Abstract
Fisetin (Fis), quercetin (Que), and myricetin (Myr) are flavonols with similar structure but different number of hydroxyl groups. The present research focused on the anti-inflammatory effect of these three flavonols in lipopolysaccharide-stimulated RAW264.7 cells. The number and site of hydroxyl group in flavonols obviously affected their anti-inflammation activity. These flavonols suppressed the overproduction of nitric oxide. Fis showed the best activity with an inhibition rate of 52% at 20 μM. Moreover, the flavonols reduced the levels of ROS, TNF-α, and IL-6. The mechanistic study showed that they inhibited the activation of NF-κB and MAPK pathways by suppressing the phosphorylation of IκBα, p65, JNK, ERK, p38, MEK, and reducing the nuclear translocation of NF-κB p65. In addition, the metabolism of the flavonols was examined. The results indicated that Fis was both methylated and glucuronidated. Que and Myr were mainly transformed into methylated products. This study highlights the anti-inflammatory activity of flavonols, particularly Fis, which has the potential for the prevention or treatment of inflammation as an adjuvant medicine or food additive.
Keywords: Fisetin, Quercetin, Myricetin, Anti-inflammation, NF-κB, MAPK
Graphical abstract
Fisetin is an excellent anti-inflammatory reagent which can be treated as potential adjuvant treatment for inflammation.
Highlights
-
•
Flavonols suppressed the production of NO and ROS.
-
•
Flavonols partially blocked the activation of NF-κB and MAPK pathways.
-
•
Fisetin is an excellent anti-inflammatory reagent.
-
•
The number of hydroxyl group in flavonols obviously affects their anti-inflammation activity.
1. Introduction
Inflammation is a kind of physiological responses in immune system that protects human bodies against tissue injury or infection (Chen et al., 2020b; Liu et al., 2021). Inflammatory response under normal condition has protective effects on body functions, but it would cause host injury when lacking control (Ayala et al., 2019). For instance, chronic inflammatory diseases have been reported to associated with rheumatoid arthritis (Tu et al., 2019), cardiovascular disease (Furman et al., 2019), and Alzheimer's disease (Qian et al., 2021). Macrophage is an important immune cell and plays a pivotal role during inflammation in host defenses against pathogens infection. Lipopolysaccharide (LPS)-induced inflammation in RAW264.7 murine macrophagies is a widely used inflammation model. The stimulated cells exhibit typical inflammatory responses by producing nitric oxide (NO) and reactive oxygen species (ROS) levels, and generating pro-inflammatory mediators such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and inducible NO synthase (iNOS) (Lim et al., 2021; Tian et al., 2021). Several signaling pathways were demonstrated to be closely associate with inflammation in LPS-induced RAW264.7 cells. The transcriptional regulator nuclear factor-κB (NF-κB) is vitally involved in the pathogenesis of various inflammatory diseases, which modulates the production of many cytokines and mediators (Chen et al., 2020a, Chen et al., 2020b). Besides, NF-κB could be activated through mitogen activated protein kinase (MAPK), such as c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38, which are regulated by mitogen-activated protein kinase (MEK) (Buchanan et al., 2010; Huang et al., 2012).
Dietary flavonols are typical and promising phytochemicals belonging to the flavonoids. They have been recognized to exert various effects such as anti-oxidantion, anti-microbe, anticancer, anti-inflammation, and anti-hypoglycemia (Barreca et al., 2021; Wang et al., 2006; Zhao et al., 2020). Fis (3,3′,4′,7-tetrahydroxyflavone), Que (3,3′,4′,5,7-pentahydroxyflavone), and Myr (3,3′,4′,5,5′,7-hexahydroxyflavone) are three structural related flavonols, which have the same 3-hydroxyflavone backbone (Fig. 1A). However, they have minor structural differences. Fis does not have a 5-OH group and Myri has a 3′-OH group when they were compared with Que. These minor differences might attribute to the difference in their bioactivities (Funakoshi-Tago et al., 2011). Fis is widely found in strawberry, onion, and grape (Arai et al., 2000). Que is a major flavonoid of many fruits and vegetables such as mango, apple and tea (Kandemir et al., 2021). Also, Myr is distributed in many foods including berry, onion, and red wine (Cao et al., 2020). These three compounds are common flavonols distributing in many foods, which would have more application value for exploring potential adjuvant treatment for inflammation. Different structures of polyphenols were observed to have various pharmacological activities, absorption, metabolism (Hollman, 2004; Tian et al., 2021). However, there is no comparative and systematic research on the anti-inflammatory effect and metabolism of Fis, Que, and Myr in vitro. Therefore, in this present work, we selected the compounds of Fis, Que, and Myr to examine their anti-inflammatory potential and the underlying mechanisms and metabolism in LPS-induced RAW264.7 macrophage model.
Fig. 1.
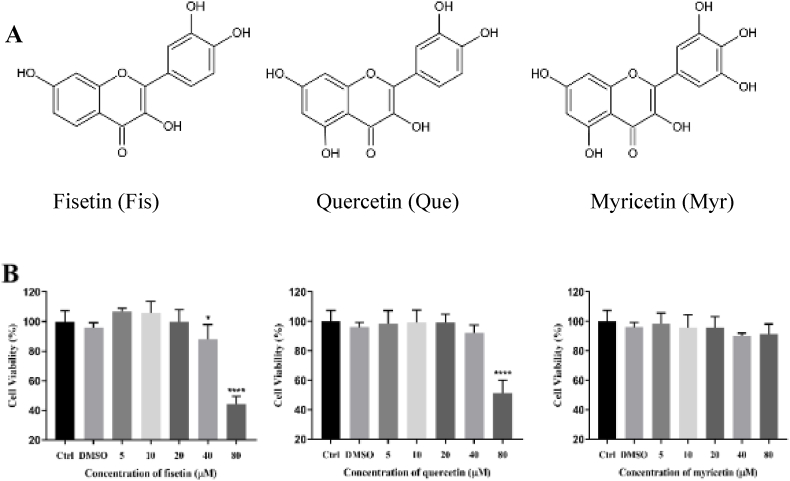
Cytotoxicity of flavonols on RAW 264.7 macrophages. (A) The chemical structures of flavonols were drawn by KingDraw software. (B) Cells were pretreated with 5, 10, 20, 40, and 80 μM of flavonols for 24 h and detected by MTT assay. Data were expressed as mean ± SD. *P < 0.05 and ****P < 0.0001 versus Control.
2. Materials and methods
2.1. Chemicals and reagents
Lipopolysaccharide (LPS) from Escherichia coli O111:B4, dimethyl sulfoxide (DMSO), and Griess reagent were purchased from Sigma Aldrich (St. Louis, MO, USA). Dichlorodihydro-fluorescein diacetate (DCFH-DA) probe (S0033S) and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were obtained from Beyotime Biotechnology (Shanghai, China). Penicillin-streptomycin (P/S), Dulbecco's Modified Eagle Medium (DMEM/high glucose), fetal bovine serum (FBS), and phosphate-buffered saline (PBS) were purchased from Gibco (Carlsbad, CA, USA). Primary antibodies against iNOS, JNK, ERK, MEK, IκBα, NFκB p65, phosphorylated (p)- JNK, p-ERK, p-MEK, p-IκBα, p-p65, GAPDH, Lamin B, and the secondary antibody were acquired from Cell Signaling Technology (Danvers, MA, United States). TNF-α and IL-6 ELISA kits were obtained from Neobioscience Technology Co., Ltd (Shenzhen, China). Fis, Que, and Myr were purchased from Tokyo Chemical Industry Co., Ltd. The HPLC purity of them was ≥97.0%.
