Abstract
Spinal muscular atrophy (SMA) is a degenerative neuromuscular disease that causes progressive muscle weakness and atrophy due to loss of the anterior horn cells of the spinal cord. Although effective treatments, such as gene therapy, have emerged in recent years, their therapeutic efficacy depends on a restricted time window of treatment initiation. For the treatment to be effective, it must be started before symptoms of the disease emerge. For this purpose, newborn screening (NBS) for SMA is conducted in many countries worldwide. The NBS program for SMA has been initiated in Japan in several regions, including the Kumamoto Prefecture. We started the NBS program in February 2021 and detected a patient with SMA after screening 13,587 newborns in the first year. Herein, we report our experience with the NBS program for SMA and discuss an issue to be approached in the future.
Keywords: Newborn screening, Real-time PCR, Spinal muscular atrophy, SMN1
Abbreviations: SMA, spinal muscular atrophy; NBS, newborn screening; SMN1, survival motor neuron 1 gene; SMN2, survival motor neuron 2 gene; PCR, polymerase chain reaction; DBS, dried blood spot; MLPA, multiplex ligation-dependent probe amplification; CHOP INTEND, Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders
1. Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive disorder caused by a mutation in the survival motor neuron 1 gene (SMN1). SMA causes progressive symmetrical limb and trunk paralysis associated with muscular atrophy owing to the degeneration of the anterior horn cells of the spinal cord [1]. Humans have a second SMN gene (SMN2) that differs from SMN1 by only five nucleotides. One of these, a substitution of cytosine to tyrosine in exon 7, leads to an increased skipping of exon 7; this results in low levels of the functional SMN protein. Therefore, the copy number of SMN2 is the most important genetic modifier of SMA disease severity.
SMA can be classified into types 0–4 according to the age of onset and the maximum motor function achieved [2]. Type 0 is rare (<1%); the most severe symptoms occur with onset during the prenatal period, and death typically occurs within 1 month of age [3]. Type 1 is the most frequent (60%) and also has severe symptoms; these begin before 6 months of age. Patients with type 1 SMA cannot sit without support and require permanent ventilator use. Type 2 is the second most frequent type (30%), with an onset between 6 and 18 months of age. Patients with type 2 SMA present with proximal predominant weakness and the inability to stand up. In patients with type 3 SMA, the onset time ranges from 18 months to adulthood; furthermore, they acquire the ability to stand and walk without support. In patients with type 4 SMA, onset occurs from the age of 30 years onwards, and patients can ambulate independently. Patients with type 3 and 4 SMA are expected to have a normal life expectancy. The estimated incidence in Japan was reported to be 0.51 (95% confidence interval, 0.32–0.71) per 10,000 live births [4]. SMA was previously considered incurable. In recent years, some treatments designed for treating SMA have emerged. The United States Food and Drug Administration approved nusinersen, onasemnogene abeparvovec, and risdiplam in 2016, 2019, and 2020, respectively [5]; these were also approved in Japan by the Japanese Ministry of Health, Labour and Welfare in 2017, 2020, and 2021, respectively. These treatments can improve the life prognosis and motor functions of infants affected with SMA. Delayed diagnosis and treatment limit the effects on clinical outcomes [2]. Newborn screening (NBS) is recommended for SMA, because it can allow early detection; hence, treatment can be started early. >95% of patients with SMA are homozygous for the deletion of SMN1 exon 7 [1]. Therefore, NBSs for SMA have been designed to detect the presence or absence of exon 7 using real-time polymerase chain reaction (PCR) in the USA and other countries [6]. NBS programs for SMA have just started in some regions of Japan, including the Kumamoto Prefecture. In this article, we report the first-year results of our ongoing NBS program for SMA and discuss issues that must be addressed in the future.
2. Material and methods
2.1. Study population
A total of 13,587 newborns who were born in the Kumamoto Prefecture between February 2021 and January 2022 were included in this study. Informed consent was obtained from the parents of the participating newborns, and dried blood spot (DBS) samples were collected 4–6 days after birth in each maternity clinic or obstetric department using a heel-prick procedure. The blood spots were blotted with filter paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan), and the filter paper was dried for at least 4 h at room temperature (15–30 °C). The samples were sent to the Newborn Screening Center at KM Biologics Co., Ltd. (Kumamoto, Japan) by mail, where a public-funded NBS was performed within 1 week of collection. Between February 2021 and September 2021, the DBSs were transferred to the Kumamoto University to assay for SMN1. From October 2021 onwards, the assay for SMN1 has been conducted by KM Biologics Co., Ltd. (Supplemental Fig. 1).
