Graphical abstract
Keywords: Ultrasonic extraction, Date fruit, Date sugar, Optimization, Characterization, Kinetic modeling
Highlights
-
•
Date sugar is extracted from date fruit powder by ultrasonication.
-
•
Operating conditions are optimized for the total sugar content and extraction yield.
-
•
Characterization of extracts obtained by modern analytical tools.
-
•
Sugar extraction is increased due to acoustic or ultrasonic cavitation.
-
•
Statistical correlation and kinetic modeling agree well with the experimental data.
Abstract
Alternative sweeteners to white sugar with a lower calorie content and glycemic index obtained through date palm fruits is of great interest to the food industry. In this study, ultrasound-assisted extraction of nutritive sugar from date fruit powder was investigated through Box-Behnken design. A maximum total sugar content (TSC) of 812 mg glucose eq./g of DFP was obtained with a sugar extraction yield (SEY) of 81.40 ± 0.27 % under the following optimal extraction conditions: extraction temperature of 60 °C, extraction time of 30 min, and L/S ratio of 7.6 mL/g. Various modern techniques were used to characterize the obtained extracts and associated residues. The results showed that the extract contained fructose, glucose, and sucrose and had good thermal stability. Furthermore, SEM and TSC analysis revealed that ultrasonic treatment of the biomass improved mass transfer diffusion due to acoustic or ultrasonic cavitation, resulting in a higher sugar yield.
1. Introduction
The most favorable and widely used sweetener is refined sugar, which mainly consists of sucrose. Refined sugar is commonly produced from sugar cane and sugar beet that are composed of a sucrose content ranging from 14 to 20 g/100 g of sugar cane and 70 to 90 g/100 g of sugar beet [1]. The refined sugar production process involves the addition of chemicals and high operating temperatures. Furthermore, increased consumption of refined sugar leads to various health problems such as diabetes, obesity, cancer, hypertension, and others [2]. There is a need to find a healthier substitute for refined sugar. It is generally considered that an ideal alternative sweetener should provide caloric-controlled carbohydrates (sugar intake) and can help with diabetes treatment, prevent dental decay, and aid in maintaining or reducing weight [3]. An ideal sweetener should be water-soluble, stable in acidic and basic pH levels, non-toxic, and metabolized normally across a wide range of temperatures.
The date palm (Phoenix dactylifera) is one of the oldest cultivated trees in the world that serves as a staple food in Arab countries. The United Arab Emirates (UAE) is one of the top ten countries in date production, where in 2020, approximately 328,669 tons of dates were produced [4]. Date fruits, which are abundantly available in the UAE, contain a high amount of sugar, ranging from 60 to 70 % by weight [5]. The simple reducing sugars present in the fruit are easily assimilated by human metabolism. Many dates in the UAE contain substantial amounts of fructose and glucose [6]. The researchers examined 11 date fruit varieties commonly grown in the UAE for their chemical composition, physical properties, amino acids, minerals, and antinutrients. The results indicated that all varieties contained a reasonably significant amount of micronutrients (K, Mg, Ca, and P), protein, glutamine, and aspartic amino acids, as well as other essential amino acids [6]. Furthermore, date fruits contain a good amount of dietary fiber ranging from 6.5 to 11.5 % (of which 84–94 % are insoluble and 6–16 % are soluble), which can help to meet the required balanced diet, i.e., having 14 g of fiber for every 1000 calories of food consumed each day [7]. The high amount of simple sugars (sucrose, fructose, and glucose) and other phytochemicals that naturally occur in date fruits make them an ideal nutritional source for the production of natural sugar. Alkaabi et al. determined the glycemic indices of five varieties of dates in healthy and diabetic subjects. The results showed low glycemic indices for all types of dates, and their consumption by diabetic patients did not result in significant postprandial glucose excursions. These findings highlight the potential benefits of dates for diabetic patients when used in a healthy balanced diet [8]. Therefore, the highly nutritious soluble date sugar extracted from date fruit would be a promising and novel food product for various applications. Moreover, it would be a suitable and superior alternative to commercially available refined sugar. Currently, liquid date sugar (date syrup or Dibbs) is obtained from date fruits by pressing and extraction with hot water. The extraction process involves mixing date fruits with water for around 1 h at 70 °C [9]. The temperature-based extraction deteriorates some of the nutrients and darkens the color of the product, generally known as extract browning [10], [11]. Specifically, conventional hot water (CHW) extractions require a higher amount of solvent, a longer extraction time, and higher operating temperatures. Consequently, boiling date fruits in water is an energy-intensive and laborious technique and exhibits a very low sugar recovery of about 40–50 % with the degradation of thermolabile constituents [12]. These challenges that limit the application of conventional extraction techniques were the driving force toward the development of new advanced extraction techniques, such as ultrasound-assisted extraction (USAE), enzyme-assisted extraction, pressurized liquid extraction, and microwave-assisted extraction. Among all these techniques, USAE has attracted more attention due to its advantages in reducing capital and operation costs and extraction time [13]. It also exhibited enhanced extraction yield by facilitating solvent penetration that effectively extracts heat-sensitive materials and biologically active molecules under mild conditions [14].
USAE is an emerging non-thermal process used to extract many compounds, such as sugars from natural sources, by deforming, disrupting, and breaking cell walls or tissues by cavitation, resulting in increased diffusion and release of elements through cell membranes and more accessible access of the solvent to cell content [15]. In addition, intense local energy and high-pressure signals generated by ultrasound waves resulted in a localized pasteurization effect without causing a substantial increase in macro-temperature [16]. Factors that significantly influence saccharide extraction yield through the USAE process are particle size, solvent-to-material ratio or solution concentration, extraction temperature, extraction time, and ultrasonic power or intensity [17]. Optimal sonication power and liquid-to-solid ratio often result in higher yields of bioactive materials at a faster extraction time [18], [19]. The influence of these USAE process variables on the yield and quality of the final product was studied and optimized for many plant materials and their associated biomasses using response surface methods (RSM) [20], [21]. There are very few studies on the ultrasonic extraction of sugar or syrup from date fruits [9], [22], [23]. However, to the best of our knowledge, there are no focused studies on the USAE of sugars from date fruit covering parametric optimization, characterization, and kinetic modeling to maximize the sugar yield and deeply understand the sonication effect on the extraction process.
In this study, the USAE of the date syrup from the date fruit powder was optimized using RSM with Box-Behnken design (BBD). Several characterization techniques such as UV–vis, HPLC, SEM, TGA, and FT-IR were performed to determine the total sugar content, sugar composition, morphological characteristics, thermal decomposition, and functional groups of the untreated date powder, along with its residue and extract after USAE. Furthermore, this work also investigates the energy calculations and its conversion to other forms during the sonication process along with the kinetic parameters by developing the mass transfer model and presents the plausible mechanism involved in the extraction of nutritional sugar from DFP through the USAE technique.
