Summary
Background
The prevalence of type 2 diabetes (T2DM) is increasing, but increasing longevity among persons with diagnosed diabetes may be is associated with more extensive and diverse types of morbidity. The extent and breadth of morbidity and how this varies across sub-groups is unclear and could have important clinical and public health implications. We aimed to estimate comorbidity profiles in people with T2DM and variations across sub-groups and over time.
Methods
We identified approximately 224,000 people with T2DM in the Discover-NOW dataset, a real-world primary care database from 2000 to 2020 covering 2.5 million people across North-West London, England, linked to hospital records. We generated a mixed prevalence and incidence study population through repeated annual cross sections, and included a broad set of 35 comorbidities covering traditional T2DM conditions, emerging T2DM conditions and other common conditions.
We estimated annual age-standardised prevalence of comorbidities, over the course of the disease in people with T2DM and several sub-groups.
Findings
Multimorbidity (two or more chronic conditions) is common in people with T2DM and increasing, but the comorbidity profiles of people with T2DM vary substantially. Nearly 30% of T2DM patients had three or more comorbidities at diagnosis, increasing to 60% of patients ten years later. Two of the five commonest comorbidities at diagnosis were traditional T2DM conditions (hypertension (37%) and ischaemic heart disease (10%)) the other three were not (depression (15%), back pain (25%) and osteoarthritis (11%)). The prevalence of each increased during the course of the disease, with more than one in three patients having back pain and one in four having depression ten years post diagnosis.
People with five or more comorbidities at diagnosis had higher prevalence of each of the 35 comorbidities. Hypertension (73%) was the commonest comorbidity at diagnosis in this group; followed by back pain (69%), depression (67%), asthma (45%) and osteoarthritis (36%). People with obesity at diagnosis had substantially different comorbidity profiles to those without, and the five commonest comorbidities were 50% more common in this group.
Interpretation
Preventative and clinical interventions alongside care pathways for people with T2DM should transition to reflect the diverse set of causes driving persistent morbidity. This would benefit both patients and healthcare systems alike.
Funding
The study was funded by the National Institute for Health and Care Excellence (NICE).
Keywords: Diabetes, Multimorbidity, Epidemiology
Research in context.
Evidence before this study
We searched PubMed for reports of population-based estimates of comorbidity profiles in people living with type 2 diabetes (T2DM) from 1st January 1990 to 30th September 2021 using the terms “diabetes mellitus” and “type 2 diabetes” along with “comorbidit* prevalence” and “multimorbidity”. Research to date has predominantly focused on comorbidity prevalence of conditions with long standing evidence o aetiological association with T2DM such as acute myocardial infarction, lower extremity amputation and stroke. There has been some estimation of comorbidity prevalence of a broader set of conditions but how this varies across sub-groups and during the course of the disease remains unclear
Added value of this study
In this observational analysis of more than 200,000 people with diabetes in North West London we found multimorbidity (two or more chronic conditions) to be common in people with T2DM with 30% of people having three or more comorbidities at diagnosis, increasing to 60% 10-years later. Two of the five commonest comorbidities at diagnosis were conditions traditionally associated with T2DM - hypertension and ischaemic heart disease however the other three were conditions not traditionally associated with T2DM, nor targeted in existing clinical guidelines – depression, back pain and osteoarthritis. Comorbidity profiles and prevalence varied substantially across sub-groups.
Implications of all the available evidence
Multimorbidity is common in people with T2DM and the most prevalent comorbidities are a diverse group including traditional, such as hypertension, and non-traditional, such as depression, T2DM conditions. Preventative measures alongside clinical care pathways for people with T2DM should transition to reflect the diverse set of causes driving persistent morbidity. This would benefit both patients and healthcare systems alike.
Alt-text: Unlabelled box
Introduction
Diabetes Mellitus (DM) and type 2 diabetes (T2DM) particularly is an increasingly urgent health priority. While the number of people living with DM has increased more than four-fold over the past 40-years to more than 460 million people today,1 all-cause mortality rates have declined substantially in several high-income countries2, 3, 4 including England.5 In parallel there has been a diversification in cause of death in people with DM illustrated by cancer overtaking cardiovascular disease (CVD) to become the leading cause of death in people with DM in England.5 Meanwhile a diversification in non-fatal conditions has also been reported.6,7
The increasing share of mortality and non-fatal conditions attributable to broader, non-vascular conditions7 is suggestive of increasing and diversifying multimorbidity in people with DM. The health needs of people with T2DM and other chronic conditions are therefore likely to be broad, complex and cut across both physical and mental health.8 Multimorbidity, living with two or more chronic conditions, is increasing in the UK9,10 yet work to date has largely focused on identifying patterns of multimorbidity in the general population.9,11
Current DM clinical guidelines,12 care pathways and secondary prevention approaches generally focus on the traditional, predominantly vascular, excess risks faced by people with DM and this is likely to have contributed to the large declines in fatal and non-fatal CVD incidence observed.2,4, 5, 6 The understanding of multimorbidity patterns and composition of specific comorbidities in people with DM, how this varies across patient groups and during the course of disease, is limited. Further knowledge of this could provide insight to provide more holistic and more personal approaches to clinical guideline development, care pathways and secondary prevention. We therefore aimed to estimate comorbidity profiles and how this varied in people with T2DM during the course of the disease.
Methods
Study design and participants
We identified patients living with T2DM in the Discover-Now Dataset from 1st January 2000 to 1st March 2020, generating a mixed prevalence and incidence population through serial annual cross sections over the study period. This is achieved by including both new incident cases and existing prevalent cases to create the study population in each year of analysis within the study period (2000-2019). Discover-Now is a new administrative real world evidence (RWE) dataset originally designed for clinical care and commissioning of services. This pseudo-anonymised dataset, described in detail elsewhere,13 is a primary care dataset covering the North-West London population of approximately 2.5 million people across 365 primary care practices linked to secondary care data from 2015. The dataset is broadly representative of the UK population by age and sex but is more diverse with regards to ethnicity and deprivation.13
The dataset is accessible via Discover-NOW Health Data Research Hub for Real World Evidence through their data scientist specialists and IG committee-approved analysts, hosted by Imperial College Health Partners.
