Summary
Background
Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid that may impact atherosclerosis, and animal experimental studies suggest EETs protect cardiac function. Plasma EETs are mostly esterified to phospholipids and part of an active pool. To address the limited information about EETs and CVD in humans, we conducted a prospective study of total plasma EETs (free + esterified) and diabetes-related CVD in the Cardiovascular Health Study (CHS).
Methods
We measured 4 EET species and their metabolites, dihydroxyepoxyeicosatrienoic acids (DHETs), in plasma samples from 892 CHS participants with type 2 diabetes. We determined the association of EETs and DHETs with incident myocardial infarction (MI) and ischemic stroke using Cox regression.
Findings
During follow-up (median 7.5 years), we identified 150 MI and 134 ischemic strokes. In primary, multivariable analyses, elevated levels of each EET species were associated with non-significant lower risk of incident MI (for example, hazard ratio for 1 SD higher 14,15-EET: 0.86, 95% CI: 0.72–1.02; p=0.08). The EETs-MI associations became significant in analyses further adjusted for DHETs (hazard ratio for 1 SD higher 14,15-EET adjusted for 14,15-DHET: 0.76, 95% CI: 0.63–0.91; p=0.004). Elevated EET levels were associated with higher risk of ischemic stroke in primary but not secondary analyses. Three DHET species were associated with higher risk of ischemic stroke in all analyses.
Interpretation
Findings from this prospective study complement the extensive studies in animal models showing EETs protect cardiac function and provide new information in humans. Replication is needed to confirm the associations.
Funding
US National Institutes of Health.
Keywords: Epoxyeicosatrienoic acids, Diabetes, Myocardial infarction, Ischemic stroke, Epidemiology
Research in context.
Evidence before this study
Animal studies have suggested that EETs play a protective role on cardiac function. EETs are converted to DHETs that have been assumed to have less biological activity than EETs. The role of plasma EETs and DHETs in the development of CVD in humans has been largely underexplored.
Added value of this study
In a large prospective study of older adults with type 2 diabetes, elevated plasma EETs were associated with lower risk of incident MI after adjustment for plasma DHETs. Several DHET metabolites were associated with higher risk of incident ischemic stroke.
Implications of all the available evidence
Findings from this prospective study complement the extensive studies in animal models showing EETs protect cardiac function and provide new information in humans.
Alt-text: Unlabelled box
Introduction
Diabetes is a major risk factor for cardiovascular disease (CVD) including coronary heart disease and stroke,1, 2, 3 and increases the risk of mortality from CVD.4 In addition, adults with type 2 diabetes are more likely to have calcium plaque, an early marker of atherosclerosis that predicts CVD.5,6 Therefore, understanding mechanisms that might impact atherosclerosis and cardiac function may inform novel efforts at reducing the burden of diabetes-related CVD.
Epoxyeicosatrienoic acids (EETs) are produced by cytochrome P450 enzymes from arachidonic acid that has been released from the membrane in response to stimuli.7 In humans, several CYP isoforms can generate EETs in vitro. However, CYP2C and CYP2J2 are the major isoforms that form an epoxide on each double bond position (5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET). All EET species can be incorporated at the sn-2 position in the phospholipids and can be released, like arachidonic acid, in response to an agonist by phospholipase A2.8 In rodents8 and in humans9 more than 90% of the plasma EET is esterified to phospholipids where they are thought to be an active pool and stable. While free (released) EETs can be substrates for partial β-oxidation or chain elongation, the major catabolic pathway of free EET is through hydrolysis to the corresponding DHETs by the cytosolic enzyme soluble epoxide hydrolase (sEH).8
EETs exhibit multiple biological activities that may influence the risk of CVD10, 11, 12, 13 and experimental data in rodents suggest a role for EETs in the pathology of atherosclerosis and cardiac function. In ApoE mice fed a high-fat diet, overexpression of CYP2J2 protects from vascular apoptosis and atherosclerosis and prevents weight gain.14 And CYP2J2 expression in diabetic db/db mice reduces the production of proinflammatory cytokines including CRP, IL-6, IL-1β and TNFα.15 Furthermore, in a rat model of metabolic syndrome, administration of an EET-agonist reduced weight gain, hyperglycemia, dyslipidemia and hypertension, normalized inflammatory cytokines, oxidative stress and improved cardiac function.16
Evidence for a role of EETs in CVD in humans is limited to cross-sectional and genetic studies. In particular, in patients referred for angiography, the extent of coronary artery disease was inversely associated with plasma levels of EETs, suggesting EETs might protect against atherosclerosis in humans.17 Several genetic polymorphisms are present in EPHX2, the gene coding for the human sEH that converts EETs into DHETs. A variant allele associated with greater sEH activity – expected to lower EETs – was associated with greater coronary artery calcium plaque in African Americans in the Coronary Artery Risk Development in Young Adults (CARDIA) study18 and with carotid plaque in Caucasians in the Diabetes Heart Study.19 The same variant allele was associated with higher risk of coronary heart disease in Caucasian participants in the Atherosclerosis Risk in Communities (ARIC) Study.20 Polymorphisms of CYP2J2 also exist and have been found associated with MI.21 In addition, a functional polymorphism in CYP2J2 was associated with greater risk of ischemic stroke in a meta-analysis in the Chinese population (Wang. 2017) but not in other populations.21,22 No study has measured EETs in a population-based study and examined the risks of MI and ischemic stroke prospectively. To address this gap, we measured plasma EETs (free + esterified) and their downstream metabolites, DHETs (free + esterified), in the Cardiovascular Health Study (CHS), a cohort of older adults. We examined whether EETs, primarily, and DHETs, secondarily, are associated with incident MI and ischemic stroke risks among participants with type 2 diabetes.