2.2. NBS program for SMA
Homozygous deletion of exon 7 of the SMN1 gene was examined by real-time PCR using the DBS samples. Newborns who were screened positive were referred to the Department of Pediatrics, Kumamoto University Hospital. There, they underwent physical examination for clinical symptoms related to SMA and were also subjected to another round of the SMN1 assay. Simultaneously, the copy numbers of exon 7 and exon 8 of the SMN1 and SMN2 genes were determined by the multiplex ligation-dependent probe amplification (MLPA) method for a definitive diagnosis. After the diagnosis of SMA, the patients were followed up and treated by the pediatric neurologists and pediatricians of the Kumamoto University Hospital.
2.3. SMN1 assay of the DBS
The SMN1 assay of the DBSs was performed using the NeoSMAAT® SMN1 kit (Sekisui medical Co., Ltd., Tokyo, Japan) using the manufacturer's protocol. Briefly, disks of 1.5 mm diameter were punched from the DBS cards; one disk was placed into each well of a 96-well reaction plate (WATSON Bio Lab, Tokyo, Japan). The reaction mixture contained the SMN1 forward primer, SMN1 reverse primer, Cy5-labeled SMN1 probe (Supplemental Fig. 2), RNaseP forward primer, RNaseP reverse primer, FAM-labeled RNaseP probe, dNTPs, and DNA polymerase. Forty microliters of this mixture were added to each well, and real-time PCR was performed using a scanning photofluorometric thermal cycler (QuantStudio® 5, ThermoFisher Scientifc, MA, Waltham, USA) as follows: 95 °C for 15 min followed by 40 cycles of melting at 95 °C for 15 s and annealing/extension at 63 °C for 1 min. RNaseP was used as the internal positive control. Cycle thresholds were initially determined by an inspection of the amplification curves and then retained in fixed positions. Threshold cycles were reported by the instrument software.
2.4. MLPA test
After obtaining informed consent, the blood of the patient who was screened as positive was sent to a contract lab (BML Inc., Tokyo, Japan). The copy numbers of exons 7 and 8 of SMN1 and SMN2 were assayed with the SALSA® MLPA® Probemix P060 SMA Carrier kit (MRC Holland, Amsterdam, Netherlands) using the manufacturer's protocol. The kit contained 21 MLPA probes, including 17 reference probes and two probes for SMN1 and SMN2 each (these were specific for exons 7 and 8 of both the genes).
2.5. Ethics
This study was approved by the Ethics Committee of the Kumamoto University (approval no. 2018). Written informed consent was obtained from the parents or legal guardians of the newborns.
3. Results
3.1. NBS for SMA
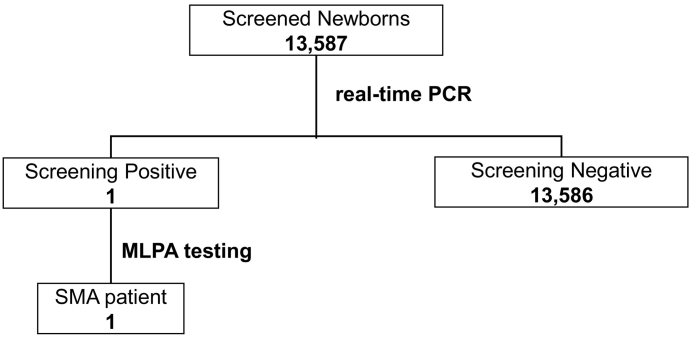
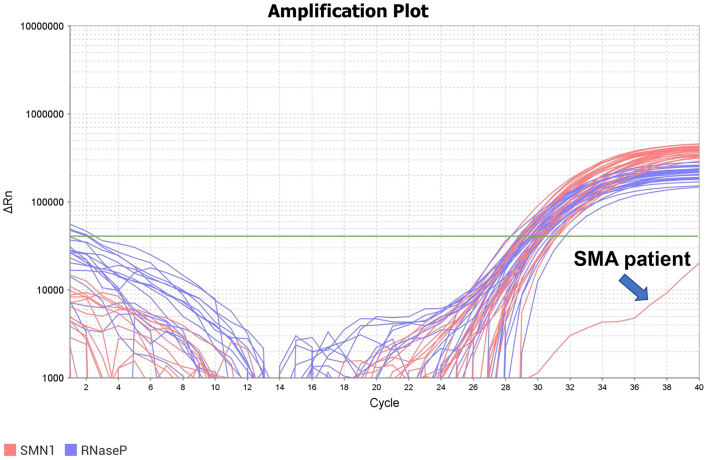
Fig. 1 summarizes the study results. During the study period, 13,587 newborns (which accounted for 96% of all births) underwent NBS for SMA; only one newborn was screened as positive (Fig. 2). He was examined for the copy numbers of exons 7 and 8 for both SMN1 and SMN2 using the MLPA method. The results showed that he had 0 copies of exons 7 and 8 for SMN1 and three copies of exons 7 and 8 each for SMN2. Thus, it was determined that he was likely to present with SMA. Currently, no newborns who were screened as negative in this NBS study have SMA or SMA-related symptoms.
