Abstract
Deubiquitinases (DUBs) are required for the reverse reaction of ubiquitination and act as major regulators of ubiquitin signaling processes. Emerging evidence suggests that these enzymes are regulated at multiple levels in order to ensure proper and timely substrate targeting and to prevent the adverse consequences of promiscuous deubiquitination. The importance of DUB regulation is highlighted by disease-associated mutations that inhibit or activate DUBs, deregulating their ability to coordinate cellular processes. Here, we describe the diverse mechanisms governing protein stability, enzymatic activity, and function of DUBs. In particular, we outline how DUBs are regulated by their protein domains and interacting partners. Intramolecular interactions can promote protein stability of DUBs, influence their subcellular localization, and/or modulate their enzymatic activity. Remarkably, these intramolecular interactions can induce self-deubiquitination to counteract DUB ubiquitination by cognate E3 ubiquitin ligases. In addition to intramolecular interactions, DUBs can also oligomerize and interact with a wide variety of cellular proteins, thereby forming obligate or facultative complexes that regulate their enzymatic activity and function. The importance of signaling and post-translational modifications in the integrated control of DUB function will also be discussed. While several DUBs are described with respect to the multiple layers of their regulation, the tumor suppressor BAP1 will be outlined as a model enzyme whose localization, stability, enzymatic activity, and substrate recognition are highly orchestrated by interacting partners and post-translational modifications.
Keywords: quality control, post-translational modifications, deubiquitinase, ubiquitin, folding, DUB activity, multiprotein complex, BAP1
Abbreviations: CTD, C-terminal domain; DEUBAD, DEUBiquitinase ADaptator; DUB, deubiquitinase; JAMM, JAB1/MPN/MOV34 metalloenzymes; MINDY, motif-interacting with ubiquitin-containing novel DUB family; MJD, Machado–Josephin domain-containing proteases; MAVS, mitochondrial antiviral signaling protein; NLS, nuclear localization signal; OTU, ovarian tumor proteases; TRAF, tumor necrosis factor receptor–associated factor; UCH, ubiquitin carboxy-terminal hydrolases; USP, ubiquitin-specific proteases; Ub, ubiquitin; UBL, ubiquitin-like; ULD, UCHL5-like domain; ZUP1, zinc finger-containing ubiquitin peptidase 1
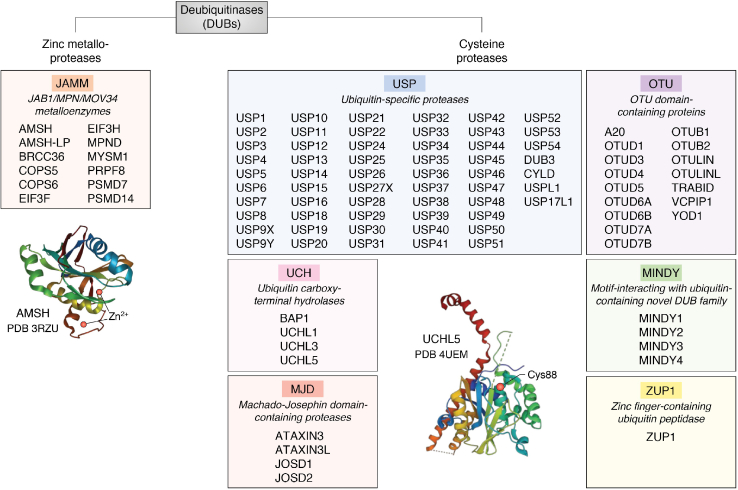
The attachment of ubiquitin (Ub) moieties to proteins is a highly conserved post-translational modification in eukaryotes. Protein ubiquitination is catalyzed by E1 Ub-activating, E2 Ub-conjugating, and E3 Ub-ligating enzymes, culminating in the modification of internal lysines or N-terminal residues of proteins (1, 2, 3, 4). Ligation of Ub to proteins regulates different signaling pathways and cellular processes by inducing changes in protein function or targeting proteins for proteasomal degradation (4, 5, 6, 7, 8, 9). Deubiquitinases (DUBs) constitute a superfamily of proteases that participate in the timely reversal of protein ubiquitination, thus controlling the functional outcomes of this post-translational modification (10, 11, 12, 13, 14, 15). In mammals, DUBs can be classified into seven major families based on sequence conservation of the catalytic domain (Fig. 1). These include the Ub carboxy-terminal hydrolases (UCH), the Ub-specific proteases (USP), the Machado–Josephin domain-containing proteases, the ovarian tumor proteases (OTU), the JAMM/MPN+ metalloproteases (JAMM), the motif interacting with Ub-containing novel DUB family, and the recently discovered Zinc finger-containing Ub peptidase 1 (12). Most DUB enzymes are cysteine proteases, which are characterized by a catalytic triad containing, notably, cysteine and histidine residues. Their mechanism of catalysis involves a nucleophilic attack mediated by the cysteine thiol side chain, which results in the cleavage of the peptide bonds. On the other hand, the JAMM/MPN+ family of DUBs are metalloproteases that use a zinc atom coordinated by histidine and aspartic acid to ensure catalysis and Ub removal (10, 11, 12, 13, 14, 15).
Figure 1.
The deubiquitinase (DUB) superfamily. DUBs are classified into seven families: USP, OTU, MJD, UCH, MINDY, JAMM, and ZUP1. The JAMM family are zinc metalloproteases DUBs. The other DUB families are cysteine proteases. JAMM, JAB1/MPN/MOV34 metalloenzymes; MINDY, motif-interacting with ubiquitin-containing novel DUB family; MJD, Machado–Josephin domain-containing proteases; OTU, OTU domain-containing proteins; UCH, ubiquitin carboxy-terminal hydrolases; USP, ubiquitin-specific proteases; ZUP1, zinc finger-containing ubiquitin peptidase 1. AMSH PDB (3RZU), UCHL5 PDB (4UEM).
In addition to representing a substantial class of proteins in higher eukaryotes, DUBs are also found in viruses, bacteria, and yeast (12, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28). Studies in eukaryotes indicate that DUBs regulate a wide spectrum of cellular processes including protein quality control, membrane receptor signaling, endocytosis, DNA-dependent processes, cell cycle regulation, differentiation, cell survival, and cell death (10, 11, 12, 13, 14, 15). Moreover, these enzymes have emerged as key factors in the cellular responses that orchestrate host–pathogen interactions (16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30).
Several mechanisms of control ensure the spatiotemporal deubiquitination of substrates and prevent unrestrained DUB catalysis. This includes mechanisms influencing gene expression levels, protein abundance, folding, and tissue distribution (10, 11, 12, 13, 14, 15). Moreover, DUBs contain a variety of domains and motifs that could be post-translationally modified to regulate their subcellular localization, conformation, protein–protein interaction, and enzymatic activity (10, 11, 12, 13, 14, 15).
In this review, we outline regulatory mechanisms responsible for controlling DUB stability, localization, and enzymatic activity as well as coordinating protein interactions and multiprotein complex assembly. While we provide examples of regulation for key DUBs in multiple families, the tumor suppressor BAP1 is outlined as a model DUB that is subjected to multiple levels of tight regulation. BAP1 is an essential DUB best known for its DUB activity toward histone H2AK119ub and the regulation of chromatin-associated processes (9, 31, 32). BAP1 is mutated in multiple cancers including malignant pleural mesothelioma, uveal melanoma, renal cell carcinoma, and intrahepatic cholangiocarcinoma, rendering this enzyme as the most frequently mutated DUB in human cancers (32, 33).
Regulation of DUBs by intramolecular interactions and self-assembly
Intramolecular interactions and DUB catalysis
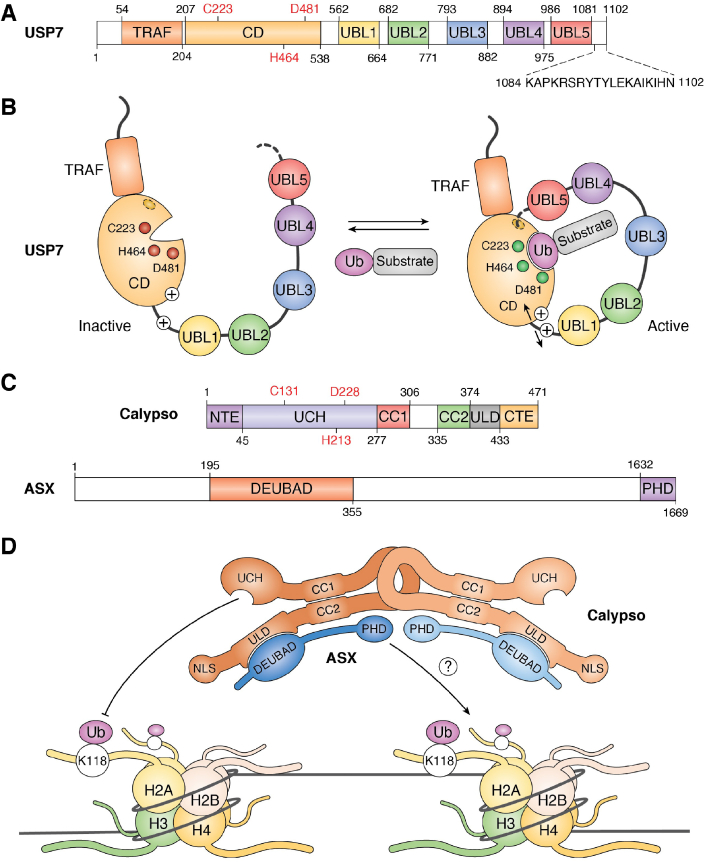
Most DUBs have modular structures and contain diverse domains, nonorganized extensions and motifs, in addition to their catalytic domains. These additional structures can engage in intramolecular and intermolecular interactions and play critical roles in coordinating DUB activity and function (12, 13, 34, 35, 36). Moreover, several DUBs contain one or multiple Ub-binding domains as well as insertions of variable lengths inside their catalytic domains (12, 34, 35). The importance of intramolecular interactions is notably provided by the Ub specific protease 7 (USP7) (also termed Herpes-Associated Ub-Specific Protease (HAUSP)), which contains a TNF receptor-associated factor (TRAF) domain, a catalytic domain in the N-terminal region as well as five Ub-like domains (UBLs) in the C-terminal region (Fig. 2A). As a member of the USP family, USP7 is characterized by architectural features known as the palm, the thumb, and the finger subdomains. While the finger subdomain is mostly associated with Ub-binding, the catalytic triad responsible for hydrolysis is localized at the junction between the palm and the thumb subdomains (35). To enable DUB enzymatic activity, USP7 uses its most distant C-terminal UBL domain to stabilize and coordinate its catalytic site (37, 38, 39) (Fig. 2B). To allow USP7 to switch between active and inactive conformations, this DUB partly relies on a long α-helix, termed the connector helix, that connects the catalytic domain to the first UBL domain (40). Furthermore, linker regions separating the UBL domains permit intramolecular flexibility and rearrangements. Indeed, the binding of Ub to USP7 promotes the localization of the C-terminal tail within an activation cleft located within the catalytic domain of USP7 (Fig. 2B). This event then favors USP7 stabilization into a DUB competent state (37, 38, 39, 41). Interestingly, an additional layer of regulation that involves interacting partners, such as the GMP-synthetase, is likely to be critical for USP7 capacity to target different substrates (42, 43). Indeed, GMP-synthetase interacts with UBL1-2-3 and stabilizes UBL4-5 at the catalytic domain of USP7 (38). Altogether, these results suggest that USP7-mediated deubiquitination is achieved by intramolecular interactions. The multiple domains of USP7 endow this DUB with the functional versatility to deubiquitinate many substrates and regulate various cellular processes including DNA damage signaling, epigenetic control of gene expression, viral infection, and immune response (42, 44).
Figure 2.
