Introduction
Acanthosis nigricans (AN) is a skin disorder characterized by hyperpigmentation and hyperkeratosis and is associated with an underlying disease or condition.1 It appears as dark, coarse, thick, and velvety plaques, usually in intertriginous areas, such as the groin, axilla, and neck folds.1,2 The pathophysiology of AN is multifactorial, resulting in proliferation of epidermal keratinocytes and dermal fibroblasts.2 The resultant histopathological changes include epidermal acanthosis with papillomatosis, hyperkeratosis, mild increase in pigmentation, with a pauci-inflammatory dermis.2,3 Multiple classification systems exist to describe this condition.2
Skin grafts are often required in burn patients for reconstruction. This is performed using split-thickness skin grafts or full-thickness skin grafts (FTSGs), depending on the anatomic area of reconstruction.4 Hyperpigmentation following grafting in burn patients is not uncommon5 and occurs due to excessive accumulation of melanin resulting from increased melanogenic activity of melanocytes in the basal layer of the epidermis.6 The following report describes a young boy with burns to the palms of both hands who required skin grafting for reconstruction. Initially thought to have conventional hyperpigmentary changes in his skin grafts, biopsy later revealed features consistent with AN.
Case report
A 2-year-old boy with a past medical history of a solitary kidney sustained deep partial- and full-thickness burns to both palms after falling into a fire pit. The wounds were initially dressed with Flamazine (Smith & Nephew) until subsequent eschar debridement, and his dressings switched to daily antibiotic ointment and Jelonet (Smith & Nephew). After 2 weeks, he was noted to have complete re-epithelization of most burns, apart from those over the ulnar aspect of the hands. He was discharged home with close follow-up.
Complete re-epithelization, including the ulnar aspect of the hands, occurred by 1 month post burn. He eventually developed contractures along his left long, ring and small fingers and his right small finger, which led to surgical release of these contractures with Z-plasties 1 year following his initial injury.
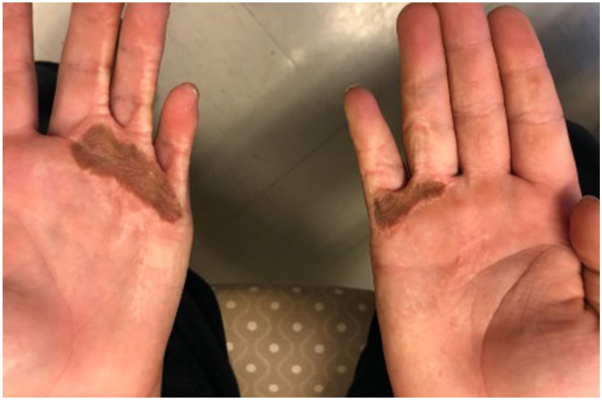
He developed further contractures during periods of rapid hand growth. His contractures began interfering with function 4 years after the initial burn, necessitating further surgical release. The patient underwent repeat scar excision with coverage of the resulting skin defects using FTSG harvested from the inguinal crease. He was noted to have 100% take of his grafts in follow-up visits. They were initially noted to be violaceous, in keeping with normal early graft appearance. There were early signs of hyperpigmentation and hyperkeratosis 1 month later, which appeared well developed on the palmar aspects of the skin grafts by 2 months after surgery (Fig 1). The hyperpigmentation worsened over several follow-up appointments, resulting in dermatology consultation 1.5 years after skin grafting (6 years following initial injury). Topical 0.5% clobetasol cream was trialed but did not improve the appearance.
Fig 1.
Ectopic acanthosis nigricans. Hyperpigmentation and velvet-like thickening of the grafted skin on both palms, associated with adjacent scarring and flexion contractures of the ulnar digits.
He was noted to have recurrence of his flexion contractures and was experiencing emotional distress due to the hyperpigmentation in follow-up 3.5 years after skin grafting (8 years after the initial injury). As such, excision of the previous skin graft from the left palm was performed. To cover the residual skin defect, an FTSG was planned. It was harvested from the left forearm in hopes of avoiding the previously noted hyperpigmentation observed with the inguinal crease-derived grafts. Intraoperatively, during tangential excision of the previous grafts, the epithelial layer was noted to be friable, thick, and easily separated from the underlying graft dermis. Suspicion arose at this point that the dark color of the grafts could be attributable to acanthosis nigricans.
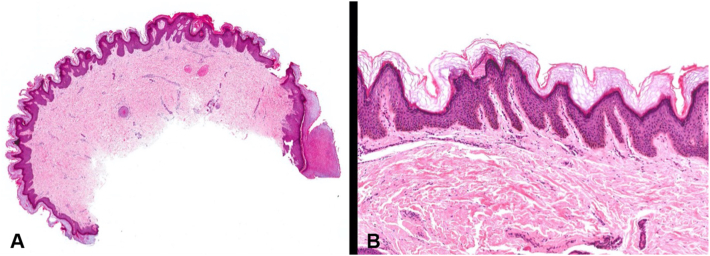
Histopathological examination of the old graft tissue revealed epidermal papillomatosis, hyperkeratosis, and mild basal hyperpigmentation (Fig 2). The epidermal changes were beyond those expected in conventional skin graft hyperpigmentation and were consistent with features of “ectopic AN.” The patient did not have any evidence of AN elsewhere on his body.
