Abstract
Background
Pathologic complete response (pCR) rates in early stage HER2-positive breast cancer improved after pertuzumab was added to neoadjuvant treatment. However, survival benefit is less-well established and seems mostly limited to node-positive patients. We used national cancer registry data to compare outcomes of patients treated with and without pertuzumab.
Methods
We identified stage II-III HER2-positive breast cancer patients treated with neoadjuvant trastuzumab-based chemotherapy between November 2013 until January 2016 from the Netherlands Cancer Registry. During that period pertuzumab was only available in the 37 hospitals that participated in the TRAIN-2 study. Missing grade and pCR-status were obtained from the Dutch Pathology Registry (PALGA) and cause of death from Statistics Netherlands. We used multiple imputation to impute missing data, multivariable logistic regression to evaluate the association between pertuzumab and pCR (ypT0/is, ypN0) and multivariable Cox regression models for overall survival and breast cancer specific survival (BCSS).
Results
We identified 1124 patients of whom 453 received pertuzumab. Baseline characteristics were comparable, although tumor grade was missing more often in patients treated without pertuzumab (12% vs. 2%). Pertuzumab improved pCR rates (41% vs 65%, adjusted odds ratio [aOR] 2.91; 95% CI:2.20–3.94). After a median follow-up of 6.0 years, 5-year BCSS rates were 95% and 98% respectively (adjusted hazard ratio [aHR]: 0.58; 95% CI:0.36–0.95). Younger patients derived more benefit from pertuzumab, but no other significant interactions were found.
Conclusion
These results support earlier data of a small survival benefit with the addition of pertuzumab to trastuzumab-based neoadjuvant chemotherapy which is most meaningful in younger patients.
Keywords: Non-metastatic breast cancer, HER2-Positive, ErbB2, Pertuzumab, Neoadjuvant chemotherapy, Survival
Abbreviations: Adj, adjuvant; aHR, adjusted hazard ratio; ALD, axillary lymph node dissection; aOR, adjusted Odds ratio; BCS, breast conserving surgery; BCSS, breast cancer specific survival; CBS, Statistics Netherlands; cN, clinical lymph node status; cT, clinical tumor stage; EMA, European Medicines Agency; EFS, even-free survival; ER, estrogen receptor; US Food and Drug Administration, (FDA); HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MARI, marking axillary lymph nodes with radioactive iodine seeds; NCR, Netherlands Cancer Registry; Neo-Adj, neoadjuvant; NST, invasive carcinoma of no special type; OS, overall survival; PALGA, Dutch Nationwide Pathology Databank; pCR, pathologic complete response; PR, progesterone receptor; SNP, sentinel node procedure; T-DM1, trastuzumab emtansine
Highlights
-
•
The benefit of adding pertuzumab to the (neo)adjuvant treatment of lymph node negative and HR+/HER2+ patients remains unclear.
-
•
Pertuzumab increases pCR rate from 41% to 65% in stage II-III HER2+ breast cancer.
-
•
5-year BCSS is 95% and 98% in patients treated without and with pertuzumab, respectively.
-
•
Patients <50 years benefit most from the addition of pertuzumab.
-
•
Survival benefit of pertuzumab is more evident in patients with higher stage, but independent of HR-status.
1. Introduction
Pertuzumab is a monoclonal antibody directed to the extracellular domain of the human epidermal growth factor receptor 2 (HER2). The addition of pertuzumab to neoadjuvant trastuzumab-based chemotherapy for stage II and III HER2-positive breast cancer is based on the results of the NeoSphere trial, which showed improvement in pathological complete response (pCR) rate from 29% to 46% [1]. Subsequent clinical trials in which patients were treated with neoadjuvant trastuzumab, pertuzumab and different chemotherapy backbones showed comparable results [[2], [3], [4]]. Another study evaluated the effect of combining pertuzumab and trastuzumab in the adjuvant setting for the duration of one year and found a small invasive disease-free survival benefit and no overall survival (OS) benefit after six years of follow up [5,6]. Long term benefit of pertuzumab seems modest and most pronounced in patients with a higher risk of breast cancer recurrence [6,7]. Therefore, the current international guidelines state that pertuzumab may be added to neoadjuvant treatment as standard of care especially in lymph node positive and/or estrogen receptor negative patients [8,9]. However, if pertuzumab should be added to the treatment of clinical stage II, lymph node negative and hormone receptor positive patients remains unclear.