Methods
Study population
The CHS cohort21 is a cohort of older adults who were recruited from four U.S. communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; Allegheny County, Pennsylvania) from a random sample generated from the Health Care Financing Administration files. Among eligible adults who were contacted, 57% agreed to participate. The cohort consists of 5201 community-dwelling men and women, aged ≥65 y, recruited in 1989–1990, plus an additional 687 predominantly black participants recruited in 1992–93. We used plasma specimens from the 1994–1995 or the 1992–1993 clinic visits to measure EETs and DHETs. Participants entered the study at the time their blood specimen was selected for the laboratory measurement. Participants with prevalent type 2 diabetes at the time of the laboratory measurement (n=892) were included in the study.
Ethics
Each field center's institutional review board approved the CHS study, and at the time of enrollment, all participants provided informed written consent to participate in the CHS study and for their blood specimen to be used in future studies. The use of specimens and data from CHS is covered by a University of Washington Human Subject Committee approval (STUDY00000109). The current study was conducted on de-identified blood specimens and data and is not human subjects.
Ascertainment of type 2 diabetes
Prevalent type 2 diabetes at the study baseline was defined by either glucose ≥126 mg/dL when participants reported fasting ≥8 hours before venipuncture; or glucose ≥200 mg/dL when fasting was <8 hours; or reported use of insulin or oral hypoglycemic medication; or from CMS records showing at least 2 inpatient (i.e., hospital, nursing home, or home health services), or 3 outpatient (outpatient or carrier health services), or ≥ 1 inpatient and ≥ 1 outpatient International Classification of Diseases, Ninth Revision, Clinical Modification Medicare claim codes for diabetes diagnosis (i.e., prefix 250.xx at any position within the claim) over a 2-year period.
Data collection
Information on lifestyle factors and clinical conditions was collected at the annual study clinic visits as previously described.21 Blood samples were collected and stored at −70 ˚C. Plasma glucose, insulin, lipids, and inflammatory biomarkers were assessed on fasting blood samples using enzymatic methods.21 Medication use was assessed annually through 1999 and again in 2005–2006 by an in-person inventory of medications used in past 2 weeks at the clinic visits.23 During years without clinic visits, medication use was assessed via semi-annual phone call. Body mass index (BMI) was calculated from study clinic visit measured body weight (kg) divided by height squared (m2). Estimated glomerular filtration rate (eGFR) was calculated with an equation from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) using serum creatinine and cystatin C.22 The same formula was used in Whites and African Americans.
Ascertainment of incident MI and ischemic stroke
Incident MI and ischemic stroke events were identified from annual clinic visits, interim 6-month phone contacts, hospital records, and Centers for Medicare & Medicaid Services (CMS) and National Death Index (NDI) data. Stroke and MI events were adjudicated centrally by a committee of physicians using standardized criteria based on data from participants, proxy interviews, medical records, physician questionnaires, death certificates, medical examiner forms, CMS hospitalizations, the NDI, and available brain imaging as described previously.24, 25, 26
Measurement of EETs
Blood was drawn in EDTA tubes and plasma was stored at −80 °C. Plasma EETs were measured using a high throughput method that we developed and published for the measurement of hydroxy and epoxy metabolites of arachidonic acid.9 Briefly, total lipids were extracted using a modified Bligh and Dyer method27; phospholipids were hydrolyzed by saponification to release fatty acids; the fatty acids were extracted by solid phase extraction and derivatized by a modified Bollinger method28; eicosanoids were then separated and quantitated by ultra-performance liquid chromatograph tandem mass spectrometry. Additional details on the published method is provided in the Supplementary Material. We quantitated the total levels in plasma of 4 EET species (14,15-EET, 11,12-EET, 8,9-EET and 5,6-EET) with the epoxide in the cis configuration. The numbering refers to the position of the epoxide bond. We also measured 4 DHETs (14,15-DHET, 11,12-DHET, 8,9-DHET and 5,6-DHET).
EETs and DHETs were measured in 96-well plates that each included a calibration curve made of commercial standards.9 Within each plate, values of each EET and DHET were normalized using the calibration curve of the appropriate standard. Due to skewed distributions, species values were log-transformed. In addition, because of plate-to-plate variability, species values on each plate were centered to the plate mean and scaled to plate standard deviation (SD). On most plates, the distributions of MIs, strokes and nonevents were balanced across the plates, therefore, plate was not likely a confounder. Finally, the laboratory assay was optimized for measuring the EET species, and DHETs measurements that did not pass quality control procedures were excluded.