2.2. Cell culture and flavonols treatment
A mouse macrophage cell line RAW264.7 was purchased from the American Type Culture Collection (ATCC, VA, USA). The RAW264.7 cells were cultured in DMEM/High Glucose with 1% P/S (v/v) and 10% (v/v) FBS and maintained in the atmosphere of 95% humidity and 5% CO2 at 37 °C. The cells were sub-cultured into a new 50 cm2 flask (Thermo Fisher Scientific, MA, USA) when they reached 80% confluence. Fis, Que, and Myr were dissolved in DMSO to yield stock solutions of 100 mM and stored at −20 °C. Then they freshly diluted with DMEM with 10% FBS to make working solutions, which the concentrations of DMSO were less than 0.1%.
2.3. Cytotoxicity assay
Cell viability was detected by MTT method with a few modifications (Miao et al., 2019). RAW 264.7 cells (1 × 104 cells/well) were seeded into 96-well plates and adhered overnight. Then, the cells were treated with DMEM or increasing concentrations of flavonols (Fis at 5, 10, and 20 μM; Que at 10, 20, and 40 μM; Myr at 20, 40, 80 μM) for 24 h. Then, the supernatant was removed and 100 μL solution of MTT (10 mg/ml) with flesh DMEM (MTT: DMEM = 1: 10) was added to each well, incubating at 37 °C for 3 h. Thereafter, the MTT media were carefully replaced with 150 μL DMSO for dissolving the formazan crystals. After shaking for 30 min, the absorbance at 490 nm was read through a microplate spectrophotometer reader (Molecular Devices, USA). And the cell viability of the control group was regarded as 100%. The experiments were performed in triplicate of each flavonol.
2.4. Measurement of NO
RAW264.7 cells were seeded in 24-well plates at a density of 4 × 104 cells/well and incubated overnight at 37 °C with 5% CO2. After pre-treatment with indicated concentrations of three flavonols for 4 h, cells were stimulated with LPS (1 μg/ml) for another 12 h. Then, 100 μL supernatants of were transformed into a new 96-well plate. And the Griess reagent was added and the 96-well plate was shaken for 30 min in dark conditions. The absorbance of the final solution was measured at 540 nm by a microplate reader. The inhibition rates of NO release were shown to express the percentage of NO production in flavonols and only LPS groups. This assay was performed in triplicate.
2.5. Enzyme linked immune Sorbent assay (ELISA)
RAW 264.7 cells were pretreated with different concentrations of Fis, Que, and Myr for 4 h and stimulated with LPS (1 μg/mL) for 12 h. The cell supernatant samples were collected to detect the cytokine levels of IL-6 and TNF-α by ELISA kits according to the manufacturers’ protocols. The absorbance at 450 nm was read through a microplate spectrophotometer reader.
2.6. Detection of intracellular ROS production
RAW 264.7 (2 × 105 cells/well) cells were seeded in 6-well plates and cultured for 12 h. Cells were pretreated with Fis at 20 μM, Que at 40 μM, and Myr at 80 μM for 4 h, and exposed to LPS (1 μg/ml) for 12 h. DCFH-DA reagent (10 mM) was diluted to 5 μM by DMEM (1:2000) in dark condition. The cells were discarded and added 1 mL DCFH-DA diluted solution, incubating at 37 °C for 20 min. Then, the solution was discarded and cells were washed three times with DMEM to remove DCFH-DA that existing on the cell surfaces. Finally, cells were collected into 1.5 mL tubes and suspended with 200 μL PBS to determine the intracellular ROS production by flow cytometer (Beckman, USA). The FlowJo software (BD, America) was conducted for analysis.
The immunofluorescence analysis was also done to investigate the ROS production. The preliminary procedure was almost the same as for flow cytometer detection. However, the cells were not need to be collected instead of filling with 300 μL PBS, and they were taken photos using DMI8 inverted fluorescent microscope (Leica, Germany).
2.7. Western blot analysis
RAW 264.7 (2 × 105 cells/well) cells were seeded in 6-well plates and cultured for 12 h. Cells were stimulated with LPS (1 μg/mL) for 30min or 12 h in the absence or presence of different concentrations of flavonols for 4 h. The supernatants were discarded after centrifugating for 6 min at 2000 rpm. And 100 μL RIPA buffer containing with 1% protease inhibitor cocktail and 1% phenylmethylsulfonyl fluoride (PMSF) (Beyotime, China) was added into harvested cells and incubated for 40 min on ice. Then they were centrifuged (1500 rpm, 15 min) at 4 °C and collected the supernatants to obtain the total protein. Nuclear protein was fractionated with nuclear extraction reagent from Nuclear and Cytoplasmic Protein Extraction kit (Beyotime, China) according to the manufacture's instruction. The concentrations of total protein and nuclear protein were determined by Thermo Pierce BCA protein assay kit (Thermo Scientific, USA). Proteins were dissolved by SDS/PAGE loading buffer and boiled for 10 min at 99 °C. Subsequently, the same amounts of protein (20 μg) were isolated by 8%–10% SDS-PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, USA). The membranes were blocked by 5% de-fatted dry milk in Tris-Buffered Saline/Tween 20 (TBST) for 1 h and probed with corresponding specific primary antibodies diluted with TBST at 1:1000 overnight at 4 °C (GAPDH, Lamin B, IκBα, p-IκBα, NFκB p65, p-p65, JNK, p- JNK, ERK, p-ERK, p38, p-p38, MEK, p-MEK, and iNOS). The membranes were washed with TBST for 3 times and incubated with the secondary antibody diluted with TBST at 1:2000 for 1 h at room temperature. After washing with TBST for another 3 times, the protein bands were visualized by an enhanced chemiluminescence detection kit and scanned by Bio-Rad ChemiDoc™ MP Imaging System (Bio-Rad, USA). The bands were quantified by Image J densitometry software.
2.8. Immunofluorescence assay
RAW 264.7 cells (1 × 104) were cultured in the confocal dishes (NEST Biotechnology Co., Ltd, China) overnight. Then, cells were pretreated with the highest concentration of selected flavonols for 4 h, followed by inducing LPS (1 μg/mL) for 30 min. After treatment and stimulation, cells were washed with cold PBS for 3 times and immediately fixed in 4% paraformaldehyde for 15 min. Cold PBS was used to washed the cells for another three times and 0.5% Triton X-100 solution was added for cell permeabilization for 20 min at room temperature. Next, cells were blocked with 3% BSA for 1 h in the cell incubator and followed by incubating with primary antibody rabbit NF-κB p65 (1: 500 dilution) for 2 h at room temperature. Cells were washed with ice-cold PBS for 3 times and incubated with secondary antibody Alexa Fluor 568 goat anti-rabbit IgG (H + L) (1:500 dilution) for 1 h at dark at room temperature. Lastly, cells were stained by 4′,6-diamidino-2-phenylindole (DAPI) for 10 min at dark for labeling cell nucleus and photographed by Leica TCS SP8 Confocal Laser Scanning Microscope System (Leica, USA).