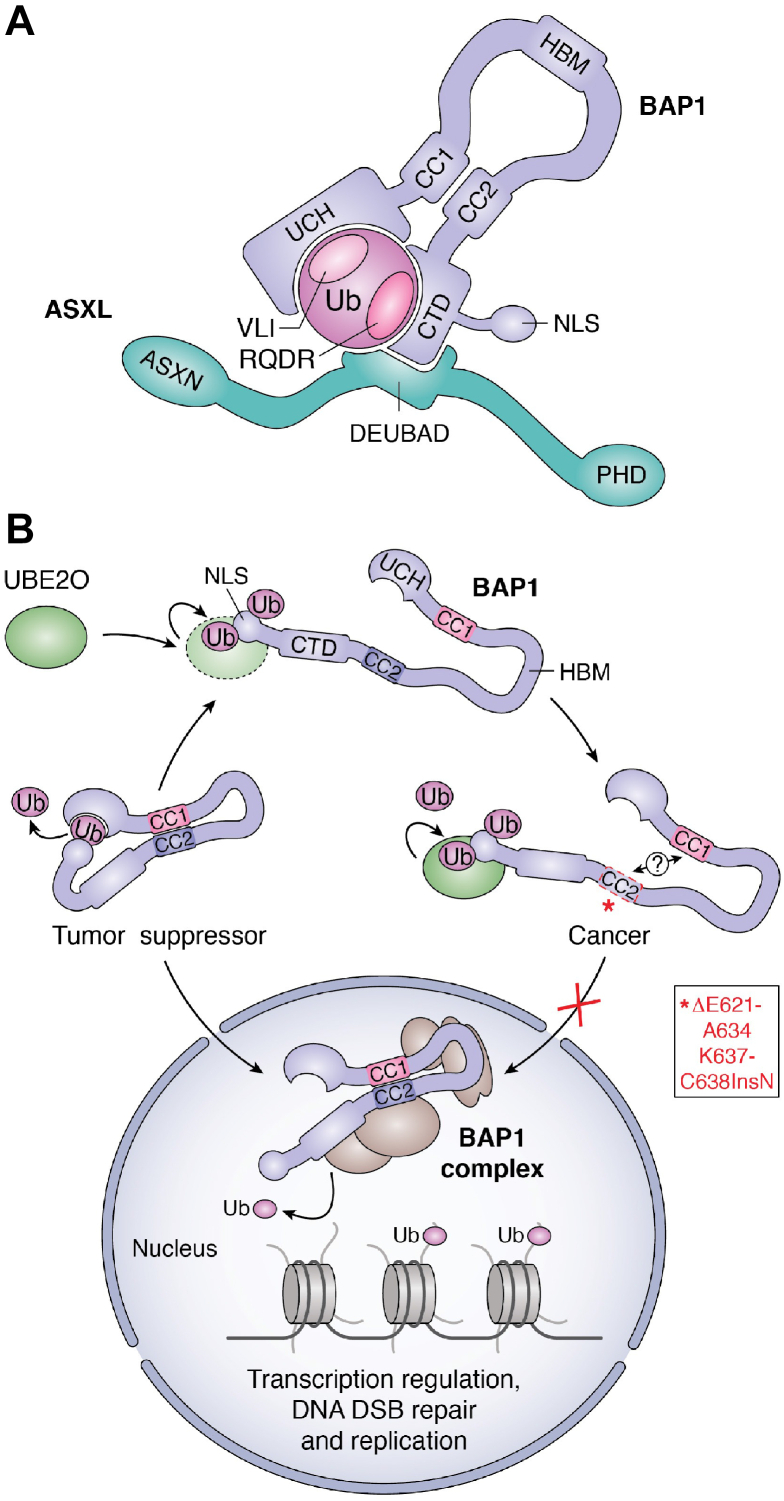
DUB regulation by intramolecular interactions and self-assembly. A, schematic representation of USP7 domain organization and boundaries. USP7 catalytic triad is shown in red as well as the C-terminal tail (K1084 to N1102) required for its activation in black. B, USP7 self-activation. USP7 adopts two conformations respectively associated with an activated and an inactivated state. The switch from the inactive to the active conformation requires USP7 C-terminal tail binding into an activating cavity located in the catalytic domain (CD). This leads to a conformational rearrangement of the UBLs that takes place in the presence of the ubiquitin-conjugated substrate. This rearrangement might also involve a long flexible charged α-helix positioned at the interface between the CD and the UBLs. Once the C-terminal tail is engaged into the activation cleft, the catalytic domain is stabilized and fully active. The “plus” signs show the charged helix. C, schematic representation of Calypso and ASX domain organization and boundaries. Calypso catalytic triad is shown in red. D, Calypso dimerization promotes its recruitment to the nucleosomes. The Drosophila Calypso and ASX proteins form a 2:2 stochiometric complex. This assembly is needed for chromatin recruitment and catalytic activity toward H2AK118ub. ASX, additional-sex comb; CC1/2, coiled-coil 1/2; CD, catalytic domain; CTE, C-terminal extension, DEUBAD, DEUBiquitinase ADaptator; NLS, nuclear localization signal; NTE, N-terminal extension; PHD, plant homeo-domain; TRAF, tumor necrosis factor receptor–associated factor; Ub, ubiquitin; UBL, ubiquitin-like; UCH, ubiquitin carboxy-terminal hydrolase; ULD, UCHL5-like domain.
Intramolecular interactions between distinct DUB domains can also be permanent, as part of the 3D structure of the enzyme. An example of a stable intramolecular interaction is provided by the UCH family DUB BAP1 (45, 46, 47, 48, 49). BAP1 is localized predominantly in the nucleus, as part of a large multiprotein complex (discussed in section 3). BAP1 and its Drosophila ortholog Calypso contain a highly conserved UCH catalytic domain adjacent to a small coiled-coil motif, followed by an insertion in the middle of the protein (termed the nonorganized regions for BAP1), and then a C-terminal domain (CTD) containing a coiled-coil motif adjacent to the nuclear localization signal (NLS) (Fig. 2C). Through coiled-coil motif interactions, the catalytic domain of BAP1/Calypso establishes a stable interaction with the CTD. The UCH–CTD interaction is important for the stimulation of BAP1/Calypso DUB activity by cofactors (45, 46, 47, 48, 49) (Fig. 2D). A similar strategy of 3D organization is employed by UCHL5 (UCH37), a component of the proteasome and the INO80 chromatin-remodeling complex (50, 51, 52, 53, 54, 55). Of note, BAP1 and UCHL5 also use a similar mechanism of regulation by their respective cofactors, involving a distinct domain termed DEUBAD (DEUBiquitinase ADaptor) found in ASXLs, ADRM1 (RPN13), and NFRKB (INO80G) (described in section 3). Overall, these studies assert the importance of intramolecular interactions between the catalytic domain and other DUB domains for the control of enzymatic activity and regulation by cofactors.
DUB oligomerization and catalysis
Another level of DUB regulation involves their oligomerization. For example, USP25 is assembled into a homotetrameric quaternary complex that inhibits its enzymatic activity (Fig. 3) (56, 57, 58). A coiled-coil insertion within the catalytic domain is extended by a disordered sequence that contacts the catalytic site in the tetramer, but not in the active dimer, precluding Ub binding (56, 57, 58). In support of this autoinhibitory mechanism, cancer-associated mutations that disrupt this intermolecular interaction lead to relief from autoinhibition, emphasizing the biological importance of USP25 oligomerization states (57). Currently, it remains unclear whether specific molecular signals, interacting partners, and/or post-translational modifications regulate the oligomerization states of USP25 and hence its enzymatic activity.
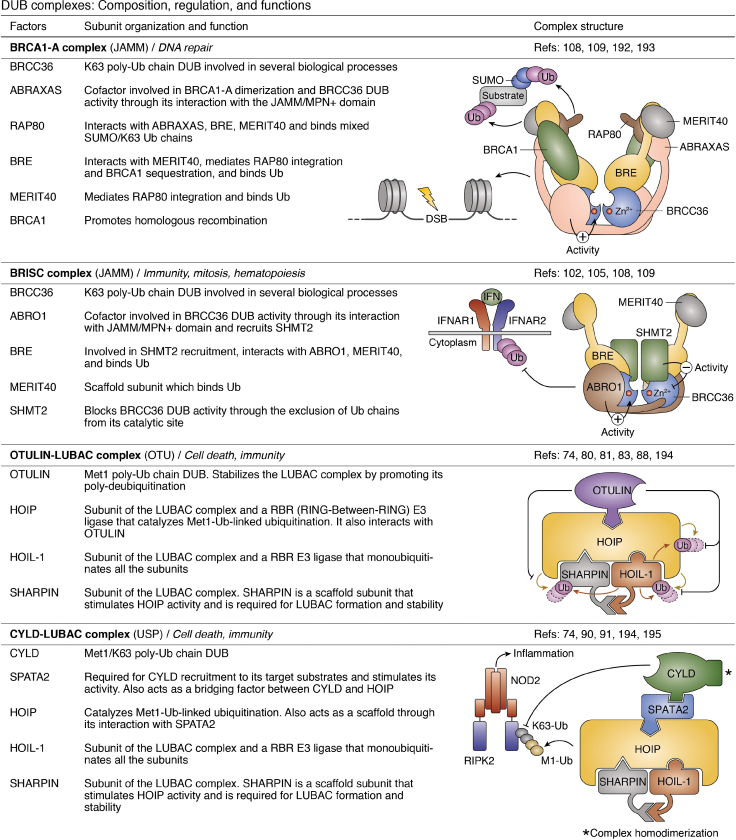
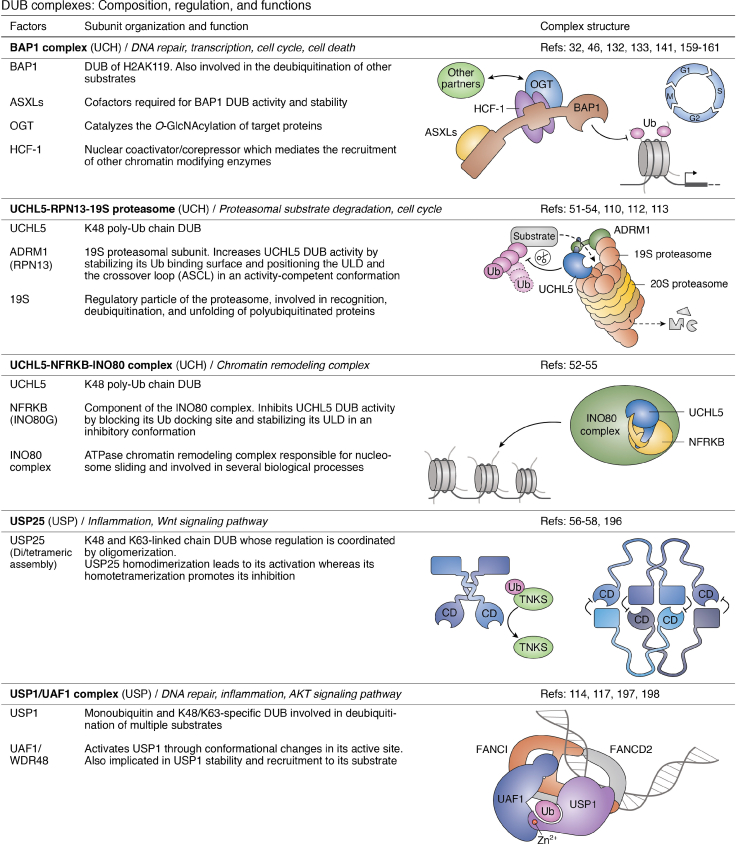
Figure 3.
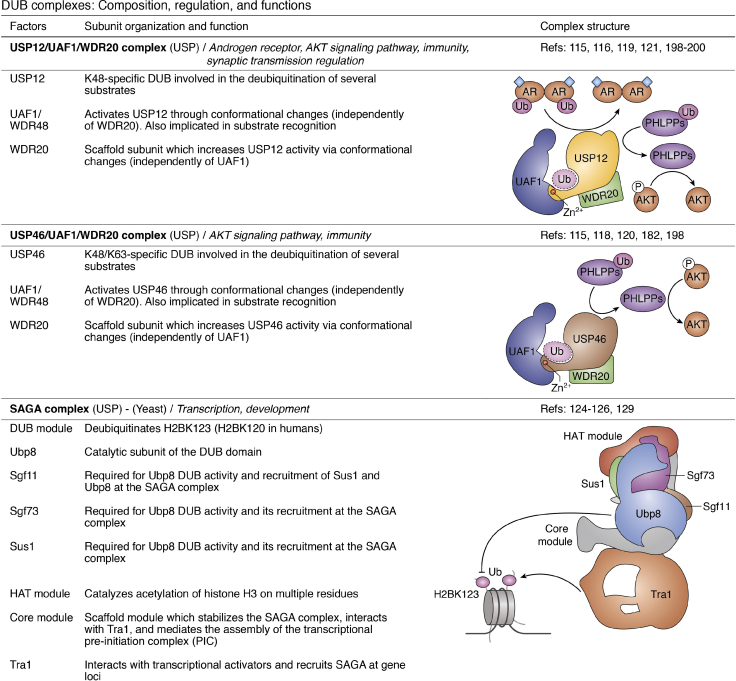
DUB complexes: composition, regulation, and complexes.32,46,51, 52, 53, 54, 55, 56, 57, 58,74,80,81,83,88,90,91,102,105,108, 109, 110,112, 113, 114, 115, 116, 117, 118, 119, 120, 121,124, 125, 126,129,132,133,141,159, 160, 161,182,192, 193, 194, 195, 196, 197, 198, 199, 200 ABRO1, abraxas brother 1; ADRM1, adhesion-regulating molecule 1; AR, androgen receptor; ASXL, additional sex-combs like; BRCA1, breast cancer 1; BAP1, BRCA1-associated protein 1; BRCC36, BRCA1/BRCA2-containing complex subunit 3; BRISC, BRCC36-containing isopeptidase complex; FANCI, Fanconi anemia complementation group I; FANCD2, Fanconi anemia complementation group D2; HAT, histone acetyltransferase; HCF-1, host cell factor 1; HOIP, HOIL-1L interaction protein; IFNAR, interferon alpha and beta receptor; JAMM, JAB1/MPN/MOV34 metalloprotease; LUBAC, linear ubiquitin assembly complex; MERIT40, mediator of RAP80 interactions and targeting subunit of 40 kDa; NFRKB, nuclear factor related to kappaB binding protein; NOD2, nucleotide binding oligomerization domain containing 2; OGT, O-linked N-acetylglucosamine transferase; PHLPP, PH domain leucine-rich repeat protein phosphatase; RAP80, receptor-associated protein 80; RBR, RING between RING; SHMT2, serine hydroxymethyltransferase 2; RIPK2, receptor interacting serine/threonine kinase 2; SAGA, Spt-Ada-Gcn5 acetyltransferase; Sgf11/73, SAGA-associated factor 11/73; Tra1, transcription-associated protein 1; TNKS, tankyrase; UAF1, USP1 associated factor 1; UCH, ubiquitin C-terminal hydrolase; USP, ubiquitin-specific protease; WDR20, WD repeat domain 20.