Statistical analysis
To assess the risk of incident MI and ischemic stroke associated with EETs and DHETs, we used Cox proportional-hazards regression.29 For each EET and DHET species we estimated the hazard ratios (HR) associated with an increase in log EET of 1 SD. The time variable in these analyses was time since the blood collection used for the EET measurement (1992–1993 or 1994–1995). In analyses of MI, we excluded participants with a history at MI at baseline. Participants remained at risk until a diagnosis of first MI, or death, or the latest date of surveillance or loss to follow-up. In analyses of ischemic stroke, we excluded participants with a history of any stroke. Participant remained at risk until first ischemic stroke diagnosis, another type of stroke, death, or loss to follow-up. The primary models included adjustments for age (linear), sex, race (African American vs. other), enrollment site, BMI (linear), smoking status (never, former, current), systolic blood pressure (linear), treated hypertension, LDL cholesterol (linear), HDL cholesterol (linear), alcohol consumption (servings/week, linear) and log C-reactive protein (CRP).
In sensitivity analyses, we further adjusted for aspirin use (yes/no) and use of any lipid lowering medication (yes/no). Interactions with age, sex, and BMI were tested by adding a multiplicative term. Schoenfeld residuals were reviewed to assess whether assumptions of proportional hazards were violated.30 Models of each EET fit with cubic splines were reviewed to assess linearity.31 Finally, we conducted analysis of models of each EET species adjusted for the corresponding DHET species (for example 14,15-EET and 14,15-DHET in the same model).
Because the EET species were highly intercorrelated and were the primary exposure, we used a threshold p-value of 0.025 (adjusting for 2 outcomes) for statistical significance.
Role of the funding source
The study sponsors did not have any role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication. RNL had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The cohort consisted of 892 CHS participants with prevalent type 2 diabetes at the clinic visit of the EET measurements, the study baseline. On average the cohort participants were 76 years old at baseline, with 53% women and 76% self-identified as white. In this older population with type 2 diabetes, 71% were treated for hypertension.
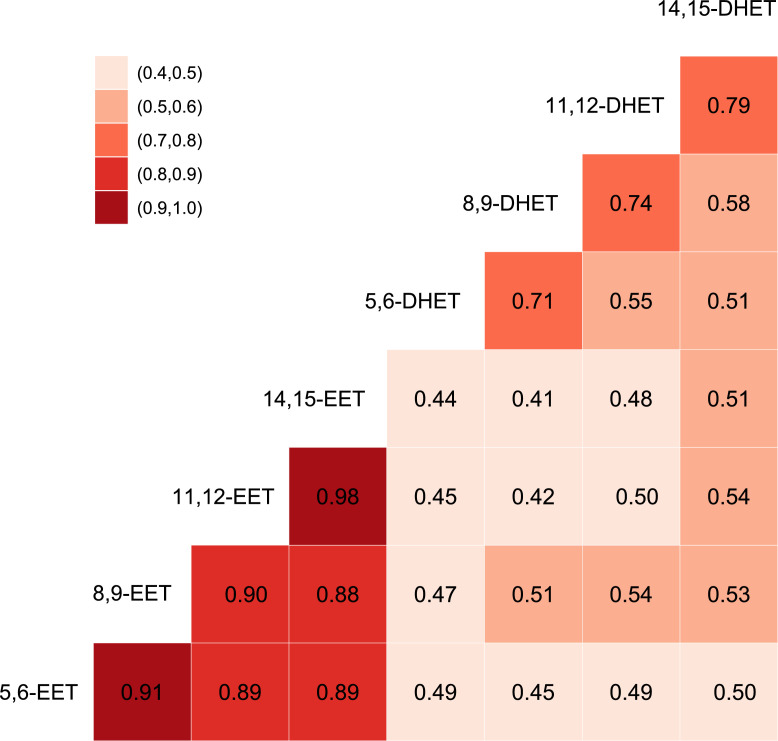
We measured plasma levels of 4 cis EET species and 4 corresponding DHETs. The levels of individual plasma EET species were highly correlated with each other, from 0.88 to 0.98 (Figure 1). Correlations between corresponding species of EETs and DHETs were 0.49 to 0.51. Scatterplots of the species associations are shown in the Supplementary Material (Figure S1).
Figure 1.
Spearman correlations between EET and DHET species measured in plasma samples on 892 participants in the Cardiovascular Health Study.