2.9. Metabolism of flavonols in RAW264.7 cells
RAW cells were cultured in 6-well plates of DMEM (10% FBS) culture medium without P/S. Different time points (5 min, 30 min, 6 h, 16h) after adding flavonols were set up to collect the cells. The concentration treatments of flavonols and LPS were the same as section 2.3. Besides, LPS did not added into cells at 5 min and 30 min, but added after pretreating flavonols for 4 h. At different time points, 500 μL of cell culture medium supernatant was removed and pipetted into a 1.5 mL centrifuge tube containing 1000 μL of methanol (HPLC grade, −20 °C). The remaining medium was immediately discarded and cells were washed with 1 mL PBS three times. Cells were then scraped using 100 μL MiliQ water once and 100 μL of methanol (HPLC grade, −20 °C) twice respectively. The solutions were combined in a 1.5 mL centrifuge tube and sonicated at 0oC for 30 min. Finally, all tubes were centrifuged at 20,627 g for 15 min at 0 °C and the supernatants were used to analyze the metabolism of flavonols in RAW264.7 cells by UPLC- LTQ Orbitrap XL hybrid FTMS system in positive ion (ESI) mode.
UPLC-MS/MS analysis was performed on a Thermo UltiMate 3000 UHPLC system and a Thermo Scientific LTQ Orbitrap XL hybrid FT Mass Spectrometer (San Jose, CA, USA). Chromatographic separation on the system was achieved on a Waters ACQUITY UPLC HSS T3 1.8 μm, 2.1 × 150 mm column (Waters Technology, USA). The mobile phase was a gradient of 0.1% formic acid in MiliQ water (A) and acetonitrile (B) at a flow rate of 0.3 mL/min. The injection volume was set at 10.0 μL. The elution gradient was set as follows: 0–5 min, 15% B; 5–10 min, 15–25% B; 10–20 min, 25–40% B; 20–25 min, 40–45% B; 25–30 min, 45-15% B. The MS detection was carried out by using electrospray ionization (ESI) on positive ionization mode with capillary temperature of 380 °C, sheath gas (N2) flow rate of 48 arbitrary units (arb), auxiliary gas flow rate of 15 arb and ion spray voltage of 4.5 kV. The acquisition time was 30 min and full MS scans were acquired in the range of m/z 80–1500 with a mass resolution of 30,000. MS1 and MS2 data including retention time, quasi-molecular ions and fragment ions information were collected using Xcalibur software (Thermo Fisher Scientific, CA, USA) for data collection and analysis. These data were combined with the characteristic fragment ions, chemical structure, and reaction characteristics of Fis, Que, and Myr to analyze their metabolism in macrophagy cells.
2.10. Statistical analysis
All data were analyzed by GraphPad Prism 7 software and shown as mean ± SD. Significant differences between the experimental groups were determined through one-way ANOVA. p < 0.05 was regarded statistically significant. Every experiment was conducted for at least three times.
3. Results
3.1. Toxicity of flavonols on RAW 264.7 cells
In order to evaluate whether flavonols have potential cytotoxicity at the texted concentrations, MTT assay was used to determine the effect of flavonols on the viability of RAW 246.7 cells. Cells were incubated with flavonols for 24 h and the results showed that the cell viability was significantly decreased when treated with over 20 uM Fis and over 40 uM Que comparing with the control group. Therefore, the safe concentrations of flavonols were Fis at 5, 10, and 20 μM, Que at 10, 20, and 40 μM, and Myr at 20, 40, 80 μM (Fig. 1B), which were used for the following experiments.
3.2. Flavonols inhibited LPS-induced NO release and pro-inflammatory proteins production in RAW264.7 cells
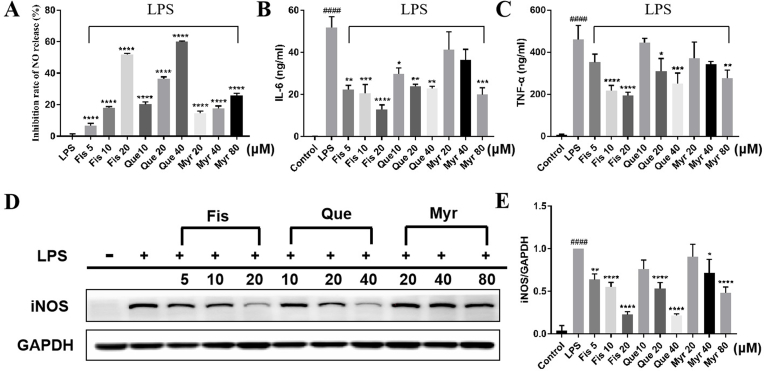
Excessive NO production is a typical sign of inflammation in activated RAW264.7 cells (Peng et al., 2020). The nitric oxide release in the LPS group was significantly higher than the control group (p < 0.0001) (data not shown). Results showed that three flavonols could dramatically inhibited NO release dose-dependently compared with the LPS group (Fig. 2A). Specifically, the inhibitory rates were 6.84%, 17.92%, and 51.92% in Fis group of 5, 10, and 20 μM, 20.25%, 36.67%, and 59.97% in Que group of 10, 20, and 40 μM, and 14.47%, 17.71%, and 25.91% in Myr group of 20, 40, 80 μM, respectively. Regarding the same concentration of 20 μM for comparison, Fis has the strongest inhibition effect (51.92%) on NO release, and Que ranked the second. Myr showed the least inhibition effect (14.47%) at 20 μM, and it only had 25.91% inhibition even at its highest concentration at 80 μM. The iNOS protein played a vital role during inflammation (Fu et al., 2014). Western blotting was performed to evaluate whether the NO release is related to the modulation of iNOS expression. The result showed that LPS significantly increased the expression of iNOS protein, which could be reversed by the three flavonols with variable effects (Fig. 2D and E). Similarly, the expression levels of iNOS were inhibited to the greatest extent by Fis at 20 μM, followed by Que, while Myr had the lowest effect. This result was consistent with that of NO inhibition.
Fig. 2.
The inhibitory effects of flavonols on inflammatory responses. RAW 264.7 cells were pretreated with different concentrations of flavonols for 4 h and then induced with LPS (1 μg/ml) for 12 h. The supernatant were used for (A) detection of NO release with Griess reagent and determination of (B) IL-6 and (C) TNF-α with ELISA kits. The cells were collected for analysis of (D, E) iNOS expression by western blotting. Data were mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 versus LPS, ####P < 0.0001 versus Control.
In addition, great amounts of pro-inflammatory proteins were produced by activating macrophages when the inflammation triggers (Su et al., 2017). LPS strongly increased the expression levels of IL-6 and TNF-α, which were reduced by flavonols in dose-dependent manners (Fig. 2B and C). Notably, these inflammatory mediators’ expression was inhibited greatly by Fis group at 20 μM treatment. Taken together, the above data implied that the three flavonols effectively suppressed LPS-stimulated inflammatory responses in macrophages, among which Fis exhibited the strongest anti-inflammatory effect.