While the example of USP25 indicates how oligomerization induces DUB autoinhibition, self-assembly can also promote DUB activity. The Drosophila ortholog of BAP1, Calypso, was recently shown to undergo dimerization (48). While this assembly is not directly required for stimulating catalytic activity, it favors the recruitment of this DUB to chromatin, whereby it can access and deubiquitinate histone H2Aub. The dimerization of Calypso requires the coiled-coil regions that are also conserved in human BAP1. Interestingly, Calypso dimerization would simultaneously position the two UCH domains near the two-ubiquitination sites of the H2A dimer within the nucleosome (Fig. 2D). Thus, Calypso/BAP1 interaction with nucleosomes and subsequent deubiquitination of H2Aub appear to be highly coordinated. Nonetheless, further studies are needed to determine how BAP1 dimerization regulates its DUB activity in vivo. In particular, it will be worthwhile to define how transcription factors and chromatin-associated proteins cooperate with the BAP1 dimer to ensure timely deubiquitination of H2AK119ub at defined genomic regions.
In summary, DUBs can undergo oligomerization, regulating intrinsic DUB activity and access to substrates, thus providing an important level of regulation. The extent to which transient or stable oligomerization can be generalized to the majority of DUBs and whether this influences their functions remain to be determined.
DUB action through diverse modes of association with E2-conjugating and E3 ligases
DUB-mediated inhibition of ubiquitination by diverting E2s from E3s
DUBs do not necessarily act only following the action of E2 Ub-conjugating enzymes and E3 Ub ligases to terminate ubiquitination reactions (10, 11, 12, 13, 14). Indeed, DUBs can actively participate, in conjunction with E2 and E3 enzymes, to orchestrate Ub-signaling events. For instance, an intricate relationship between DUBs and E2s is exemplified by OTUB1, a DUB involved in DNA damage signaling and immune regulation. OTUB1 inhibits chromatin-associated ubiquitination events mediated by the E3 Ub ligase RNF168, which occur during the cellular response to DNA double-strand breaks (59). Mechanistically, OTUB1 interacts with and inhibits several E2 Ub-conjugating enzymes, including UBC13 and UBCH5. These events are mediated in a DUB catalytic activity–independent manner and result in the inhibition of Ub chain elongation. This occurs through binding of the DUB to E2∼Ub thioester intermediates, thus diverting them from their cognate E3 Ub ligases (59, 60, 61). In addition, OTUB1-E2 interactions stimulate OTUB1-mediated cleavage of K48-linked Ub chains through conformational changes of this DUB. Interestingly, this is further modulated by E2 charging and free Ub, providing a potential mechanism for the coordination of signaling processes with Ub metabolism (60, 62).
DUBs also assemble into multiprotein complexes wherein they simultaneously interact with both E2 Ub-conjugating enzymes and E3 Ub ligases. For instance, the DUB Ataxin3 (AT3), whose polyglutamine expansion underlies the Machado–Joseph neurodegenerative disorder (63), interacts with UBC7 and UBCH7 E2 Ub-conjugating enzymes as well as the E3 Ub ligase Parkin. Parkin promotes autophagy-mediated clearance of damaged mitochondria, and its mutation or deregulation is also involved in the pathogenesis of Parkinson’s disease (64, 65). AT3 deubiquitinates Parkin and their interaction is strongly promoted by Parkin auto-ubiquitination. AT3 subsequently stabilizes an unproductive UBCH7-Parkin complex that limits Parkin autoubiquitination, possibly through inhibition of the E2 release from Parkin and its subsequent charging by Ub. In addition, the E2-mediated transfer reaction might be diverted toward Ub ligation to AT3 itself (64, 65). While the significance of AT3 interactions with E2s/Parkin remains incompletely understood, the above findings emphasize the intricate partnerships between Ub ligation and deubiquitination in fine-tuning Ub-signaling events.
Consistent with the variety of cellular processes in which DUBs limit the access of E3s to cognate E2s, the zinc finger DUB A20 (also known as tumor necrosis factor, alpha-induced protein 3) constitutes an important regulator of inflammation. A20 negatively regulates the nuclear factor kappa B (NF-κB) and inflammation through multiple mechanisms (66, 67, 68, 69), including the targeting of receptor-interacting protein 1 of the TNF receptor signaling pathway for proteasomal degradation through its own E3 Ub ligase activity (67). A20 blocks the interactions between the E3 Ub ligases TRAF6, TRAF2, and cIAP1 and the corresponding E2 Ub-conjugating enzymes UBC13 and UBCH5C. This ensures the targeting of the latter enzymes for proteasomal degradation (69).
In summary, in addition to Ub removal from proteins, DUBs can also limit substrate ubiquitination by interfering with E2s and E3s, providing additional means to tightly regulate ubiquitin-signaling events.
DUBs modulating ubiquitination through direct interaction with E3s
A notable example of direct DUB-E3 coordination is provided by the LUBAC (linear Ub chain assembly complex) E3 Ub ligase, which is known to interact with two DUBs; OTULIN (OTU DUB with linear linkage specificity) and CYLD (cylindromatosis). LUBAC is an E3 Ub ligase complex composed of three interacting partners: SHARPIN (Shank-associated RH domain-interacting protein), HOIP (HOIL-1-interacting protein), and HOIL-1 (heme-oxidized IRP2 Ub ligase-1) (Fig. 3) (70, 71, 72, 73, 74). It functions as a linear Met1-Ub chain conjugating E3 complex that regulates innate immune signaling, notably by promoting NF-κB activation (72, 75, 76, 77, 78). HOIP is activated through association with its interacting partners, which triggers the ligation of linear Ub chains on receptor-interacting protein 1 (RIPK1) and NEMO (IKKγ), two downstream activators of NF-κB signaling (72, 73, 77, 79). Furthermore, HOIL-1 catalyzes the monoubiquitination of LUBAC subunits, which primes for HOIP-mediated linear Ub chain extension, therefore dampening LUBAC activity (80). Meanwhile, OTULIN specifically cleaves the Met1-Ub chains generated by LUBAC (81, 82). The PIM (PUB-interaction motif) domain of OTULIN interacts specifically with the PUB domain of HOIP to ensure DUB-E3 interaction (83, 84). Strikingly, while several studies have shown that OTULIN could act on LUBAC-regulated substrates to promote or inhibit downstream signaling (81, 85, 86, 87), this DUB seems to act preferentially on components of the LUBAC itself, preventing its autoubiquitination and leading to an increase in its E3-ligase activity (88). It is possible that cell-type and cellular contexts as well as potential Ub-independent functions of OTULIN might account for the apparent discrepancies (89). Thus, further studies are required to fully establish how OTULIN regulates LUBAC-associated signaling events and related cellular processes.
CYLD, which acts specifically toward Met1-Ub and poly-Ub K63 chains, binds indirectly to LUBAC through spermatogenesis-associated protein 2 (SPATA2) (90). SPATA2 is not only required for CYLD recruitment to LUBAC and its substrates (TNFR1 and NOD2) but also for CYLD activation, thus ensuring a spatiotemporal regulation of this DUB (Fig. 3) (90). Furthermore, in order to bind SPATA2, it seems that CYLD needs to be in a dimeric conformation, thereby forming a heterotetrameric complex (90). Surprisingly, a mechanism of interaction similar to that used by OTULIN is also at play. Indeed, a PUB–PIM interaction is engaged with SPATA2, which bridges HOIP with CYLD. Thus, CYLD and OTULIN are mutually exclusive for their interaction with LUBAC and might be finely regulated to exert their functions (90, 91). It will be interesting to define how these DUBs are dynamically interchanged on LUBAC, depending on cellular contexts, and how this coordination regulates cell survival and inflammation.
Finally, it is worth mentioning that DUBs can act cooperatively to stabilize and promote the activity of E3 Ub ligases. For example, the ubiquitin-specific proteases USP7 and USP11 interact with and stabilize several components of the PRC1 complex, the E3 Ub ligase responsible for H2AK119 monoubiquitination, and transcriptional repression of polycomb group target genes, including the INK4a tumor suppressor (92). Remarkably, depletion of USP7 also influences the association of USP11 with chromatin, suggesting an intimate relationship between these two DUBs in PRC1 regulation (92).
In summary, DUBs can dynamically interact with E3 Ub ligases, and this can affect their own enzymatic activity as well as the protein stability or activity of E3 Ub ligases. This not only adds a layer of selectivity for the ubiquitination of substrates but also allows the fine-tuning of signaling events.
Regulation of DUBs through assembly into large heteromeric and multienzymatic complexes
DUB assembly in mutually exclusive complexes
Several DUBs are assembled into large stable multiprotein complexes found in several tissues and subcellular compartments (12, 13, 35) (Fig. 3). Indeed, a systematic purification and mass spectrometry identification of DUB complexes and interacting partners indicated that these enzymes are associated with a plethora of proteins and enzymes covering a wide spectrum of cellular functions and processes (93). Association into large complexes endows DUBs with additional interaction interfaces to regulate substrate recruitment and modulation of enzymatic specificity and activity. BRCC36 provides a prominent example of a DUB assembled in two distinct complexes in a mutually exclusive manner. BRCC36 is a member of the JAMM family DUBs that exhibit a preference for K63-linked Ub chains (94). BRCC36 is found in the BRCA1-A complex and the BRCC36 isopeptidase complex (BRISC), which are involved in DNA repair and immune signaling, respectively (95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106) (Fig. 3). Structural studies indicate that BRCC36 and ABRAXAS2 or ABRO1 form heterodimers, which ensure enzymatic activity and association with additional cofactors including RAP80 and BRCA1 (in the BRCA1-A complex) or the serine hydroxymethyltransferase 2 (SHMT2) (in the BRISC complex) (107, 108, 109). Of note, the dimeric form of SHMT2 interacts with the BRISC complex and blocks the catalytic site of BRCC36, thereby preventing deubiquitination. However, SHMT2 can also undergo tetramerization, which results in its dissociation from BRISC and the subsequent activation of BRCC36 (106). Thus, BRCC36 exemplifies how a DUB can be regulated by its interacting partners to mediate distinct cellular processes. Nonetheless, several questions await further studies to fully establish how BRCC36 is regulated. In particular, it remains unknown the manner by which BRCC36 molecules are allocated to nuclear (BRCA1-A) or cytoplasmic (BRISC) complexes. In addition, how BRCC36-mediated deubiquitination is coordinated with other functions of its associated complexes also remains incompletely understood.
Similar to BRCC36, UCHL5 is a component of two distinct multiprotein assemblies. Indeed, UCHL5 interacts, in a mutually exclusive manner, with the proteasome or the INO80 ATPase chromatin remodeling complex (Fig. 3) (51, 52, 53, 54, 110, 111). UCHL5 uses its ULD (UCHL5-like domain) to interact with the DEUBAD domain of the proteasome subunit RPN13, promoting a conformational change that activates UCHL5 (53, 55). Notably, the active site cross-over loop, normally localized above the catalytic cysteine inside the Ub-binding site of UCHL5, is repositioned to allow deubiquitination (53, 55). Functionally, UCHL5 recruitment to the proteasome ensures poly-Ub chain debranching, enhancing proteasomal degradation, and promoting proper cell cycle progression (112, 113). Additionally, UCHL5 can also bind the DEUBAD of NFRKB, a subunit of the INO80 chromatin-remodeling complex (52, 53, 55). However, in contrast to RPN13, NFRKB inhibits UCHL5 DUB activity. A spatial rearrangement of an NFRKB loop with the ULD and UCH domains prevents substrate docking and deubiquitination (53, 55).