In the Netherlands, pertuzumab was first added to the neoadjuvant treatment of HER2-positive breast cancer in 2014 in the TRAIN-2 trial in which 37 hospitals participated [4]. Patients participating in TRAIN-2 were randomized to receive dual HER2-blockade with either an anthracycline-containing or an anthracycline-free chemotherapy regime of the same duration [4]. Since pertuzumab was not reimbursed outside the study until January of 2016, most patients who were treated in hospitals that did not participate in the study received chemotherapy and trastuzumab only. This provided an opportunity to evaluate pathological complete response rate and five-year survival in early stage HER2-positive breast cancer patients treated with trastuzumab-based chemotherapy with or without pertuzumab in a quasi-experimental setting.
2. Materials & methods
2.1. Patient and data collection
Patient data was obtained from the Netherlands Cancer Registry (NCR). The NCR has nationwide coverage in all Dutch hospitals and data is collected by trained and experienced data managers. We identified all patients with clinical stage II and III HER2-positive breast cancer who received neoadjuvant treatment with chemotherapy plus trastuzumab with or without pertuzumab during the inclusion period of the TRAIN-2 study (November 2013 until January 2016). All patients included in the TRAIN-2 study received pertuzumab as part of their neoadjuvant trial regimen. Completing one year of HER2-blockade with trastuzumab was standard of care for patients treated within and without the TRAIN-2 study. Patient and tumor characteristics, neoadjuvant treatment schedule, pathological stage at surgery, adjuvant treatment, survival status, and date of last follow-up are all part of standard data collected by the NCR. Breast cancer specific survival (BCSS) was obtained by linking the data to cause of death data by Statistics Netherlands (CBS). For missing pathology data (tumor grade, ER and/or PR, pT-status and pN-status we linked the data with the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA) [10]. Cases with missing tumor grade of which a baseline biopsy was available were scored by a dedicated breast pathologist (JS). We defined estrogen receptor (ER) and progesterone receptor (PR) positivity as at least 10% positive nuclear staining as stated in the Dutch National Guidelines [11]. HER2-positivity was defined as an immunohistochemistry score of 3+, or 2+ with amplification by in situ hybridization. Pathology findings were not centrally reviewed.
2.2. Endpoints
Pathological complete response was defined as complete disappearance of all invasive tumor cells of the breast and axilla (ypT0/is, N0), with either presence or absence of ductal or lobular carcinoma in situ. BCSS was defined time from diagnosis of primary breast cancer until death from breast cancer or censoring other causes of death. OS was defined as the time between the date of diagnosis of primary breast cancer until the date of death from any cause or last follow-up date. Patients who were alive were censored at time of last follow-up visit. Breast cancer recurrences are not standardly registered in the NCR registration and therefore not available for other time-to-event analysis.
2.3. Statistical analyses
Missing data was imputed using multiple imputation with ten imputations based on the maximum percentage missing data (10%). More details can be found in the supplements (p.2). Results based on the imputed data set are presented in the main text, but complete case analysis results are available in the supplements. Univariable logistic regression was used to identify potential associations between patient and tumor characteristics and pCR. Factors that were identified to be associated with pCR or survival in previous studies were included in the multivariate model, as were factors with P < 0.05 in univariable analysis. To evaluate differential treatment benefit between subgroups, pairwise interactions with pertuzumab were included in the regression models. OS and BCSS were estimated with the Kaplan Meier method. Cause-specific Cox proportional hazard regression models were used for survival analyses. An univariable sensitivity analysis was performed treating for death from other causes as competing risk. We considered P values of <0.05 as statistically significant. All tests were two-sided. Statistical analyses were performed with SPSS 25.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.