3.3. Flavonols suppressed LPS-induced ROS production in RAW264.7 cells
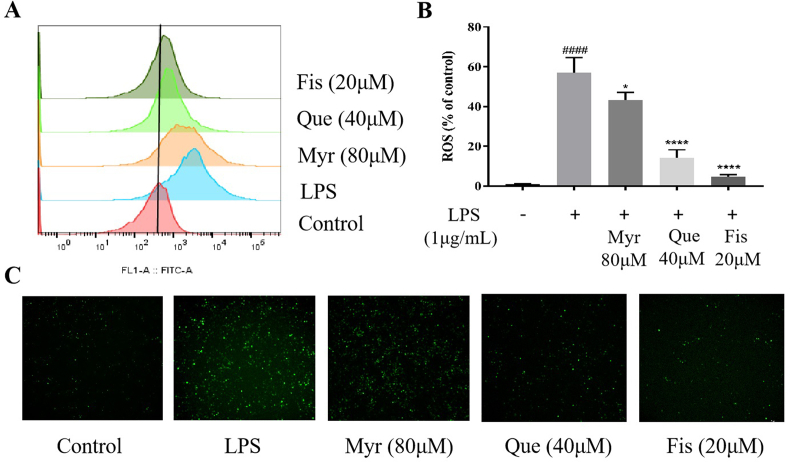
It was reported that inflammation is closely related to oxidative stress, with increased intracellular ROS levels. ROS was regarded as one of the inflammatory mediators (Ji et al., 2018). Flow cytometry analysis was performed to text the ROS levels under LPS (1 μg/mL, 12 h) induction. LPS stimulated excessive ROS production in cells compared with the control group, while the ROS production was decreased in the presence of all selected flavonols, especially in Fis group at 20 μM (Fig. 3A and B). Moreover, the fluorescence of ROS in cells captured by Lecia DMI8 microscope showed the same tendency as the results of flow cytometry (Fig. 3C). These data confirmed that the three flavonols had the ability to reverse LPS-induced ROS production to some extent. Notably, Fis could almost completely inhibit the ROS production.
Fig. 3.
Effects of flavonols on intracellular ROS in LPS-induced RAW264.7 cells. Cells were pretreated with flavonols for 4 h and induced with LPS (1 μg/ml) for 12 h, then they were incubated with DCFH-DA. Positive cells were (A, B) counted by cell flow cytometry and (C) visualized by Leica DMI8 fluorescence. Values were mean ± SD. *P < 0.05 and ****P < 0.0001 versus LPS, ####P < 0.0001 versus Control.
3.4. Flavonols inhibited the activation of MAPK signaling pathways in LPS-induced RAW264.7 cells
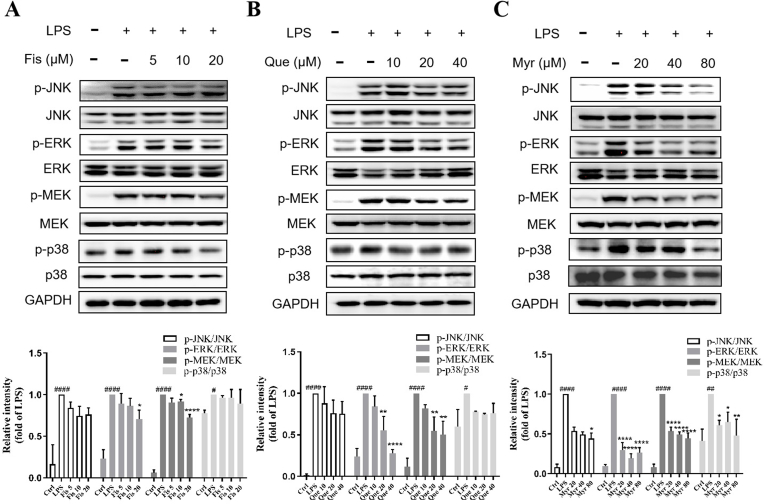
To further explore whether the anti-inflammatory effects of flavonols are associated with MAPK signaling pathways, the expressions of phosphorylation of JNK, ERK, p38, and MEK proteins were investigated by western blotting assays. RAW264.7 cells were pre-treated with flavonols and stimulated with LPS (1.0 μg/ml) for 30 min. As shown in Fig. 4A, the ratios of p-ERK/total ERK and p-MEK/total MEK were up-regulated substantially by LPS but inhibited by Fis at 20 μM. However, the ratios of p-JNK/total JNK and p-p38/total p38 slightly decreased with no significant differences between LPS group and Fis group. The ratios of p-ERK/total ERK and p-MEK/total MEK were remarkably lower in 20 μM and 40 μM Que groups than LPS group (Fig. 4B). However, the ratios f p-JNK/total JNK and p-p38/total p38 only slightly decreased in Que group with no significant differences compared to LPS group. Notably, Myr could down-regulated p-JNK, p-ERK, p-MEK, and p38 protein expressions apparently (Fig. 4C). These results suggest that anti-inflammatory activities of Fis, Que, and Myr might be through blocking the activation of MAPK signaling pathways.
Fig. 4.
Effects of flavonols on the activation of MAPK signaling pathways in LPS-induced RAW264.7 cells. Cells were pretreated with flavonols for 4 h and induced with LPS (1 μg/ml) for 30 min. The protein expression levels of p-JNK, JNK, p-ERK, ERK, p-MEK, MEK, p-p38, and p38 in Fis (A), Que (B), and Myr (C) were determined by western blotting. Values were mean ± SD. *P < 0.05, **P < 0.01, and ****P < 0.0001 versus LPS, ####P < 0.0001 versus Control.