Fig. 1.
Flowchart of newborn screening for SMA.
SMA: spinal muscular atrophy.
Fig. 2.
Amplification plots for SMN1 and RNaseP.
Green line shows the threshold line.
SMN1: survival motor neuron 1 gene.
3.2. Patient with SMA detected in this NBS
One male newborn was diagnosed with SMA through NBS. The outcomes of this case are summarized in Table 1. He was born by cesarean section at 42 weeks and 0 days of gestation. There were no abnormalities from pregnancy to birth. No blood relatives had SMA. A DBS was acquired for NBS 5 days after birth. He was judged as positive after screening and visited the Kumamoto University Hospital 14 days after birth. He presented with no SMA-related symptoms, such as muscle weakness or tongue fasciculations. Moreover, results of the copy number analysis using the MLPA method were presented 19 days after birth: he had 0 copies of exons 7 and 8 for SMN1 and three copies of exons 7 and 8 each for SMN2. Based on these results, it was determined that he was likely to develop SMA; thus, he was administered with onasemnogen abeparvovec 42 days after birth. His Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) score immediately prior to administration was 48/64 (range, 0 to 64; higher scores indicated a better motor function). At the age of 11 months, he was able to stand by himself and had normal motor development, with a CHOP INTEND score of 60/64.
Table 1.
Outcomes of a patient with SMA detected by the NBS.
| Age at DBS collection | 5 days |
| Age at screening result known | 13 days |
| Condition at first visit | normal |
| Age at SMA diagnosis | 19 days |
| SMN2 copy number | 3 |
| Age at treatment | 42 days |
| CHOP INTEND score just before treatment | 48/64 |
| Age at last visit | 11 months |
| CHOP INTEND score at last visit | 60/64 |
SMA = Spinal muscular atropy.
NBS = Newborn screening.
DBS = Dried blood spot.
CHOP INTEND = Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders.
4. Discussion
In this study, an NBS system for detecting a homozygous deletion of exon 7 in SMN1 with real-time PCR screened 13,587 newborns and identified one who was likely to develop SMA. He was treated with gene therapy before he could develop the disease symptoms. The previously reported frequency of SMA in Japan is 1:20,000 [4]. The frequency in the present study was higher than that reported previously. Further data accumulation is needed to evaluate patient frequency because of the limited number of participants screened in this study and the short study period (1 year).
In this study, no falsely positive newborns were detected. Furthermore, the assay used could not detect the SMN2 exon 7-SMN1 exon 8 hybrid; however, this should not affect the detection of a homozygous deletion of SMN1 exon 7. Ninety-six percent of patients with SMA have this deletion; the remaining 3%–4% have a compound heterozygosity for the deletion of SMN1 exon 7 and other mutations in SMN1 [7]. Therefore, it is important to understand that screening that targets SMN1 exon 7 is not likely to detect 5% of the patients with SMA.
Many other modifiers of SMA have been reported. For example, the plastin-3 gene and the neurocalcin delta gene have been identified as protective modifier genes in some phenotypically discordant SMA families [8]. The SMN2 exon 7-SMN1 exon 8 hybrid may be associated with a milder phenotype; the function of such hybrid genes is expected to be similar to that of SMN2 [9].