3. Results
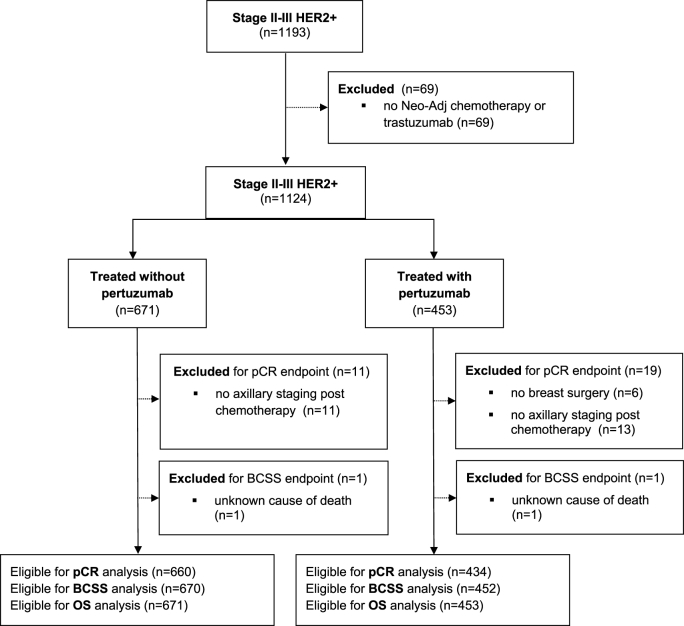
We identified 1124 patients who received neoadjuvant trastuzumab-based chemotherapy for stage II and III HER2-positive breast cancer between November 2013 and January 2016 from the Netherlands Cancer Registry (Fig. 1). Patient characteristics are summarized in Table 1. Four hundred fifty-three patients received pertuzumab as part of their neoadjuvant regimen while 671 did not. The vast majority of patients (423 of 453) receiving pertuzumab participated in the TRAIN-2 study. Ten percent of patients participating in TRAIN-2 were treated in non-teaching hospitals. No statistically significant differences between the two cohorts were found for age, clinical tumor stage, clinical nodal stage and hormone receptor status. However, since more participating centers were open for enrollment in the TRAIN-2 study in 2015 date of diagnosis differs between the groups. Also, tumor grade was missing in more patients in the group that did not receive pertuzumab as this was routinely registered within the trial. Additionally, more patients treated with pertuzumab received an anthracycline-free chemotherapy regimen as per the design of the TRAIN-2 study [4].
Fig. 1.
Flow diagram of included patients. Abbreviations: Neo-Adj, neoadjuvant; pCR, pathological complete response; BCSS, breast cancer specific survival; OS, overall survival.
Table 1.
Patient and treatment characteristics.
| No Pertuzumab (n = 671) |
Pertuzumab (n = 453) |
||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | 0.18 | ||||
| ≤50 | 363 | 54.1% | 264 | 58.3% | |
| >50 | 308 | 45.9% | 189 | 41.7% | |
| Year of diagnosis | <0.001 | ||||
| 2013 | 64 | 9.5% | 5 | 1.1% | |
| 2014 | 310 | 46.2% | 136 | 30.0% | |
| 2015a | 297 | 44.3% | 312 | 68.9% | |
| WHO-performance status | |||||
| 0 | 313 | 46.6% | 272 | 60.0% | <0.001 |
| 1 | 46 | 6.9% | 33 | 7.3% | |
| 2 | 1 | 0.1% | 1 | 0.2% | |
| 4 | 1 | 0.1% | 0 | 0.0% | |
| Unknown | 310 | 46.2% | 147 | 32.5% | |
| Clinical tumor stage | 0.98 | ||||
| cT0-2 | 469 | 69.9% | 317 | 70.0% | |
| cT3-4 | 202 | 30.1% | 136 | 30.0% | |
| Clinical nodal stage | 0.70 | ||||
| N0 | 217 | 32.3% | 157 | 34.7% | |
| N+ | 450 | 67.1% | 293 | 64.7% | |
| Unknown | 4 | 0.6% | 3 | 0.7% | |
| Hormone receptor status | 0.65 | ||||
| Positive (ER or PR ≥ 10%) | 421 | 62.7% | 273 | 60.3% | |
| Negative (ER and PR < 10%) | 249 | 37.1% | 180 | 39.7% | |
| Unknown | 1 | 0.1% | 0 | 0% | |
| Tumor grade | <0.001 | ||||
| Grade 1-2 | 216 | 32.2% | 217 | 47.9% | |
| Grade 3 | 376 | 56.0% | 225 | 49.7% | |
| Unknown | 79 | 11.8% | 11 | 2.4% | |
| Histology | 0.47 | ||||
| NST | 640 | 95.4% | 433 | 95.6% | |
| Lobular | 27 | 4.0% | 19 | 4.2% | |
| Other | 4 | 0.6% | 1 | 0.2% | |