We identified people with T2DM using both diagnostic (C10) and management (66A) Read and Oxford Medical Information System codes for DM14 and glucose lowering therapy (GLT) prescription data. Diagnosis date was identified as the first T2DM clinical readcode in their primary care record (which may have been prior to the study start date) or the first GLT prescription providing there were at least 2 GLT prescriptions on 2 different dates. People with type 1 diabetes (T1DM) were excluded if identified via the algorithm of i) DM diagnosis <30 years old AND insulin prescribed within 3 months of diagnosis AND non-insulin GLTs not prescribed or prescribed for <3 months.
People diagnosed with T2DM at 18 years of age or older entered the study population on the latest date of: i) their first T2DM event in primary care or prescription of GLT or ii) 1st January 2000 (study start period) if they were diagnosed before the study start date. People diagnosed aged under 18 years old entered the population on the latest date of i) the date they become 18 years or older or ii) 1st January 2000 (study start period) if they were diagnosed and became 18 before the study start date.
Patients remained in the study population until the earliest of: i) death; ii) transfer out of the North-West London region; iii) 1st March 2020 (end of study period).
Comorbidity groupings
We included a deliberately broad set of 35 comorbidities that we grouped into 3 categories; i) conditions associated with T2DM with evidence of an aetiological association with T2DM and captured in existing National Institute for Health and Care Excellence (NICE) guidelines12 aim to prevent, termed ‘traditional conditions’ throughout this analysis; ii) conditions with increasing association to T2DM in published literature7 which captures conditions with emerging association with T2DM but are not currently included in T2DM clinical guidelines and secondary prevention approaches – hereon termed ‘emerging conditions for this analysis; iii) other associated conditions that are either prevalent in the DM or general population and/or a substantial burden to patients and UK health system, hereon termed ‘other associated conditions’. The comorbidities and definitions are outlined in detail (Supplementary Table 1). Briefly the comorbidities were amputations, asthma, atrial fibrillation (AF), back pain, blindness, DM-related cancers15 (colorectal, pancreatic, gallbladder, breast, hepatocellular and endometrial), all-other cancers, Charcot osteoarthropathy, chronic obstructive pulmonary disease (COPD), dementia, depression, epilepsy, foot ulcer, frailty, heart failure, hypertension, hypoglycaemia, hypothyroidism, ischaemic heart disease (IHD), learning disability, liver disease, macular degeneration, myocardial infarction (MI), osteoarthritis, osteoporosis, peripheral vascular disease (PVD), chronic kidney disease (CKD), all other kidney disease, nephropathy, retinopathy, severe mental illness, severe pancreatitis, stroke and tuberculosis.
Comorbidities were identified through both primary care (clinical read codes) and secondary care (ICD-10 codes) as outlined in Appendix Table 1. Primary and secondary diagnoses from inpatient admissions, outpatient appointment and Emergency Department (ED) episodes were identified in assessing for the presence of any of our 35 specific comorbidities. Secondary care amputation data used the OPCS Classification of Interventions and Procedures codes, with specific codes were used (Supplemental Table 1).
Statistical analysis
We estimated crude and age-standardised, according to 5-year age bands in the 2013 European Reference Population,16 prevalence of comorbidities using 2 time perspectives – i) calendar years from 2000 to 2019 with each calendar year as discrete annual periods comprising those living with T2DM with heterogenous duration and ii) duration post T2DM diagnosis in discrete years 0–10. We stratified the results according to several pre-defined sub-groups of interest (Appendix Table 2). These included demographic strata: age, ethnicity, deprivation (according to Index of Multiple Deprivation (IMD) Decile); clinical strata, those with any of the following conditions at T2DM diagnosis: CVD (acute myocardial infarction (AMI), IHD, stroke or heart failure), hypertension, renal disease or obesity; low and high multimorbidity – those with less than two or five or more comorbidities (in addition to T2DM) respectively at T2DM diagnosis.
We further developed an online visualisation platform to enable more comprehensive exploration of the results which is hosted at https://dmcomorbidities.lcp.uk.com/ when the paper is published. All data management was performed in SQL, analyses conducted in R version 3.6.1 and figures generated in MS Excel.
Ethics statement
This study was approved by the Discover Data Access Group (DRAG).
Role of funding source
The study was funded by the National Institute for Health and Care Excellence (NICE). Three authors (KH, JE, PJ) are employees of NICE and were involved in the development of the study and finalising of the manuscript.
JP-S, TP and PJ conceived of the idea for the paper. SH, RP and JP-S led the analysis with input from LZ, TP, RS, KH, EG and JE. SH, RP, LZ, TP, JP-S all had access to the study dataset. JP-S wrote the first draft of the manuscript. All authors critically reviewed and contributed to the final manuscript.
Results
Study participants
There were approximately 224,000 unique individuals with T2DM living in North-West London and in the Discover-Now dataset between 1st January 2000 and 31st December 2019 with a total of 22,523 (10.1%) patients excluded due to meeting exclusion criteria (Supplemental Figure 1). The number of people living with T2DM in the Discover-Now dataset increased progressively over the study period from approximately 38,000 in 2000 to 167,000 in 2019, with median age increasing from 60 to 62, a slight majority of the population being, and the most common ethnicity being Asian or Asian British throughout the study period (Table 1a & 1b).
Table 1a.