DUBs can also compete for specific partners. For instance, UAF1 (USP1-associated factor 1), first identified as a factor that activates USP1 DUB activity (114), was later shown to interact with and activates, in a mutually exclusive manner, several DUBs including USP12 and USP46 (Fig. 3) (115, 116, 117, 118, 119). UAF1 contains three domains with an N-terminal β-propeller domain, a central ancillary domain, and a C-terminal SUMO-like domain. The β-propeller domain of UAF1 binds to the distal end of the USP finger and promotes structural rearrangements in the Ub-binding site, increasing its overall activity. Adding more complexity, USP12 and USP46 display a high degree of homology (88%) and can both interact with an additional coactivator named WDR20 (116, 119, 120, 121, 122). Composed of multiple WD40-repeat motifs, WDR20 interacts with the back of the finger and the palm subdomains of USP12/46, which triggers conformational modifications in the catalytic center of these DUBs. These events enhance the activity of USP12 and USP46, independently of UAF1 (119, 120). Thus, UAF1 and WDR20 represent allosteric activators that mediate, through multiple structural rearrangements, the synergistic activation of USP12 and USP46. Of note, both UAF1 and WDR20 are subjected to multiple post-translational modifications, including phosphorylation and ubiquitination (PhosphoSite database (123)), but the significance of these modifications with respect to modulation of DUB activity remains to be established.
DUB assembly in multiprotein complexes with distinct enzymatic activities
DUBs can also be integrated into large multiprotein complexes with several subcomplex modules. This is the case for the highly conserved Spt-Ada-Gcn5 acetyltransferase (SAGA) transcription coactivator complex, which has multiple structural and functional modules including a core module, a histone acetyltransferase module and a DUB module (Fig. 3) (124). In yeast, the SAGA DUB module is composed of the DUB Ubp8 and three subunits, Sgf11, Sus1, and Sgf73, that are all required for deubiquitination of H2BK123 (K120 in mammals), a modification associated with gene transcription by RNA polymerase II (124, 125, 126, 127). Indeed, the four subunits establish extensive contacts with each other, illustrating the importance of complex assembly for DUB activity (125, 126). In addition, the DUB module creates contacts with nucleosomes through the zinc finger domain of Sgf11 which interacts with the H2A/H2B acidic patch, while Ubp8 forms contacts with H2B and Ub (125, 126). Moreover, structural studies indicate that positioning of the multiple SAGA modules within the supercomplex ensure coordinated deubiquitination and acetylation of nucleosomes to promote gene transcription (125, 128, 129, 130, 131). Thus, the SAGA multiprotein complex provides a notable example of how DUB enzymatic activity is tightly controlled by associated proteins. In addition, it also shows how a DUB, as being part of a multifunctional complex, can exert its function in concert with additional activities.
The DUB BAP1 assembles several multiprotein complexes containing diverse chromatin-associated factors (Fig. 3). These include the additional sex combs–like proteins (ASXL1/ASXL2/ASXL3), the host cell factor 1, the O-linked N-acetylglucosamine transferase, and the lysine demethylase KDM1B/LSD2 (93, 132, 133). While host cell factor 1, O-linked N-acetylglucosamine transferase, and KDM1B are known regulators of transcription and other chromatin-associated processes (134, 135, 136, 137, 138, 139, 140, 141), their exact coordination with BAP1 DUB activity remains incompletely understood. Nonetheless, BAP1 might deubiquitinate and stabilize these cofactors or their associated proteins to regulate transcription (140, 141). BAP1 also associates with transcription factors, including FOXK1, FOXK2, and Yin Yang1 (YY1), which ensure the recruitment of this DUB to chromatin (133, 142). Of interest, BAP1 forms mutually exclusive complexes with ASXLs, which play important roles in maintaining BAP1 stability and promoting its DUB activity. Notably, similar to the RPN13-UCHL5 interaction, these factors also use their DEUBAD to interact with the CTD of BAP1 (see above). These interactions are necessary for histone H2AK119ub deubiquitination and transcription regulation by this DUB. The UCH and CTD domains of BAP1 as well as the DEUBAD form a composite Ub-binding interface that interacts with the hydrophobic and the charged patches of ubiquitin, thus ensuring catalysis (Fig. 4A) (46). Notably, cancer-associated mutations of BAP1 that inhibit its interaction with ASXLs also engender abrogation of its DUB activity toward H2AK119ub and reduce cell proliferation. How BAP1 activity is dynamically controlled by its partners in diverse ASXL complexes and contexts remains a fundamental question with respect to understanding the regulation of this tumor suppressor.
Figure 4.
Regulation of BAP1 by intramolecular interactions and self-deubiquitination. A, BAP1 intramolecular interactions are required for its ASXLs-mediated regulation. Interactions between the UCH-CC1 and the CTD domains of BAP1 as well as between the CTD and the DEUBAD of ASXLs are essential for the formation of the CUBI (composite ubiquitin-binding interface). This composite interface enables binding of the VLI hydrophobic patch and the RQDR charged patch of ubiquitin to BAP1 UCH domain and CTD, respectively. This assembly results in the activation of BAP1 DUB activity. B, regulation of BAP1 by self-deubiquitination. UBE2O is a E2 Ub-conjugating-E3 Ub ligase hybrid enzyme that multimonoubiquitinates BAP1 in its NLS region. The CC2 region of BAP1 interacts with the UCH-CC1 domains. This event brings the NLS close enough to the UCH catalytic domain facilitating NLS deubiquitination. BAP1 is then able to translocate into the nucleus and act as a tumor suppressor. Cancer mutations localized in the CC2 domain promote BAP1 association with UBE2O and lead to the disruption of BAP1 self-deubiquitination. As a consequence, BAP1 is sequestered in the cytoplasm. BAP1, BRCA1-associated protein 1; CC1/2, coiled-coil 1/2; CTD, C-terminal domain; DEUBAD, DEUBiquitinase ADaptator; HBM, HCF-1 binding motif; NLS, nuclear localization signal; PHD, plant homeo-domain; Ub, ubiquitin; UCH, ubiquitin carboxy-terminal hydrolase.
In summary, DUB function is intimately orchestrated by interacting partners that regulate protein stability or activity. Several DUBs are integrated into large complexes with multiple functions. However, how DUBs are regulated within such complexes in response to developmental, physiological, or stress-associated signaling remains an area of active investigation.
Regulation of DUBs by ubiquitination and self-deubiquitination
Regulation of DUB catalytic activity by ubiquitination
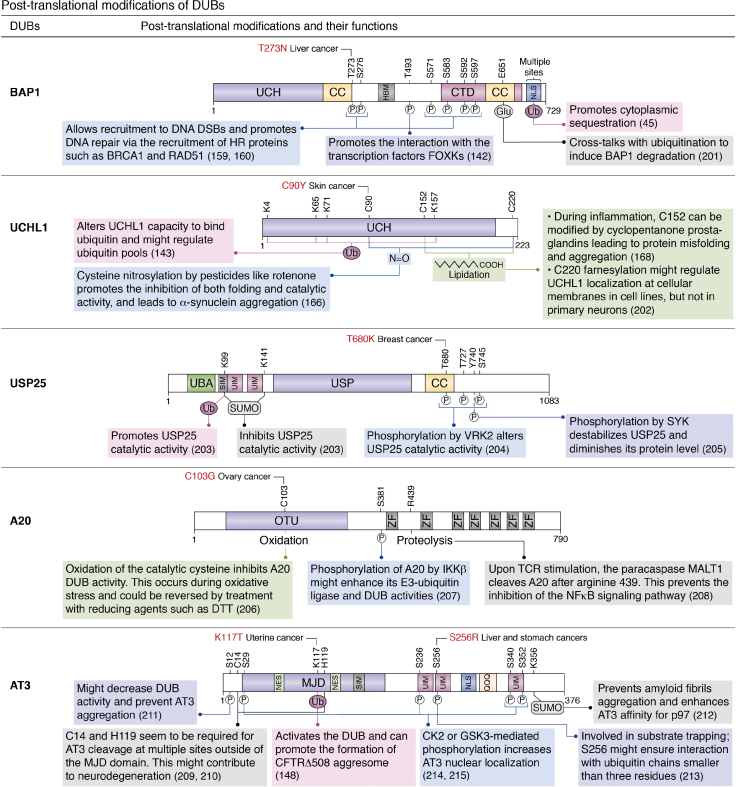
Several DUBs were shown to be regulated by ubiquitination targeting the catalytic domain or other regions of the enzyme. The biological significance and exact mechanisms controlling these ubiquitination events are far from being fully established. Nonetheless, over the years, valuable information has been gained regarding the regulation imposed by this post-translational modification on interactions and activity of specific DUBs. For instance, UCHL1 is multimonoubiquitinated in its catalytic domain, blocking its capacity to bind Ub and substrates (Fig. 5) (143). Importantly, mutation of residues that abolish Ub-binding by UCHL1 also inhibits its monoubiquitination. This intriguing dependency of DUB ubiquitination on Ub-binding suggests that there may exist an intermediate step of noncovalent Ub transfer occurring between the catalytic domain of UCHL1 and the E2-E3 complex. Additionally, mutation of the catalytic cysteine results in increased monoubiquitination of UCHL1, suggesting that its ubiquitination state is reversed by self-deubiquitination through intramolecular interactions. Thus, it is possible that the equilibrium between ubiquitination and deubiquitination regulates UCHL1 DUB activity to ensure a tight control of its activity toward substrates (143).
Figure 5.
Post-translational modifications of DUBs.45,142,143,148,159,160,166,168,201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215 AT3, ataxin3; BAP1, BRCA1-associated protein 1; CC, coiled-coil; CFTR, cystic fibrosis transmembrane conductance regulator; CK2, casein kinase 2, CTD, C-terminal domain; DTT, dithiothreitol; DUB, deubiquitinase; FOXK, forkhead box class K; Glu, glutamylation; GSK3, glycogen synthase kinase 3; HBM, HCF-1 binding motif; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MJD, Machado–Joseph deubiquitinases; NFκB, nuclear factor kappa B; NES, nuclear exporting signal; NLS, nuclear localization signal; P, phosphorylation; QQQ, poly-Q region; SUMO, SUMOylation; SIM, SUMO-interacting motif; Ub, ubiquitin; UBA, Ub-associated domain; UCH, Ub-C terminal hydrolase; UIM, Ub-interacting motif; USP, ubiquitin carboxyl-terminal hydrolase; ZF, zing finger.
The DUB AT3 described above (section 2A) is also associated with ubiquitination-dependent quality control mechanisms (Fig. 5) (144, 145, 146). AT3 is constitutively ubiquitinated near the catalytic site, and this ubiquitination state is increased following proteasome inhibition or when the unfolded protein response is induced by treatment with dithiothreitol. The ubiquitinated form of AT3 is more effective in cleaving Ub chains, indicating that Ub modification increases AT3 catalytic activity (147, 148). AT3 ubiquitination might thus constitute a feedback mechanism that links Ub metabolism to DUB activity. However, AT3 regulation appears to be more complex, as the catalytic activity of this DUB also regulates its own stability and cellular localization (149, 150). While the significance of these events remains unclear, they suggest that AT3 ubiquitination plays an important role in controlling its activity and stability.
Regulation of DUB localization by ubiquitination
Self-deubiquitination of BAP1 provides a notable example of DUB regulation at the level of subcellular localization (Fig. 4B). The E2-conjugating/E3-ligase hybrid UBE2O is a substoichiometric component of BAP1 complexes (93, 132, 133). UBE2O monoubiquitinates multiple lysines on the BAP1 NLS, thereby inducing its cytoplasmic sequestration (45). These ubiquitination events are actively counteracted by BAP1 self-deubiquitination which, importantly, could only be observed when the BAP1 UCH domain interacts with the CTD (Fig. 4B). Indeed, the UCH–CTD interaction brings the NLS of BAP1 close enough to the catalytic domain to promote self-deubiquitination (45). Thus, UBE2O-mediated ubiquitination of BAP1 might constitute a quality control mechanism that prevents the nuclear import of an improperly folded DUB. Consistent with this notion, it was later found that UBE2O targets orphan proteins that fail to assemble into their cognate multiprotein complexes (151, 152). It will therefore be interesting to determine whether similar mechanisms of regulation target other DUBs, safeguarding their proper folding, localization, and stabilization.