| Neo-Adj chemotherapy | |||||
| Anthracycline based | 530 | 79.0% | 238 | 52.5% | <0.001 |
| Anthracycline free | 141 | 21.0% | 215 | 47.5% | |
| Breast Surgery | 0.001 | ||||
| BCS | 353 | 52.6% | 253 | 55.8% | |
| Mastectomy | 318 | 47.4% | 192 | 42.4% | |
| None | 0 | 0% | 8 | 1.8% | |
| Axillary staging | <0.001 | ||||
| SNP/MARI | 186 | 27.7% | 215 | 47.5% | |
| ALD | 251 | 37.4% | 120 | 26.5% | |
| Unknown method | 209 | 31.7% | 95 | 21.0% | |
| None | 25 | 3.7% | 23 | 5.1% | |
| Radiotherapy | 0.70 | ||||
| Yes | 549 | 81.8% | 366 | 80.8% | |
| No | 122 | 18.2% | 87 | 19.2% | |
| Adj. chemotherapy | 0.82 | ||||
| Yes | 13 | 1.9% | 7 | 1.5% | |
| No | 658 | 98.1% | 446 | 98.5% | |
| Endocrine therapy (overall) | 0.36 | ||||
| Yes | 381 | 56.8% | 244 | 53.9% | |
| No | 290 | 43.2% | 209 | 46.1% | |
| Endocrine therapy (HR+) | 1.00 | ||||
| Yes | 375 | 89.1% | 243 | 89.0% | |
| No | 46 | 10.9% | 30 | 11.0% | |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; NST, invasive carcinoma of no special type; Neo-Adj, neoadjuvant; BCS, breast conserving surgery; SNP, sentinel node procedure; MARI, marking axillary lymph nodes with radioactive iodine seeds; ALD, axillary lymph node dissection; Adj, adjuvant; HR+, hormone receptor positive.
Includes January 2016.
3.1. Pathologic complete response
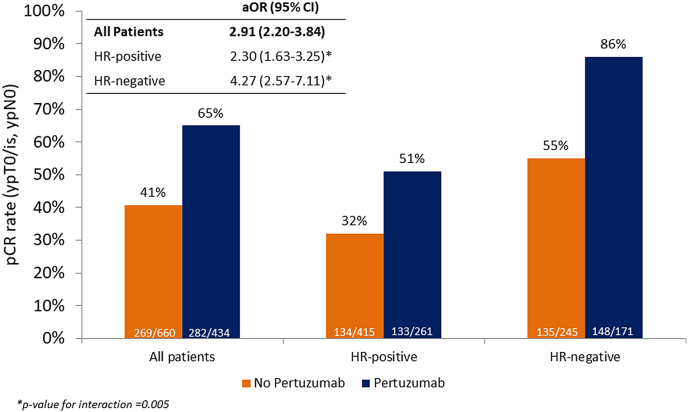
Pathological complete response rates according to treatment group and hormone receptor status are shown in Fig. 2. Overall, 551 of 1104 (49.9%) patients reached a pCR after neoadjuvant systemic treatment. Of the patients treated without pertuzumab, 269 of 660 (40.7%) reached a pCR, versus 282 of 432 (65%) patients treated with pertuzumab (adjusted odds ratio (aOR) 2.91; 95% CI 2.20–3.84). This difference was more pronounced in hormone-receptor negative patients (aOR 4.27, 95% CI 2.57–7.11) compared to hormone-receptor positive patients (aOR 2.30. 95% CI 1.63–3.25, p for interaction = 0.005). Analyses in other subgroups are presented in the supplements in which no formal interaction was identified although patients with tumor grade 3 more often reach a pCR numerically (p.4). Odds ratio's across other subgroups as age and clinical disease stage indicate no clear benefit differences.
Fig. 2.
Bar chart of pCR with adjusted odds ratios for pCR – overall and according to hormone receptor status. Abbreviations: pCR, pathological complete response; HR-positive, hormone receptor positive; HR-negative, hormone receptor negative; aOR, adjusted odds ratio: analyzed in multiple imputated data set and adjusted for age, clinical tumor stage, clinical nodal status, tumor grade, anthracyclines (yes/no), histology and in case of all patients: hormone receptor status.