2. Materials and methods
2.1. Samples and reagents
The date fruit powder (DFP) was obtained from the local dates processing factory in Abu Dhabi, United Arab Emirates. Generally, the Sukkari variety of date fruits was sun-dried for 2–3 days, followed by a grinding process to obtain the powdered date fruit. The as-obtained DFP was screened using an Electromagnetic Sieve Shaker (Model: Retsch GmbH AS-200 @ 50 Hz). The particle size of the DFP ranged between 1000 and 63 µm. More precisely, about 80.6 % of the DFP was moderately fine particle sizes (500–250 µm), while 6.4 %, 8.1 %, and 4.9 % were coarse (>500 µm), fine (<250–125 µm) and very fine particle sizes (<125 µm), respectively. Noticeably, in this study, DFP with moderate particle sizes were used to investigate the effect of process parameters on the Ultrasound-Assisted Extraction of nutritious sugar constituents from the commercially available DFP. Furthermore, the nutritional analysis of the obtained powder was also assessed, and according to the date-processing industry, the as-produced DFP is comprised of 14 % fats, 10 % carbohydrates, and 7 % fibers”. Solvents and other standards such as acetonitrile (HPLC grade), fructose, glucose, sucrose, phenol, and sulfuric acid were purchased from Sigma Aldrich (USA) and used as such without further purification. Deionized (DI) water (conductivity 0.05 µS/cm) was used throughout this work for sample preparation and washing purposes.
2.2. Ultrasonic-assisted extraction
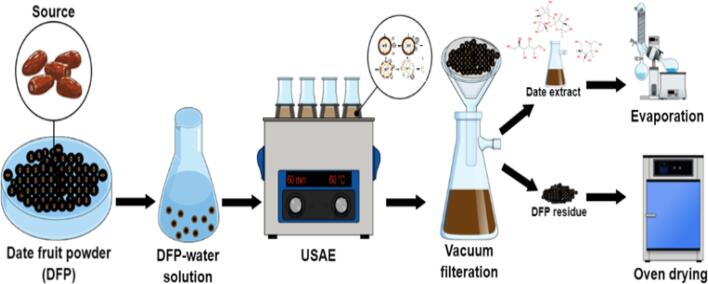
Extraction of nutritional sugar from DFP was carried out in a batch mode using a bath sonicator (USC 2100 THD, VWR, UK) that has a constant frequency of 45 kHz and an ultrasonic power density of 12 Watts per Liter. As the main aim of this research work was to optimize the process parameters such as liquid to solid (L/S) ratio, operational temperature and processing time to maximize the nutritious date sugar extraction yield. Therefore, the acoustic frequency (45 kHz) and the ultrasonic power density (i.e. 50 % sonication power = 6 W/L) were constant throughout the experiments. Briefly, a fixed amount of DFP (5 g) was dissolved in a specific amount of deionized (DI) water according to the studied range of L/S ratios (5–20 mL/g). The as-prepared mixture was placed inside the ultrasound bath after setting the operating conditions such as 50 % sonication power (level 6 = 6 W/L), process temperature (30–75 °C), and extraction time (5–95 min). At the end of each USAE run, the as-obtained solution was vacuum filtered to separate the extract from the biomass (spent DFP). The filtered extract was analyzed to measure the TSC and nutritional sugar content, while the definite amount of extract was processed in a rotary evaporator (R-215, Buchi, Switzerland) at a temperature and pressure of 75 °C and 100 m bar for about 20 min to remove the solvent and estimate the total extraction yield, respectively. Subsequently, the DFP residue (DFPR) obtained after vacuum filtration was oven-dried at 105 °C for 24 h and stored for further analysis. Each experimental run was performed in duplicates. Fig. 1 shows the schematic illustration of the ultrasonication-assisted extraction (USAE) process for the extraction of valuable nutrients from date fruit powder.
Fig. 1.
Schematic illustration of ultrasonication-assisted extraction (USAE) process for the production of nutritional sugar from date fruit powder.
2.3. Experimental design
2.3.1. Single-factor experimental design
The performance of sugar extraction from DFP using the USAE technique is affected by various operating parameters. In this study, the main parameters of the process such as temperature, extraction time, and L/S ratio were considered to study their influence on the USAE of date sugars and to determine the effective ranges for the optimization experiment to maximize the sugar extraction yield (SEY) and total sugar content (TSC) in the as-obtained extracts of the USAE process. Briefly, sugar extract was produced from DFP under the following conditions: temperature (30, 45, 60, and 75 °C), time (5, 35, 65, 95 min), and L/S ratio (5, 10, 15, 20 mL/g). The parametric study was adopted by varying a single parameter while fixing the remaining variables. Response variables such as total sugar content (TSC) and sugar extraction yield (SEY) were determined for different operating conditions using the phenol–sulfuric acid method. A detailed discussion on the estimation of TSC (mg glucose eq./g of DFP) and SEY (%) is presented in Section 2.4.
2.3.2. Box–Behnken experimental design
According to the results of the single factor experiments, the range of each factor was fixed, and the RSM with BBD was used to optimize the USAE conditions to achieve a maximum TSC in the extract. BBD with three factors and three levels (−1, 0, and +1) was used. A total of 15 experiments were carried out, including three replicates at the center points. The independent variables were extraction temperature (, °C), extraction time (, min), and liquid to solid (L/S) ratio (, mL/g), and their coded and uncoded levels are presented in Table 1. The total sugar content (TSC, mg/g of DFP) and the sugar extraction yield (SEY, %) were taken as responses for this optimization study. Analysis of variance (ANOVA) (with a 95 % confidence interval) was carried out to evaluate the effect of the independent variables. Regression analysis was performed to fit the experimental data to the second-order empirical polynomial model and establish the relationship between the independent variables and the responses. The statistical model was used to determine the optimal conditions for the maximum extraction yield, which was then experimentally validated.
Table 1.
RSM-BBD of USAE of sugars from DFP.