Regulation of DUBs by other post-translational modifications
Regulation of DUB activity and function by phosphorylation
Owing to their involvement in controlling a wide spectrum of cellular processes, DUBs are regulated by other post-translational modifications including phosphorylation, acetylation, limited proteolysis, hydroxylation, and oxidation (11, 153). Selected examples of DUBs indicate the diversity of post-translational modifications that regulate these enzymes (Fig. 5). Phosphorylation is widespread in eukaryotes and regulates a large variety of cellular signaling processes. Several examples demonstrate how phosphorylation regulates DUB stability, activity, or recruitment, further reinforcing the notion that phosphorylation-mediated signaling is intimately associated with deubiquitination. For instance, the stability of USP4, a DUB that regulates transforming growth factor-β signaling, is regulated by phosphorylation (154). AKT-mediated phosphorylation of USP4 prevents its ubiquitination and promotes its accumulation at the plasma membrane, thereby stabilizing transforming growth factor-β receptor and downstream signaling in the regulation of epithelial to mesenchymal transition (154). Another example of tight control of DUB stability by phosphorylation is observed for USP7, which deubiquitinates and stabilizes the E3 Ub ligase MDM2. Phosphorylation of USP7 by casein kinase 2 promotes its stability, thereby maintaining higher levels of MDM2. This, in turn, promotes p53 degradation, hence maintaining this transcription factor at basal protein levels (155). However, in response to genotoxic stress, dephosphorylation of USP7 by PPM1G results in its destabilization, leading to MDM2 degradation, stabilization of p53, and upregulation of the p53 transcriptional response (155).
Phosphorylation can also directly regulate DUB enzymatic activity. For instance, phosphorylation of OTUD5 catalytic domain induces conformational changes thereby promoting substrate binding and facilitating deubiquitination (156). Another interesting example is provided by OTUD4 whose phosphorylation occurs near the catalytic domain. OTUD4 phosphorylation, in conjunction with an adjacent Ub-binding domain, promote deubiquitination of K63-linked Ub chains. This mechanism is used by OTUD4 to target MyD88 for deubiquitination and to dampen NF-κB activation in response to Toll-like receptor signaling (157). The DUB OTULIN (described above) can also be subjected to phosphorylation events. Phosphorylation of OTULIN tyrosine 56 (Y56P) in the PIM domain prevents its binding with HOIP-LUBAC, therefore impairing OTULIN-mediated LUBAC regulation (83). During genotoxic stress, this phosphorylation event seems to be required for the association with and deubiquitination of β-catenin. This, in turn, leads to the noncanonical activation of the Wnt signaling pathway (158).
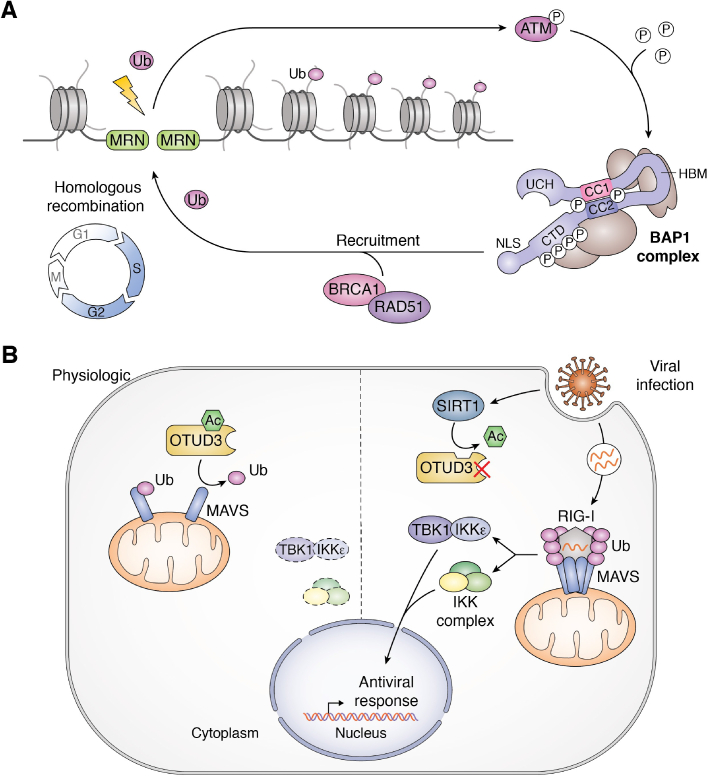
Phosphorylation is also critical for the BAP1-mediated response to genotoxic stress. BAP1 promotes the recruitment of BRCA1 and RAD51 to sites of DNA double-strand breaks (DSBs) and promotes homologous recombination–mediated DNA repair (9, 32, 159, 160, 161). Moreover, BAP1 is directly recruited to genomic regions in the vicinity of DSBs, which promotes the deubiquitination of H2AK119ub on the chromatin (9, 32, 159, 160, 161) (Fig. 6A). BAP1 is phosphorylated on six serine/threonine residues following ionizing radiation and mutation of these residues inhibits BAP1 recruitment to DSB sites. However, whether BAP1 phosphorylation involves DUB conformational changes or recruitment of additional proteins remain to be determined.
Figure 6.
DUB regulation by post-translational modifications. A, phosphorylation of BAP1 during the DNA damage response. Upon ionizing-irradiation, DNA double-stand breaks (DSBs) are recognized by the MRN complex resulting in ATM activation. ATM then phosphorylates BAP1 leading to its recruitment at the DSB sites. In turn, BAP1 recruits homologous recombination (HR) proteins such as BRCA1 and RAD51 to promote HR. B, acetylation of OTUD3 in innate immunity. Under normal conditions, OTUD3 is acetylated on its lysine 129 (K129-Ac). K129-Ac enhances OTUD3 DUB activity toward the poly-K63 ubiquitin chains of the immune protein MAVS. This mechanism prevents the aggregation of MAVS and activation of the innate immune response. However, upon viral infection, the deacetylase SIRT1 is recruited to OTUD3 to catalyze its deacetylation and inactivation. As a consequence, MAVS becomes polyubiquitinated and interacts with RIG-I, leading to its aggregation. This ultimately leads to the activation of downstream immune signaling pathways involved in the antiviral response. ATM, ataxia telangiectasia mutated; BAP1, BRCA1-associated protein 1; IKK, IκB kinase; MAVS, mitochondrial anti-viral signaling protein; MRN, MRE11/RAD50/NBS1; OTUD3, OTU-domain containing protein 3; RIG-I, retinoic acid-inducible gene I; SIRT1, sirtuin 1; TBK1, TANK-binding kinase 1; Ub, ubiquitin.
Altogether, the studies described above emphasize the versatility of phosphorylation in regulating the activity of DUBs and their interaction with other factors. In addition, a survey of the DUB repertoire of phosphorylation sites (PhosphoSite database) shows that DUBs are extensively phosphorylated on multiple domains. This suggests that an intricate crosstalk between multiple signaling pathways tightly orchestrates DUB function.
Regulation of DUB activity and function by acetylation
Another prominent example of DUB regulation by post-translational modifications is illustrated by acetylation of OTUD3 in the context of innate immunity surveillance (162). Following infection by RNA viruses, the mitochondrial antiviral-signaling protein MAVS undergoes K63-linked polyubiquitination, which is essential for its multimerization and activation of the host antiviral response (163, 164, 165). Remarkably, acetylation of OTUD3 on lysine 129 (K129) triggers deubiquitination of K63-polyubiquitination chains on MAVS thereby blocking the antiviral response (162) (Fig. 6B). While OTUD3 acetylation promotes its DUB activity, its deacetylation by SIRT1 inhibits MAVS deubiquitination following viral infection, thus promoting activation of innate immune signaling (162). K129 is found in the variable loop of the OTU catalytic domain, i.e., within an acetylation motif conserved throughout OTUD3 orthologues. Acetylation of this residue abolishes its positive charge, promoting substrate binding. Thus, OTUD3 provides a noteworthy example of direct regulation of DUB catalytic activity by acetylation, with important implications for the antiviral response.
Together, these investigations emphasize the versatility of phosphorylation or acetylation in mediating DUB regulation and suggest that an intricate crosstalk might take place between signaling pathways in order to provide a strict regulation of DUB function.
Regulation of DUB by atypical post-translational modifications
In addition to mechanisms involving classical post-translational modifications, other biochemical processes that coordinate DUB activity have been proposed. For instance, UCHL1, a DUB highly expressed in the brain and whose deregulation is associated with neurodegeneration, is subjected to nitrosylation (166). Moreover, its catalytic activity might be affected under conditions of pronounced oxidative stress (Fig. 5). Notably, in vitro studies indicated that oxidation of the three cysteines C90, C152, and C220 inhibits the enzyme and induces significant conformational changes that prevent Ub-recognition and, hence, removal from substrates (166). In addition, C152, within the substrate recognition cross-over loop, might act as a scavenger of reactive oxygen species, possibly protecting the catalytic cysteine, although this needs further demonstration in vivo (167). Finally, UCHL1 could also be modified by lipidation (Fig. 5). Under inflammatory conditions, C152 could be subjected to the covalent ligation of cyclopentanone prostaglandins, causing DUB misfolding and neurotoxicity (168, 169).
Consistent with the variety of post-translational modifications regulating DUBs, USP1 is regulated by autocleavage-mediated inactivation during exposure to ultraviolet radiations (170). In particular, USP1 contains an internal Ub-like diglycine (Gly-Gly) motif, which is essential for DUB recognition and catalysis. The autocleavage of USP1 permits the accumulation of monoubiquitinated PCNA and execution of translesion synthesis across UV-induced pyrimidine dimers (170). Of note, USP1 is also regulated by reversible oxidation of cysteine upon oxidative stress, a mechanism proposed to promote PCNA ubiquitination in response to oxidative DNA damage (171). Finally, the DUB Cezanne (OTUD7B) is hydroxylated by the asparaginyl β-hydroxylase factor inhibiting HIF1 (FIH1) within a domain similar to the Ub-associated domain, and this modification blocks Ub-binding, although the significance of this event remains unknown (172).
In summary, the above examples illustrate the relevance of post-translational modifications and their crosstalk in the dynamic regulation of DUB function.
Concluding remarks
As outlined in this review, the proper regulation of Ub signaling is of crucial importance for the maintenance of cellular homeostasis. Indeed, humans express more than 600 E3-Ub ligases and about 100 DUBs that regulate thousands of Ub-modified sites (173, 174, 175). A substantial body of evidence now indicates that DUBs mediate multiple, highly orchestrated mechanisms to counterbalance ubiquitination events and regulate Ub signaling. We have summarized diverse control systems that regulate the catalytic activity of DUBs as well as their interaction with E3 ligases and substrates. Deregulation of DUB function is therefore expected to profoundly perturb cellular processes. Indeed, mutation of DUB genes or dysfunction of their protein homeostasis are increasingly associated with major pathologies such as neurodegenerative diseases (USP25, UCHL1, OTUB1, and AT3) (176, 177, 178, 179, 180, 181), cancers (BAP1, CYLD, USP46, USP28, and PSMD14) (32, 182, 183, 184, 185, 186) and inflammation (A20, CYLD, USP7, and USP47) (187, 188, 189, 190, 191). To properly target these enzymes in the clinic, several wide-ranging questions remain to be addressed: (i) What is the full spectrum of substrates modified by each DUB? (ii) What is the complete DUB interactome and how dynamic is it? (iii) To what extent ubiquitination and deubiquitination reactions are spatiotemporally interconnected? and (iv) How the inhibition of DUBs affects physiological processes at the organismal level? Undoubtedly, with the development of new tools and biotechnologies, there are many exciting prospects for the upcoming decades.
Conflicts of interest
The authors declare that they have no conflicts of interest with the content of this article.
Acknowledgments
We thank Dr Nathalie Labrecque and Dr Elliot Drobetsky for comments and suggestions on the manuscript.
Author contributions
B. E., C. M., and E. B. A conceptualization; B. E. and C. M. data curation; E. B. A. writing-original draft; B. E., C. M., M. E., C. E. R., E. M., and E. B. A. writing-review and editing.
Funding and additional information
This work was supported by the Canadian Institutes of Health Research.