3.2. Breast cancer specific survival and overall survival
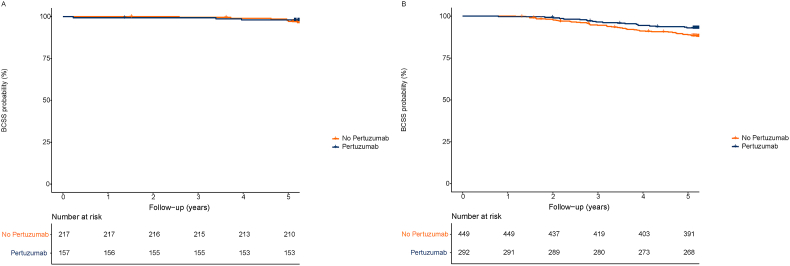
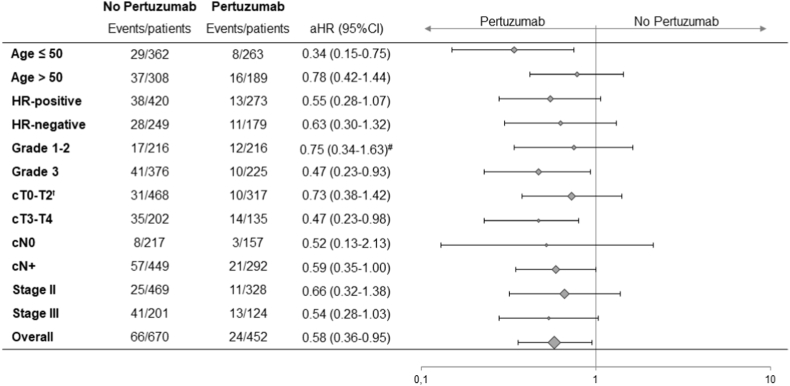
During a median follow-up of 6.0 years, 90 patients had died of breast cancer, 11 patients died of other causes and 2 patients of unknown causes. Kaplan-Meier plots for lymph node negative and positive patients are presented in Fig. 3. Kaplan-Meier plots for OS and BCSS overall are presented in the supplements (p.5). BCSS rate after five years was 92% in patients treated with trastuzumab–based chemotherapy and 95% when pertuzumab was added (adjusted hazard ratio [aHR] 0.58; CI 95% 0.36–0.95). This was confirmed in a competing risk analysis, which showed a similar hazard ratio (0.57; CI 95% 0.36–0.90). Subgroup analyses showed a greater BCSS benefit for pertuzumab in patients younger than 50 years (aHR 0.34; CI 95% 0.15–0.75, p for interaction = 0.04) (Fig. 4). No statistically significant interaction was found across other subgroups and adjusted hazard ratios showed overlapping CIs. However, pertuzumab benefit is numerically more evident in lymph node positive disease and even more pronounced in stage III patients (Fig. 3 and supplements, p.5). Five-year OS was 90% (95% CI 88.2%–92.7%) in patients treated without and 95% (95% CI, 92.5%–96.6%) in patients treated with pertuzumab (aHR: 0.51, 95% CI 0.32–0.82). Results for complete cases and subgroup analyses for OS are presented in the supplements (p.6-7).
Fig. 3.
Kaplan-Meier curves for breast cancer specific survival by lymph node status. A. Breast cancer specific survival per treatment group in clinical lymph node negative (cN0) patients; B. Breast cancer specific survival per treatment group in clinical lymph node positive (cN+) patients.
Fig. 4.
Forest Plot for Breast Cancer Specific Survival overall and within subgroups. Abbreviations: HR-positive, hormone receptor positive; HR-negative, hormone receptor negative; cT, clinical tumor stage; cN, clinical lymph node status; aHR, adjusted hazard ratio.
ꝉTx patients are also included in cT0-2. #aHR for complete case analysis
4. Discussion
We evaluated pCR rates, BCSS and OS in patients treated with and without neoadjuvant pertuzumab in a nationwide cohort. Patients treated with pertuzumab had higher pCR rates, BCSS and OS. Increase in pCR rate was largest in hormone receptor negative patients, while long term benefit seemed independent of hormone receptor status. Overall, younger patients and with lymph node positive breast cancer seem to benefit most of dual HER2-blockade with pertuzumab with regard to BCSS.
Our findings are in line with the results of previous clinical trials and the current international breast cancer guidelines. In the NeoSphere study, 5-year progression-free survival was 86% in patients treated with trastuzumab plus pertuzumab in combination with docetaxel, versus 81% in patients treated without pertuzumab. Since this study was powered for pCR as primary endpoint the event-rate was too low for formal comparison between treatment arms [7]. In the APHINITY trial, a small improvement in invasive-disease free survival was observed with the addition of pertuzumab to adjuvant trastuzumab-containing chemotherapy after six years of follow-up (91% vs 88%). This effect was most pronounced in the lymph node positive subgroup. No overall survival benefit has been demonstrated so far [6]. Other clinical trials in which pertuzumab was added to various chemotherapy backbones had 3-year event free-survival (EFS)-rates ranging from 92 to 94% and 5-year EFS ranging from 82% to 94% [[12], [13], [14], [15]]. Previously published comparative real world studies only evaluated pCR or only assessed recurrence-rates in patients achieving a pCR [[16], [17], [18], [19]].