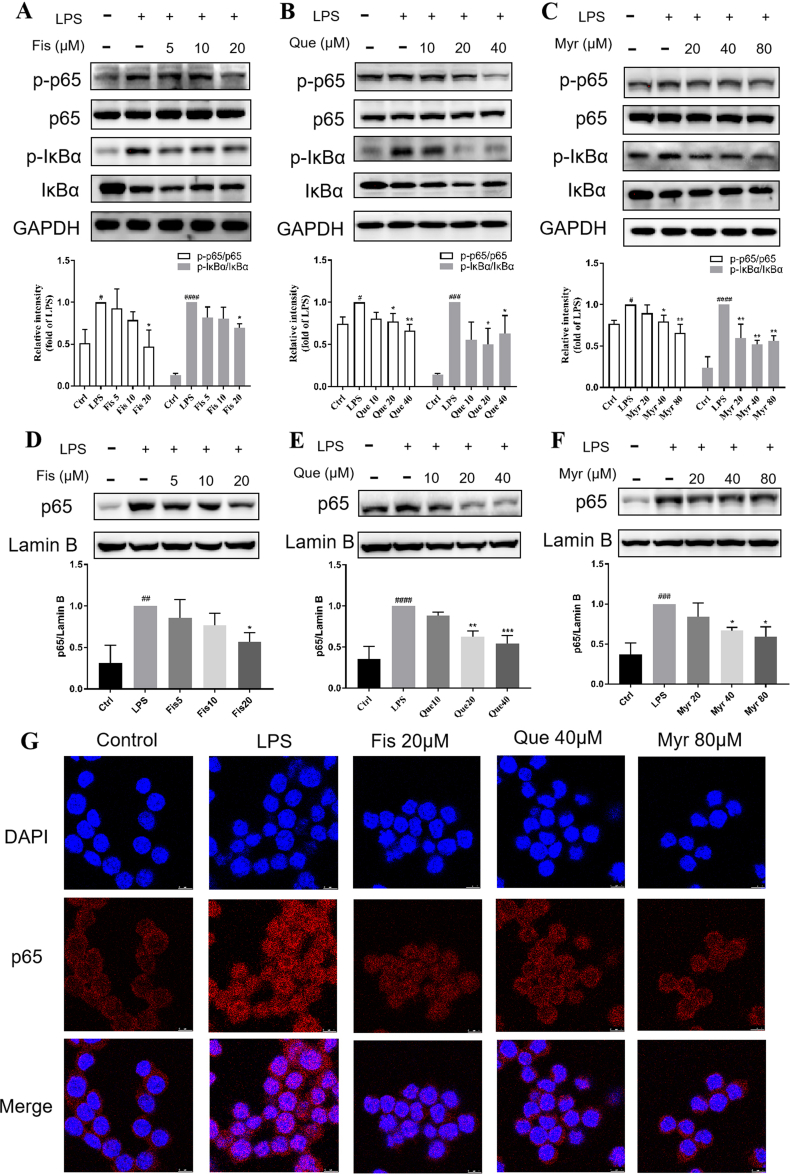
3.5. Flavonols inhibited the activation of NF-κB signaling pathways in LPS-induced RAW264.7 cells
NF-κB is a typical transcription factor for regulating inflammatory genes expression in LPS-induced inflammation (Ding et al., 2022). To further reveal the molecular mechanisms of inflammatory suppression of flavonols, the activity of NF-κB signaling pathway was investigated by Western blot. The expressions of phosphorylated-p65 and IκBα proteins were increased in LPS-induced RAW264.7 cells, but they were reversed by Fis treatment at the highest concentration at 20 μM (Fig. 5A). These proteins were also markedly declined at the middle and high dose of Que treatment (Fig. 5B). In addition, Myr could decrease these proteins expressions in a dose-dependent manner (Fig. 5C). The nuclear translocation of p65 protein is an important step for the function of NF-κB pathway, so the nuclear cell fractions were separated for the determination of p65 (Tu et al., 2019). Results showed that the nuclear expression levels of NF-κB p65 were dramatically increased in the LPS stimulation compared with control group, while flavonols pretreatment inhibited the LPS-induced nuclear accumulation of NF-κB p65. The inhibition effects were significant in Fis and Que groups at 20 μM (Fig. 5D and E) and Myr group at 40 and 80 μM in (Fig. 5F). An immunofluorescence assay was performed to confirm the nuclear translocation of NF-κB p65. The red color intensity which presented the p65 fluorescence was accumulated in the nucleus after LPS induction, but it was reversed by flavonols treatment, which was nearly the same intensity as the control group (Fig. 5G). Overall, these data implied that three flavonols significantly inhibited the activation of LPS-stimulated NF-κB signaling pathway.
Fig. 5.
Effects of flavonols on the activation of NF-κB signaling pathways in LPS-induced RAW264.7 cells. Cells were pretreated with flavonols for 4 h and induced with LPS (1 μg/ml) for 30 min. The protein expression levels of p-IκBα, IκBα, p-p65, and p65 were measured by western blotting from total cell lysates in Fis (A), Que (B), and Myr (C). The level of nuclear p65 in cells treated with Fis (D), Que (E), and Myr (F) was tested by western blotting. Lamin B was used as internal loading controls. Immunofluorescence staining of nuclear p65 was observed by confocal microscopy (G). Red fluorescence represented p65 protein and blue fluorescence (DAPI) indicated nuclei. Values were mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 versus LPS, #P < 0.05, ##P < 0.01, ###P < 0.001, and ####P < 0.0001 versus Control. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
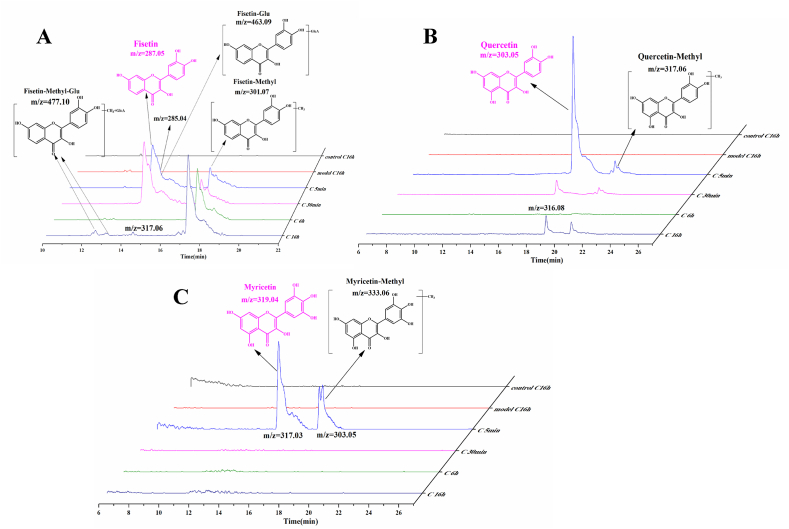
3.6. Metabolism of flavonols in RAW264.7 cells
To further reveal the anti-inflammatory effect of flavonols, it is necessary to explore the metabolism of flavonols in macrophages. Medium with flavonols were added into cells and collected them at different time points (5 min, 30 min, 6 h, and 16h) followed by UPLC-MS/MS analysis. In the first 30 min, the metabolism of the three flavonols were examined in macrophages. LPS was then added into cells after 4 h. The result showed that Fis, Que, and Myr quickly entered the cells at only 5 min and degraded at different times. As shown in Fig. 6A, the methylation reaction was speculated in Fis in the molecular weight (Mw) of 301.07 at 5 min. The intensity of methylated Fis was increased continuously and accumulated to the maximum value at 16 h. Glucuronidation of Fis (Mw: 463.09) was detected later. Finally, Fis was both methylated and glucuronidated according to the Mw of 477.10. Que was metabolized rapidly from 5 min to 30 min and mainly transformed into methylated Que (Mw: 317.06) (Fig. 6B). Myr was metabolized quickly as well and then transformed to methylated Myr (Mw: 333.06). However, methylated Myr product was almost undetectable at 30 min (Fig. 6C).
Fig. 6.
The metabolism of flavonols in RAW264.7 cells. Fis (A), Que (B), and Myr (C).
