous urticaria, and there are ongoing trials investigating the efficacy of mepolizumab (NCT03494881), benralizumab (ARROYO, NCT 04612725) and lirentelimab (NCT03436797) in this condition.62, 63, 64 Drug reaction with eosinophilia and systemic symptoms (DRESS) has been treated successfully with mepolizumab and benralizumab.65,66 Mepolizumab has been successfully used in allergic bronchopulmonary aspergillosis,67 chronic eosinophilic pneumonia,68 eosinophilic bronchitis,69 and in one patient with concomitant severe eosinophilic asthma, EGID, and AERD.70 There are ongoing trials investigating the use of mepolizumab in eosinophilic fasciitis (NCT04305678), COPD with frequent exacerbations and eosinophilia (MATINEE, NCT04133909), and episodic angioedema with eosinophilia (Gleich syndrome, NCT04128371) as well as trials investigating the use of benralizumab in atopic dermatitis (NCT04605094, NCT03563066) and bullous pemphigoid (FJORD, NCT04612790).
Summary
There are 20 years of experience in the use of anti-IL-5 therapy for eosinophilic diseases and a nascent but growing level of experience in the use of anti-Siglec-8 therapy. There is already FDA approval of anti-IL-5 biologics for severe eosinophilic asthma and mepolizumab for EGPA, idiopathic HES, and CRSwNP. Despite initial positive results, the FDA has not yet granted approval for benralizumab in CRSwNP, and preliminary unpublished results from phase 3 trials of lirentelimab in EoE and EGID are disappointing. Despite this, there are still several ongoing trials which will potentially result in additional FDA approved indications for eosinophil targeting biologic therapy in the coming years, which will provide new therapeutic options for these often debilitating diseases. This review provides one of the most comprehensive summaries of eosinophil directed biologic therapy, and it will be important for the practicing allergist-immunologist to stay up to date with the accelerating release of new trials.
Abbreviations
HES, hypereosinophilic syndrome; CRSwNP, chronic rhinosinusitis with nasal polyposis; EGPA, eosinophilic granulomatosis with polyangiitis; EOE, eosinophilic esophagitis; EGID, eosinophilic gastrointestinal disease; IL-5, interleukin-5; IL-5R, interleukin 5 receptor; Siglec-8, sialic acid-binding immunoglobulin-like lectin 8; NK, natural killer; ADCC, antibody dependent cellular cytotoxicity, AEC, absolute eosinophil count; IV, intravenous; ICS, inhaled corticosteroid; OCS, oral corticosteroid; SC, subcutaneous, SGRQ, St. George respiratory questionnaire, ACQ, asthma control questionnaire; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SABA, short acting beta agonist; FEF25-75, forced expiratory flow at 25–75% of forced vital capacity; ASUI, asthma symptom utility index; FDA, Food and Drug Administration; LABA, long acting beta agonist; PDGFRA, platelet derived growth factor receptor A; BVAS, Birmingham vasculitis activity score; VAS, visual analog scale; SNOT-22, sinonasal outcome test-22; CHQ, children's health questionnaire; AERD, aspirin exacerbated respiratory disease.
Funding
Not applicable.
Author contributions and consent for publication
MMP performed literature review and drafting the manuscript. JTL and TP both are responsible for study design and critical review of the manuscript. All authors have read and consent to approval of the final manuscript.
Availability of data and materials
All abstracted data is available to the readers in the tables of the manuscript and references.
Ethics
The authors report no ethical concerns in this literature review.