Edited by George DeMartino
References
- 1.Komander D., Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 2.Yau R., Rape M. The increasing complexity of the ubiquitin code. Nat. Cell Biol. 2016;18:579–586. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 3.Swatek K.N., Komander D. Ubiquitin modifications. Cell Res. 2016;26:399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rape M. Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol. 2018;19:59–70. doi: 10.1038/nrm.2017.83. [DOI] [PubMed] [Google Scholar]
- 5.Popovic D., Vucic D., Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 6.Senft D., Qi J., Ronai Z.A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer. 2018;18:69–88. doi: 10.1038/nrc.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer-Schwesinger C. The ubiquitin-proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 2019;15:393–411. doi: 10.1038/s41581-019-0148-1. [DOI] [PubMed] [Google Scholar]
- 8.Hnia K., Clausen T., Moog-Lutz C. Shaping striated muscles with ubiquitin proteasome system in Health and disease. Trends Mol. Med. 2019;25:760–774. doi: 10.1016/j.molmed.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Barbour H., Daou S., Hendzel M., Affar E.B. Polycomb group-mediated histone H2A monoubiquitination in epigenome regulation and nuclear processes. Nat. Commun. 2020;11:5947. doi: 10.1038/s41467-020-19722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eletr Z.M., Wilkinson K.D. Regulation of proteolysis by human deubiquitinating enzymes. Biochim. Biophys. Acta. 2014;1843:114–128. doi: 10.1016/j.bbamcr.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahtoe D.D., Sixma T.K. Layers of DUB regulation. Trends Biochem. Sci. 2015;40:456–467. doi: 10.1016/j.tibs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Clague M.J., Urbe S., Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 2019;20:338–352. doi: 10.1038/s41580-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 13.Mevissen T.E.T., Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 2017;86:159–192. doi: 10.1146/annurev-biochem-061516-044916. [DOI] [PubMed] [Google Scholar]
- 14.Snyder N.A., Silva G.M. Deubiquitinating enzymes (DUBs): regulation, homeostasis, and oxidative stress response. J. Biol. Chem. 2021;297:101077. doi: 10.1016/j.jbc.2021.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange S.M., Armstrong L.A., Kulathu Y. Deubiquitinases: from mechanisms to their inhibition by small molecules. Mol. Cell. 2022;82:15–29. doi: 10.1016/j.molcel.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Bodda C., Reinert L.S., Fruhwurth S., Richardo T., Sun C., Zhang B.C., et al. HSV1 VP1-2 deubiquitinates STING to block type I interferon expression and promote brain infection. J. Exp. Med. 2020;217 doi: 10.1084/jem.20191422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dzimianski J.V., Beldon B.S., Daczkowski C.M., Goodwin O.Y., Scholte F.E.M., Bergeron E., et al. Probing the impact of nairovirus genomic diversity on viral ovarian tumor domain protease (vOTU) structure and deubiquitinase activity. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J.I., Sollars P.J., Baver S.B., Pickard G.E., Leelawong M., Smith G.A. A herpesvirus encoded deubiquitinase is a novel neuroinvasive determinant. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng D., Chen G., Guo B., Cheng G., Tang H. PLP2, a potent deubiquitinase from murine hepatitis virus, strongly inhibits cellular type I interferon production. Cell Res. 2008;18:1105–1113. doi: 10.1038/cr.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berglund J., Gjondrekaj R., Verney E., Maupin-Furlow J.A., Edelmann M.J. Modification of the host ubiquitome by bacterial enzymes. Microbiol. Res. 2020;235:126429. doi: 10.1016/j.micres.2020.126429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Negrate G., Faustin B., Welsh K., Loeffler M., Krajewska M., Hasegawa P., et al. Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-kappaB, suppresses IkappaBalpha ubiquitination and modulates innate immune responses. J. Immunol. 2008;180:5045–5056. doi: 10.4049/jimmunol.180.7.5045. [DOI] [PubMed] [Google Scholar]
- 22.Pruneda J.N., Bastidas R.J., Bertsoulaki E., Swatek K.N., Santhanam B., Clague M.J., et al. A Chlamydia effector combining deubiquitination and acetylation activities induces Golgi fragmentation. Nat. Microbiol. 2018;3:1377–1384. doi: 10.1038/s41564-018-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruneda J.N., Durkin C.H., Geurink P.P., Ovaa H., Santhanam B., Holden D.W., et al. The molecular basis for ubiquitin and ubiquitin-like specificities in bacterial effector proteases. Mol. Cell. 2016;63:261–276. doi: 10.1016/j.molcel.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rytkonen A., Poh J., Garmendia J., Boyle C., Thompson A., Liu M., et al. SseL, a Salmonella deubiquitinase required for macrophage killing and virulence. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3502–3507. doi: 10.1073/pnas.0610095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert A.F., Nguyen J.V., Franklin T.G., Geurink P.P., Roberts C.G., Sanderson D.J., et al. Identification and characterization of diverse OTU deubiquitinases in bacteria. EMBO J. 2020;39 doi: 10.15252/embj.2020105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin D., Bhattacharya A., Cheng Y.L., Alonso M.C., Mehdipour A.R., van der Heden van Noort G.J., et al. Bacterial OTU deubiquitinases regulate substrate ubiquitination upon Legionella infection. Elife. 2020;9 doi: 10.7554/eLife.58277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan M., Wang X., Huang C., Xu D., Wang Z., Zhou Y., et al. A bacterial effector deubiquitinase specifically hydrolyses linear ubiquitin chains to inhibit host inflammatory signalling. Nat. Microbiol. 2019;4:1282–1293. doi: 10.1038/s41564-019-0454-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Zhan Q., Wang X., Li P., Liu S., Gao G., et al. Insights into catalysis and regulation of non-canonical ubiquitination and deubiquitination by bacterial deamidase effectors. Nat. Commun. 2020;11:2751. doi: 10.1038/s41467-020-16587-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X., Chen Z.J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 2011;12:35–48. doi: 10.1038/nri3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schluter D., Schulze-Niemand E., Stein M., Naumann M. Ovarian tumor domain proteases in pathogen infection. Trends Microbiol. 2021;30:22–33. doi: 10.1016/j.tim.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Affar E.B., Carbone M. BAP1 regulates different mechanisms of cell death. Cell Death Dis. 2018;9:1151. doi: 10.1038/s41419-018-1206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masclef L., Ahmed O., Estavoyer B., Larrivee B., Labrecque N., Nijnik A., et al. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 2021;28:606–625. doi: 10.1038/s41418-020-00709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carbone M., Harbour J.W., Brugarolas J., Bononi A., Pagano I., Dey A., et al. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov. 2020;10:1103–1120. doi: 10.1158/2159-8290.CD-19-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nijman S.M., Luna-Vargas M.P., Velds A., Brummelkamp T.R., Dirac A.M., Sixma T.K., et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Komander D., Clague M.J., Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 36.Faesen A.C., Luna-Vargas M.P., Geurink P.P., Clerici M., Merkx R., van Dijk W.J., et al. The differential modulation of USP activity by internal regulatory domains, interactors and eight ubiquitin chain types. Chem. Biol. 2011;18:1550–1561. doi: 10.1016/j.chembiol.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Montalvan A., Bouwmeester T., Joberty G., Mader R., Mahnke M., Pierrat B., et al. Biochemical characterization of USP7 reveals post-translational modification sites and structural requirements for substrate processing and subcellular localization. FEBS J. 2007;274:4256–4270. doi: 10.1111/j.1742-4658.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- 38.Faesen A.C., Dirac A.M., Shanmugham A., Ovaa H., Perrakis A., Sixma T.K. Mechanism of USP7/HAUSP activation by its C-terminal ubiquitin-like domain and allosteric regulation by GMP-synthetase. Mol. Cell. 2011;44:147–159. doi: 10.1016/j.molcel.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 39.Rouge L., Bainbridge T.W., Kwok M., Tong R., Di Lello P., Wertz I.E., et al. Molecular understanding of USP7 substrate recognition and C-terminal activation. Structure. 2016;24:1335–1345. doi: 10.1016/j.str.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Kim R.Q., van Dijk W.J., Sixma T.K. Structure of USP7 catalytic domain and three Ubl-domains reveals a connector alpha-helix with regulatory role. J. Struct. Biol. 2016;195:11–18. doi: 10.1016/j.jsb.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim R.Q., Geurink P.P., Mulder M.P.C., Fish A., Ekkebus R., El Oualid F., et al. Kinetic analysis of multistep USP7 mechanism shows critical role for target protein in activity. Nat. Commun. 2019;10:231. doi: 10.1038/s41467-018-08231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozhidaeva A., Bezsonova I. USP7: structure, substrate specificity, and inhibition. DNA Repair (Amst) 2019;76:30–39. doi: 10.1016/j.dnarep.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steger M., Demichev V., Backman M., Ohmayer U., Ihmor P., Muller S., et al. Time-resolved in vivo ubiquitinome profiling by DIA-MS reveals USP7 targets on a proteome-wide scale. Nat. Commun. 2021;12:5399. doi: 10.1038/s41467-021-25454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rawat R., Starczynowski D.T., Ntziachristos P. Nuclear deubiquitination in the spotlight: the multifaceted nature of USP7 biology in disease. Curr. Opin. Cell Biol. 2019;58:85–94. doi: 10.1016/j.ceb.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mashtalir N., Daou S., Barbour H., Sen N.N., Gagnon J., Hammond-Martel I., et al. Autodeubiquitination protects the tumor suppressor BAP1 from cytoplasmic sequestration mediated by the atypical ubiquitin ligase UBE2O. Mol. Cell. 2014;54:392–406. doi: 10.1016/j.molcel.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Daou S., Hammond-Martel I., Mashtalir N., Barbour H., Gagnon J., Iannantuono N.V., et al. The BAP1/ASXL2 histone H2A deubiquitinase complex regulates cell proliferation and is disrupted in cancer. J. Biol. Chem. 2015;290:28643–28663. doi: 10.1074/jbc.M115.661553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahtoe D.D., van Dijk W.J., Ekkebus R., Ovaa H., Sixma T.K. BAP1/ASXL1 recruitment and activation for H2A deubiquitination. Nat. Commun. 2016;7 doi: 10.1038/ncomms10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foglizzo M., Middleton A.J., Burgess A.E., Crowther J.M., Dobson R.C.J., Murphy J.M., et al. A bidentate Polycomb Repressive-Deubiquitinase complex is required for efficient activity on nucleosomes. Nat. Commun. 2018;9:3932. doi: 10.1038/s41467-018-06186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De I., Chittock E.C., Grotsch H., Miller T.C.R., McCarthy A.A., Muller C.W. Structural basis for the activation of the deubiquitinase Calypso by the Polycomb protein ASX. Structure. 2019;27:528–536.e4. doi: 10.1016/j.str.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Morrow M.E., Kim M.I., Ronau J.A., Sheedlo M.J., White R.R., Chaney J., et al. Stabilization of an unusual salt bridge in ubiquitin by the extra C-terminal domain of the proteasome-associated deubiquitinase UCH37 as a mechanism of its exo specificity. Biochemistry. 2013;52:3564–3578. doi: 10.1021/bi4003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao T., Song L., Xu W., Demartino G.N., Florens L., Swanson S.K., et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat. Cell Biol. 2006;8:994–1002. doi: 10.1038/ncb1460. [DOI] [PubMed] [Google Scholar]
- 52.Yao T., Song L., Jin J., Cai Y., Takahashi H., Swanson S.K., et al. Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Mol. Cell. 2008;31:909–917. doi: 10.1016/j.molcel.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vander Linden R.T., Hemmis C.W., Schmitt B., Ndoja A., Whitby F.G., Robinson H., et al. Structural basis for the activation and inhibition of the UCH37 deubiquitylase. Mol. Cell. 2015;57:901–911. doi: 10.1016/j.molcel.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X., Walters K.J. Structural plasticity allows UCH37 to be primed by RPN13 or locked down by INO80G. Mol. Cell. 2015;57:767–768. doi: 10.1016/j.molcel.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahtoe D.D., van Dijk W.J., El Oualid F., Ekkebus R., Ovaa H., Sixma T.K. Mechanism of UCH-L5 activation and inhibition by DEUBAD domains in RPN13 and INO80G. Mol. Cell. 2015;57:887–900. doi: 10.1016/j.molcel.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu B., Sureda-Gomez M., Zhen Y., Amador V., Reverter D. A quaternary tetramer assembly inhibits the deubiquitinating activity of USP25. Nat. Commun. 2018;9:4973. doi: 10.1038/s41467-018-07510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauer F., Klemm T., Kollampally R.B., Tessmer I., Nair R.K., Popov N., et al. Differential oligomerization of the deubiquitinases USP25 and USP28 regulates their activities. Mol. Cell. 2019;74:421–435.e10. doi: 10.1016/j.molcel.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Gersch M., Wagstaff J.L., Toms A.V., Graves B., Freund S.M.V., Komander D. Distinct USP25 and USP28 oligomerization states regulate deubiquitinating activity. Mol. Cell. 2019;74:436–451.e7. doi: 10.1016/j.molcel.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakada S., Tai I., Panier S., Al-Hakim A., Iemura S., Juang Y.C., et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]