Participant characteristics as a function of plasma EETs and DHETs
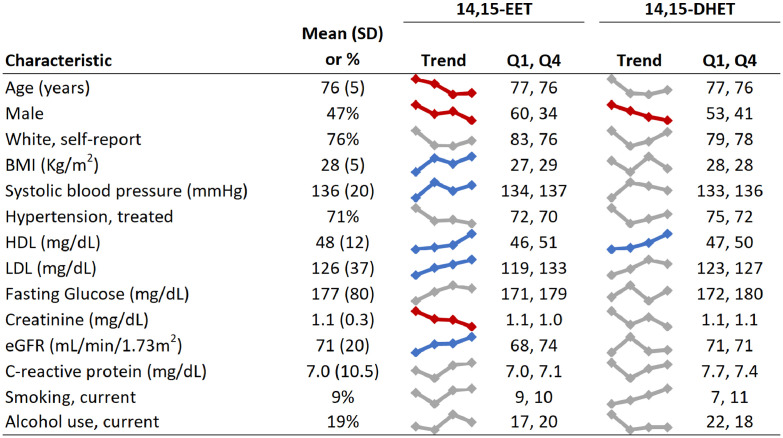
Figure 2 shows the distribution of participant characteristics in the whole cohort and across increasing quartiles of 14,15-EET and 14,15-DHET levels. Men had lower levels of 14,15-EET than women. In the whole cohort, the mean levels were on average 0.41 standard deviations (SD) lower in men than women. We observed a positive association of 14,15-EET with HDL and LDL levels. Overall in the whole cohort, the correlations were 0.12 with HDL and 0.14 with LDL. 14,15-EET was also related to higher eGFR and lower creatinine, markers of better kidney function. The other EET species, as expected from the high correlations, showed similar associations or lack of associations (not shown). 14,15-DHET showed generally similar associations with sex, HDL and LDL as 14,15-EET.
Figure 2.
Distribution of participant characteristics in quartiles of 14,15-EET and 14,15-DHET in 892 participants in the Cardiovascular Health Study.
The figure shows overall mean of each characteristics in the cohort, a small plot of values of each characteristic across quartiles of each metabolite (Trend), and mean values of each characteristic in quartile 1 (Q1) and quartile 4 (Q4).
Plasma EETs, DHETs and risk of incident MI
During a median 7.5 years of follow-up (up to 21.5 years), we identified 150 incident MIs. After adjusting for age, sex, race, enrollment sites, HDL, LDL, BMI, systolic blood pressure, treated hypertension, alcohol consumption, smoking and CRP, elevated plasma levels of EET species were found associated with a non-significant lower risk of MI (Table 1). For example, a one SD higher in log 14,15-EET was associated with a hazard ratio (HR) of MI of 0.86 (95% CI: 0.72, 1.02; p-value = 0.08 [likelihood ratio test]). Most DHET species were not associated with MI risk with HRs close to 1. Further adjustment for aspirin use or lipid lowering medications did not change the association results (not shown). In addition, we found no significant interaction of EETs and DHETs with age, sex and BMI (Supplementary Table S1).
Table 1.
Association of plasma EETs and DHETs with incident myocardial infarction.
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| HRa | 95% CI | p | HRa | 95% CI | p | |
| 5,6-EET | 0.87 | (0.73, 1.04) | 0.14 | 0.84 | (0.71, 1.00) | 0.06 |
| 8,9-EET | 0.92 | (0.77, 1.09) | 0.33 | 0.87 | (0.73, 1.03) | 0.11 |
| 11,12-EET | 0.90 | (0.76, 1.06) | 0.21 | 0.86 | (0.72, 1.01) | 0.06 |
| 14,15-EET | 0.90 | (0.76, 1.06) | 0.21 | 0.86 | (0.72, 1.02) | 0.08 |
| 5,6-DHET | 0.96 | (0.80, 1.14) | 0.63 | 0.94 | (0.78, 1.13) | 0.50 |
| 8,9-DHET | 1.03 | (0.86, 1.24) | 0.75 | 0.99 | (0.82, 1.20) | 0.94 |
| 11,12-DHET | 1.17 | (1.00, 1.36) | 0.04 | 1.14 | (0.98, 1.33) | 0.10 |
| 14,15-DHET | 1.06 | (0.89, 1.27) | 0.54 | 1.03 | (0.86, 1.24) | 0.73 |
HR: Hazard ratio of incident MI associated with one SD higher fatty acid levels. Model 1 includes adjustments for age, sex, enrollment site, race. Model 2 includes model 1 adjustments and HDL cholesterol, LDL cholesterol, body mass index, systolic blood pressure, treated hypertension, alcohol consumption, current smoking and log of C-Reactive Protein.
We observed borderline departures from the assumption of proportional hazard over time in the analyses of EETs and MI, which led us to conduct sensitivity analyses restricted to the first 10 years of follow-up (Table S2). In these analyses, we observed slightly stronger, statistically significant, associations of each EET species with lower risk of incident MI. For example, the HR for a one SD higher 14,15-EET was 0.79 (95% CI: 0.66–0.95; p-value = 0.01 [likelihood ratio test]).