PCR is approved by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) as surrogate endpoint in early breast cancer trials. The prognostic value of pCR is found across breast cancer subtypes, but is highest in triple negative and HER2-positive breast cancer [[20], [21], [22]]. However, a large and more recent published meta-analysis including 54 randomized trials did not find a strong association between pCR and disease-free survival [23]. Although the analysis was not based on individual patient data and results were not stratified within HER2-positive subgroups the findings stress the need for supportive real-world evidence of survival benefit for agents approved and widely prescribed following trials powered for surrogate endpoints.
So, real-world data are important to assess the validity of clinical trial results in clinical practice. However, analyses of treatment effects based on real-world data suffer from bias including confounding-by-indication. A strength of our study is that use of pertuzumab was largely based on whether a hospital participated in the TRAIN-2 trial, rather than on individual patient characteristics. This design reduces bias resulting from confounding-by-indication. Residual confounding was addressed in multivariable analyses adjusting for differences in baseline characteristics. Importantly, patients treated with and without pertuzumab were diagnosed in the same time period, precluding changes in treatment guidelines over time to influence the results. Another strength of our study is the prospectively defined dataset routinely collected by the nationwide Netherlands Cancer Registry.
Some limitations need to be acknowledged. First, residual confounding still exists, as in any observational study despite the unique setting and adjustment for baseline characteristics. Although the majority of eligible patients in the participating centers were included in the trial, we have limited information on why some patients did not participate. Importantly, for these analyses we only used data that was extracted from the Netherlands Cancer Registry rather than the TRAIN-2 trial database. This resulted in a substantial percentage of missing data on important prognostic factors as tumor grade and performance-status. Secondly, we do not have information on breast cancer recurrence as this was not routinely registered in the NCR. Third, differences in chemotherapy backbone and duration exist between patients treated with or without pertuzumab, as anthracycline-free schedules were largely restricted to patients treated in the TRAIN-2 trial with pertuzumab. In addition, the anthracycline-free regimen within the trial consisted of nine cycles taxane-carboplatin and was generally of longer duration than anthracycline-containing schedules used outside the study [4]. Finally, adjuvant treatment with T-DM1 was not yet incorporated in guidelines during the study and this may limit the extrapolation of the long-term survival results to current day practice.
Our study confirms previous findings that pertuzumab improves pCR and long-term survival in patients with stage II and III HER2-positive breast cancer. The 25% increase in pCR rate and 5% increase in 5-year BCSS must be weighed against the well-known safety profile of pertuzumab. The most limiting side effect is diarrhea, which is seen in up to 46% of patients when combined with docetaxel and trastuzumab. This can be burdensome and may lead to dehydration, electrolyte imbalances and sometimes hospitalization [1,24]. In addition, the costs of pertuzumab increase health care expenses [25]. Therefore, a high need remains to select patient in whom pertuzumab can be omitted. The Adapt trial successfully showed that patients with small lymph node negative disease treated with only trastuzumab and taxanes have excellent survival rates [26]. In addition, translational studies on molecular subtype suggest that HER2-enriched subtype or IHC 3+ tumors are most sensitive to dual HER2-blockade whereas basal-like tumors do not seem to benefit at all [[27], [28], [29]]. Further studies are needed to confirm these findings.
Also, patients who have excellent response to (dual) HER2-blockade could be excellent candidates for chemotherapy de-escalation to avoid toxicity even more. In Neosphere 11% of patients treated with only trastuzumab and pertuzumab reached a pCR. Therefore multiple chemotherapy de-escalation studies are executed. In PHERGAIN patients received trastuzumab and pertuzumab and chemotherapy was only added if the response was less than a reduction in maximum standardized uptake value of 40% from baseline after cycle two. The corresponding pCR rate in responders, thus not receiving neoadjuvant chemotherapy, was 38%. Early response defined as ≥30% Ki-67 decrease or <500 invasive tumor cells in biopsy after three weeks resulted in a pCR rate of 45% in HR-negative patients treated with targeted treatment only in the WSG-ADAPT-HER2+/HR– [30]. In the multicenter phase 2 TRAIN-3 trial (NCT03820063) patients are treated with chemotherapy plus trastuzumab and pertuzumab and response is monitored by MRI every three cycles. In case of a radiologic complete response on MRI breast patients are referred for early surgery.