Demographic characteristics of those with T2DM in the Discover-NOW population in selected years 2000–2019.
| Year | 2000 | 2005 | 2010 | 2015 | 2019 |
|---|---|---|---|---|---|
| Patients | 38,391 | 70,207 | 1,07,791 | 1,44,144 | 1,67,478 |
| Gender | |||||
| Female (%) | 44.4 | 44.6 | 44.4 | 44.9 | 45.5 |
| Male (%) | 55.6 | 55.4 | 55.6 | 55.1 | 54.5 |
| Age | |||||
| Mean Age (years) | 58.9 | 60.3 | 61.6 | 61.7 | 62 |
| Median Age (years) | 60 | 61 | 62 | 62 | 62 |
| Age bands | |||||
| Age Category Under 25 n(%) | 97 (0.3) | 170 (0.2) | 233 (0.2) | 280 (0.2) | 301 (0.2) |
| Age Category 25–34 n(%) | 1091 (2.8) | 1800 (2.6) | 2711 (2.5) | 3904 (2.7) | 3979 (2.4) |
| Age Category 35–44 n(%) | 4400 (11.0) | 7257 (10.3) | 10,332 (9.6) | 13,635 (9.5) | 15,366 (9.2) |
| Age Category 45–54 n(%) | 8238 (21.5) | 14,714 (21) | 21,222 (19.7) | 27,779 (19.3) | 31,265 (18.7) |
| Age Category 55–64 n(%) | 11,067 (28.8) | 17,496 (24.9) | 26,461 (24.5) | 36,397 (25.3) | 43,679 (26.1) |
| Age Category 65–74 n(%) | 9217 (24) | 18,235 (26) | 25,455 (23.6) | 31,963 (22.2) | 38,199 (22.8) |
| Age Category 75–84 n(%) | 3543 (9.2) | 8642 (12.3) | 16,433 (15.2) | 22,971 (15.9) | 25,679 (15.3) |
| Age Category 85+ n(%) | 738 (1.9) | 1893 (2.7) | 4954 (4.6) | 7215 (5) | 9010 (5.4) |
| Duration | |||||
| Mean Diabetes duration (years) | 6.6 | 7 | 8.2 | 9.1 | 9.7 |
| Median Diabetes duration (years) | 5 | 5.1 | 6.8 | 7.4 | 7.9 |
| Diabetes duration <2 years (%) | 23 | 19.4 | 16.4 | 15.6 | 15.3 |
| Diabetes duration 2–5 years (%) | 25.9 | 27.8 | 21.5 | 20.2 | 19.2 |
| Diabetes duration 5–10 years (%) | 30.8 | 28.9 | 31.9 | 26.5 | 25.3 |
| Diabetes duration 10+ years (%) | 20.4 | 23.9 | 30.2 | 37.7 | 40.3 |
Table 1b.
Ethnicity and deprivation characteristics of those with T2DM in the Discover-NOW population in selected years 2000–2019.
| Year | 2000 | 2005 | 2010 | 2015 | 2019 |
|---|---|---|---|---|---|
| Patients | 38,391 | 70,207 | 1,07,791 | 1,44,144 | 1,67,478 |
| Ethnicity | |||||
| Asian or Asian British Ethnicity (%) | 40.8 | 41.7 | 42.3 | 44.9 | 47.1 |
| Black or black British Ethnicity (%) | 10.7 | 11.1 | 11.4 | 11.3 | 11.4 |
| Mixed Ethnicity (%) | 4.3 | 4.3 | 4.2 | 4.4 | 4.6 |
| White Ethnicity (%) | 30 | 32 | 31.9 | 29.1 | 26.2 |
| Other ethnic groups Ethnicity (%) | 3.7 | 3.8 | 4.1 | 4.7 | 5.3 |
| Ethnicity Not Stated (%) | 10.5 | 7.2 | 6.1 | 5.5 | 5.3 |
| IMD Decile | |||||
| IMD Decile 1 (%) | 5.2 | 5 | 5 | 4.9 | 5 |
| IMD Decile 2 (%) | 9.9 | 10.1 | 10.2 | 10.4 | 10.7 |
| IMD Decile 3 (%) | 16.3 | 16.4 | 16.7 | 17.1 | 17.7 |
| IMD Decile 4 (%) | 14.5 | 14.2 | 14.1 | 14.2 | 14.5 |
| IMD Decile 5 (%) | 14.4 | 14.3 | 14.1 | 14.3 | 14.4 |
| IMD Decile 6 (%) | 12.2 | 11.9 | 11.7 | 11.7 | 11.5 |
| IMD Decile 7 (%) | 8.6 | 8.5 | 8.6 | 8.6 | 8.3 |
| IMD Decile 8 (%) | 5.3 | 5.5 | 5.5 | 5.4 | 5.2 |
| IMD Decile 9 (%) | 4.2 | 4.2 | 4.2 | 4 | 3.7 |
| IMD Decile 10 (%) | 2.6 | 3 | 3 | 2.8 | 2.5 |
Increasing comorbidities over time & commonest comorbidities
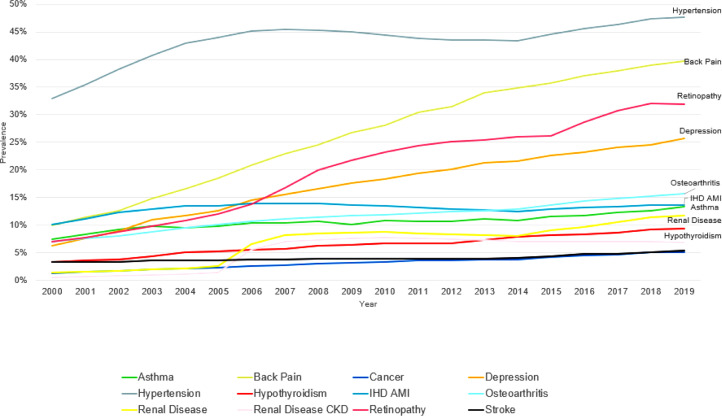
Comorbidities were increasingly common during the study period with all of the 9 commonest comorbidities (prevalence over 3%) increasing in prevalence from 2000 to 2019. Hypertension was the commonest comorbidity throughout the study period, reaching a prevalence of 47.6% in 2019. While traditional DM-conditions retinopathy (31.9%), IHD/AMI (13.6%), and renal disease (11.7%) were some of the commonest comorbidities, there was a diverse range of comorbidities. Back pain (39.7%), Depression (25.7%) and osteoarthritis (15.7%) were 2nd, 4th and 5th commonest comorbidities in people with T2DM over the last 10-years of the study period (Figure 1). Depression was the most common emerging condition and back pain the most common other associated condition.