| Run # | Temperature (°C) - X1 | Time (min) - X2 | L/S ratio (mL/g DFP) - X3 | Total yield (%) | TSC (mg glucose Eq./g DFP) - Y | SEY (%) |
|---|---|---|---|---|---|---|
| 1 | 30 (−1) | 30 (−1) | 8 (0) | 82.5 | 595.9 | 60.93 |
| 2 | 60 (+1) | 30 (−1) | 8 (0) | 80.4 | 856.5 | 85.18 |
| 3 | 30 (−1) | 90 (+1) | 8 (0) | 80.6 | 638.6 | 65.07 |
| 4 | 60 (+1) | 90 (+1) | 8 (0) | 79.0 | 693.1 | 69.78 |
| 5 | 30 (−1) | 60 (0) | 6 (−1) | 75.3 | 661.9 | 67.12 |
| 6 | 60 (+1) | 60 (0) | 6 (−1) | 75.7 | 692.8 | 70.78 |
| 7 | 30 (−1) | 60 (0) | 10 (+1) | 82.9 | 542.7 | 53.58 |
| 8 | 60 (+1) | 60 (0) | 10 (+1) | 80.7 | 627.8 | 64.26 |
| 9 | 45 (0) | 30 (−1) | 6 (−1) | 74.2 | 734.8 | 77.15 |
| 10 | 45 (0) | 90 (+1) | 6 (−1) | 76.3 | 553.8 | 54.18 |
| 11 | 45 (0) | 30 (−1) | 10 (+1) | 82.2 | 684.5 | 66.87 |
| 12 | 45 (0) | 90 (+1) | 10 (+1) | 81.7 | 558.1 | 55.41 |
| 13 | 45 (0) | 60 (0) | 8 (0) | 79.9 | 717.7 | 73.00 |
| 14 | 45 (0) | 60 (0) | 8 (0) | 79.5 | 717.1 | 72.00 |
| 15 | 45 (0) | 60 (0) | 8 (0) | 79.5 | 680.4 | 67.97 |
2.4. Characterization
2.4.1. Total sugar content (TSC)
The total sugar in the aqueous extract was determined using the phenol sulfuric acid method [24]. In this method, sugars react in the presence of strong acids to generate furan derivatives that condense with phenol to form stable yellow-gold compounds. Briefly, diluted extract samples were transferred to test tubes, where 0.05 mL of 80 % phenol was added following the addition of 5 mL of concentrated sulfuric acid. The solution was thoroughly mixed using a vortex mixer and allowed to cool to room temperature. Subsequently, the absorbance of the sample at 490 nm was determined using a UV/Vis spectrophotometer (DR 5000, Lange, USA). Total sugar concentrations were determined using a standard glucose calibration curve (Fig. S1). The total sugar content was calculated using Eq. (1) in mg of glucose equivalence per g of DFP:
| (1) |
2.4.2. Sugar composition analysis
The compositional analysis of sugars in the date sugar extract was determined using HPLC (Ultimate 3000, Thermo Scientific, USA). The date sugar extract was filtered using a 0.45 µm syringe filter prior to HPLC analysis. Quantitative estimation of glucose, fructose, and sucrose was performed using a ZORBAX carbohydrate column (4.6x150 mm, 5 µm; Agilent Technologies, California, USA) connected to the guard column (Agilent, USA). The samples were analyzed with a refractive index (RI) detector using an isocratic mobile phase of acetonitrile: water (75:25, v/v) retained at 1 mL/min. Analyzes were performed by injecting the sample volume of approximately 5 µL at the column oven temperature of 35 °C. The identification of the peak was recorded by comparison with the standard calibration curves with a correlation coefficient (R2) of 0.999 (Figs. S2-S4). The sugar extraction yield (SEY) and the total yield (TY) were calculated using Equations 2 and 3, respectively.
| (2) |
| (3) |
2.4.3. FT-IR and TGA analysis
FT-IR spectra for DFP, DFPR, date sugar extract and other standard sugars (fructose, glucose, and sucrose) were obtained using the FT-IR instrument (ATR FTIR, Bruker ALPHA, UK) in the range of 4000–400 cm−1. Each spectrum was corrected against the background spectrum of the air and was obtained by taking an average of 32 scans. Spectra were collected using the OPUS software (version 4.0, Bruker, France) provided by the equipment manufacturer. Thermogravimetric analysis (TGA) was also performed for the fresh DFP sample, DFPR, and date sugar extract using a thermogravimetric analyzer (TGA 4000, Perkin Elmer, USA). For the fresh DFP sample and the DFP residue, approximately 25 mg of sample was placed in the crucible and heated from 30 to 500 °C at 10 °C/min under a nitrogen atmosphere [25]. For the date sugar extract, two heating cycles were performed. The first heating cycle was from 30 to 160 °C at a heating rate of 10 °C/min. The sample was then cooled to 30 °C and the second heating cycle was from 30 to 600 °C at a heating rate of 10 °C/min to assess the true thermal properties.
2.4.4. Morphological analysis
Fresh DFP and DFPR after USAE were mounted on aluminium stubs with double-sided carbon adhesive tape and coated with gold–palladium to prevent sample charging. The surface morphology of the fresh and residual DFP was studied with SEM (FEI, Quanta 3D FIB, USA) operated at an accelerating voltage of 20 kV and image magnifications of 120, 650, and 1000 X to evaluate the sonication effect.
2.5. Statistical analysis and kinetic modeling
Minitab software v 19.1 (Minitab Inc, Pennsylvania, USA) was used to perform statistical analysis and the ANOVA test, where p-values 0.05 were considered statistically significant. All analyzes were performed in duplicates, and their values were expressed as mean standard deviation (SD). The as-developed statistical correlation was used to develop the kinetic extraction model based on the diffusional mode of the mass transfer mechanism. The simplified form of the second-order rate law was applied to investigate the kinetics of USAE of sugar from DFP, as shown in Equation 4 [26].
| (4) |
where: k is the second-order reaction rate (L/g.min), while PCt (g/L) and PCe (g/L) are the concentration of total phenolics in the liquid extracts at time t and the equilibrium condition, respectively.
2.6. Energy calculations in a sonicator
The USAE process utilizes electrical energy (Ee) which is converted into other forms of energy during its operation. Briefly, an ultrasonicator converts electrical energy into mechanical energy due to the oscillation of piezoelectric crystals. Subsequently, the ultrasonic waves were propagated into the liquid medium by converting mechanical energy into acoustic energy. The waves then propagate in the liquid medium, causing the molecules to oscillate around their mean position. This oscillation occurs until the average distance between the molecules exceeds a critical molecular distance. After this point, the acoustic energy (Ea) was converted into cavitation or sonication energy (Es) (causing bubbles formation) followed by the heat generation. Zaib and Ahmad [27] and Mamvura et al [28] discussed in detail the energy transformation during the ultrasonication process. Herein, the three main energy conversions i.e. electrical to acoustical to cavitation (sonication) are considered into account and were estimated by equations 5, 6, and 7, respectively.
| (5) |
| (6) |
| (7) |
where; P is the power supplied to the sonicator in watts (W), t is operation time in seconds, m is the mass of water in the sonicator, Cp is the specific heat capacity of water (4.2 J/g/K), ΔT is the change in water temperature with time.