In conclusion, our data supports the current international treatment guidelines to add pertuzumab to trastuzumab-based neoadjuvant chemotherapy in patients with stage II and III HER2-positive breast cancer in both hormone receptor-positive and hormone receptor-negative patients. Younger patients and those with a higher risk of breast cancer recurrence seem to benefit most from pertuzumab. More research on predictive biomarkers is needed to further investigate if there is a subgroup of patients in whom pertuzumab can be safely omitted. Great responders to HER2-directed therapy could be candidates for chemotherapy-free treatment regimens.
Funding information
This research was funded by a donation from the Team Westland foundation (to GSS). Team Westland played no role in the design or execution of this study, nor in the execution of the analysis, interpretation of data, writing the manuscript or in the decision to submit for publication.
Author contributions
Study concept and design: MSvR, GSS, AvdV, MCL. Quality and control of data and algorithms AvdV and MCL. Data analysis and interpretation: all authors. Statistical analyses: AvdV, MCL, EvW. Manuscript preparation: AvdV. Manuscript editing: AvdV, MCL, GSS. Manuscript review and approval: all authors.
Compliance with ethical standards
The review board of the Netherlands Cancer Registry and Statistics Netherlands approved this study and it was conducted in accordance with the Declaration of Helsinki. The TRAIN-2 clinical trial was approved by the medical ethics committee of the Netherlands Cancer Institute and all participants signed written informed consent before any study related-procedure.
Declaration of competing interest
GSS reports institutional research support from Agendia, AstraZeneca, Merck, Novartis, Roche and Seagen and consultancy fees paid to the institution from Biovica and Seagen. All other authors declare that they have no conflicts of interest.
Acknowledgements
We thank the staff and data managers of the Netherlands Comprehensive Cancer Organization (IKNL), Statistics Netherlands (CBS), the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA), and the staff of the core facility molecular pathology and biobanking (CFMPB) of the Netherlands Cancer Institute for the collection of the data and support during data analyses. We thank the Dutch Breast Cancer Research Group (BOOG) as the study sponsor and Roche as the funder of the investigator-initiated TRAIN-2 study for making pertuzumab available for study patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.07.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gianni L., Pienkowski T., Im Y.H., Roman L., Tseng L.M., Liu M.C., et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 2.Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Hegg R., et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 3.Swain S.M., Ewer M.S., Viale G., Delaloge S., Ferrero J.M., Verrill M., et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Ramshorst M.S., van der Voort A., van Werkhoven E.D., Mandjes I.A., Kemper I., Dezentje V.O., et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630–1640. doi: 10.1016/S1470-2045(18)30570-9. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G., Procter M., de Azambuja E., Zardavas D., Benyunes M., Viale G., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccart M., Procter M., Fumagalli D., de Azambuja E., Clark E., Ewer M.S., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 Years' follow-up. J Clin Oncol. 2021;39(13):1448–1457. doi: 10.1200/JCO.20.01204. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L., Pienkowski T., Im Y.H., Tseng L.M., Liu M.C., Lluch A., et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 9.Korde L.A., Somerfield M.R., Carey L.A., Crews J.R., Denduluri N., Hwang E.S., et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–1505. doi: 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casparie M., Tiebosch A.T., Burger G., Blauwgeers H., van de Pol A., van Krieken J.H., et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29(1):19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nationaal Borstkanker Overleg Nederland . 2012. Dutch breast cancer guideline.https://richtlijnendatabase.nl/richtlijn/borstkanker/pathologie/receptorbepaling.html receptorbepaling [Internet] [cited 30 Jun 2022]. Available from: [Google Scholar]
- 12.Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Waldron-Lynch M., et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Dang C.E.M., Delaloge S., et al. 2021. Pertuzumab/trastuzumab in early stage HER2-positive breast cancer: 5-year and final analysis of the BERENICE trial. ESMO Breast Cancer Virtual Congress Abstract 43O. Presented May 7, 2021. [Google Scholar]