Figure 1.
Age standardised comorbidity prevalence between 2000 and 2019 in the type 2 diabetes population. Co-morbidities with average prevalence of 3% or above included.
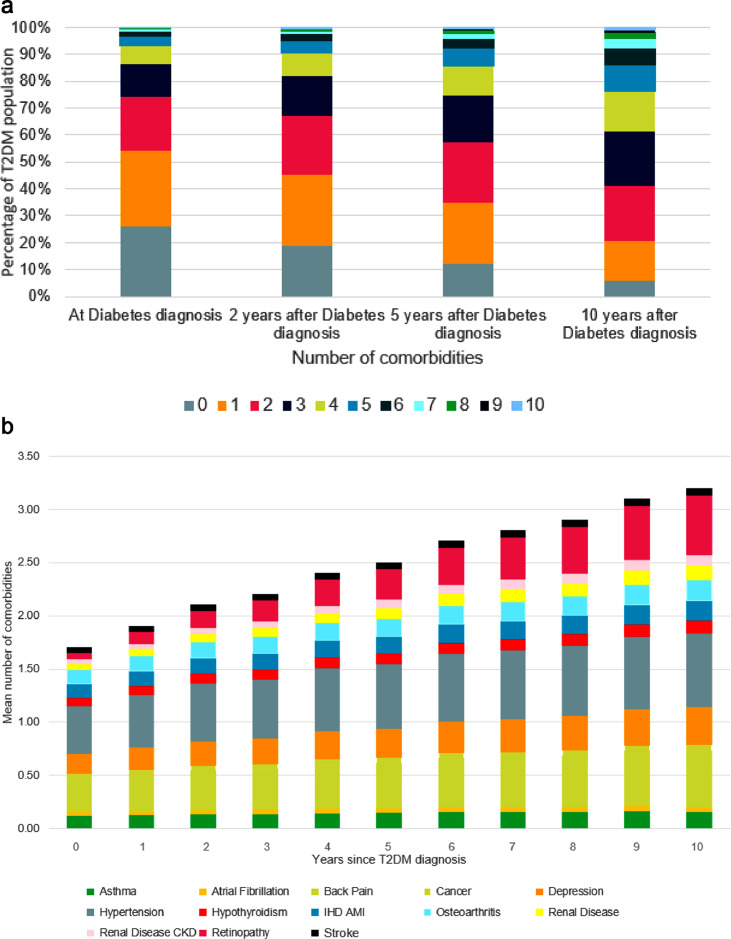
The number of comorbidities in people with T2DM also increased over duration of T2DM. Approximately 55% of patients had 0 or 1 comorbidities at diagnosis of T2DM while approximately 5% had 5 or more comorbidities. Ten years after diagnosis, those numbers had reversed with just 20% of patients still having 0 or 1 comorbidities while approximately 25% of patients had 5 or more comorbidities, some 5-fold increase compared to at diagnosis (Figure 2a). Hypertension (37%), back pain (25%) and depression (15%) were the commonest comorbidities at diagnosis. Ten years post diagnosis, the commonest comorbidities were similar to diagnosis with the exception of retinopathy which increased in prevalence substantially from 4.8% at diagnosis to 41.3% 10-years post diagnosis (Figure 2b).
Figure 2.
(a) Percentage of T2DM population with 0–10 comorbidities at diagnosis and 2, 5 and 10 years after diagnosis of type 2 diabetes. (b) Proportional contribution to co-morbidity burden of common co-morbidities in the type 2 diabetes population by years since diagnosis over 2000–2019. Co-morbidities with average prevalence of 3% or above included.
Hypertension was present in the 5 commonest comorbidity combinations in the T2DM population. These combinations were hypertension and back pain (24%), hypertension and retinopathy (22%), hypertension and IHD (17%), hypertension and osteoarthritis (16%) and hypertension and depression (15%). The commonest combinations of 3 comorbidities at diagnosis were hypertension & back pain (38%) in those with T2DM who either had IHD or depression respectively. In those with T2DM who had IHD or depression, there were 5 comorbidity combinations that were prevalent at a rate of more than 20% across the population (Supplemental Table 4) (Table 2).
Table 2.
Age standardised comorbidity prevalence in the T2DM population at diagnosis and 2, 5 and 10 years after diagnosis.