Submission declaration
The authors declare this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Declaration of competing interest
The authors report no conflicts of interest, financial or otherwise.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Rothenberg M.E., Hogan S.P. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Bochner B.S., Book W., Busse W.W., et al. Workshop report from the national institutes of health taskforce on the research needs of eosinophil-associated diseases (TREAD) J Allergy Clin Immunol. 2012;130:587–596. doi: 10.1016/j.jaci.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochner B.S. Novel therapies for eosinophilic disorders. Immunol Allergy Clin. 2015;35:577–598. doi: 10.1016/j.iac.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roufosse F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front Med. 2018;5:49. doi: 10.3389/fmed.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migita M., Yamaguchi N., Mita S., et al. Characterization of the human IL-5 receptors on eosinophils. Cell Immunol. 1991;133:484–497. doi: 10.1016/0008-8749(91)90120-z. [DOI] [PubMed] [Google Scholar]
- 6.Bochner B.S. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiwamoto T., Kawasaki N., Paulson J.C., Bochner B.S. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legrand F., Cao Y., Wechsler J.B., et al. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic disorders: receptor expression and targeting using chimeric antibodies. J Allergy Clin Immunol. 2019;143:2227–2237 e10. doi: 10.1016/j.jaci.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keating G.M. Mepolizumab: first global approval. Drugs. 2015;75:2163–2169. doi: 10.1007/s40265-015-0513-8. [DOI] [PubMed] [Google Scholar]
- 10.Markham A. Reslizumab: first global approval. Drugs. 2016;76:907–911. doi: 10.1007/s40265-016-0583-2. [DOI] [PubMed] [Google Scholar]
- 11.Matera M.G., Calzetta L., Rinaldi B., Cazzola M. Pharmacokinetic/pharmacodynamic drug evaluation of benralizumab for the treatment of asthma. Expet Opin Drug Metabol Toxicol. 2017;13:1007–1013. doi: 10.1080/17425255.2017.1359253. [DOI] [PubMed] [Google Scholar]
- 12.Kolbeck R., Kozhich A., Koike M., et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344–13453 e2. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Youngblood B.A., Brock E.C., Leung J., et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youngblood B.A., Brock E.C., Leung J., et al. AK002, a humanized sialic acid-binding immunoglobulin-like lectin-8 antibody that induces antibody-dependent cell-mediated cytotoxicity against human eosinophils and inhibits mast cell-mediated anaphylaxis in mice. Int Arch Allergy Immunol. 2019;180:91–102. doi: 10.1159/000501637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Silva L., Hassan N., Wang H.Y., et al. Heterogeneity of bronchitis in airway diseases in tertiary care clinical practice. Can Respir J. 2011;18:144–148. doi: 10.1155/2011/430317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aleman F., Lim H.F., Nair P. Eosinophilic endotype of asthma. Immunol Allergy Clin. 2016;36:559–568. doi: 10.1016/j.iac.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Bel E.H., Wenzel S.E., Thompson P.J., et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 18.Ortega H.G., Liu M.C., Pavord I.D., et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 19.Pavord I.D., Korn S., Howarth P., et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 20.Ortega H.G., Yancey S.W., Mayer B., et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–556. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 21.Lugogo N., Domingo C., Chanez P., et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Therapeut. 2016;38:2058–20570 e1. doi: 10.1016/j.clinthera.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Khurana S., Brusselle G.G., Bel E.H., et al. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin Therapeut. 2019;41:2041–20456 e5. doi: 10.1016/j.clinthera.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A., Pouliquen I., Austin D., et al. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr Pulmonol. 2019;54:1957–1967. doi: 10.1002/ppul.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A., Ikeda M., Geng B., et al. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J Allergy Clin Immunol. 2019;144:1336–13342 e7. doi: 10.1016/j.jaci.