Because the EETs and DHETs were positively correlated, we considered the possibility that DHET might confound the association of EETs with MI and conducted sensitivity analysis that included both EET and corresponding DHET in the same models. In these analyses, the EETs became significantly associated with lower risk of MI and the DHET associations shifted toward higher risk (Table S3). For example, with both 14,15-EET and 14,15-DHET in the same model, 14,15-EET was associated with lower risk of MI (HR: 0.76, 95% CI: 0.63–0.91; p-value =0.004 [likelihood ratio test]), and 14,15-DHET with non significant higher risk of MI (HR: 1.21, 95% CI: 1.00–1.47; p-value =0.05 [likelihood ratio test]).
Plasma EETs, DHETs and risk of incident ischemic stroke
We identified 134 incident ischemic strokes during the follow-up. Elevated plasma levels of 3 of the DHET species were significantly associated with higher risk of ischemic stroke (Table 2). For example, 1 SD higher levels of 14,15-DHET were associated with a 37% higher risk of incident ischemic stroke (95% CI: 17–61%, p=2.7×10–4 [likelihood ratio test]) after adjustments for other risk factors. Further adjustment for use of aspirin or lipid lowering medications did not change the associations with ischemic stroke (not shown). We found no departure from linearity for any of the associations and did not see any interaction with age, sex and BMI (Supplementary Table S1).
Table 2.
Association of plasma EETs and DHETs with incident ischemic stroke.
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| HRa | 95% CI | p | HRa | 95% CI | p | |
| 5,6-EET | 1.22 | (1.02, 1.46) | 0.03 | 1.21 | (1.01, 1.45) | 0.04 |
| 8,9-EET | 1.24 | (1.03, 1.50) | 0.02 | 1.24 | (1.03, 1.50) | 0.03 |
| 11,12-EET | 1.29 | (1.06, 1.57) | 0.01 | 1.28 | (1.05, 1.56) | 0.02 |
| 14,15-EET | 1.24 | (1.03, 1.50) | 0.03 | 1.23 | (1.01, 1.49) | 0.04 |
| 5,6-DHET | 1.22 | (1.02, 1.46) | 0.03 | 1.20 | (1.00, 1.45) | 0.05 |
| 8,9-DHET | 1.35 | (1.13, 1.62) | 8.7×10–4 | 1.33 | (1.10, 1.59) | 0.003 |
| 11,12-DHET | 1.30 | (1.14, 1.48) | 7.1×10–5 | 1.29 | (1.12, 1.48) | 2.7×10–4 |
| 14,15-DHET | 1.36 | (1.15, 1.61) | 2.7×10–4 | 1.37 | (1.17, 1.61) | 2.7×10–4 |
HR: Hazard ratio of incident ischemic stroke associated with one SD higher fatty acid levels. The adjustments in models 1 and 2 were the same as listed in Table 1.
We also observed bordeline departures from the assumption of proportional hazard over time in the analysis of 3 EET species and ischemic stroke. In sensitivity analyses restricted to the first 10 years of follow-up, the associations of EETs with incident ischemic stroke moved toward the null, with p-values of 0.19 to 0.51 [likelihood ratio test] (Table S4). In these analyses, the 3 DHETs species were still associated with higher risk of ischemic stroke (for example, HR for 14,15-DHET: 1.31, 95% CI: 1.07–1.60; p-value =0.01 [likelihood ratio test]).
In sensitivity analyses with both EETs and DHETs, the associations of EETs with ischemic stroke moved to the null (Table S5). For example, the HR of ischemic stroke associated with 1 SD higher 14,15-EET was 1.05 (95% CI: 0.85–1.30; p-value =0.66 [likelihood ratio test]) when adjusted for 14,15-DHET while 14,15-DHET itself remained associated with higher risk of ischemic stroke (HR: 1.33, 95% CI: 1.10–1.61; p-value =0.003 [likelihood ratio test]).
Discussion
EETs and MI
We conducted a large prospective study of EETs and incident MI and ischemic stroke in a cohort of older adults with prevalent type 2 diabetes. In primary analysis, we observed that plasma levels of EETs were associated with non-significant lower risk of incident MI. In a sensitivity analysis restricting the follow-up up to 10 years, the associations of EETs with lower risk of MI became significant, raising the possibilities that “shorter” follow-ups are needed to detect the association of EETs with lower MI risk. In addition, the EETs associations with lower MI risk became significant with adjustment for levels of DHETs. Further studies will be required to confirm an association of EETs with lower risk of incident MI.
There is considerable experimental evidence from animals and in vitro studies with human cardiomyocytes that EETs play a protective role in ischemia and reperfusion and cardiac remodeling leading to better cardiac function following stress conditions including diabetes.13,32 Experiments with cardiomyocytes in culture have shown that supplementation with 14,15-EET reduces high glucose-induced hypertrophy33; and reduction of EETs by CYP2J2 inhibition reduces the survival of adult ventricular myocytes submitted to reactive oxygen species (ROS),34 a component of diabetes pathogenesis.35 In experimental models of diabetes in rodents, mice with cardiac hypertrophy show a decrease in 14,15-EET33; overexpression of CYP2J2 in mice attenuates diabetes-induced myocardial hypertrophy36; and treatment of diabetic rats with a sEH inhibitor attenuates the reduction in cardiac outputs, left ventricular hypertrophy and fibrosis induced by hyperglycemia.37 These experimental models provide strong evidence that EETs protect the diabetic heart, although how this might translate to a reduced risk of MI in humans is not known. Findings from our prospective study complement the animal models and provide preliminary evidence in support of the hypothesis that EETs protect from MI in humans.