3. Results and discussion
3.1. Single-factor experiments
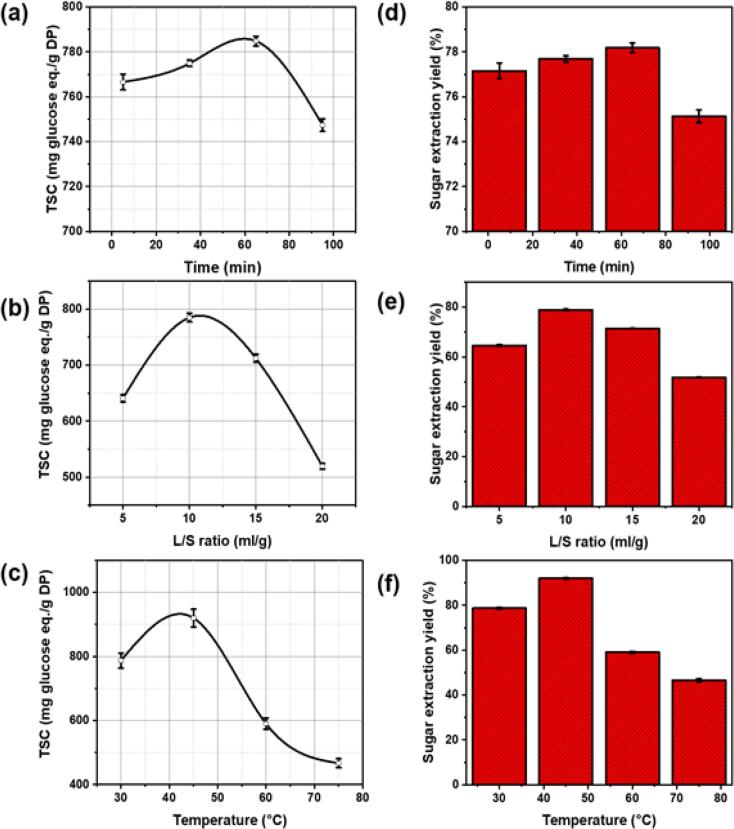
The effect of a single factor on TSC and sugar yield in the extract obtained using the USAE method was investigated. As shown in Fig. 2, the extraction time, L/S ratio, and process temperature showed a considerable effect on the TSC of the extract and the sugar yield. Fig. 2 (a and d) shows the effect of extraction time on TSC and SEY. Under the conditions of 30 °C and L/S = 10 mL/g, the TSC increased slightly with the increase in the extraction time from 5 to 65 min due to the enhanced solubility of the sugars in the solvent, resulting in higher release and diffusivity of saccharides from the DFP. However, a prolonged extraction time beyond 65 min resulted in a small decrease in TSC and SEY due to impurity dissolution and a lower extraction rate [29]. The optimal extraction time that resulted in the highest TSC and SEY was around 65 min. Furthermore, the sugar extraction was also carried out by varying the L/S ratios (5, 10, 15, and 20 mL/g) at a fixed temperature of 30 °C and an optimal extraction time of 65 min (Fig. 2 (b and e)). TSC and SEY increased with increasing L/S ratio from 5 to 10 mL/g due to increased concentration gradient and decreased viscosity of the extraction solvent, resulting in the dissolution of more sugar molecules in water. However, excessive extraction medium above 10 mL/g resulted in a decrease in TSC detected in the extract obtained and SEY. This could be due to the large amount of solvent that hinders the transfer of ultrasonic energy, thus inhibiting the dissolution of saccharides from biomass [30]. Therefore, the optimal L/S ratio that resulted in the maximum TSC and SEY was 10 mL/g for the extraction of nutritional sugar from DFP using the USAE technique. Additionally, the process temperature also has a significant impact on the extraction yield and the mass transfer mechanism. For this purpose, the extraction of valuable nutrients through the USAE was investigated for a wider range of temperatures (30, 45, 60, 75 °C) at an optimized extraction time and an L/S ratio of 65 min and 10 mL/g, respectively. Fig. 2 (c and f) demonstrated the initial increase in TSC and SEY with the increase in the extraction temperature from 30 to 45 °C as a result of the enhanced solubility. However, prolonged exposure to high temperatures above 45 °C caused a rapid decrease in TSC and SEY due to the possible depletion of thermolabile and other sugar constituents and the generation of impurities such as Maillard reaction products [31], [32]. The maximum TSC and SEY were detected within the following ranges: 30–90 min, 6–10 mL/g, and 30–60 °C, which were considered for further optimization studies using the RSM method.
Fig. 2.
Effect of the three factors studied on TSC (mg glucose eq./g of DFP) and extraction yield (%). (a, b) extraction time (min), (c, d) L/S ratio (mL/g), and (e, f) extraction temperature (°C).
3.2. RSM optimization of extraction conditions
The parameters studied and the response values are listed in Table 1. The TSC ranged between 542.7 and 856.5 mg glucose eq./g of DFP, while the estimated SEY under various operating conditions ranged between 53.58 and 85.18 %. The runs from 13 to 15 correspond to center points, which show low variation in the responses (TSC and SEY), indicating good experimental repeatability. Statistical analysis of the model was performed using analysis of variance (ANOVA). The ANOVA calculations for the quadratic model of RSM-BBD between the targeted responses and the USAE parameters are shown in Table S1. The model results for the linear and squared effects of some variables were significant (p < 0.05). However, all interaction effects were insignificant (p > 0.05). The insignificant parameters were omitted and the coefficients of significant parameters were considered. The experimental data showed a good fit to the model equations developed with a p-value < 0.05. Based on the regression analysis of the experimental data, the TSC (mg glucose eq./g of DFP) of the date sugar extract and their associated sugar extraction yield (SEY, %) can be expressed by equations 8 and 9, respectively:
| (8) |
| (9) |
where; , , and are the coded variables for the extraction temperature, time, and the L/S ratio, respectively. Fig. S5 shows the Pareto chart, which confirms that the coefficients of , , and are significantly based on the p-values. The rest of the coefficients were insignificant, including the L/S ratio and other square and two-way interaction terms. The normality plot demonstrates that the residuals of the data are normally distributed (Fig. S6). Moreover, model validation was measured by the lack of fit testing, where the p-value was 0.107, indicating that the model was adequate to predict the extraction yield of date pulp. The R2 value (96.5 %) close to 1 suggests a good fit between the response values of the developed models and the experimental data. Furthermore, a low value of the coefficient of variation (0.86) illustrates a high degree of precision and reliability of the experimental data.
The 3D surface plots and 2D contour plots provide information on the interaction between two parameters when other variables remain unchanged at the zero level. Figs. S7 (a) and (b) illustrate the effect of extraction temperature and time on the TSC in the obtained extract when the L/S ratio was fixed at 8 mL/g. The TSC increased as the extraction temperature varied from 30 to 60 °C at a shorter extraction time. Subsequently, TSC decreased at high extraction temperature for a prolonged extraction time, which could be attributed to saccharide decomposition [31]. Figs. S7 (c) and (d) present the effect of extraction temperature and L/S ratio on the TSC of the extract, which was insignificant. It is evident from Figs. S7 (e) and (f) that the extraction time and the L/S ratio exhibit a quadratic effect on the TSC of the extract. However, based on ANOVA, their interactive effect on TSC was insignificant.
3.3. Validation of the predicted model and kinetic analysis
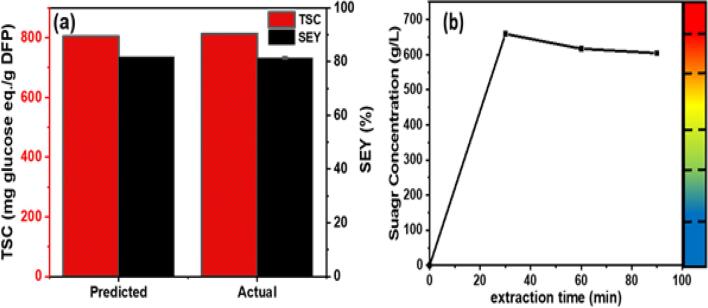
To verify the validity of DFP the model, the USAE of sugar was performed under optimal conditions (60 °C, 30 min, and an L/S ratio of 7.6 mL/g) according to the model. The experimentally obtained extract has a TSC of 819.07 ± 2.6 mg glucose eq./g DFP with the associated SEY of 81.40 ± 0.27 %, which is in good agreement with the TSC and SEY values predicted by the proposed models (806 mg glucose eq./g DFP and 81.84 %) as shown in Fig. 3(a). Furthermore, the statistical correlation developed (Equation 8) for the TSC was used to develop the mass transfer model and estimate the kinetic parameters for the extraction of sugar constituents from DFP using the USAE technique. Detailed information on how to obtain regression equations and the diffusional model of mass transfer has been discussed in the literature [33].
| (8) |
Fig. 3.
(a) Predicted and actual TSC and SEY at optimized conditions, (b) Kinetics of extraction of sugar from DFP through USAE technique (color code indicates the concentration of sugars in the bulk solution (g/L)).