| Time period | At Index | 2 years after diagnosis | 5 years after diagnosis | 10 years after diagnosis |
|---|---|---|---|---|
| Patients | 1,89,068 | 1,57,182 | 1,14,841 | 61,074 |
| Conditionsa | Age standardised Prevalence (CI) | Age standardised Prevalence (CI) | Age standardised Prevalence (CI) | Age standardised Prevalence (CI) |
| Amputation | 0.2% (0.2–0.3) | 0.3% (0.3–0.3) | 0.4% (0.4–0.5) | 0.6% (0.5–0.7) |
| Amputation below knee | 0.2% (0.1–0.2) | 0.2% (0.2–0.2) | 0.3% (0.2–0.3) | 0.4% (0.3–0.4) |
| Asthma | 10.1% (9.9–10.2) | 10.7% (10.5–10.8) | 11.6% (11.4–11.8) | 12.0% (11.7–12.2) |
| Atrial Fibrillation | 3.1% (3.0–3.2) | 3.2% (3.1–3.3) | 3.2% (3.1–3.3) | 3.3% (3.2–3.5) |
| Back Pain | 25.3% (25.1–25.6) | 28.7% (28.5–29.0) | 32.9% (32.6–33.3) | 38.8% (38.3–39.3) |
| Blindness | 1.5% (1.5–1.6) | 1.7% (1.6–1.8) | 2.1% (2.0–2.2) | 2.6% (2.5–2.8) |
| Cancer | 3.6% (3.5–3.7) | 3.8% (3.7–3.9) | 4.0% (3.9–4.1) | 4.3% (4.1–4.5) |
| Charcot Osteoarthropathy | 0.0% (0.0–0.0) | 0.0% (0.0–0.0) | 0.1% (0.1–0.1) | 0.2% (0.1–0.2) |
| Chronic Obstructive Pulmonary Disease | 2.3% (2.3–2.4) | 2.4% (2.4–2.5) | 2.5% (2.4–2.6) | 2.5% (2.4–2.6) |
| Dementia | 1.0% (1.0–1.1) | 1.2% (1.1–1.2) | 1.2% (1.2–1.3) | 1.4% (1.4–1.5) |
| Depression | 15.3% (15.1–15.5) | 17.9% (17.7–18.1) | 21.0% (20.7–21.2) | 26.6% (26.2–27.0) |
| Diabetes related cancers | 1.4% (1.3–1.4) | 1.5% (1.4–1.5) | 1.5% (1.5–1.6) | 1.6% (1.5–1.7) |
| Epilepsy | 1.0% (0.9–1.0) | 1.0% (0.9–1.0) | 1.0% (1.0–1.1) | 1.2% (1.2–1.3) |
| Foot Ulcer | 0.2% (0.2–0.2) | 0.3% (0.3–0.4) | 0.5% (0.4–0.5) | 1.1% (1.0–1.2) |
| Frailty | 0.4% (0.3–0.4) | 0.4% (0.4–0.5) | 0.6% (0.5–0.6) | 0.8% (0.8–0.9) |
| Heart Failure | 2.4% (2.3–2.5) | 2.6% (2.5–2.6) | 2.7% (2.6–2.8) | 3.1% (3.0–3.2) |
| Hypertension | 36.8% (36.5–37.0) | 42.1% (41.8–42.4) | 46.4% (46.0–46.8) | 51.0% (50.5–51.6) |
| Hypothyroidism | 6.4% (6.3–6.5) | 7.1% (6.9–7.2) | 7.7% (7.5–7.9) | 8.7% (8.4–8.9) |
| IHD AMI2 | 10.2% (10.1–10.3) | 11.5% (11.3–11.6) | 12.4% (12.2–12.6) | 13.8% (13.5–14.1) |
| Learning Disability | 0.7% (0.7–0.7) | 0.7% (0.6–0.7) | 0.7% (0.7–0.8) | 1.4% (1.3–1.5) |
| Year | At index | 2 years after diagnosis | 5 years after diagnosis | 10 years after diagnosis |
|---|---|---|---|---|
| Liver Disease | 0.9% (0.9–0.9) | 1.0% (1.0–1.1) | 1.2% (1.2–1.3) | 1.5% (1.4–1.6) |
| Macular Degeneration | 0.7% (0.6–0.7) | 0.8% (0.8–0.9) | 0.9% (0.9–1.0) | 1.0% (0.9–1.1) |
| Osteoarthritis | 11.1% (10.9–11.2) | 12.1% (12.0–12.3) | 13.1% (12.9–13.3) | 14.3% (14.0–14.6) |
| Osteoporosis | 1.8% (1.7–1.8) | 1.9% (1.8–1.9) | 2.0% (2.0–2.1) | 2.3% (2.1–2.4) |
| Other cancers | 2.2% (2.2–2.3) | 2.4% (2.3–2.4) | 2.5% (2.4–2.6) | 2.7% (2.6–2.8) |
| Peripheral Vascular Disease | 1.1% (1.0–1.1) | 1.3% (1.2–1.3) | 1.5% (1.4–1.5) | 1.8% (1.6–1.9) |
| Renal Disease | 4.7% (4.6–4.8) | 5.9% (5.7–6.0) | 7.6% (7.5–7.8) | 10.0% (9.8–10.3) |
| Renal Disease CKD 2 | 3.7% (3.6–3.8) | 4.6% (4.5–4.7) | 6.1% (5.9–6.2) | 7.8% (7.5–8.0) |
| Renal Disease Nephropathy | 0.2% (0.2–0.2) | 0.4% (0.3–0.4) | 0.6% (0.5–0.6) | 0.9% (0.9–1.0) |
| Renal Disease Other | 0.8% (0.7–0.8) | 0.9% (0.8–0.9) | 1.0% (0.9–1.0) | 1.3% (1.2–1.4) |
| Retinopathy | 4.8% (4.7–4.9) | 12.4% (12.2–12.6) | 22.5% (22.2–22.8) | 41.3% (40.8–41.8) |
| Severe mental illness | 2.7% (2.6–2.8) | 2.8% (2.8–2.9) | 3.0% (2.9–3.1) | 2.8% (2.6–2.9) |
| Severe Pancreatitis Secondary3 | 0.2% (0.2–0.2) | 0.2% (0.2–0.2) | 0.1% (0.1–0.2) | 0.2% (0.1–0.2) |
| Stroke | 3.5% (3.4–3.6) | 3.8% (3.7–3.9) | 4.1% (3.9–4.2) | 4.7% (4.5–4.8) |
Age standardised condition prevalence (95% CIs), for primary and secondary care. Abbreviations: CKD is chronic kidney disease, IHD is ischaemic heart disease and AMI is acute myocardial infarction. Secondary care from 2015 only.