It is worthwhile to consider major differences between our study and short-term experimental studies. Inherent to a prospective study design, plasma EETs were measured at one time point and participants were then followed for many years; this is in contrast to the well documented short-term effects of EETs acting as paracrines or autocrines. In addition, we measured total EETs in plasma, both esterified and free EETs. We have observed that 90% of circulating EETs is esterified to phospholipids9; and EETs esterified in the membrane appear to be part an active pool that can be released by phospholipase A2 in response to stimuli.7 Therefore, the question we addressed was whether the reservoir of EETs, reflected in the plasma levels, was associated with incident MI. Given the limited information on the effects of EETs in humans, the study findings make a significant contribution to the field.
EETs and ischemic stroke
In contrast to MI, EETs were not associated with lower risk of ischemic stroke. Higher levels of plasma EETs were in fact associated with higher risk of ischemic stroke in primary analyses. However, the association of EETs with stroke was not robust; it was not detected with shorter (10 year) follow-up, and it moved toward the null when adjusting for DHETs. It is therefore possible that the EET association with stroke was confounded by DHET levels. While some risk factors might be more important in stroke (e.g. blood pressure) and others in MI (e.g. LDL cholesterol), ischemic stroke and MI share the common pathophysiology of atherosclerosis. If EETs were to act on atherosclerosis, we would have expected similar associations with MI and ischemic stroke. However, in this population of older adults with highly prevalent subclinical disease, the effects of EETs, if causal, might be more on cardiac function than atherosclerosis, as suggested by the experimental data.13
DHET and ischemic stroke
EETs released by stimuli are rapidly converted to DHETs by sEH. DHETs are less active than EETs and the conversion to DHET ends the EET signal.38,39 This may explain why we observed some evidence of association of EETs, but not DHETs, with lower risk of incident MI. In contrast, the DHET species were associated with increased risk of incident ischemic stroke in all analyses, and the associations were independent of EET levels. Interestingly, lowering DHETs by inhibition of sEH in experimental studies in rodents results in a reduction in infarct size and better functional recovery from experimental stroke.40, 41, 42, 43 In addition, in primary cultured cortical neurons, a functional genetic variant that increases sEH activity increases cell death induced in cortical neurons by oxygen-glucose deprivation and reoxygenation, while another variant that decreases sEH activity is protective.44 In humans, genetic studies have shown that polymorphisms in sEH that lower the ratio of EET/DHET are associated with higher risk of ischemic stroke.45 The relevance of these studies to the risk of incident ischemic stroke is not known. In addition, while the consequences of variation in sEH activity have generally been ascribed to changes in EETs, it is conceivable that concomitant changes in DHETs may have played a role. Our study suggests that elevated 14,15-DHET plasma levels are associated with higher risk of incident ischemic stroke independent of EETs among older adults with type 2 diabetes. Replication of these findings is needed and an investigation of DHET biological activities is warranted.
Well established protocols exist to modify EET levels in animal studies and cell experiments. In addition, inhibitors of sEH are currently being evaluated for safety and efficacy in small clinical trials. For example the sEH inhibitor GSK2256294 was shown to attenuate endothelial dysfunction in patients with chronic obstructive pulmonary disease.46 The same inhibitor was found to be safe and raise serum EET levels compared to placebo in patients with aneurysmal subarachnoid hemorrhage.47 Should our study findings be replicated in other cohorts, they would indicate that therapy using sEH inhibitors should likely be most beneficial to people at high risk of CVD. It would be interesting to explore if dietary intake of n6 fatty acids and background diet might influence downstream EET formation from arachidonic acid, as this would have the potential to improve CVD prevention in the general population.
Strengths and limitations
Strengths of our study include the prospective design that minimised reverse causation and selection bias; adjustment for many covariates reduced the possibility of residual confounding; CHS is a general, community-based, prospective cohort, enhancing generalisability. In addition, we avoided to convert EETs into DHETs during our measurement; the measurement of EETs mostly esterified to phospholipids allowed us to measure both EETs and DHETS and to distinguish their associations with MI and stroke risks. Our study also has limitations. The study was observational and this design precludes conclusions about causality. The associations of EETs with MI did not reach statistical significance in primary analysis and therefore could be due to chance. However, the findings are supported by known biologic effects of EETs and animal experimental studies. The laboratory measurement showed plate to plate variability and to avoid the possibility that it could introduce bias, we adjusted sample values to the mean levels of the plate they were on. This method may have made it difficult to detect true associations. In addition, for this reason, we were not able to provide absolute levels of plasma EETs and DHETs. Finally, our study was conducted in an older population with type 2 diabetes, on average 76 at the time of EETs measurements, and the associations need to be replicated in younger populations.