When X1 = 60, X2 = 90, X3 = 10,
| (9) |
When X1 = 30, X2 = 30, X3 = 6,
| (10) |
The values for the second-order extraction rate (k = -0.00048 L g-1min−1) and the equilibrium concentration of total phenolics in the liquid extract (PCe = 579.84 g/L) were calculated by solving equations 9 and 10 simultaneously. Therefore, the kinetic diffusional model for the extraction of valuable constituents from DFP through the USAE process is represented by Equation 11.
| (11) |
Moreover, the developed correlation was used to examine the mass transfer dynamics of the diffused sugar molecules from DFP by displaying a kinetic model curve as shown in Fig. 3(b). It was observed that the concentration of sugar molecules changed with time during the sonication-based sugar extraction process. Briefly, the developed kinetic equation demonstrated a maximum sugar concentration of 659.26 g/L for the extraction time of 30 min, while a gradual decrease in sugar concentration was observed with the increase in the extraction time (Fig. 3(b)). The results of the kinetic model were in good agreement with the experimental data, and hence, the developed correlation could be used effectively to design the commercial-scale sugar extraction process from DFP through the ultrasonication-assisted extraction technique.
3.4. Sugar composition analysis
The compositional analysis of individual sugar molecules in the extract obtained under optimal conditions (60 °C, 30 min, and L/S ratio of 7.6 mL/g) was performed using HPLC. Fig. S8 shows the types and concentrations of sugars present in the date sugar extract, including fructose, glucose, and sucrose. An equivalent concentration (∼10 mg/mL) of reducing sugars (fructose and glucose) was detected in the extract. Furthermore, the concentration of disaccharide (sucrose) was the maximum (∼85 mg/mL). HPLC results indicate that the sugar content, including the three simple sugars, was 0.70 g/g of DFP. However, the phenol–sulfuric acid method revealed a TSC value of 0.806 g/g DFP, which included all possible carbohydrates. This indicates the presence of other nutritious soluble polysaccharides.
3.5. FT-IR and TGA
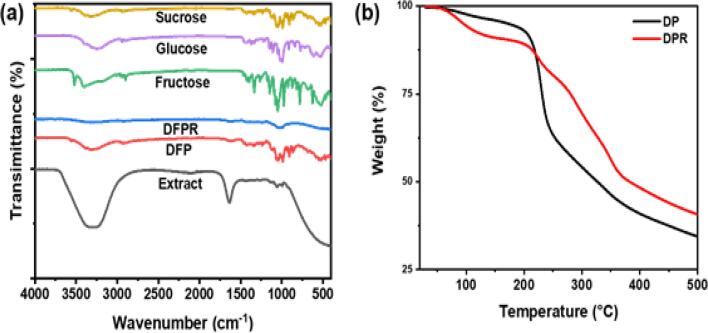
The thermal decomposition behavior of fresh DFP, DFPR, and date sugar extract was studied using the TGA technique in the temperature range of 30–500 °C. Fig. 4 (b) and Fig. S9 present the TGA thermograms, showing the three main degradation stages that biomass undergoes, namely drying (up to 200), and devolatilization (200–500 °C) and degradation of char (above 500 °C) [34], [35], [36]. The first degradation stage exhibits ∼9.01 % weight loss in DFP and ∼8.67 % weight loss in DFPR, which was observed up to 210 and 140 °C, respectively. This weight loss corresponds to the evaporation of physically absorbed water and lower molecular weight compounds. The second decomposition zone (pyrolytic stage) was observed in the range of 210–375 °C and 375–500 °C for DFP and 140–375 °C and 375–500 °C for DFPR, where hemicellulose, pectin, and cellulose devolatilization occurred with a maximum weight loss of ∼56.6 % for DFP and ∼50.6 % for DFPR, respectively. At 500 °C, the final biomass weight that will be converted to char was ∼8.8 % and 10.2 % for DFP and DFPR, respectively. The TGA results showed a significant difference between the thermal degradation of DFP and DFPR, indicating the thermal sensitivity of DFP as a result of the presence of thermolabile components, such as phytochemicals and saccharides. This shows the significant impact of temperature on the analytes of interest during the extraction process.
Fig. 4.
(a) FT-IR spectra of fresh fructose, glucose, sucrose, DFP, DFPR, extract, (b) TGA thermogram of fresh DFP and DFPR.
The FT-IR method was used to determine the functional groups present in DFP, DFPR, and extract, along with other sugar standards such as fructose, glucose, and sucrose, which are commonly found in date fruits and the results are presented in Fig. 4 (a). A wideband centered at 3300 cm−1 can be attributed to the stretching vibrations of the OH groups and the water. This indicates that hydroxyl groups are involved in the formation of hydrogen bonds [37]. There are hydroxyl groups of phenols and carboxylic acids in the region between 3000 and 2800 cm−1, reflecting the hydrophobic properties of organic substances. The band at 2850 cm−1 was due to the stretching vibrations of the CHO group. The band at 1650 cm−1 was attributed to the stretching vibration of C C aromatic, the antisymmetric vibration of COO−, as well as the deformation of the primary amide N—H, and the bending vibrations of OH-water. A little shoulder at around 1730 cm−1 indicates the stretching vibrations of a carboxylic group (C O) of acids, ketones, and aldehydes. Polysaccharides (C—O stretch) absorb between 1170 and 1050 cm−1. Several bands were visible in the fingerprint region between 1600 and 900 cm−1 [38]. The fingerprint regions of the FT-IR spectra of DFP extract and sucrose standard samples were relatively similar. However, in the same region, the FT-IR spectrum of the DFPR does not detect any prominent peaks and hence confirms the absence of specific functional groups attributed to glucose, fructose, and sucrose. Compared to the FT-IR analysis of pure DFP, some functional groups, such as ketones, aldehydes, and those in the fingerprint region, disappeared in the FT-IR spectrum of DFPR, which confirms the successful extraction of sugars and other phytochemicals. However, the overall spectra were identical, demonstrating that no chemical deformation occurred and that sonication did not change the chemical integrity of the material.