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Castro M., Zangrilli J., Wechsler M.E., et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 26.Corren J., Weinstein S., Janka L., Zangrilli J., Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Bjermer L., Lemiere C., Maspero J., Weiss S., Zangrilli J., Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Bleecker E.R., FitzGerald J.M., Chanez P., et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta 2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 29.FitzGerald J.M., Bleecker E.R., Nair P., et al. Benralizumab, an anti-interleukin-5 receptor a monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 30.Nair P., Wenzel S., Rabe K.F., et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 31.Busse W.W., Bleecker E.R., FitzGerald J.M., et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7:46–59. doi: 10.1016/S2213-2600(18)30406-5. [DOI] [PubMed] [Google Scholar]
- 32.FitzGerald J.M., Bleecker E.R., Bourdin A., et al. Two-year integrated efficacy and safety analysis of benralizumab in severe asthma. J Asthma Allergy. 2019;12:401–413. doi: 10.2147/JAA.S227170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korn S., Bourdin A., Chupp G., et al. Integrated safety and efficacy among patients receiving benralizumab for up to 5 years. J Allergy Clin Immunol Pract. 2021;9:4381–43892 e4. doi: 10.1016/j.jaip.2021.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Menzies-Gow A., Gurnell M., Heaney L.G., et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2022;10:47–58. doi: 10.1016/S2213-2600(21)00352-0. [DOI] [PubMed] [Google Scholar]
- 35.Busse W., Chupp G., Nagase H., et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143:190–200 e20. doi: 10.1016/j.jaci.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Curtis C., Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol. 2016;50:240–251. doi: 10.1007/s12016-015-8506-7. [DOI] [PubMed] [Google Scholar]
- 37.Dispenza M.C., Bochner B.S. Diagnosis and novel approaches to the treatment of hypereosinophilic syndromes. Curr Hematol Malig Rep. 2018;13:191–201. doi: 10.1007/s11899-018-0448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326–331. doi: 10.1182/asheducation-2018.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shomali W., Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94:1149–1167. doi: 10.1002/ajh.25617. [DOI] [PubMed] [Google Scholar]
- 40.Rothenberg M.E., Klion A.D., Roufosse F.E., et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 41.Roufosse F.E., Kahn J.E., Gleich G.J., et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol. 2013;131:461–467. doi: 10.1016/j.jaci.2012.07.055. e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roufosse F., Kahn J.E., Rothenberg M.E., et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;146:1397–1405. doi: 10.1016/j.jaci.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuang F.L., Legrand F., Makiya M., et al. Benralizumab for PDGFRA-negative hypereosinophilic syndrome. N Engl J Med. 2019;380:1336–1346. doi: 10.1056/NEJMoa1812185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakiela B., Szczeklik W., Plutecka H., et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg-Strauss syndrome patients. Rheumatology (Oxford) 2012;51:1887–1893. doi: 10.1093/rheumatology/kes171. [DOI] [PubMed] [Google Scholar]
- 45.Jennette J.C., Falk R.J., Bacon P.A., et al. 2012 Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 46.Wechsler M.E., Akuthota P., Jayne D., et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921–1932. doi: 10.1056/NEJMoa1702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachert C., Wagenmann M., Hauser U., Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–842. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 48.Tomassen P., Vandeplas G., Van Zele T., et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449. doi: 10.1016/j.jaci.2015.12.1324. -+ [DOI] [PubMed] [Google Scholar]
- 49.Van Zele T., Holtappels G., Gevaert P., Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2014;28:192–198. doi: 10.2500/ajra.2014.28.4033. [DOI] [PubMed] [Google Scholar]
- 50.Han J.K., Bachert C., Fokkens W., et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:1141–1153. doi: 10.1016/S2213-2600(21)00097-7. [DOI] [PubMed] [Google Scholar]