4. Discussion
RAW 264.7 macrophages are transformed from the Abelson leukemia virus, which is an ideal model for studying inflammatory effect. And LPS can fully activate macrophages to trigger inflammation, which is mediated by NO release (Hwang et al., 2019; Malayil et al., 2022). NO is a kind of free radicals to regulate human body functions, but excessive NO synthesized by iNOS will lead to autoimmune disorders, cytotoxicity, and inflammation (Zhang et al., 2019). In the present study, the Griess assay and Western blot analysis implied that the NO overproduction was related to iNOS overexpression when LPS triggered. Flavonols suppressed the production of NO, which were highly consistent with the down-regulation of iNOS protein. In addition, Fis treatment presented the greatest inhibition effect (Fig. 2A, 2D-E). Excessive NO release could stimulate proinflammatory cytokines and these cytokines mediators could promote the NO production, which formed a positive feedback loop leading to increased inflammation (Zhang et al., 2020). All selected flavonols markedly inhibited the proinflammatory cytokines of IL-6 and TNF-α (Fig. 2B and C).
Oxidative stress played a critical role in inflammatory diseases, of which ROS level was an important mediator. Excessive ROS production disrupts the balance of oxidant/antioxidant in the human bodies, leading to damages of lipids, protein, and DNA (Wang et al., 2021b). DCFH-DA cell-permeable fluorescent probe is commonly used to determine intracellular ROS levels in cells or tissues by flow cytometry or confocal microscopy (Shen et al., 2018). The flow cytometry and fluorescence images showed that flavonols especially Fis dramatically decreased the ROS generations in LPS-induced macrophages, indicating flavonols could showed anti-inflammation on RAW264.7 cells by reducing oxidative stress (Fig. 3). The structure-activity relationship (SAR) could reveal the antioxidant characteristics of these structurally related flavonols (Zhong et al., 2022). The anti-oxidant potencies of dietary flavonoids are reported to be closely associated with the site and number of hydroxylations on the A and B rings. However, pyrogallol structure could form superoxide anions in the presence of stable free radicals, which could be good pro-oxidants (Acker et al., 1996). Thus, flavonoids containing a pyrogallol group (such as Myr) might have weaker antioxidant activity because of the counteraction effect. In addition, hydroxy group position would have influence on the stability of flavonols-protein complex, contributing to varying degrees of action on the downstream signaling pathways (Wang et al., 2021a). Taking together, these flavonoids, particularly Fis, can be good antioxidants to scavenge free radicals and ameliorate inflammation (Chen et al., 2019).
MAPKs and NF-κB signaling pathways are activated through LPS stimulation to regulate the expression of pro-inflammatory cytokines. MAPKs belong to serine-threonine protein kinases (Chen et al., 2017). The phosphorylation of MAPKs activates the transcription factor activator protein 1 (AP1), which then translocates to nucleus, causing the expression of pro-inflammatory genes such as TNF-α (Wu and Schauss, 2012). The main components of MAPK signaling pathways, i.e. JNK, ERK, p38, and MEK and their phosphorylation forms were examined. Flavonols significantly inhibited JNK, ERK, p38, and MEK phosphorylation in LPS-induced RAW264.7 cells (Fig. 4), which implied that the inhibition effect of MAPK may be involved in suppressing anti-inflammatory effect by flavonols. The transcription factor NF-κB has been proved to be an up-stream signal in inflammatory cascade reaction which can increase the chemokines and pro-inflammatory cytokines expression including IL-6, TNF-α, iNOS, etc. (Liu and Malik, 2006; Park et al., 2007). NF-κB exists in an inactive form in resting cells which is bound with IκB-α protein in the cytoplasm. However, the IκB-α would be activated and degrades upon LPS stimulation, then NF-κB would translocate into the nucleus and promote the expression of variable pro-inflammatory genes (Gilroy et al., 2004; Pham et al., 2017). Therefore, the effects of flavonols on NF-κB were examined. The results presented that flavonols remarkably attenuated the phosphorylation of IκB-α and p65 and inhibited the accumulation of NF-κB p65 in nucleus (Fig. 5). It has been demonstrated that MAPKs modulated NF-κB pathway. Inhibiting MAPK pathway could suppress the NF-κB activation (Surh et al., 2001; Zhong et al., 2012). We assume that the suppression of inflammatory cytokines and mediators of the three flavonols were probably via inhibiting MAPK and NF-κB signaling pathways. And the suppression of NF-κB might be, at least partially, attributed to the inhibition of MAPK. In addition to inhibiting the activation of MAPK and NF-κB signaling pathways, some signal cascades also involved in flavonoids anti-inflammation effects such as JAK/STAT signaling pathway and the inflammasome formation. Flavonoids could regulate the activities of other signaling molecules, such as tyrosine kinase, phosphoinositol kinase, and protein kinase C (Maleki et al., 2019). In addition, other binding targets of flavonoids were found in anti-inflammatory responses, including G-protein coupled receptors, estrogen receptors, and aryl hydrocarbon receptors (Safe et al., 2021). Based on our results, we hypothesized that the regulation of signaling pathways by the flavonols, could be attributed to the antioxidant property and direct interaction with related proteins in the pathways.
To further explore the anti-inflammation mechanisms of flavonols, the metabolism of the three flavonols in RAW264.7 cells was explored. Several metabolic products of Fis were formed by methylation and glucuronidation. Both Que and Myr were transformed into methylated products. The glucuronidation of Que and Myr was undetectable (Fig. 6). Methylation and glucuronidation were discovered for other flavonoids, such as catechin, luteolin, and catechin (Hai et al., 2020; Manach et al., 2004). Some studies have claimed that the metabolites and conjugates are bioactive forms of flavonols arising from their aglycone forms on absorption. One research has presented that the hydroxyl groups at 3′site of Que was methylated to isorhamnetin. Isorhamnetin could absorbed more adequately and eliminated more slowly than parent Que form, which showed stronger antifibrotic effect on hepatic stellate cells than Que (Ganbolda et al., 2019). Also, methylated Myr could increase the activity of enhancing the life span from Caenorhabditis elegans (C. elegans). Methylated Myr enhanced stress resistance of C. elegans, which showed dependent on DAF-16 transcription factor (Büchter et al., 2015). The metabolism of Fis in vivo played a vital role in anticancer activities. Methylated Fis presented more cytotoxic than the parent Fis in tumor cells and glucuronidated Fis had longer terminal half-lives than Fis (Touil et al., 2011). These findings indicated that the anti-inflammatory effect of flavonols might be improved through adding glucuronide group and methyl group on functionally position sites. Thus, the methylated and glucuronidated derivatives of three flavonols are needed to be synthesized to test whether the derivatives can improve the biological effect and how they work against inflammation in further study.