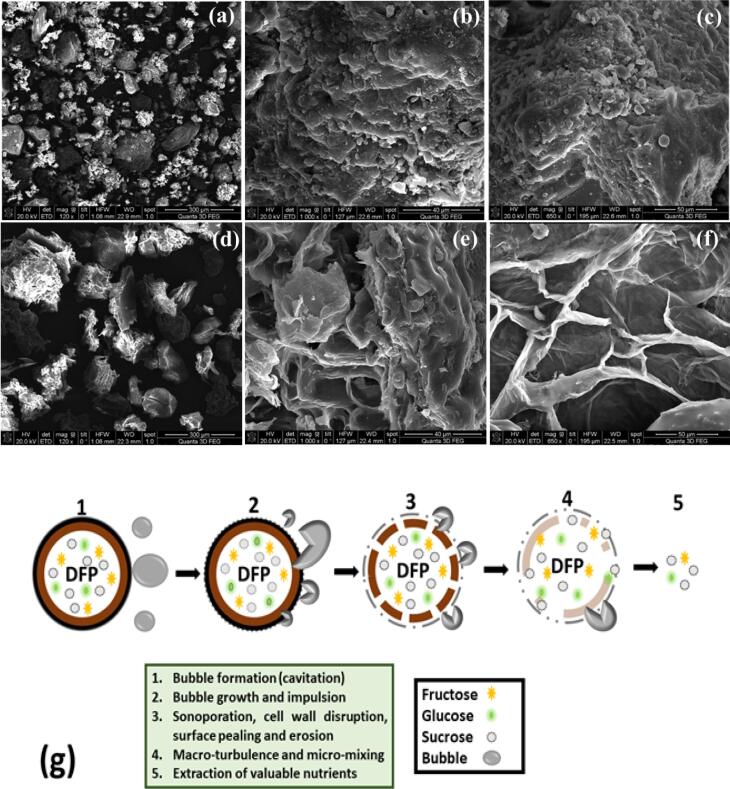
3.6. Morphological analysis and extraction mechanism
A scanning electron microscope was used to determine the microscopic characteristics of the raw biomass (DFP) and the residue (DFPR) obtained after the sonication treatment. SEM micrographs of DFP and DFPR samples at different magnifications are presented in Fig. 5 (a-f). At lower and higher magnification levels (Fig. 5 (b and c), the untreated DFP sample does not show structural surface damage. However, the DFPR sample showed considerable effective damage to the surface as shown in Fig. 5 (e and f). This could be due to the acoustic cavitation produced by the ultrasonic wave, which affects the liquid medium and breaks the cell walls [39]. The SEM images taken at a higher magnification level in Fig. 5 (e) show that the DFPR surface contains some holes that might have been generated by the cavitation bubbles induced by the ultrasonic wave. As a result, USAE achieved extraction yields higher than those of conventional methods, primarily due to the effective cell disruption that facilitated easy penetration of the solvents and enhanced dissolution of the analytes of interest.
Fig. 5.
(a-c) SEM with different magnification images of fresh untreated DFP and (d-f) SEM with different magnification images of ultra-sonication treated DFPR (g) plausible extraction mechanism for the extraction of nutritional constitutes in DI water through ultrasonication-assisted technique.
Ultrasound-assisted extraction is based on the working principle of acoustic or ultrasonic cavitation. USAE is achieved by employing high-power, low-frequency ultrasound waves in a slurry consisting of biomass in the desired solvent [40]. In this work, DFP was used as raw biomass to extract and dissolve nutritional constituents in DI water. Fig. 5 (g) shows a schematic illustration of the USAE mechanism involved in the recovery of valuable nutrients from DFP. Briefly, high-energy ultrasound waves propagate in the liquid medium (slurry) by creating high-pressure/low-pressure cycles, known as acoustic or ultrasonic cavitation. Bubble growth (cavitation) increases with time and implodes on the surface of solids (biomass). Interparticular collision, impulsion, and microjet formation resulted in surface peeling, erosion, particle breakdown, cell disruption, and sonoporation, that is, the formation of pores on the cell wall and the DFP cell membrane [41]. Remarkably, cavitation and impulsion also initiate macro-turbulences and micro-mixing of valuable nutrients from DFP into DI water [42]. However, cell disruption and sonoporation enhance the cell wall permeability and mass transfer diffusion rate in the boundary layer surrounding the solid (DFP) matrix. Furthermore, penetration into the DFP particles and transport of nutritional components from solid to liquid was also intensified by the mechanical effects of ultrasound-induced cavitation, such as temperature gradients, shock waves, shear force, liquid jets, etc. [43], [44]. In conclusion, USAE is an efficient and cost-effective process for the extraction of bioactive compounds such as vitamins, antioxidants, polyphenols, and polysaccharides compared to conventional hot water extraction techniques [45].
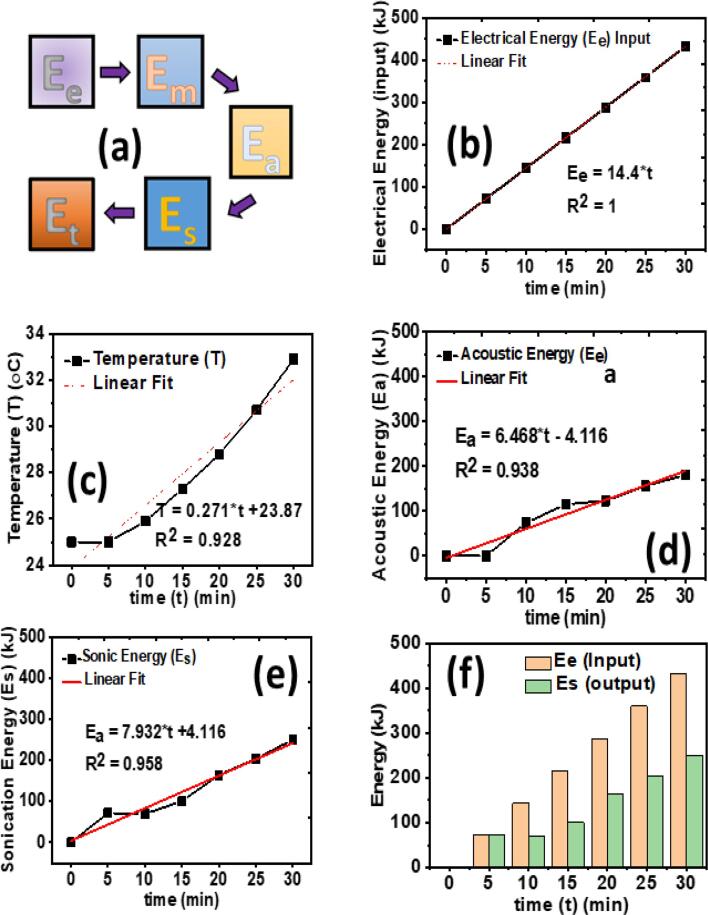
3.7. Energy transformation during sonication
The electrical energy consumption and its transformation during extraction of nutritious sugar from DFP via sonication technique were calculated as discussed in Section 2.6. Briefly, the sonication process transforms electrical energy (Ee) into other kinds of energies such as mechanical energy (Em), acoustical energy (Ea), cavitation/sonication energy (Es), and thermal energy (Et) as shown in Fig. 6 (a). However, during sonication, the transformation of Ee to Ea and Es are the important steps responsible for the extraction of valuable nutrients, and hence, their respective values were estimated in this research work using equations 5–7, respectively. Fig. 6 (b) shows the relationship between the electrical energy consumption with time during sonication of DFP. A linear increase in energy consumption with time was observed at a frequency of 45 kHz, and the corresponding linear equation with R2 is displayed in the inset of Fig. 6 (b). Manas et al [46] also reported the linear relationship of electrical power input by using distilled water at 40 °C with the ultrasonic frequency of 20 kHz. Furthermore, the acoustical energy (Ea) was calculated by using the rate of temperature change for the extraction time of 30 min at the same operating conditions (45 kHz) as shown in Fig. 6 (c and d), respectively. It can be observed that for the first 15 min, there is a negligible temperature rise. However, with the increase in extraction time, the acoustical energy increased linearly having an R2 of 0.938. It was reported that the rate of temperature and acoustic energy increased with the increase in sonication frequency [47]. The rate of temperature change was used to calculate the cavitation or sonication energy (Es). Fig. 6 (e) demonstrates the relationship between time and the sonication energy delivered to the system. It can be inferred that like other energy types, Es also exhibited the linear trend with the R2 of 0.958. However, with the increase in time, Es decreases due to an increase in the system’s temperature. A similar trend was observed for the conversion of Ee to Es with respect to the extraction time (Fig. 6 (f)). It can be observed that at the end of extraction time (30 min), >50 % of energy was being lost by the sonicator. The analogous findings have been reported in the published literature [27]. This may be because of the rise in the system’s temperature as a result of the acoustic energy (losses) over time [48]. In summary, time is a limiting factor and must be optimized to prevent energy losses and ensure to transfer most of electrical energy into sonication energy to enhance the sonication performance for various demanding applications.