- 60.Wiener R., Zhang X., Wang T., Wolberger C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature. 2012;483:618–622. doi: 10.1038/nature10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juang Y.C., Landry M.C., Sanches M., Vittal V., Leung C.C., Ceccarelli D.F., et al. OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol. Cell. 2012;45:384–397. doi: 10.1016/j.molcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiener R., DiBello A.T., Lombardi P.M., Guzzo C.M., Zhang X., Matunis M.J., et al. E2 ubiquitin-conjugating enzymes regulate the deubiquitinating activity of OTUB1. Nat. Struct. Mol. Biol. 2013;20:1033–1039. doi: 10.1038/nsmb.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- 64.Durcan T.M., Kontogiannea M., Thorarinsdottir T., Fallon L., Williams A.J., Djarmati A., et al. The Machado-Joseph disease-associated mutant form of ataxin-3 regulates parkin ubiquitination and stability. Hum. Mol. Genet. 2011;20:141–154. doi: 10.1093/hmg/ddq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Durcan T.M., Kontogiannea M., Bedard N., Wing S.S., Fon E.A. Ataxin-3 deubiquitination is coupled to Parkin ubiquitination via E2 ubiquitin-conjugating enzyme. J. Biol. Chem. 2012;287:531–541. doi: 10.1074/jbc.M111.288449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boone D.L., Turer E.E., Lee E.G., Ahmad R.C., Wheeler M.T., Tsui C., et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 67.Wertz I.E., O'Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 68.Heyninck K., Beyaert R. A20 inhibits NF-kappaB activation by dual ubiquitin-editing functions. Trends Biochem. Sci. 2005;30:1–4. doi: 10.1016/j.tibs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Shembade N., Ma A., Harhaj E.W. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirisako T., Kamei K., Murata S., Kato M., Fukumoto H., Kanie M., et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tokunaga F., Nakagawa T., Nakahara M., Saeki Y., Taniguchi M., Sakata S., et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 72.Gerlach B., Cordier S.M., Schmukle A.C., Emmerich C.H., Rieser E., Haas T.L., et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 73.Ikeda F., Deribe Y.L., Skanland S.S., Stieglitz B., Grabbe C., Franz-Wachtel M., et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujita H., Tokunaga A., Shimizu S., Whiting A.L., Aguilar-Alonso F., Takagi K., et al. Cooperative domain formation by homologous motifs in HOIL-1L and SHARPIN plays A crucial role in LUBAC stabilization. Cell Rep. 2018;23:1192–1204. doi: 10.1016/j.celrep.2018.03.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niu J., Shi Y., Iwai K., Wu Z.H. LUBAC regulates NF-kappaB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. EMBO J. 2011;30:3741–3753. doi: 10.1038/emboj.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brazee P., Dada L.A., Sznajder J.I. Role of linear ubiquitination in health and disease. Am. J. Respir. Cell Mol. Biol. 2016;54:761–768. doi: 10.1165/rcmb.2016-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stieglitz B., Morris-Davies A.C., Koliopoulos M.G., Christodoulou E., Rittinger K. LUBAC synthesizes linear ubiquitin chains via a thioester intermediate. EMBO Rep. 2012;13:840–846. doi: 10.1038/embor.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smit J.J., van Dijk W.J., El Atmioui D., Merkx R., Ovaa H., Sixma T.K. Target specificity of the E3 ligase LUBAC for ubiquitin and NEMO relies on different minimal requirements. J. Biol. Chem. 2013;288:31728–31737. doi: 10.1074/jbc.M113.495846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smit J.J., Monteferrario D., Noordermeer S.M., van Dijk W.J., van der Reijden B.A., Sixma T.K. The E3 ligase HOIP specifies linear ubiquitin chain assembly through its RING-IBR-RING domain and the unique LDD extension. EMBO J. 2012;31:3833–3844. doi: 10.1038/emboj.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuseya Y., Fujita H., Kim M., Ohtake F., Nishide A., Sasaki K., et al. The HOIL-1L ligase modulates immune signalling and cell death via monoubiquitination of LUBAC. Nat. Cell Biol. 2020;22:663–673. doi: 10.1038/s41556-020-0517-9. [DOI] [PubMed] [Google Scholar]
- 81.Keusekotten K., Elliott P.R., Glockner L., Fiil B.K., Damgaard R.B., Kulathu Y., et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell. 2013;153:1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rivkin E., Almeida S.M., Ceccarelli D.F., Juang Y.C., MacLean T.A., Srikumar T., et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature. 2013;498:318–324. doi: 10.1038/nature12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elliott P.R., Nielsen S.V., Marco-Casanova P., Fiil B.K., Keusekotten K., Mailand N., et al. Molecular basis and regulation of OTULIN-LUBAC interaction. Mol. Cell. 2014;54:335–348. doi: 10.1016/j.molcel.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaeffer V., Akutsu M., Olma M.H., Gomes L.C., Kawasaki M., Dikic I. Binding of OTULIN to the PUB domain of HOIP controls NF-kappaB signaling. Mol. Cell. 2014;54:349–361. doi: 10.1016/j.molcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 85.Fiil B.K., Damgaard R.B., Wagner S.A., Keusekotten K., Fritsch M., Bekker-Jensen S., et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol. Cell. 2013;50:818–830. doi: 10.1016/j.molcel.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuo Y., Feng Q., Jin L., Huang F., Miao Y., Liu J., et al. Regulation of the linear ubiquitination of STAT1 controls antiviral interferon signaling. Nat. Commun. 2020;11:1146. doi: 10.1038/s41467-020-14948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu Y., Wang H., Dai H., Zhu Q., Cui C.P., Sun X., et al. OTULIN allies with LUBAC to govern angiogenesis by editing ALK1 linear polyubiquitin. Mol. Cell. 2021;81:3187–3204.e7. doi: 10.1016/j.molcel.2021.05.031. [DOI] [PubMed] [Google Scholar]
- 88.Heger K., Wickliffe K.E., Ndoja A., Zhang J., Murthy A., Dugger D.L., et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature. 2018;559:120–124. doi: 10.1038/s41586-018-0256-2. [DOI] [PubMed] [Google Scholar]
- 89.Weinelt N., van Wijk S.J.L. Ubiquitin-dependent and -independent functions of OTULIN in cell fate control and beyond. Cell Death Differ. 2021;28:493–504. doi: 10.1038/s41418-020-00675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elliott P.R., Leske D., Hrdinka M., Bagola K., Fiil B.K., McLaughlin S.H., et al. SPATA2 links CYLD to LUBAC, activates CYLD, and controls LUBAC signaling. Mol. Cell. 2016;63:990–1005. doi: 10.1016/j.molcel.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Draber P., Kupka S., Reichert M., Draberova H., Lafont E., de Miguel D., et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13:2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maertens G.N., El Messaoudi-Aubert S., Elderkin S., Hiom K., Peters G. Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J. 2010;29:2553–2565. doi: 10.1038/emboj.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sowa M.E., Bennett E.J., Gygi S.P., Harper J.W. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cooper E.M., Cutcliffe C., Kristiansen T.Z., Pandey A., Pickart C.M., Cohen R.E. K63-specific deubiquitination by two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J. 2009;28:621–631. doi: 10.1038/emboj.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim H., Chen J., Yu X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science. 2007;316:1202–1205. doi: 10.1126/science.1139621. [DOI] [PubMed] [Google Scholar]
- 96.Sobhian B., Shao G., Lilli D.R., Culhane A.C., Moreau L.A., Xia B., et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science. 2007;316:1198–1202. doi: 10.1126/science.1139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B., Matsuoka S., Ballif B.A., Zhang D., Smogorzewska A., Gygi S.P., et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu X., Kim J.A., Castillo A., Huang M., Liu J., Wang B. NBA1/MERIT40 and BRE interaction is required for the integrity of two distinct deubiquitinating enzyme BRCC36-containing complexes. J. Biol. Chem. 2011;286:11734–11745. doi: 10.1074/jbc.M110.200857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong Y., Hakimi M.A., Chen X., Kumaraswamy E., Cooch N.S., Godwin A.K., et al. Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol. Cell. 2003;12:1087–1099. doi: 10.1016/s1097-2765(03)00424-6. [DOI] [PubMed] [Google Scholar]
- 100.Wu J., Liu C., Chen J., Yu X. RAP80 protein is important for genomic stability and is required for stabilizing BRCA1-A complex at DNA damage sites in vivo. J. Biol. Chem. 2012;287:22919–22926. doi: 10.1074/jbc.M112.351007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin Z., Menendez D., Resnick M.A., French J.E., Janardhan K.S., Jetten A.M. RAP80 is critical in maintaining genomic stability and suppressing tumor development. Cancer Res. 2012;72:5080–5090. doi: 10.1158/0008-5472.CAN-12-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng H., Gupta V., Patterson-Fortin J., Bhattacharya S., Katlinski K., Wu J., et al. A BRISC-SHMT complex deubiquitinates IFNAR1 and regulates interferon responses. Cell Rep. 2013;5:180–193. doi: 10.1016/j.celrep.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Donaghy R., Han X., Rozenova K., Lv K., Jiang Q., Doepner M., et al. The BRISC deubiquitinating enzyme complex limits hematopoietic stem cell expansion by regulating JAK2 K63-ubiquitination. Blood. 2019;133:1560–1571. doi: 10.1182/blood-2018-10-877563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan K., Li L., Wang X., Hong R., Zhang Y., Yang H., et al. The deubiquitinating enzyme complex BRISC is required for proper mitotic spindle assembly in mammalian cells. J. Cell Biol. 2015;210:209–224. doi: 10.1083/jcb.201503039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ren G., Zhang X., Xiao Y., Zhang W., Wang Y., Ma W., et al. ABRO1 promotes NLRP3 inflammasome activation through regulation of NLRP3 deubiquitination. EMBO J. 2019;38 doi: 10.15252/embj.2018100376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walden M., Tian L., Ross R.L., Sykora U.M., Byrne D.P., Hesketh E.L., et al. Metabolic control of BRISC-SHMT2 assembly regulates immune signalling. Nature. 2019;570:194–199. doi: 10.1038/s41586-019-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeqiraj E., Tian L., Piggott C.A., Pillon M.C., Duffy N.M., Ceccarelli D.F., et al. Higher-order assembly of BRCC36-KIAA0157 is required for DUB activity and biological function. Mol. Cell. 2015;59:970–983. doi: 10.1016/j.molcel.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rabl J., Bunker R.D., Schenk A.D., Cavadini S., Gill M.E., Abdulrahman W., et al. Structural basis of BRCC36 function in DNA repair and immune regulation. Mol. Cell. 2019;75:483–497.e9. doi: 10.1016/j.molcel.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rabl J. BRCA1-A and BRISC: multifunctional molecular machines for ubiquitin signaling. Biomolecules. 2020;10:1503. doi: 10.3390/biom10111503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qiu X.B., Ouyang S.Y., Li C.J., Miao S., Wang L., Goldberg A.L. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J. 2006;25:5742–5753. doi: 10.1038/sj.emboj.7601450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamazaki J., Iemura S., Natsume T., Yashiroda H., Tanaka K., Murata S. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 2006;25:4524–4536. doi: 10.1038/sj.emboj.7601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Randles L., Anchoori R.K., Roden R.B., Walters K.J. The proteasome ubiquitin receptor hRpn13 and its interacting deubiquitinating enzyme Uch37 are required for proper cell cycle progression. J. Biol. Chem. 2016;291:8773–8783. doi: 10.1074/jbc.M115.694588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deol K.K., Crowe S.O., Du J., Bisbee H.A., Guenette R.G., Strieter E.R. Proteasome-bound UCH37/UCHL5 debranches ubiquitin chains to promote degradation. Mol. Cell. 2020;80:796–809.e9. doi: 10.1016/j.molcel.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohn M.A., Kowal P., Yang K., Haas W., Huang T.T., Gygi S.P., et al. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol. Cell. 2007;28:786–797. doi: 10.1016/j.molcel.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 115.Cohn M.A., Kee Y., Haas W., Gygi S.P., D'Andrea A.D. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J. Biol. Chem. 2009;284:5343–5351. doi: 10.1074/jbc.M808430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dharadhar S., Clerici M., van Dijk W.J., Fish A., Sixma T.K. A conserved two-step binding for the UAF1 regulator to the USP12 deubiquitinating enzyme. J. Struct. Biol. 2016;196:437–447. doi: 10.1016/j.jsb.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rennie M.L., Arkinson C., Chaugule V.K., Toth R., Walden H. Structural basis of FANCD2 deubiquitination by USP1-UAF1. Nat. Struct. Mol. Biol. 2021;28:356–364. doi: 10.1038/s41594-021-00576-8. [DOI] [PubMed] [Google Scholar]