- 51.Bachert C., Han J.K., Desrosiers M.Y., et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2021;149:1309–1317. doi: 10.1016/j.jaci.2021.08.030. [DOI] [PubMed] [Google Scholar]
- 52.Furuta G.T. Management of eosinophilic esophagitis from childhood to adulthood. Gastroenterol Hepatol (NY) 2012;8:683–685. [PMC free article] [PubMed] [Google Scholar]
- 53.Liacouras C.A., Furuta G.T., Hirano I., et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. e6; quiz 1-2. [DOI] [PubMed] [Google Scholar]
- 54.Assa'ad A.H., Gupta S.K., Collins M.H., et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 55.Spergel J.M., Rothenberg M.E., Collins M.H., et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–463. doi: 10.1016/j.jaci.2011.11.044. 63 e1-3. [DOI] [PubMed] [Google Scholar]
- 56.Markowitz J.E., Jobe L., Miller M., Frost C., Laney Z., Eke R. Safety and efficacy of reslizumab for children and adolescents with eosinophilic esophagitis treated for 9 years. J Pediatr Gastroenterol Nutr. 2018;66:893–897. doi: 10.1097/MPG.0000000000001840. [DOI] [PubMed] [Google Scholar]
- 57.Allakos . 2021. Allakos Announces Topline Phase 3 Data from the ENIGMA 2 Study and Phase 2/3 Data from the KRYPTOS Study in Patients with Eosinophilic Gastrointestinal Diseases.https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-enigma-2-study-and-phase [Google Scholar]
- 58.Egan M., Furuta G.T. Eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018;121:162–167. doi: 10.1016/j.anai.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Gonsalves N. Eosinophilic gastrointestinal disorders. Clin Rev Allergy Immunol. 2019;57:272–285. doi: 10.1007/s12016-019-08732-1. [DOI] [PubMed] [Google Scholar]
- 60.Pesek R.D., Reed C.C., Muir A.B., et al. Increasing rates of diagnosis, substantial Co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114:984–994. doi: 10.14309/ajg.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dellon E.S., Peterson K.A., Murray J.A., et al. Anti-siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383:1624–1634. doi: 10.1056/NEJMoa2012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bergmann K.C., Altrichter S., Maurer M. Benefit of benralizumab treatment in a patient with chronic symptomatic dermographism. J Eur Acad Dermatol Venereol. 2019;33:e413–e415. doi: 10.1111/jdv.15720. [DOI] [PubMed] [Google Scholar]
- 63.Magerl M., Terhorst D., Metz M., et al. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J Dtsch Dermatol Ges. 2018;16:477–478. doi: 10.1111/ddg.13481. [DOI] [PubMed] [Google Scholar]
- 64.Maurer M., Altrichter S., Metz M., Zuberbier T., Church M.K., Bergmann K.C. Benefit from reslizumab treatment in a patient with chronic spontaneous urticaria and cold urticaria. J Eur Acad Dermatol Venereol. 2018;32:e112–e113. doi: 10.1111/jdv.14594. [DOI] [PubMed] [Google Scholar]
- 65.Ange N., Alley S., Fernando S.L., Coyle L., Yun J. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract. 2018;6:1059–1060. doi: 10.1016/j.jaip.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 66.Schmid-Grendelmeier P., Steiger P., Naegeli M.C., et al. Benralizumab for severe DRESS in two COVID-19 patients. J Allergy Clin Immunol Pract. 2020;9:481–483. doi: 10.1016/j.jaip.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolebeyan A., Mohammadi O., Vaezi Z., Amini A. Mepolizumab as possible treatment for allergic bronchopulmonary aspergillosis: a review of eight cases. Cureus. 2020;12:e9684. doi: 10.7759/cureus.9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brenard E., Pilette C., Dahlqvist C., et al. Real-life study of mepolizumab in idiopathic chronic eosinophilic pneumonia. Lung. 2020;198:355–360. doi: 10.1007/s00408-020-00336-3. [DOI] [PubMed] [Google Scholar]
- 69.Takeshita Y., Nobuyama S., Kanetsuna Y., et al. Eosinophilic bronchiolitis successfully treated with mepolizumab. J Allergy Clin Immunol Pract. 2020;8:1159–11561 e1. doi: 10.1016/j.jaip.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 70.Caruso C., Colantuono S., Pugliese D., et al. Severe eosinophilic asthma and aspirin-exacerbated respiratory disease associated to eosinophilic gastroenteritis treated with mepolizumab: a case report. Allergy Asthma Clin Immunol. 2020;16:27. doi: 10.1186/s13223-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All abstracted data is available to the readers in the tables of the manuscript and references.