A four-month-old boy was negative for SMA in a Belgian NBS. He developed SMA and harbored a compound heterozygous deletion of SMN1 exon 7 and a missense mutation (c.815A > G [p.Tyr272Cys]) in SMN1 [10]. The newborn with potential SMA detected in this study had three copies of SMN2. The copy number of SMN2 is a predictor of SMA symptoms [11]. The lower the copy number of SMN2, the earlier and more severe the disease symptoms tend to develop. If the SMN2 copy number is three or less, treatment should be started immediately after diagnosis [12]. Even among patients with two copies of SMN2, those who started receiving treatment after the onset of symptoms tended to achieve motor milestones later in life [13].
Our patient had three copies of SMN2 and was asymptomatic at the age of 42 days when he underwent gene therapy. Five out of 10 newborns diagnosed with SMA, who harbored two copies of SMN2 (as detected in an NBS in Germany), had already developed symptoms (such as hypotonia) at the first visit [13]. In Taiwan, newborns with two copies of SMN2 showed muscle weakness 4 days after birth [14]. Even if no SMA-related symptoms are observed, patients with SMA can rapidly develop neuronal loss immediately after birth [15]; in fact, neurological damage may progress from before birth [16]. Thus, treatment should be initiated as soon as possible.
Onasemnogen abeparvovec serves as a recombinant adeno-associated virus serotype 9 vector-based gene therapy medication; a single intravenous infusion is designed to deliver a full-length functional copy of the human SMN gene via a self-complementary adeno-associated virus serotype 9 vector. Baker et al. administered onasemnogen abeparvovec to a patient with two copies of SMN2, which were detected at 11 days of age through an NBS in Wisconsin [17]. They could perform gene therapy >30 days earlier than we could. Differences in the time when newborns with SMA received this therapy resulted from differences in the DBS collection times and time intervals between DBS collection and reporting of the screening results. When the patient was detected in our NBS, it took 13 days from birth to the reporting of the screening results. DBS collection was also performed 3–5 days later than that in Wisconsin. It is presently difficult in Japan to perform this collection earlier, because SMA screening uses the same DBS samples collected in public-funded NBSs that screen for other inherited metabolic diseases (such as amino acid disorders and organic acidemia). To address this issue, the assay for SMN1 is now conducted at the same facility that conducts public-funded NBSs (i.e. the Newborn Screening Center at KM Biologics Co., Ltd.). Thus, by eliminating the transportation time, the time for reporting of the results has reduced by 3–5 days. Moreover, the Wisconsin screening system included SMN2 gene copy number measurements using the same DBS. In our system, blood samples are collected for the MLPA test for definitive diagnosis, which includes SMN1 and SMN2 gene copy number measurements, during the outpatient visit after the first SMN1 screening. The MLPA test is performed by a different clinical laboratory, and it takes about 5 days to obtain the results (including the time required to transport the specimen). In addition to the MLPA method, measurement of the SMN2 copy number by droplet digital PCR [17,18] and real-time PCR [19] using DBSs has also been reported. Using these methods to measure the SMN2 copy number in the DBSs may shorten the time taken for diagnosis.
Currently, three therapies have been approved for the treatment of SMA [5]. Among these, onasemnogen abeparvovec has been preferentially used for patients discovered by NBS in recent years [20]; this is because of its sustained efficacy after a single dose [21] and the fact that lifetime treatment with it is cost-efficient [22]. There are two shipping points for onasemnogen abeparvovec, one in Europe and the other in the United States. However, Japan is distant from both points; accordingly, it takes 10–14 days from order placement to delivery. During this time, the disease may develop and progress even if the symptoms have not appeared [15]. Nusinersen sodium (known as the antisense oligonucleotide) can be administered early, even in Japan; in fact, it can be administered ahead of the scheduled onasemnogen abeparvovec dose. Its usage may improve the prognosis after onasemnogen abeparvovec administration. Nusinersen sodium has been administered in patients with asymptomatic SMA in many countries [23] and has resulted in good clinical outcomes [13,24]. A combination therapy of nusinersen sodium and onasemnogen abeparvovec [25] has also been reported to cause no significant side effects. In the future, administration of nusinersen sodium prior to onasemnogen abeparvovec may be considered as the standard treatment in countries such as Japan where acquiring onasemnogen abeparvovec takes time.