Fig. 6.
(a) The energy transformation chain during ultrasonic treatment, Relationship of time with (b) electrical energy consumption (Ee, input), (c) system’s (sonicator’s) temperature, (d) acoustic energy (Ea, losses), (e) sonication energy (Es, output), and (f) conversion of electrical energy ‘Ee’ into sonication energy ‘Es’.
4. Conclusion
In summary, an ultrasound-assisted extraction technique was applied to extract highly nutritious date sugar from date fruit powder. A BBD-based response surface approach was used to optimize the extraction conditions for maximum sugar production. A statistical correlation was also used to develop the mass transfer model and estimate the kinetics of sugar extraction from DFP. In addition, single-factor experiments were performed to determine the ranges of the parameters studied. The results showed a maximum TSC of 812 mg glucose eq./g DFP under the following optimal extraction conditions: temperature of 60 °C, extraction time of 30 min, and L/S ratio of 7.6 mL/g. The extract and residue were characterized using different techniques such as TGA, SEM, FT-IR, and HPLC. The results showed that the extract contained fructose, glucose, and sucrose and exhibited good thermal stability. Furthermore, SEM analysis indicated that the ultrasonic effect improved the sugar extraction efficiency. Date sugar extract is a potential nutritious alternative to refined white sugar. The study concluded that the ultrasound-assisted extraction process is an efficient and cost-effective method to produce highly nutritious sugar from date powder.
CRediT authorship contribution statement
Jawaher AlYammahi: Methodology, Software, Formal analysis, Data curation, Writing – original draft. Abdul Hai: Methodology, Formal analysis, Data curation, Writing – review & editing. Rambabu Krishnamoorthy: Software, Formal analysis. Thanigaivelan Arumugham: Visualization, Data curation. Shadi W. Hasan: Validation, Writing – review & editing. Fawzi Banat: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the project grant CIRA-2019-028 under the Competitive Internal Research Award scheme of Khalifa University, UAE.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106107.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- 1.Tomasik P. CRC Press; London: 2003. Chemical and Functional Properties of Food Saccharides. [Google Scholar]
- 2.Johnson R.K., Yon B.A. Weighing in on added sugars and health. J. Am. Diet. Assoc. 2010 doi: 10.1016/j.jada.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 3.L.O.B. Nabors, Alternative sweeteners: Fourth edition, 2016.
- 4.FAOSTAT, (n.d.).
- 5.Samarawira I. Date palm, potential source for refined sugar. Econ. Bot. 1983;37:181–186. doi: 10.1007/BF02858783. [DOI] [Google Scholar]
- 6.Rambabu K., Bharath G., Hai A., Banat F., Hasan S.W., Taher H., Zaid H.F.M. Nutritional quality and physico-chemical characteristics of selected date fruit varieties of the United Arab Emirates. Processes. 2020;8 doi: 10.3390/pr8030256. [DOI] [Google Scholar]
- 7.Ghnimi S., Umer S., Karim A., Kamal-eldin A. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017;6:1–10. doi: 10.1016/j.nfs.2016.12.001. [DOI] [Google Scholar]
- 8.Alkaabi J.M., Al-Dabbagh B., Ahmad S., Saadi H.F., Gariballa S., Al Ghazali M. Glycemic indices of five varieties of dates in healthy and diabetic subjects. Nutr. J. 2011;10:59. doi: 10.1186/1475-2891-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamal S.M.A., El Sharnouby A. liquid sugar extraction from date palm (Phoenix dactylifera L.) fruits. J. Food Process. Technol. 2014;5 doi: 10.4172/2157-7110.1000402. [DOI] [Google Scholar]
- 10.Dhenge R., Langialonga P., Alinovi M., Lolli V., Aldini A., Rinaldi M. Evaluation of quality parameters of orange juice stabilized by two thermal treatments (helical heat exchanger and ohmic heating) and non-thermal (high-pressure processing) Food Control. 2022 doi: 10.1016/j.foodcont.2022.109150. [DOI] [Google Scholar]
- 11.Pingret D., Fabiano-Tixier A.-S., Chemat F. Degradation during application of ultrasound in food processing: A review. Food Control. 2013;31:593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- 12.Hao W., Fei Wang S., Zhao J., Ping Li S. Effects of extraction methods on immunology activity and chemical profiles of Lycium barbarum polysaccharides. J. Pharm. Biomed. Anal. 2020;185 doi: 10.1016/j.jpba.2020.113219. [DOI] [PubMed] [Google Scholar]
- 13.Watrelot A.A., Bouska L. Optimization of the ultrasound-assisted extraction of polyphenols from Aronia and grapes. Food Chem. 2022;386 doi: 10.1016/j.foodchem.2022.132703. [DOI] [PubMed] [Google Scholar]
- 14.Ojha K.S., Aznar R., O’Donnell C., Tiwari B.K. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. TrAC – Trends Anal. Chem. 2020;122 doi: 10.1016/j.trac.2019.115663. [DOI] [Google Scholar]
- 15.P.E.S.M. Francisco J. Barba, Giancarlo Cravotto, Farid Chemat, José Manuel Lorenzo Rodriguez, design and optimization of innovative food processing techniques assisted by ultrasound, 2021. 10.1016/b978-0-12-818275-8.09999-2.
- 16.Jiménez-Sánchez C., Lozano-Sánchez J., Segura-Carretero A., Fernández-Gutiérrez A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017;57:501–523. doi: 10.1080/10408398.2013.867828. [DOI] [PubMed] [Google Scholar]
- 17.Gam D.H., Kim S.Y., Kim J.W. Optimization of ultrasound-assisted extraction condition for phenolic compounds, antioxidant activity, and epigallocatechin gallate in lipid-extracted microalgae. Molecules. 2020;25:1–17. doi: 10.3390/molecules25030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A.R. Anggraini, J. Oliver, Ingredients extraction by physicochemical methods in food, 2019. 10.1017/CBO9781107415324.004.
- 19.Gościnna K., Pobereżny J., Wszelaczyńska E., Szulc W., Rutkowska B. Effects of drying and extraction methods on bioactive properties of plums. Food Control. 2021;122 doi: 10.1016/j.foodcont.2020.107771. [DOI] [Google Scholar]
- 20.Zhu C.P., Zhai X.C., Li L.Q., Wu X.X., Li B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015;177:139–146. doi: 10.1016/j.foodchem.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Chen H.M., Fu X., Luo Z.G. Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem. 2015;168:302–310. doi: 10.1016/j.foodchem.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 22.Entezari M.H., Nazari S.H., Haddad Khodaparast M.H. The direct effect of ultrasound on the extraction of date syrup and its micro-organisms. Ultrason. Sonochem. 2004;11:379–384. doi: 10.1016/j.ultsonch.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Djaoud K., Arkoub-Djermoune L., Remini H., Sait S., Tazarourte M., Hadjal S., Romero A., Madani K., Boulekbache-Makhlouf L. Syrup from common dates variety (Phoenix dactylifera L.): Optimization of sugars extraction and their quantification by high performance liquid chromatography. Curr. Nutr. Food Sci. 2019;15:1–13. doi: 10.2174/1573401315666190115160950. [DOI] [Google Scholar]
- 24.N.S. Suzanne, Food Analysis Laboratory Manual, 2010.