Conclusion
We have shown in a large population of older adults with type 2 diabetes that elevated plasma levels of EETs may be associated with reduced risk of incident MI while levels of DHETs are associated with increased risk of ischemic stroke.
Contributors
Rozenn N. Lemaitre contributed to conceptualisation, funding acquisition, verification of the underlying data, data interpretation, and original draft.
Paul N. Jensen contributed to data curation, verification of the underlying data, formal analysis and reviewing and editing the manuscript.
Maxwell Zeigler contributed to investigation, methodology and reviewing and editing the manuscript.
Amanda M Fretts contributed to reviewing and editing the manuscript
Jason G. Umans contributed to funding acquisition, resources, data interpretation and reviewing and editing the manuscript.
Barbara V. Howard contributed to data interpretation and reviewing and editing the manuscript.
Colleen M. Sitlani contributed to formal analysis, data interpretation and reviewing and editing the manuscript.
Barbara McKnight contributed to data curation, formal analysis, data interpretation and reviewing and editing the manuscript.
Sina A. Gharib contributed to reviewing and editing the manuscript.
Irena B. King contributed to data interpretation and reviewing and editing the manuscript.
David S. Siscovick contributed to data interpretation and reviewing and editing the manuscript.
Bruce M. Psaty contributed to data interpretation and reviewing and editing the manuscript.
Nona Sotoodehnia contributed to data curation, data interpretation and reviewing and editing the manuscript.
Rheem A. Totah contributed to funding acquisition, investigation, methodology, data interpretation and reviewing and editing the manuscript.
All authors read and approved the final version of the manuscript.
Data sharing statement
The Data and Material Distribution Agreement (DMDA) we signed with CHS does not allow us to transfer CHS data to outside investigators. Further information on the CHS study can be found at https://chs-nhlbi.org/.
Declaration of interests
The following authors declare funding from the U.S. National Institute of Health (NIH): RNL, BMcK, NS,PNJ, BMP, AMF, JGU, CS, and IBK.
The other authors disclosed no conflict of interest.
Acknowledgements
This Cardiovascular Heart Study research was supported by contracts HHSN268201200036C, HHSN 268200800007C, HHSN268201800001C, N01HC 55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021 D00006, and grants U01HL080295, U01HL130114, R01HL130880 and R01HL096706 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org/. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104189.
Appendix. Supplementary materials
References
- 1.Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in Type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dal Canto E, Ceriello A, Ryden L, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26(2_suppl):25–32. doi: 10.1177/2047487319878371. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes. 2016;7(18):449–461. doi: 10.4239/wjd.v7.i18.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoni AG, Kramer H, Watson K, Post WS. Diabetes and clinical and subclinical CVD. Glob Heart. 2016;11(3):337–342. doi: 10.1016/j.gheart.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis JP, Allen NB, Bancks MP, et al. Duration of diabetes and prediabetes during adulthood and subclinical atherosclerosis and cardiac dysfunction in middle age: the CARDIA study. Diabetes Care. 2018;41(4):731–738. doi: 10.2337/dc17-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 8.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zeigler M, Whittington D, Sotoodehnia N, Lemaitre RN, Totah RA. A sensitive and improved throughput UPLC-MS/MS quantitation method of total cytochrome P450 mediated arachidonic acid metabolites that can separate regio-isomers and cis/trans-EETs from human plasma. Chem Phys Lipids. 2018;216:162–170. doi: 10.1016/j.chemphyslip.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaspera R, Totah RA. Epoxyeicosatrienoic acids: formation, metabolism and potential role in tissue physiology and pathophysiology. Expert Opin Drug Metab Toxicol. 2009;5(7):757–771. doi: 10.1517/17425250902932923. [DOI] [PubMed] [Google Scholar]
- 12.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–S56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliwarga T, Evangelista EA, Sotoodehnia N, Lemaitre RN, Totah RA. Regulation of CYP2J2 and EET levels in cardiac disease and diabetes. Int J Mol Sci. 2018;19(7):1916–1935. doi: 10.3390/ijms19071916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Wang T, He X, et al. CYP2J2 overexpression increases EETs and protects against HFD-induced atherosclerosis in ApoE-/- Mice. J Cardiovasc Pharmacol. 2016;67(6):491–502. doi: 10.1097/FJC.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Xu X, Chen C, et al. CYP2J2 attenuates metabolic dysfunction in diabetic mice by reducing hepatic inflammation via the PPARgamma. Am J Physiol Endocrinol Metab. 2015;308(4):E270–E282. doi: 10.1152/ajpendo.00118.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Huang X, Gao J, et al. Improved endogenous epoxyeicosatrienoic acid production mends heart function via increased PGC 1alpha-mitochondrial functions in metabolic syndrome. J Pharmacol Sci. 2018;138(2):138–145. doi: 10.1016/j.jphs.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Oni-Orisan A, Edin ML, Lee JA, et al. Cytochrome P450-derived epoxyeicosatrienoic acids and coronary artery disease in humans: a targeted metabolomics study. J Lipid Res. 2016;57(1):109–119. doi: 10.1194/jlr.M061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Q, Doris PA, Pollizotto MV, et al. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190(1):26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Burdon KP, Lehtinen AB, Langefeld CD, et al. Genetic analysis of the soluble epoxide hydrolase gene, EPHX2, in subclinical cardiovascular disease in the Diabetes Heart Study. Diab Vasc Dis Res. 2008;5(2):128–134. doi: 10.3132/dvdr.2008.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CR, North KE, Bray MS, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006;15(10):1640–1649. doi: 10.1093/hmg/ddl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marciante KD, Totah RA, Heckbert SR, et al. Common variation in cytochrome P450 epoxygenase genes and the risk of incident nonfatal myocardial infarction and ischemic stroke. Pharmacogenet Genom. 2008;18(6):535–543. doi: 10.1097/FPC.0b013e3282fd1287. [DOI] [PubMed] [Google Scholar]
- 22.Fava C, Montagnana M, Almgren P, et al. The common functional polymorphism -50G>T of the CYP2J2 gene is not associated with ischemic coronary and cerebrovascular events in an urban-based sample of Swedes. J Hypertens. 2010;28(2):294–299. doi: 10.1097/HJH.0b013e328333097e. [DOI] [PubMed] [Google Scholar]
- 23.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 24.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 25.Longstreth WT, Jr., Bernick C, Fitzpatrick A, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56(3):368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 26.Mittelmark MB, Psaty BM, Rautaharju PM, et al. Prevalence of cardiovascular diseases among older adults. The Cardiovascular Health Study. Am J Epidemiol. 1993;137(3):311–317. doi: 10.1093/oxfordjournals.aje.a116678. [DOI] [PubMed] [Google Scholar]
- 27.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 28.Bollinger JG, Thompson W, Lai Y, et al. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal Chem. 2010;82(16):6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox D. Regression models and life-tables. J R Stat Soc Ser B (Methodol) 1972;34(2):187–220. [Google Scholar]
- 30.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 31.Harrell FE. Springer-Verlag; New York: 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- 32.Romashko M, Schragenheim J, Abraham NG, McClung JA. Epoxyeicosatrienoic acid as therapy for diabetic and ischemic cardiomyopathy. Trends Pharmacol Sci. 2016;37(11):945–962. doi: 10.1016/j.tips.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Yang C, Qiu H, et al. 14,15-EET involved in the development of diabetic cardiac hypertrophy mediated by PPARs. Prostaglandins Other Lipid Mediat. 2022;159:106620–106626. doi: 10.1016/j.prostaglandins.2022.106620. [DOI] [PubMed] [Google Scholar]
- 34.Evangelista EA, Lemaitre RN, Sotoodehnia N, Gharib SA, Totah RA. CYP2J2 expression in adult ventricular myocytes protects against reactive oxygen species toxicity. Drug Metab Dispos. 2018;46(4):380–386. doi: 10.1124/dmd.117.078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng ML, Fu Y, Wu CW, Zhang Y, Ren H, Zhou SS. Signaling pathways related to oxidative stress in diabetic cardiomyopathy. Front Endocrinol (Lausanne) 2022;13:907757–907776. doi: 10.3389/fendo.2022.907757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma B, Xiong X, Chen C, et al. Cardiac-specific overexpression of CYP2J2 attenuates diabetic cardiomyopathy in male streptozotocin-induced diabetic mice. Endocrinology. 2013;154(8):2843–2856. doi: 10.1210/en.2012-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alaeddine LM, Harb F, Hamza M, et al. Pharmacological regulation of cytochrome P450 metabolites of arachidonic acid attenuates cardiac injury in diabetic rats. Transl Res. 2021;235:85–101. doi: 10.1016/j.trsl.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Zeldin DC, Wei S, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys. 1995;316(1):443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- 39.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46(6):842–848. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpkins AN, Rudic RD, Schreihofer DA, et al. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174(6):2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Koerner IP, Noppens R, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27(12):1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuloaga KL, Krasnow SM, Zhu X, et al. Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS One. 2014;9(5):e97529. doi: 10.1371/journal.pone.0097529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koerner IP, Jacks R, DeBarber AE, et al. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27(17):4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fava C, Montagnana M, Danese E, et al. Homozygosity for the EPHX2 K55R polymorphism increases the long-term risk of ischemic stroke in men: a study in Swedes. Pharmacogenet Genom. 2010;20(2):94–103. doi: 10.1097/FPC.0b013e3283349ec9. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Cheriyan J, Gutterman DD, et al. Mechanisms of vascular dysfunction in COPD and effects of a novel soluble epoxide hydrolase inhibitor in smokers. Chest. 2017;151(3):555–563. doi: 10.1016/j.chest.2016.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martini RP, Siler D, Cetas J, Alkayed NJ, Allen E, Treggiari MM. A double-blind, randomized, placebo-controlled trial of soluble epoxide hydrolase inhibition in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2022;36(3):905–915. doi: 10.1007/s12028-021-01398-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.