5. Conclusions
We started an NBS for SMA in Japan, and identified one patient likely to develop SMA; he was administered with gene therapy prior to the onset of symptoms. In the future, we should review and redevelop the current system to achieve one that enables earlier diagnosis and treatment. Moreover, we should acquire a better screening and treatment system that enables individuals with SMA to undergo treatment within the appropriate time window.
The following are the supplementary data related to this article.
Algorithm of the NBS for SMA.
NBS: newborn survey, SMA: spinal muscular atrophy.
PCR primers and probe for the real-time PCR assay of SMN1.
The manufacturer did not disclose the actual sequences of the primers and probe used in the assay kit. This figure shows example 3 in JP6850402. The primer set was designed to specifically amplify SMN1 by the sequence of the 3′ end of the forward primer as C.
PCR: polymerase chain reaction, SMN1: survival motor neuron 1 gene.
Funding
This work was supported in part by a Health and Labor Sciences Research Grant for Research on Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare, Japan [grant number JPMH20FC1025]; a Grant-in-Aid for the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development [AMED; grant numbers JP19ek0109276, JP20ek0109482]; and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan [Japan Society for the Promotion of Science [JSPS] KAKENHI; grant number JP20K08207].
Authors contributions
TS, JK, and KN were responsible for the design of the research. SY, TS, and SO contributed to measurements and data collection. TS, JK, KS, SY, SO, and KN checked and analyzed the data. TS, JK, and KS wrote the manuscript. JK and KN supervised this study. All authors read and approved the final manuscript for submission. All authors have agreed to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work (even ones in which the authors were not personally involved) are appropriately investigated and resolved and that the resolution is documented in the literature.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful to Ms. Fumiko Nozaki, Ms. Naomi Yano, Ms. Ayuko Tateishi, and Ms. Hiroko Nasu for providing technical support related to this study.
Data availability
Data will be made available on request.
References
- 1.Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., Le Paslier D., Frézal J., Cohen D., Weissenbach J., Munnich A., Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 2.Kolb S.J., Kissel J.T. Spinal muscular atrophy. Neurol. Clin. 2015;33:831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grotto S., Cuisset J.M., Marret S., Drunat S., Faure P., Audebert-Bellanger S., Desguerre I., Flurin V., Grebille A.G., Guerrot A.M., Journel H., Morin G., Plessis G., Renolleau S., Roume J., Simon-Bouy B., Touraine R., Willems M., Frébourg T., Verspyck E., Saugier-Veber P. Type 0 spinal muscular atrophy: further delineation of prenatal and postnatal features in 16 patients. J. Neuromuscul. Dis. 2016;3:487–495. doi: 10.3233/JND-160177. [DOI] [PubMed] [Google Scholar]
- 4.Ito M., Yamauchi A., Urano M., Kato T., Matsuo M., Nakashima K., Saito K. Epidemiological investigation of spinal muscular atrophy in Japan. Brain Dev. 2022;44 doi: 10.1016/J.BRAINDEV.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Ramdas S., Servais L. New treatments in spinal muscular atrophy: an overview of currently available data. Expert. Opin. Pharmacother. 2020;21:307–315. doi: 10.1080/14656566.2019.1704732. [DOI] [PubMed] [Google Scholar]