5. Conclusion
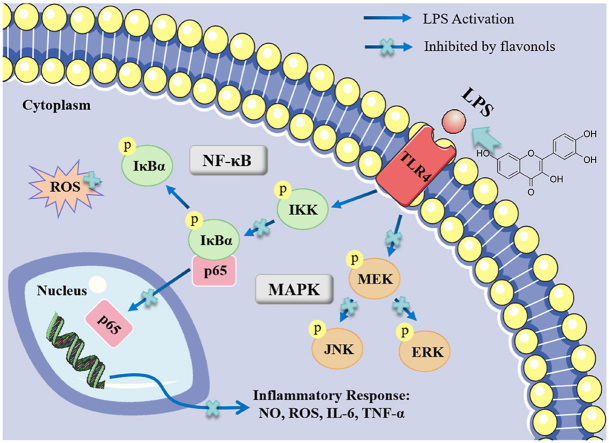
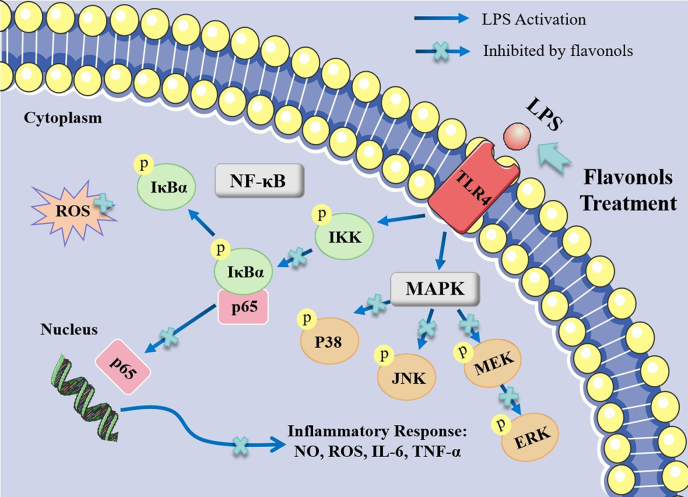
This research proved that the flavonols Fis, Que and Myr exerted anti-inflammatory effects in LPS-induced RAW 264.7 cells. Fis, Que, and Myr can inhibit NO and ROS production in LPS-stimulated RAW264.7 cells. These flavonols reduced the expression levels of ROS, TNF-α and IL-6 and suppressed the activation of NF-κB and MAPK pathways by reducing the levels of phosphorylation of IκBα, p65, JNK, ERK, MEK, and the nuclear translocation of NF-κB p65 (Fig. 7). The three flavonols, particularly Fis, has the potential for the prevention or treatment of inflammation as an adjuvant medicine or food additive.
Fig. 7.
The proposed molecular mechanisms of flavonols against LPS-induced inflammation.
Data availability
The data presented in this study are available on request from the corresponding author.
Funding
This research was supported by the National Natural Science Foundation of China (32072212)), China, the Research Fund of the University of Macau (SRG2019-00154-ICMS, MYRG2018-00169-ICMS)), Macau SAR, China, the Macao Science and Technology Development Fund, Macau SAR (0098/2020/A, 0024/2020/A1)), Macau SAR, China, the 2020 Guangdong Provincial Science and Technology Innovation Strategy Special Fund (Guangdong-Hong Kong-Macau Joint Lab) (2020B1212030006), Guangdong, China and the State Key Laboratory of Quality Research in Chinese Medicine (UM) Internal Research Grant (IRG) (QRCM-IRG2022-008), Macau SAR, China.
CRediT authorship contribution statement
Ruting Zhong: Investigation, Data curation, Writing – original draft. Lingchao Miao: Investigation, Data curation, Writing – original draft. Haolin Zhang: Investigation, Conceptualization, Writing – original draft. Lihua Tan: Investigation, Conceptualization, Writing – original draft. Yuxin Zhao: Formal analysis, Visualization. Yanbei Tu: Formal analysis, Visualization. Miguel Angel Prieto: Resources, Software. Jesus Simal-Gandara: Writing – review & editing, Supervision. Lei Chen: Data curation. Chengwei He: Funding acquisition, Writing – review & editing, Supervision. Hui Cao: Funding acquisition, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor. Professor A.G. Marangoni
Contributor Information
Chengwei He, Email: chengweihe@um.edu.mo.
Hui Cao, Email: hui_cao0830@yahoo.com.
References
- Acker S.A., Berg D.J., Tromp M.N., Griffioen D.H., Bennekom W.P., Vijgh W.J., et al. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996;20(3):331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Arai Y., Watanabe S., Kimira M., Shimoi K., Mochizuki R., Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130(9):2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- Ayala T.S., Tessaro F., Jannuzzi G.P., Bella L.M., Ferreira K.S., Martins J.O. High glucose environments interfere with bone marrow-derived macrophage inflammatory mediator release, the TLR4 pathway and glucose metabolism. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-47836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca D., Trombetta D., Smeriglio A., Mandalari G., Romeo O., Felice M.R., et al. Food flavonols: nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021;117:194–204. [Google Scholar]
- Buchanan M.M., Mark H., Watkins L.R., Hang Y. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchter C., Ackermann D., Honnen S., Arnold N., Havermann S., Koch K., et al. Methylated derivatives of myricetin enhance life span in Caenorhabditis elegans dependent on the transcription factor DAF-16. Food Funct. 2015;6(10):3383–3392. doi: 10.1039/c5fo00463b. [DOI] [PubMed] [Google Scholar]
- Cao H., Yi L., Zhong J., Högger P., Wang M., Prieto M.A., et al. Investigation of new products and reaction kinetics for myricetin in DMEM via an in situ UPLC–MS–MS analysis. Food Front. 2020;1:243–252. [Google Scholar]
- Chen G.L., Fan M.X., Wu J.L., Li N., Guo M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019;277:706–712. doi: 10.1016/j.foodchem.2018.11.040. [DOI] [PubMed] [Google Scholar]
- Chen L., Fan X.Y., Lin X.J., Qian L., Zengin G., Delmas D., et al. Phenolic extract from Sonchus oleraceus L. protects diabetes-related liver injury in rats through TLR4/NF-κB signaling pathway. eFood. 2020;1(1):77–84. [Google Scholar]
- Chen L., Yao M.J., Fan X.Y., Lin X.J., Randolph A., Aline S., et al. Dihydromyricetin attenuates streptozotocin-induced liver injury and inflammation in rats via regulation of NF-κB and AMPK signaling pathway. eFood. 2020;1(2):188–195. [Google Scholar]
- Chen Y.F., Ji N., Pan S.L., Zhang Z., Wang R., Qiu Y.L., et al. Roburic acid suppresses NO and IL-6 production via targeting NF-κB and MAPK pathway in RAW264.7 Cells. Inflammation. 2017;40(6):1959–1966. doi: 10.1007/s10753-017-0636-z. [DOI] [PubMed] [Google Scholar]
- Ding J., Mei S., Gao L., Wang Q., Ma H., Chen X. Tea processing steps affect chemical compositions, enzyme activities, and antioxidant and anti-inflammatory activities of coffee leaves. Food Front. 2022 doi: 10.1002/fft2.136. [DOI] [Google Scholar]
- Fu S.P., Li S.N., Wang J.F., Li Y., Xie S.S., Xue W.J., et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-κB activation. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi-Tago M., Nakamura K., Tago K., Mashino T., Kasahara T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharm. 2011;11(9):1150–1159. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganbolda M., Shimamotob Y., Ferdousic F., Tominagab K., Isoda H. Antifibrotic effect of methylated quercetin derivatives on TGFβ-induced hepatic stellate cells. Biochem. Biophys. Rep. 2019;20 doi: 10.1016/j.bbrep.2019.100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy D.W., Lawrence T., Perretti M., Rossi A.G. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3(5):401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Hai Y., Zhang Y.X., Liang Y.Z., Ma X.Y., Qi X., Xiao J.B., et al. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020;1:420–434. [Google Scholar]
- Hollman P.C.H. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004;42:74–83. [Google Scholar]