Variation in comorbidity profile across patient sub-groups
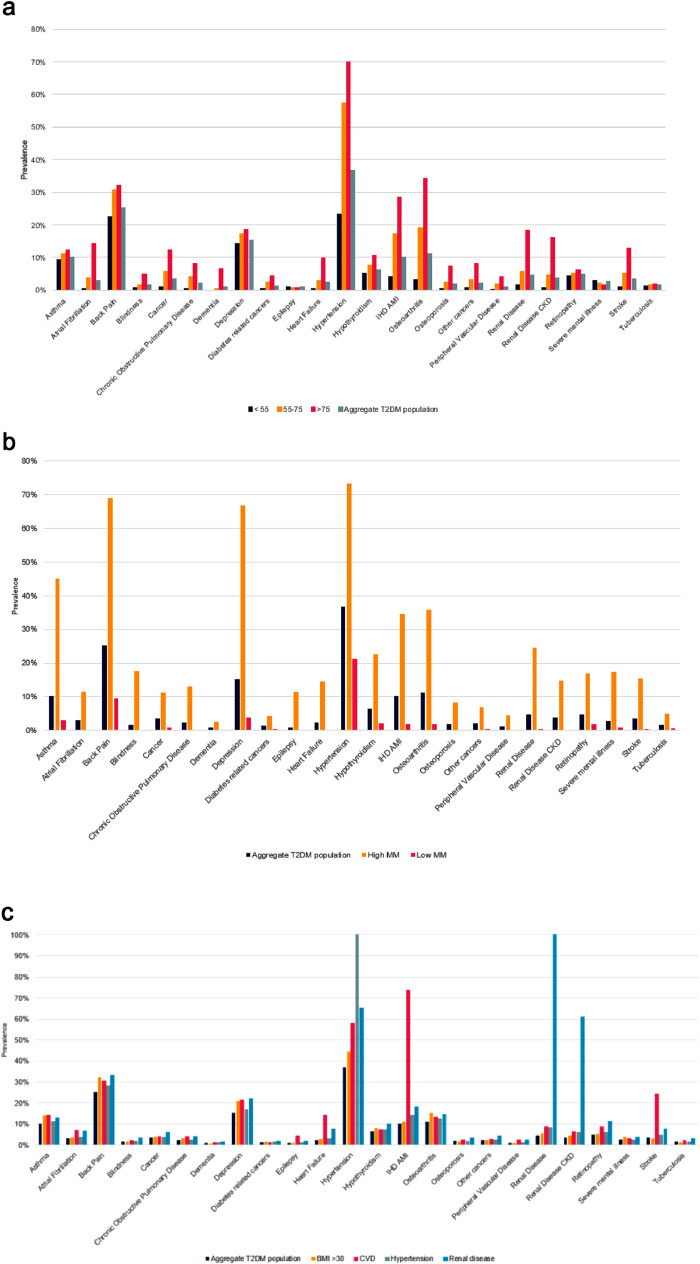
The prevalence of comorbidities increased with age at diagnosis. For those diagnosed with T2DM in older age (>75 years), the prevalence of osteoarthritis and IHD/AMI were two of the more common conditions in comparison to the younger diagnosis group (<55 years). Similarly, older adults had a prevalence of osteoarthritis and IHD/AMI of 34.2% and 28.5% at diagnosis respectively compared to 3.4% and 4.0% in the younger adult group (Figure 3a).
Figure 3.
(a) Age standardised comorbidity prevalence in year of diagnosis of T2DM population stratified by age at diagnosis. Co-morbidities with average prevalence of 1% or above included. (b) Prevalence of age-standardised comorbidities in the WSIC T2DM population in year of T2DM diagnosis stratified by multimorbidity groups and aggregate T2DM population. Co-morbidities with average prevalence of 1% or above included. (c) Prevalence of age-standardised comorbidities in the WSIC T2DM population in year of T2DM diagnosis stratified by having high BMI, CVD, Hypertension or renal disease at diagnosis and aggregate T2DM population. Co-morbidities with average prevalence of 1% or above included.
Differences across population (at diagnosis)
Those with high multimorbidity (5 or more comorbidities at diagnosis) had higher prevalence of every comorbidity at diagnosis compared to those with low multimorbidity (less than 2 comorbidities at diagnosis) with prevalence several-fold as high in each instance. While hypertension (73%) was the commonest comorbidity at diagnosis in the high multimorbidity group, the remainder of the five commonest comorbidities were not traditional DM-conditions with back pain (69%), depression (67%), asthma (45%) and osteoarthritis (36%). This compared with prevalence of these five conditions at diagnosis of just 21%, 9%, 4%, 3% and 2% in the low multimorbidity group. These differences persisted through the duration of the disease (Figure 3b).
Large differences in comorbidity profiles were found according to comorbidities at T2DM diagnosis. Those with any of our predefined comorbidities at diagnosis (CVD, renal disease, obesity, or hypertension) had substantially higher prevalence of the wide range of comorbidities compared to those who did not. Those with pre-existing CVD or renal disease at diagnosis had comorbidity profiles dominated by vascular diseases however this was much less the case for those with pre-existing obesity or hypertension. Along with hypertension, back pain and depression, osteoarthritis and asthma made up the five commonest comorbidities and were 50% more common compared to those without obesity. While less common, severe mental illness (4%) was almost twice as prevalent at diagnosis in those with obesity compared to those without (2.1%) (Figure 3c).
Differences across deprivation and ethnicity groups
Comorbidity prevalence and profiles were generally similar in the most and least deprived IMD quintiles (Supplementary Figure 2) with similar prevalence of hypertension (37% v 36%), back pain (25% v 26%) and depression (16% v 14%). Some less common comorbidities such as severe mental illness and COPD were approximately 40% higher in more deprived groups however at 2.9% (compared with 2.0%) and 2.6% (compared with 1.9%) respectively.
There were several differences in comorbidity prevalence by ethnicity (Supplemental Figure 3). Hypertension was the commonest comorbidity at diagnosis across all ethnic groups (from 33% to 41%) but there were noticeable differences in specific comorbidities. Depression was almost twice as prevalent in white people (21%) compared to Asian (12%), hypothyroidism was twice as prevalent in Asian people compared to black people while severe mental illness, cancer, stroke and CKD were all higher in black people with 2.6 fold higher prevalence of severe mental illness compared to Asian people.
Comorbidity prevalence and composition were generally similar between men and women (Supplementary Figure 4) with prevalence of blindness, hypertension and tuberculosis at similar levels (c. 2%, 37% and 2% respectively). Depression, hypothyroidism and osteoarthritis prevalence were 2-3 times higher in women than men at 19.6% (compared with 11.6%), 10.2% (compared with 3.1%) and 14.2% (compared with 8.4%). In contrast, IHD, other cancers and retinopathy prevalence was 1.5 fold higher in men than women 12.6% (compared with 7.6%), 3.3% (compared with 1.3%) and 5.4% (compared with 4.1%).
Results of comorbidity prevalence across all sub-groups studied are available in interactive and dynamic form at https://dmcomorbidities.lcp.uk.com.