Fig 2.
Histopathological examination shows a papillomatous lesion juxtaposed to normal background acral skin (far right) with subjacent dermal scarring (A. Hematoxylin and eosin, 10×). Papillomatosis was accompanied by mild epidermal acanthosis and a thickened orthokeratin layer (B. Hematoxylin and eosin, 40×, and C. Hematoxylin and eosin, 100×).
Discussion
Historically, AN has been clinically classified via multiple systems. Curth7 first described AN as benign, malignant, obesity-associated (pseudo-AN), or syndromic. Hernandez-Pérez8 simplified classification, distinguishing paraneoplastic AN from simple AN of various subtypes. Burke et al9 created a scale that classified AN according to the severity based on the number of affected sites. Sinha and Schwartz3 differentiated AN into 8 different types (Table I) including obesity associated, syndromic, medication induced, malignant, acral, unilateral, benign AN, and mixed type.
Table I.
Classification of acanthosis nigricans as described by Sinha and Schwartz3
| Type of acanthosis nigricans3 | Features and findings2,3 |
|---|---|
| Obesity associated |
|
| Syndromic |
|
| Medication induced |
|
| Malignant |
|
| Acral |
|
| Unilateral |
|
| Benign |
|
| Mixed type |
|
To our knowledge, no other case report has documented the occurrence of AN in skin grafts on the volar aspect of the hand, and this report is only the second to describe AN on the hand after skin grafting. Previously, Wu and Cunningham10 described a case of a 12-year-old boy who developed AN following FTSG from the groin to repair bilateral syndactyly. In that case, the patient had a past medical history significant for exogenous obesity, nonalcoholic steatohepatitis, dyslipidemia, and insulin resistance. In contrast, our patient did not have any identifiable predisposing factors that would be associated with AN. Specifically, he was not obese and had no evidence of insulinemia, took no AN-associated medication, exhibited bilateral involvement, and had no known AN-associated syndrome. Although our patient’s AN occurred on his hands, the involved skin was derived from the groin. Additionally, unlike acral AN, the AN observed involved the volar aspect and not the dorsal (knuckle) of the hand. While his past medical history was significant for a solitary kidney, there is no documented association between this and AN development. Additionally, there was no family history of AN. As such, it is challenging to classify the subtype of AN in our case. Surgeons and dermatologists should be aware of this exceptionally rare complication of skin grafting and should consider AN on their differential when observing hyperpigmentation in skin grafts. In such cases, clinical follow-up is advisable to monitor for other signs of insulin resistance or other conditions associated with AN.
In summary, this case report documents the occurrence of ectopic acanthosis nigricans in a skin graft used to resurface the volar aspect of a hand after burn injury. Clinicians should be suspicious of AN in cases of hyperpigmentation post skin grafting that does not respond to traditional treatments and measures.
Conflict of interest
None disclosed.
Footnotes
Funding sources: Supported by the Division of Plastic Surgery Dalhousie University.
IRB approval status: Not applicable.
Consent: Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Brady M.F., Rawl P. StatPearls. StatPearls Publishing; 2021. Acanthosis nigricans. [Google Scholar]
- 2.Das A., Datta D., Kassir M., et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19(8):1857–1865. doi: 10.1111/jocd.13544. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S., Schwartz R.A. Juvenile acanthosis nigricans. J Am Acad Dermatol. 2007;57(3):502–508. doi: 10.1016/j.jaad.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Moon S.-H., Lee S.-Y., Jung S.-N., et al. Use of split thickness plantar skin grafts in the treatment of hyperpigmented skin-grafted fingers and palms in previously burned patients. Burns. 2011;37(4):714–720. doi: 10.1016/j.burns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Kubota Y., Mitsukawa N., Chuma K., et al. Hyperpigmentation after surgery for a deep dermal burn of the dorsum of the hand: partial-thickness debridement followed by medium split-thickness skin grafting vs full-thickness debridement followed by thick split-thickness skin grafting. Burn Trauma. 2016;4:1–11. doi: 10.1186/s41038-016-0039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukada S. The melanocytes and melanin in human skin autografts. Plast Reconstr Surg. 1974;53(2):200–207. doi: 10.1097/00006534-197402000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Curth H.O. Classification of acanthosis nigricans. Int J Dermatol. 1976;15(8):592–593. doi: 10.1111/j.1365-4362.1976.tb04895.x. [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Pérez E. On the classification of acanthosis nigricans. Int J Dermatol. 1984;23(9):605–606. doi: 10.1111/j.1365-4362.1984.tb05698.x. [DOI] [PubMed] [Google Scholar]
- 9.Burke J.P., Hale D.E., Hazuda H.P., Stern M.P. A quantitative scale of acanthosis nigricans. Diabetes Care. 1999;22(10):1655–1659. doi: 10.2337/diacare.22.10.1655. [DOI] [PubMed] [Google Scholar]
- 10.Wu J.C., Cunningham B.B. Ectopic acanthosis nigricans occurring in a child after syndactyly repair. Cutis. 2008;81(1):22–24. [PubMed] [Google Scholar]