- 6.Hale K., Ojodu J., Singh S. Landscape of spinal muscular atrophy newborn screening in the United States: 2018-2021. Int. J. Neonatal. Screen. 2021;7 doi: 10.3390/IJNS7030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercuri E., Finkel R.S., Muntoni F., Wirth B., Montes J., Main M., Mazzone E., Vitale M., Snyder B., Quijano-Roy S., Bertini E., Davis R.H., Meyer O.H., Simonds A.K., Schroth M.K., Graham R.J., Kirschner J., Iannaccone S.T., Crawford T., Woods S., Qian Y., Sejersen T., Tiziano F.D., Tizzano E., Swoboda K., Swoboda K., Laing N., Kayoko S., Prior T., Chung W.K., Wu S.M., Coleman C., Gee R., Glanzman A., Kroksmark A.K., Krosschell K., Nelson L., Rose K., Stępień A., Vuillerot C., Dubousset J., Farrington D., Flynn J., Halanski M., Hasler C., Miladi L., Reilly C., Roye B., Sponseller P., Hurst R., Tarrant S., Barja S., Foust K., Kyle B., Rodan L., Roper H., Seffrood E., Szlagatys-Sidorkiewicz A. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018;28:103–115. doi: 10.1016/J.NMD.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Wirth B. Spinal muscular atrophy: in the challenge lies a solution. Trends Neurosci. 2021;44:306–322. doi: 10.1016/j.tins.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Niba E.T.E., Nishio H., Wijaya Y.O.S., Lai P.S., Tozawa T., Chiyonobu T., Yamadera M., Okamoto K., Awano H., Takeshima Y., Saito T., Shinohara M. Clinical phenotypes of spinal muscular atrophy patients with hybrid SMN gene. Brain and Development. 2021;43:294–302. doi: 10.1016/j.braindev.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Boemer F., Caberg J.H., Beckers P., Dideberg V., di Fiore S., Bours V., Marie S., Dewulf J., Marcelis L., Deconinck N., Daron A., Blasco-Perez L., Tizzano E., Hiligsmann M., Lombet J., Pereira T., Lopez-Granados L., Shalchian-Tehran S., van Assche V., Willems A., Huybrechts S., Mast B., van Olden R., Dangouloff T., Servais L. Three years pilot of spinal muscular atrophy newborn screening turned into official program in Southern Belgium. Sci. Rep. 2021;11:19922. doi: 10.1038/S41598-021-99496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calucho M., Bernal S., Alías L., March F., Venceslá A., Rodríguez-Álvarez F.J., Aller E., Fernández R.M., Borrego S., Millán J.M., Hernández-Chico C., Cuscó I., Fuentes-Prior P., Tizzano E.F. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018;28:208–215. doi: 10.1016/j.nmd.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Glascock J., Sampson J., Haidet-Phillips A., Connolly A., Darras B., Day J., Finkel R., Howell R.R., Klinger K., Kuntz N., Prior T., Shieh P.B., Crawford T.O., Kerr D., Jarecki J. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J. Neuromuscul. Dis. 2018;5:145–158. doi: 10.3233/JND-180304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vill K., Kölbel H., Schwartz O., Blaschek A., Olgemöller B., Harms E., Burggraf S., Röschinger W., Durner J., Gläser D., Nennstiel U., Wirth B., Schara U., Jensen B., Becker M., Hohenfellner K., Müller-Felber W. One year of newborn screening for SMA – results of a German pilot project. J. Neuromuscul. Dis. 2019;6:503–515. doi: 10.3233/JND-190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien Y.-H., Chiang S.-C., Weng W.-C., Lee N.-C., Lin C.-J., Hsieh W.-S., Lee W.-T., Jong Y.-J., Ko T.-M., Hwu W.-L. Presymptomatic diagnosis of spinal muscular atrophy through newborn screening. J. Pediatr. 2017;190:124–129.e1. doi: 10.1016/j.jpeds.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 15.Swoboda K.J., Prior T.W., Scott C.B., McNaught T.P., Wride M.C., Reyna S.P., Bromberg M.B. Natural history of denervation in SMA: Relation to age,SMN2 copy number, and function. Ann. Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos D.M., d’Ydewalle C., Gabbeta V., Dakka A., Klein S.K., Norris D.A., Matson J., Taylor S.J., Zaworski P.G., Prior T.W., Snyder P.J., Valdivia D., Hatem C.L., Waters I., Gupte N., Swoboda K.J., Rigo F., Frank Bennett C., Naryshkin N., Paushkin S., Crawford T.O., Sumner C.J. Age-dependent SMN expression in disease-relevant tissue and implications for SMA treatment. J. Clin. Invest. 2019;129:4817. doi: 10.1172/JCI124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker M.W., Mochal S.T., Dawe S.J., Wiberley-Bradford A.E., Cogley M.F., Zeitler B.R., Piro Z.D., Harmelink M.M., Kwon J.M. Newborn screening for spinal muscular atrophy: the Wisconsin first year experience. Neuromuscul. Disord. 2021 doi: 10.1016/j.nmd.2021.07.398. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L., Lin R., Gallagher S., Zayac A., Butchbach M.E.R., Hung P. Development and validation of a 4-color multiplexing spinal muscular atrophy (SMA) genotyping assay on a novel integrated digital PCR instrument. Sci. Rep. 2020;10:19892. doi: 10.1038/s41598-020-76893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar B., Barton S., Kordowska J., Eaton R.B., Counihan A.M., Hale J.E., Comeau A.M. Novel modification of a confirmatory SMA sequencing assay that can be used to determine SMN2 copy number. Int. J. Neonatal. Screen. 2021;7 doi: 10.3390/IJNS7030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng S., Lee B.H., Ciafaloni E. Parent perceptions in choosing treatment for infants with spinal muscular atrophy diagnosed through newborn screening. J. Child Neurol. 2022;37:43–49. doi: 10.1177/08830738211040292. [DOI] [PubMed] [Google Scholar]
- 21.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., Kissel J.T., Nagendran S., L’Italien J., Sproule D.M., Wells C., Cardenas J.A., Heitzer M.D., Kaspar A., Corcoran S., Braun L., Likhite S., Miranda C., Meyer K., Foust K.D., Burghes A.H.M., Kaspar B.K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 22.Shih S.T.F., Farrar M.A., Wiley V., Chambers G. Newborn screening for spinal muscular atrophy with disease-modifying therapies: a cost-effectiveness analysis. J. Neurol. Neurosurg. Psychiatry. 2021;92 doi: 10.1136/JNNP-2021-326344. [DOI] [PubMed] [Google Scholar]
- 23.Dangouloff T., Vrščaj E., Servais L., Osredkar D., Adoukonou T., Aryani O., Barisic N., Bashiri F., Bastaki L., Benitto A., Ben Omran T., Bernert G., Bertini E., Borde P., Born P., Boustani R.-M., Butoianu N., Castiglioni C., Catibusic F., Chan S., Chien Y.H., Christodoulou K., Dejsuphong D., Farrar M., Filip D., Goemans N., Guinhouya K., Haberlova J., Hadzsiev K., Hovhannesyan K., Isohanni P., Radovic N.I., Jacquier D., Jalloh A., Jedrzejowska M., Kandawasvika G., Kaputu C., Kawatu N., Kernohan K., Kirschner J., Klink B., Kodsy S., Kouame-Assouan A.-E., Kravljanac R., Kreile M., Litvinenko I., McMillan H., Mesa S., Mohamed I., Kanzoska L.M., Nevo Y., Nguefack S., Nkole K., O’Grady G., O’Rourke D., Oskoui M., Piazzon F., Poddighe D., Prasauskiene A., Prieto J., Rasmussen M., Razafindrasata S., Saha N., Saito K., Sakadi F., Sangare M., Schroth M., Shalkevich L., Shatillo A., Suthar R., Szabo L., Tatishvili N., Tazir M., Tizzano E., Topaloglu H., Tulinius M., van der Pol L., Vazquez G., Vlodavets D., Wanigasinghe J., Wilmshurst J., Xiong H., Zafeiriou D., Zamba E. Newborn screening programs for spinal muscular atrophy worldwide: where we stand and where to go. Neuromuscul. Disord. 2021;31:574–582. doi: 10.1016/j.nmd.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 24.De Vivo D.C., Bertini E., Swoboda K.J., Hwu W.-L., Crawford T.O., Finkel R.S., Kirschner J., Kuntz N.L., Parsons J.A., Ryan M.M., Butterfield R.J., Topaloglu H., Ben-Omran T., Sansone V.A., Jong Y.-J., Shu F., Staropoli J.F., Kerr D., Sandrock A.W., Stebbins C., Petrillo M., Braley G., Johnson K., Foster R., Gheuens S., Bhan I., Reyna S.P., Fradette S., Farwell W. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul. Disord. 2019;29:842–856. doi: 10.1016/j.nmd.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada Y., Rao V.K., Arya K., Kuntz N.L., DiDonato C.J., Napchan-Pomerantz G., Agarwal A., Stefans V., Katsuno M., Veerapandiyan A. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve. 2020;62:550–554. doi: 10.1002/mus.27034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Algorithm of the NBS for SMA.
NBS: newborn survey, SMA: spinal muscular atrophy.
PCR primers and probe for the real-time PCR assay of SMN1.
The manufacturer did not disclose the actual sequences of the primers and probe used in the assay kit. This figure shows example 3 in JP6850402. The primer set was designed to specifically amplify SMN1 by the sequence of the 3′ end of the forward primer as C.
PCR: polymerase chain reaction, SMN1: survival motor neuron 1 gene.
Data Availability Statement
Data will be made available on request.