Discussion
Our study used a large, representative administrative dataset to estimate the comorbidity profiles of more than 220,000 people with T2DM over a 20-year period. To our knowledge this is the first study to take a more holistic view of comorbidities in people with T2DM, analyse how this varies across specific sub-groups and over the course of disease There are three major findings, some of which are expected and some were not.
There are several key findings. First, multimorbidity is common in those with T2DM and increasing as with the general population.9,17 The mean number of comorbidities amongst those included in our study increased from 1.3 in 2000 to 3.5 in 2019, while this increased over the duration of the disease too from 1.7 at diagnosis to 3.2 10-years later. Less expected is the composition and commonest comorbidities in people with T2DM. While hypertension and IHD, traditional conditions associated with T2DM, were the 1st and 5th most common comorbidities, back pain, depression and osteoarthritis being 2nd, 3rd and 4th commonest comorbidities was surprising given they are not traditional T2DM conditions and only one is an emerging condition. The increasing prevalence of established T2DM conditions such as CKD and retinopathy during the course of disease was expected though the rate of increase of retinopathy, from 4.8% at diagnosis to 41.3% 10-years later is stark.
Second, comorbidity profiles of people with T2DM vary substantially. At diagnosis the interquartile range of comorbidities across the patient group was from 0.0 to 3.0 comorbidities. This increased 10-years post diagnosis to 2.0 to 4.0 comorbidities respectively. As would be expected, comorbidity prevalence was generally higher in older age groups, partially for conditions such as atrial fibrillation and cancers, but this was not uniform with a much smaller gradient of depression prevalence across ages while severe mental illness was more common in younger adults. Comorbidity prevalence was generally similar for both men and, while prevalence was higher for conditions such as IHD in men and hypothyroidism in women. Patients who had obesity at diagnosis, the largest risk factor for T2DM, at were found to have 40% higher prevalence of several non-traditional DM conditions, such as asthma, COPD, depression, heart failure and osteoarthritis, compared with T2DM patients who were not obese at diagnosis, while also having a lower prevalence of traditional vascular conditions. Building on these results could enable redefining both clinical care pathways and preventative approaches to be more patient-centred, cognisant of the vast differences in health needs and inequalities in outcomes in people with T2DM.
Third, the prevalence of comorbidities were surprisingly similar between the most and least deprived quintiles. There were some exceptions in less common conditions such as severe mental illness and COPD where prevalence was 40% higher in more deprived groups. Contrastingly, there were larger differences in which comorbidities were commonest and respective prevalence rates across ethnicities reflecting further the vast differences in unmet health need across people with T2DM.
There are several implications of these findings. The diverse conditions which those with T2DM live with, impact their health status and morbidity, and how this varies across people with T2DM makes the case for more refined, personalised and holistic based guidelines. There are currently few care pathways or guidelines that capture both the breadth and comprehensive nature of the comorbidities faced by those with T2DM while cognisant of how unique this is for each patient sub-group. These findings could inform the development of more holistic, comorbidity based clinical guidelines for people with T2DM that cater for patient's total health needs rather than on a disease by disease basis to reduce inequalities within this patient group. Second, the findings of the breadth of morbidity, including non-traditional DM conditions despite not having evidence of a pathophysiological association with T2DM, could provide a framework to evaluate therapeutics and wider health interventions more holistically both from patients’ and healthcare providers perspectives. Finally, these results could be further developed, building on QRisk18 and QDiabetes19 which are clinical risk prediction tools to inform use of preventative measures such as anti hypertensives and statins. An enhanced approach, building on our study, could enable population and individual level risk stratification tools for people with T2DM that are both more comprehensive in scope and more granular in risk estimation.
While there is relatively scarce evidence characterising the comorbidity profiles of people with T2DM to date, our findings are consistent with prior work finding that multimorbidity is common and increases with age,9 over time11 and in people with T2DM.8 Our finding that hypertension is the commonest comorbidity in people with T2DM is consistent with prior work,8,20, 21, 22, 23 however our study builds on prior analysis in including a broader set of comorbidities that are perhaps less associated with the pathophysiology of T2DM (such as back pain, the 2nd commonest comorbidity at diagnosis of T2DM) yet have substantial impacts on lived morbidity, more granular sub-groups of patients including according to specific comorbidities at diagnosis. More broadly our findings of diversification in morbidity profile of people with T2DM over both calendar time and duration of T2DM are consistent with prior work finding a diversification of cause of death in people with DM2,4,5 and complications7,24
A significant strength of our study is the large dataset enabling estimation of comorbidity patterns in more than 220,000 patients while the inclusion of T2DM duration allows more granular insight than prior work. Our study is broader in scope of comorbidities than prior work, going further than traditional DM conditions alone. Despite this, the comorbidities included in this study are not exhaustive and many people living with T2DM are likely to live with several other conditions, including obstructive sleep apnoea and certain infections. Some additional comorbidities, such as hyperlipidaemia, may impact T2DM control and specific complications.
Linking records from primary and secondary care and the specific costs associated with each allowed for more accurate costing of individual patient impacts than previously. We used a pragmatic approach including an algorithm to identify those with T2DM in our population and to exclude those who are likely to have T1DM. While this is an imperfect approach and T1DM is occasionally diagnosed later than 30-years old, any misclassification is likely to be limited to a very small number in the population and this approach has been used in similar RWE datasets previously.23,25 increases the sensitivity of our approach in identifying those with T2DM in the population, however this does not capture those with undiagnosed T2DM which is estimated to account for around 20% of all DM in the UK26 thus the findings here could most accurately be described as being relevant to the diagnosed T2DM population. While we excluded those identifiable with T1DM via a previously used algorithm, this, like many RWE approaches, may be imperfect. The missingness of risk factor data improved over time however remained at levels too high to consider incorporating confounders (e.g., body mass index, smoking status) into any statistical models, nor to develop causal models to assess aetiology.