- 14.van der Voort A., van Ramshorst M.S., van Werkhoven E.D., Mandjes I.A., Kemper I., Vulink A.J., et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual ERBB2 blockade in patients with ERBB2-positive breast cancer: a secondary analysis of the TRAIN-2 randomized, phase 3 trial. JAMA Oncol. 2021;7(7):978–984. doi: 10.1001/jamaoncol.2021.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurvitz S.A., Martin M., Jung K.H., Huang C.-S., Harbeck N., Valero V., et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2–positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37(25):2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasching P.A., Hartkopf A.D., Gass P., Häberle L., Akpolat-Basci L., Hein A., et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat. 2019;173(2):319–328. doi: 10.1007/s10549-018-5008-3. [DOI] [PubMed] [Google Scholar]
- 17.Boér K., Kahán Z., Landherr L., Csőszi T., Máhr K., Ruzsa Á., et al. Pathologic complete response rates after neoadjuvant pertuzumab and trastuzumab with chemotherapy in early stage HER2-positive breast cancer - increasing rates of breast conserving surgery: a real-world experience. Pathol Oncol Res. 2021;27 doi: 10.3389/pore.2021.1609785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Santiago S., Saura C., Ciruelos E., Alonso J.L., de la Morena P., Santisteban Eslava M., et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (The NEOPETRA Study) Breast Cancer Res Treat. 2020;184(2):469–479. doi: 10.1007/s10549-020-05866-1. [DOI] [PubMed] [Google Scholar]
- 19.O'Shaughnessy J., Robert N., Annavarapu S., Zhou J., Sussell J., Cheng A., et al. Recurrence rates in patients with HER2+ breast cancer who achieved a pathological complete response after neoadjuvant pertuzumab plus trastuzumab followed by adjuvant trastuzumab: a real-world evidence study. Breast Cancer Res Treat. 2021;187(3):903–913. doi: 10.1007/s10549-021-06137-3. [DOI] [PubMed] [Google Scholar]
- 20.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 21.Broglio K.R., Quintana M., Foster M., Olinger M., McGlothlin A., Berry S.M., et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):751–760. doi: 10.1001/jamaoncol.2015.6113. [DOI] [PubMed] [Google Scholar]
- 22.Davey M.G., Browne F., Miller N., Lowery A.J., Kerin M.J. Pathological complete response as a surrogate to improved survival in human epidermal growth factor receptor-2-positive breast cancer: systematic review and meta-analysis. BJS Open. 2022;6(3) doi: 10.1093/bjsopen/zrac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conforti F., Pala L., Sala I., Oriecuia C., De Pas T., Specchia C., et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ (Clinical research ed) 2021;375 doi: 10.1136/bmj-2021-066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bines J., Procter M., Restuccia E., Viale G., Zardavas D., Suter T., et al. Incidence and management of diarrhea with adjuvant pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive breast cancer. Clin Breast Cancer. 2020;20(2):174–181. doi: 10.1016/j.clbc.2019.06.016. e3. [DOI] [PubMed] [Google Scholar]
- 25.Borges A., Pereira F., Redondo P., Antunes L., Vieira C., Antunes P., et al. The addition of neoadjuvant pertuzumab for the treatment of HER2+ breast cancer: a cost estimate with real-world data. Health Econ Rev. 2021;11(1):33. doi: 10.1186/s13561-021-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolaney S.M., Guo H., Pernas S., Barry W.T., Dillon D.A., Ritterhouse L., et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2019;37(22):1868–1875. doi: 10.1200/JCO.19.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchini G., Kiermaier A., Bianchi G.V., Im Y.H., Pienkowski T., Liu M.C., et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017;19(1):16. doi: 10.1186/s13058-017-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baselga J., Cortés J., Im S.-A., Clark E., Ross G., Kiermaier A., et al. Biomarker analyses in cleopatra: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2–positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 29.Nitz U., Gluz O., Graeser M., Christgen M., Kuemmel S., Grischke E.M., et al. De-escalated neoadjuvant pertuzumab plus trastuzumab therapy with or without weekly paclitaxel in HER2-positive, hormone receptor-negative, early breast cancer (WSG-ADAPT-HER2+/HR-): survival outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2022 doi: 10.1016/S1470-2045(22)00159-0. [DOI] [PubMed] [Google Scholar]
- 30.Nitz U.A., Gluz O., Christgen M., Grischke E.M., Augustin D., Kuemmel S., et al. De-escalation strategies in HER2-positive early breast cancer (EBC): final analysis of the WSG-ADAPT HER2+/HR- phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab +/- weekly paclitaxel. Ann Oncol. 2017;28(11):2768–2772. doi: 10.1093/annonc/mdx494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.