- 25.Hai A., Bharath G., Ali I., Daud M., Othman I., Rambabu K., Haija M.A., Hasan S.W., Banat F. Pyrolysis of date seeds loaded with layered double hydroxide: kinetics, thermodynamics, and pyrolytic gas properties. Energy Convers. Manag. 2022;252 doi: 10.1016/j.enconman.2021.115127. [DOI] [Google Scholar]
- 26.Pan Z., Qu W., Ma H., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012;19:365–372. doi: 10.1016/j.ultsonch.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Zaib Q., Ahmad F. Experimental modeling to optimize the sonication energy in water. Measurement. 2020;163 doi: 10.1016/j.measurement.2020.108039. [DOI] [Google Scholar]
- 28.Mamvura T.A., Iyuke S.E., Paterson A.E. Energy changes during use of high-power ultrasound on food grade surfaces, South African. J. Chem. Eng. 2018;25:62–73. doi: 10.1016/j.sajce.2017.12.001. [DOI] [Google Scholar]
- 29.Nuerxiati R., Abuduwaili A., Mutailifu P., Wubulikasimu A. Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep) 2019;141:431–443. doi: 10.1016/j.ijbiomac.2019.08.112. [DOI] [PubMed] [Google Scholar]
- 30.Lin T., Liu Y., Lai C., Yang T., Xie J., Zhang Y. The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind. Crops Prod. 2018;125:150–159. doi: 10.1016/j.indcrop.2018.08.078. [DOI] [Google Scholar]
- 31.J. Gu, H. Zhang, J. Zhang, C. Wen, J. Zhou, H. Yao, Y. He, H. Ma, Y. Duan, Optimization, characterization, rheological study and immune activities of polysaccharide from Sagittaria sagittifolia L., Carbohydr. Polym. 246 (2020). 10.1016/j.carbpol.2020.116595. [DOI] [PubMed]
- 32.Guerrouj K., Sánchez-Rubio M., Taboada-Rodríguez A., Cava-Roda R.M., Marín-Iniesta F. Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food Bioprod. Process. 2016;99:20–28. doi: 10.1016/j.fbp.2016.03.007. [DOI] [Google Scholar]
- 33.Baah Appiah-Nkansah N., Li J., Zhang K., Zhang M., Wang D. Study on mass transfer kinetics of sugar extraction from sweet sorghum biomass via diffusion process and ethanol yield using SSF. Processes. 2019;7:137. doi: 10.3390/pr7030137. [DOI] [Google Scholar]
- 34.Rambabu K., Thanigaivelan A., Bharath G., Sivarajasekar N., Banat F., Show P.L. Biosorption potential of Phoenix dactylifera coir wastes for toxic hexavalent chromium sequestration. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.128809. [DOI] [PubMed] [Google Scholar]
- 35.Carrier M., Loppinet-Serani A., Denux D., Lasnier J.M., Ham-Pichavant F., Cansell F., Aymonier C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy. 2011;35:298–307. doi: 10.1016/j.biombioe.2010.08.067. [DOI] [Google Scholar]
- 36.Mishra R.K., Mohanty K. Characterization of non-edible lignocellulosic biomass in terms of their candidacy towards alternative renewable fuels. Biomass Convers. Biorefinery. 2018;8:799–812. doi: 10.1007/s13399-018-0332-8. [DOI] [Google Scholar]
- 37.Moghbeli S., Jafari S.M., Maghsoudlou Y., Dehnad D. Influence of pectin-whey protein complexes and surfactant on the yield and microstructural properties of date powder produced by spray drying. J. Food Eng. 2019;242:124–132. doi: 10.1016/j.jfoodeng.2018.08.025. [DOI] [Google Scholar]
- 38.Habchi A., Kalloum S., Bradai L. Follow the degradation of organic matter during composting of date palm (phoenix dactylifera L) waste by physicochemical properties, UV-visible and FT-IR analysis. Int. J. Environ. Anal. Chem. 2020;7319 doi: 10.1080/03067319.2020.1761347. [DOI] [Google Scholar]
- 39.Xie P.J., Huang L.X., Zhang C.H., You F., Zhang Y.L. Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food Bioprod. Process. 2015;93:29–38. doi: 10.1016/j.fbp.2013.10.004. [DOI] [Google Scholar]
- 40.Deng Y., Wang W., Zhao S., Yang X., Xu W., Guo M., Xu E., Ding T., Ye X., Liu D. Ultrasound-assisted extraction of lipids as food components: mechanism, solvent, feedstock, quality evaluation and coupled technologies – a review. Trends Food Sci. Technol. 2022;122:83–96. doi: 10.1016/j.tifs.2022.01.034. [DOI] [Google Scholar]
- 41.Yahya N.A., Attan N., Wahab R.A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018;112:69–85. doi: 10.1016/j.fbp.2018.09.002. [DOI] [Google Scholar]
- 42.Carrillo-Hormaza L., Duque L., López-Parra S., Osorio E. High-intensity ultrasound-assisted extraction of Garcinia madruno biflavonoids: mechanism, kinetics, and productivity. Biochem. Eng. J. 2020;161 doi: 10.1016/j.bej.2020.107676. [DOI] [Google Scholar]
- 43.Batghare A.H., Roy K., Moholkar V.S. Investigations in physical mechanism of ultrasound-assisted antisolvent batch crystallization of lactose monohydrate from aqueous solutions. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105127. [DOI] [PubMed] [Google Scholar]
- 44.Ferarsa S., Zhang W., Moulai-Mostefa N., Ding L., Jaffrin M.Y., Grimi N. Recovery of anthocyanins and other phenolic compounds from purple eggplant peels and pulps using ultrasonic-assisted extraction. Food Bioprod. Process. 2018;109:19–28. doi: 10.1016/j.fbp.2018.02.006. [DOI] [Google Scholar]
- 45.Dong W., Chen Q., Wei C., Hu R., Long Y., Zong Y., Chu Z. Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantas P., Pagan R., Raso J. Predicting lethal effect of ultrasonic waves under pressure treatments on listeria monocytogenes ATCC 15313 by power measurements. J. Food Sci. 2000;65:663–667. doi: 10.1111/j.1365-2621.2000.tb16069.x. [DOI] [Google Scholar]
- 47.Beckett M.A., Hua I. Impact of ultrasonic frequency on aqueous sonoluminescence and sonochemistry. J. Phys. Chem. A. 2001;105:3796–3802. doi: 10.1021/jp003226x. [DOI] [Google Scholar]
- 48.Capelo-Martínez J.L. Wiley; 2008. Ultrasound in Chemistry. 10.1002/9783527623501. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.