Administrative datasets do have limitations. The missingness of risk factors, while improving over time, is far higher than in randomised controlled trials and is likely to have biases with measurement of cardiometabolic risk factors likely to be higher in those with chronic diseases and according to health seeking behaviour. Similarly primary care read codes for comorbidities are imperfect with large variation in clinician coding over time which is imperfect. This identification was improved by the additional use of secondary care data from 2015 onwards to also identify the presence of comorbidities (using a coding system similar to the WHO ICD-10 system which has advantages of specificity compared to primary care read codes). These approaches are imperfect and therefore prevalence estimates, particularly for rarer comorbidities, may be under-estimates. The increases observed in prevalence of some comorbidities such as back pain, retinopathy and CKD may in part be impacted by changes in physician diagnostic criteria, improved testing and identification of chronic diseases and coding behaviour (including due to changes in remuneration, such as QoF incentives), while T2DM duration also increased during the study period and may have confounded increases in comorbidity prevalence over time. Our study did not have a comparator, non-T2DM population, which renders it difficult to determine how these patterns of prevalence are or are not specific to this chronic disease population.
Finally, the Discover-NOW dataset includes people living in North West London and while this population is age and sex representative of the UK population and we age-standardised the findings to the European Standard Population, the distribution of ethnicities and other demographic factors mean that there would be value in repeating this study in other populations. Prevalence of specific comorbidities, such as tuberculosis is likely to vary substantially globally for example
Care pathways and preventative measures for people with T2DM should transition to reflect the increasing and diverse set of diseases driving persistent morbidity. This more holistic approach would benefit patients, reduce inequalities in morbidity in this patient group and reduce acute demand on healthcare systems.
Contributors
JP-S, TP and PJ conceived of the idea for the paper. SH, RP and JP-S led the analysis with input from LZ, TP, RS, KH, EG and JE. JP-S wrote the first draft of the manuscript. All authors critically reviewed and contributed to the final manuscript.
Data sharing statement
The Discover-NOW data that supports the findings of this study is available from Imperial College Health Partners via approval from the Discover Data Access Group (DRAG) under certain restrictions.
Declaration of interests
JP-S reports personal fees from Novo Nordisk A/S and Pfizer Ltd outside of the submitted work and is chair-elect of the Royal Society for Public Health. All other authors report no competing interests.
Acknowledgements
NICE funded the study and three co-authors (KH, JE & PJ) are NICE employees and were involved in the development of the study and finalising the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101584.
Appendix. Supplementary materials
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Cheng YJ, Srinivasan M, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430–2440. doi: 10.1016/S0140-6736(18)30314-3. [DOI] [PubMed] [Google Scholar]
- 3.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 4.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000 to 2011 in all-cause and cause-specific mortality in Type 1 and Type 2 diabetes: a cohort study of more than one million people. Diabetes Care. 2016;39(6):1018–1026. doi: 10.2337/dc15-2308. [DOI] [PubMed] [Google Scholar]
- 5.Pearson-Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021 doi: 10.1016/S2213-8587(20)30431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 7.Pearson-Stuttard J, Cheng YJ, Bennett J, et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021 doi: 10.1016/S2213-8587(21)00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowakowska M, Zghebi SS, Ashcroft DM, et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019;17(1):145. doi: 10.1186/s12916-019-1373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 10.Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ. 2020;368:l6964. doi: 10.1136/bmj.l6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Head A, Fleming K., Kypridemos C., Schofield P., Pearson-Stuttard J., O'Flaherty M. Inequalities in incident and prevalent multimorbidity in England, 2004–2019: a population-based, descriptive study. Lancet. 2021:489–497. doi: 10.1016/S2666-7568(21)00146-X. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence. NICE clinical guidelines (NG28) - Type 2 diabetes in adults. https://wwwniceorguk/guidance/ng28. Accessed 11 July 2021.
- 13.Bottle A, Cohen C, Lucas A, et al. How an electronic health record became a real-world research resource: comparison between London's whole systems integrated care database and the clinical practice research datalink. BMC Med Inform Decis Mak. 2020;20(1):71. doi: 10.1186/s12911-020-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract. 2010;60(572):e128–e136. doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson-Stuttard J, Papadimitriou N, Markozannes G, et al. Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomisation studies. Cancer Epidemiol Biomarkers Prev. 2021 doi: 10.1158/1055-9965.EPI-20-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eurostat . Publications Office of the European Union; Luxembourg: 2013. Revision of the European standard population report of Eurostat's task force. [Google Scholar]
- 17.Agborsangaya CB, Ngwakongnwi E, Lahtinen M, Cooke T, Johnson JA. Multimorbidity prevalence in the general population: the role of obesity in chronic disease clustering. BMC Public Health. 2013;13:1161. doi: 10.1186/1471-2458-13-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ. 2007;335(7611):136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippisley-Cox J, Coupland C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ. 2017;359:j5019. doi: 10.1136/bmj.j5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zghebi SS, Steinke DT, Rutter MK, Ashcroft DM. Eleven-year multimorbidity burden among 637,255 people with and without type 2 diabetes: a population-based study using primary care and linked hospitalisation data. BMJ Open. 2020;10(7) doi: 10.1136/bmjopen-2019-033866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS, Shin AM, Kim MK, Kim YN. Comorbidity study on type 2 diabetes mellitus using data mining. Korean J Intern Med. 2012;27(2):197–202. doi: 10.3904/kjim.2012.27.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safieddine B, Sperlich S, Epping J, Lange K, Geyer S. Development of comorbidities in type 2 diabetes between 2005 and 2017 using German claims data. Sci Rep. 2021;11(1):11149. doi: 10.1038/s41598-021-90611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelinek HF, Osman WM, Khandoker AH, et al. Clinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab Emirates. BMJ Open Diabetes Res Care. 2017;5(1) doi: 10.1136/bmjdrc-2017-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4(6):537–547. doi: 10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- 25.Gray J, Orr D, Majeed A. Use of Read codes in diabetes management in a south London primary care group: implications for establishing disease registers. BMJ. 2003;326(7399):1130. doi: 10.1136/bmj.326.7399.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes UK. Diabetes Prevalence https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-prevalence-201920202019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.