- Huang G.J., Huang S.S., Deng J.S. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.H., Ma J.N., Park J.H., Jung H.W., Park Y.K. Anti-inflammatory and antioxidant effects of MOK, a polyherbal extract, on lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2019;43(1):26–36. doi: 10.3892/ijmm.2018.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Dai Z., Sun S., Ma X., Yang Y., Tso P., et al. Hydroxyproline attenuates dextran sulfate sodium-induced colitis in mice: involvment of the NF-κB signaling and oxidative stress. Mol. Nutr. Food Res. 2018;62(21) doi: 10.1002/mnfr.201800494. [DOI] [PubMed] [Google Scholar]
- Kandemir K., Tomas M., McClements D.J., Capanoglu E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2021;119:192–200. [Google Scholar]
- Lim H., Parkm J.Y., Abekura F., Choi H., Kim H.D., Magae J., et al. 4-O-methylascochlorin attenuates inflammatory responses induced by lipopolysaccharide in RAW 264.7 macrophages. Int. Immunopharm. 2021;90 doi: 10.1016/j.intimp.2020.107184. [DOI] [PubMed] [Google Scholar]
- Liu H.F., Liang J.X., Xiao G.S., Ma L.K., Wang Q. Dendrobine suppresses lipopolysaccharide-induced gut inflammation in a co-culture of intestinal epithelial Caco-2 cells and RAW264.7 macrophages. eFood. 2021;2(2):92–99. [Google Scholar]
- Liu S.F., Malik A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Malayil D., House N.C., Puthenparambil D., Job J.T., Narayanankutty A. Borassus flabellifer haustorium extract prevents pro-oxidant mediated cell death and LPS-induced inflammation. Drug Chem. Toxicol. 2022;45(4):1716–1722. doi: 10.1080/01480545.2020.1858854. [DOI] [PubMed] [Google Scholar]
- Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299 doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Miao L.C., Tao H.X., Peng Y., Wang S.P., Zhong Z.F., El-Seedi H., et al. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019;290:239–245. doi: 10.1016/j.foodchem.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Park H.J., Kim I.T., Won J.H., Jeong S.H., Park E.Y., Nam J.H., et al. Anti-inflammatory activities of ent-16αH,17-hydroxy-kauran-19-oic acid isolated from the roots of Siegesbeckia pubescens are due to the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB inactivation. Eur. J. Pharmacol. 2007;558(1–3):185–193. doi: 10.1016/j.ejphar.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Peng Z., Hu X., Li X., Jiang X., Deng L., Hu Y., Bai W. Protective effects of cyanidin-3-O-glucoside on UVB-induced chronic skin photodamage in mice via alleviating oxidative damage and anti-inflammation. Food Front. 2020;1:213–223. [Google Scholar]
- Pham T.H., Kim M.S., Le M.Q., Song Y.S., Bak Y., Ryu H.W., et al. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC dependent AP-1 and NF-ĸB signaling. Phytomedicine. 2017;24:96–103. doi: 10.1016/j.phymed.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Qian X.H., Song X.X., Liu X.L., Chen S., Tang H.D. Inflammatory pathways in Alzheimer's disease mediated by gut microbiota. Ageing Res. Rev. 2021;68 doi: 10.1016/j.arr.2021.101317. [DOI] [PubMed] [Google Scholar]
- Safe S., Jayaraman A., Chapkin R.S., Howard M., Mohankumar K., Shrestha R. Flavonoids: structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021;37:147–162. doi: 10.1007/s43188-020-00080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Cao Y., Jiang Y., Wei Y.H., Liu H.L. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: implication of an antioxidation-independent mechanism. Redox Biol. 2018;18:138–157. doi: 10.1016/j.redox.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Cao J., Huang J., Liu S., Im D.S., Yoo J.W., et al. The in vitro and in vivo anti-inflammatory effects of a phthalimide PPAR-γ agonist. Mar. Drugs. 2017;15(1):7. doi: 10.3390/md15010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh Y.J., Chun K.S., Cha H.H., Han S.S., Keum Y.S., Park K.K., et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001;480:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Tian C.L., Liu X., Chang Y., Wang R.X., Lv T.M., Cui C.C., et al. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr. J. Bot. 2021;137:257–264. [Google Scholar]
- Touil Y.S., Auzeil N., Boulinguez F., Saighi H., Regazzetti A., Scherman D., et al. Fisetin disposition and metabolism in mice: identification of geraldol as an active metabolite. Biochem. Pharmacol. 2011;82(11):1731–1739. doi: 10.1016/j.bcp.2011.07.097. [DOI] [PubMed] [Google Scholar]
- Tu Y.B., Wang K., Wan J.B., He C.W. Anti-inflammatory effects of Glycine tabacina extract in LPS-stimulated macrophages and collagen-induced arthritis mice. J. Funct.Foods. 2019;62 [Google Scholar]
- Wang L.S., Tu Y.C., Lian T.W., Hung J.T., Yen J.H., Wu M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006;54(26):9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- Wang X., Cao Y.J., Chen S.Y., Lin J.C., Bian J.S., Huang D.J. Anti-inflammation activity of flavones and their structure−activity relationship. J. Agric. Food Chem. 2021;69(26):7285–7302. doi: 10.1021/acs.jafc.1c02015. [DOI] [PubMed] [Google Scholar]
- Wang Z.C., Liu X.Y., Bao Y.R., Wang X.Q., Zhai J.Y., Zhan X.B., et al. Characterization and anti-inflammation of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on LPS-induced RAW 264.7 cells. Carbohydr. Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117129. [DOI] [PubMed] [Google Scholar]
- Wu X., Schauss A.G. Mitigation of inflammation with foods. J. Agric. Food Chem. 2012;60:6703–6717. doi: 10.1021/jf3007008. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li C.Y., Jia X.J., Wang K., Tu Y.B., Wang R.C., et al. In vitro and in vivo anti-inflammatory effects of Polyphyllin VII through downregulating MAPK and NF-κB pathways. Molecules. 2019;24(5):875. doi: 10.3390/molecules24050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.L., Chen J., Liao H.J., Li C.H., Chen M.S. Anti-inflammatory effect of lipophilic grape seed proanthocyanidin in RAW 264.7 cells and a zebrafish model. J. Funct.Foods. 2020;75 [Google Scholar]
- Zhao C., Wan X.Z., Zhou S., Cao H. Natural polyphenols: a potential therapeutic approach to hypoglycemia. eFood. 2020;1(2):107–118. [Google Scholar]
- Zhong R.T., Farag M.A., Chen M.W., He C.W., Xiao J.B. Recent advances in the biosynthesis, structure-activity relationships, formulations, pharmacology, and clinical trials of fisetin. eFood. 2022;1–2(3):e3. [Google Scholar]
- Zhong Y., Liu T., Guo Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38MAPK and NF-κB pathways in rat vascular smooth muscle cells. Inflamm. Res. 2012;61(1):61–67. doi: 10.1007/s00011-011-0389-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.