- 118.Yin J., Schoeffler A.J., Wickliffe K., Newton K., Starovasnik M.A., Dueber E.C., et al. Structural insights into WD-repeat 48 activation of ubiquitin-specific protease 46. Structure. 2015;23:2043–2054. doi: 10.1016/j.str.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 119.Li H., Lim K.S., Kim H., Hinds T.R., Jo U., Mao H., et al. Allosteric activation of ubiquitin-specific proteases by beta-propeller proteins UAF1 and WDR20. Mol. Cell. 2016;63:249–260. doi: 10.1016/j.molcel.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu H., Zhang T., Wang F., Yang J., Ding J. Structural insights into the activation of USP46 by WDR48 and WDR20. Cell Discov. 2019;5:34. doi: 10.1038/s41421-019-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kee Y., Yang K., Cohn M.A., Haas W., Gygi S.P., D'Andrea A.D. WDR20 regulates activity of the USP12 x UAF1 deubiquitinating enzyme complex. J. Biol. Chem. 2010;285:11252–11257. doi: 10.1074/jbc.M109.095141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dahlberg C.L., Juo P. The WD40-repeat proteins WDR-20 and WDR-48 bind and activate the deubiquitinating enzyme USP-46 to promote the abundance of the glutamate receptor GLR-1 in the ventral nerve cord of Caenorhabditis elegans. J. Biol. Chem. 2014;289:3444–3456. doi: 10.1074/jbc.M113.507541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hornbeck P.V., Kornhauser J.M., Latham V., Murray B., Nandhikonda V., Nord A., et al. 15 years of PhosphoSitePlus(R): integrating post-translationally modified sites, disease variants and isoforms. Nucl. Acids Res. 2019;47:D433–D441. doi: 10.1093/nar/gky1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soffers J.H.M., Workman J.L. The SAGA chromatin-modifying complex: the sum of its parts is greater than the whole. Genes Dev. 2020;34:1287–1303. doi: 10.1101/gad.341156.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morgan M.T., Haj-Yahya M., Ringel A.E., Bandi P., Brik A., Wolberger C. Structural basis for histone H2B deubiquitination by the SAGA DUB module. Science. 2016;351:725–728. doi: 10.1126/science.aac5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Samara N.L., Datta A.B., Berndsen C.E., Zhang X., Yao T., Cohen R.E., et al. Structural insights into the assembly and function of the SAGA deubiquitinating module. Science. 2010;328:1025–1029. doi: 10.1126/science.1190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fuchs G., Oren M. Writing and reading H2B monoubiquitylation. Biochim. Biophys. Acta. 2014;1839:694–701. doi: 10.1016/j.bbagrm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Wang H., Dienemann C., Stutzer A., Urlaub H., Cheung A.C.M., Cramer P. Structure of the transcription coactivator SAGA. Nature. 2020;577:717–720. doi: 10.1038/s41586-020-1933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Papai G., Frechard A., Kolesnikova O., Crucifix C., Schultz P., Ben-Shem A. Structure of SAGA and mechanism of TBP deposition on gene promoters. Nature. 2020;577:711–716. doi: 10.1038/s41586-020-1944-2. [DOI] [PubMed] [Google Scholar]
- 130.Herbst D.A., Esbin M.N., Louder R.K., Dugast-Darzacq C., Dailey G.M., Fang Q., et al. Structure of the human SAGA coactivator complex. Nat. Struct. Mol. Biol. 2021;28:989–996. doi: 10.1038/s41594-021-00682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grant P.A., Winston F., Berger S.L. The biochemical and genetic discovery of the SAGA complex. Biochim. Biophys. Acta Gene Regul. Mech. 2021;1864:194669. doi: 10.1016/j.bbagrm.2020.194669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Machida Y.J., Machida Y., Vashisht A.A., Wohlschlegel J.A., Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J. Biol. Chem. 2009;284:34179–34188. doi: 10.1074/jbc.M109.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu H., Mashtalir N., Daou S., Hammond-Martel I., Ross J., Sui G., et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol. Cell Biol. 2010;30:5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hart G.W., Slawson C., Ramirez-Correa G., Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fang R., Barbera A.J., Xu Y., Rutenberg M., Leonor T., Bi Q., et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol. Cell. 2010;39:222–233. doi: 10.1016/j.molcel.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vogel J.L., Kristie T.M. The novel coactivator C1 (HCF) coordinates multiprotein enhancer formation and mediates transcription activation by GABP. EMBO J. 2000;19:683–690. doi: 10.1093/emboj/19.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wysocka J., Myers M.P., Laherty C.D., Eisenman R.N., Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narayanan A., Nogueira M.L., Ruyechan W.T., Kristie T.M. Combinatorial transcription of herpes simplex virus and varicella zoster virus immediate early genes is strictly determined by the cellular coactivator HCF-1. J. Biol. Chem. 2005;280:1369–1375. doi: 10.1074/jbc.M410178200. [DOI] [PubMed] [Google Scholar]
- 139.Tyagi S., Chabes A.L., Wysocka J., Herr W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol. Cell. 2007;27:107–119. doi: 10.1016/j.molcel.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 140.Misaghi S., Ottosen S., Izrael-Tomasevic A., Arnott D., Lamkanfi M., Lee J., et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol. Cell Biol. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ruan H.B., Han X., Li M.D., Singh J.P., Qian K., Azarhoush S., et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okino Y., Machida Y., Frankland-Searby S., Machida Y.J. BRCA1-associated protein 1 (BAP1) deubiquitinase antagonizes the ubiquitin-mediated activation of FoxK2 target genes. J. Biol. Chem. 2015;290:1580–1591. doi: 10.1074/jbc.M114.609834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meray R.K., Lansbury P.T., Jr. Reversible monoubiquitination regulates the Parkinson disease-associated ubiquitin hydrolase UCH-L1. J. Biol. Chem. 2007;282:10567–10575. doi: 10.1074/jbc.M611153200. [DOI] [PubMed] [Google Scholar]
- 144.Chai Y., Berke S.S., Cohen R.E., Paulson H.L. Poly-ubiquitin binding by the polyglutamine disease protein ataxin-3 links its normal function to protein surveillance pathways. J. Biol. Chem. 2004;279:3605–3611. doi: 10.1074/jbc.M310939200. [DOI] [PubMed] [Google Scholar]
- 145.Burnett B.G., Pittman R.N. The polyglutamine neurodegenerative protein ataxin 3 regulates aggresome formation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4330–4335. doi: 10.1073/pnas.0407252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong X., Pittman R.N. Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum. Mol. Genet. 2006;15:2409–2420. doi: 10.1093/hmg/ddl164. [DOI] [PubMed] [Google Scholar]
- 147.Todi S.V., Winborn B.J., Scaglione K.M., Blount J.R., Travis S.M., Paulson H.L. Ubiquitination directly enhances activity of the deubiquitinating enzyme ataxin-3. EMBO J. 2009;28:372–382. doi: 10.1038/emboj.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Todi S.V., Scaglione K.M., Blount J.R., Basrur V., Conlon K.P., Pastore A., et al. Activity and cellular functions of the deubiquitinating enzyme and polyglutamine disease protein ataxin-3 are regulated by ubiquitination at lysine 117. J. Biol. Chem. 2010;285:39303–39313. doi: 10.1074/jbc.M110.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berke S.J., Chai Y., Marrs G.L., Wen H., Paulson H.L. Defining the role of ubiquitin-interacting motifs in the polyglutamine disease protein, ataxin-3. J. Biol. Chem. 2005;280:32026–32034. doi: 10.1074/jbc.M506084200. [DOI] [PubMed] [Google Scholar]
- 150.Todi S.V., Laco M.N., Winborn B.J., Travis S.M., Wen H.M., Paulson H.L. Cellular turnover of the polyglutamine disease protein ataxin-3 is regulated by its catalytic activity. J. Biol. Chem. 2007;282:29348–29358. doi: 10.1074/jbc.M704126200. [DOI] [PubMed] [Google Scholar]
- 151.Yanagitani K., Juszkiewicz S., Hegde R.S. UBE2O is a quality control factor for orphans of multiprotein complexes. Science. 2017;357:472–475. doi: 10.1126/science.aan0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nguyen A.T., Prado M.A., Schmidt P.J., Sendamarai A.K., Wilson-Grady J.T., Min M., et al. UBE2O remodels the proteome during terminal erythroid differentiation. Science. 2017;357 doi: 10.1126/science.aan0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y., Wang F. Post-translational modifications of deubiquitinating enzymes: expanding the ubiquitin code. Front. Pharmacol. 2021;12:685011. doi: 10.3389/fphar.2021.685011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang L., Zhou F., Drabsch Y., Gao R., Snaar-Jagalska B.E., Mickanin C., et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-beta type I receptor. Nat. Cell Biol. 2012;14:717–726. doi: 10.1038/ncb2522. [DOI] [PubMed] [Google Scholar]
- 155.Khoronenkova S.V., Dianova, Ternette N., Kessler B.M., Parsons J.L., Dianov G.L. ATM-dependent downregulation of USP7/HAUSP by PPM1G activates p53 response to DNA damage. Mol. Cell. 2012;45:801–813. doi: 10.1016/j.molcel.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang O.W., Ma X., Yin J., Flinders J., Maurer T., Kayagaki N., et al. Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat. Struct. Mol. Biol. 2012;19:171–175. doi: 10.1038/nsmb.2206. [DOI] [PubMed] [Google Scholar]
- 157.Zhao Y., Mudge M.C., Soll J.M., Rodrigues R.B., Byrum A.K., Schwarzkopf E.A., et al. OTUD4 is a Phospho-activated K63 deubiquitinase that regulates MyD88-dependent signaling. Mol. Cell. 2018;69:505–516.e5. doi: 10.1016/j.molcel.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang W., Li M., Ponnusamy S., Chi Y., Xue J., Fahmy B., et al. ABL1-dependent OTULIN phosphorylation promotes genotoxic Wnt/beta-catenin activation to enhance drug resistance in breast cancers. Nat. Commun. 2020;11:3965. doi: 10.1038/s41467-020-17770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu H., Pak H., Hammond-Martel I., Ghram M., Rodrigue A., Daou S., et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl. Acad. Sci. U. S. A. 2014;111:285–290. doi: 10.1073/pnas.1309085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ismail I.H., Davidson R., Gagne J.P., Xu Z.Z., Poirier G., Hendzel M.J. Germ-line mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014;74:4282–4294. doi: 10.1158/0008-5472.CAN-13-3109. [DOI] [PubMed] [Google Scholar]
- 161.Bononi A., Giorgi C., Patergnani S., Larson D., Verbruggen K., Tanji M., et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature. 2017;546:549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Z., Fang X., Wu X., Ling L., Chu F., Li J., et al. Acetylation-dependent deubiquitinase OTUD3 controls MAVS activation in innate antiviral immunity. Mol. Cell. 2020;79:304–319.e7. doi: 10.1016/j.molcel.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 163.Hou F., Sun L., Zheng H., Skaug B., Jiang Q.X., Chen Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cai X., Chen J., Xu H., Liu S., Jiang Q.X., Halfmann R., et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu B., Zhang M., Chu H., Zhang H., Wu H., Song G., et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination. Nat. Immunol. 2017;18:214–224. doi: 10.1038/ni.3641. [DOI] [PubMed] [Google Scholar]
- 166.Kumar R., Jangir D.K., Verma G., Shekhar S., Hanpude P., Kumar S., et al. S-nitrosylation of UCHL1 induces its structural instability and promotes alpha-synuclein aggregation. Sci. Rep. 2017;7:44558. doi: 10.1038/srep44558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Puri S., Hsu S.D. Cross-over loop cysteine C152 acts as an antioxidant to maintain the folding stability and deubiquitinase activity of UCH-L1 under oxidative stress. J. Mol. Biol. 2021;433:166879. doi: 10.1016/j.jmb.2021.166879. [DOI] [PubMed] [Google Scholar]
- 168.Koharudin L.M., Liu H., Di Maio R., Kodali R.B., Graham S.H., Gronenborn A.M. Cyclopentenone prostaglandin-induced unfolding and aggregation of the Parkinson disease-associated UCH-L1. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6835–6840. doi: 10.1073/pnas.1002295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu H., Li W., Rose M.E., Hickey R.W., Chen J., Uechi G.T., et al. The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post-ischemic neuronal injury. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang T.T., Nijman S.M., Mirchandani K.D., Galardy P.J., Cohn M.A., Haas W., et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 2006;8:339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 171.Lee J.G., Baek K., Soetandyo N., Ye Y. Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat. Commun. 2013;4:1568. doi: 10.1038/ncomms2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mader J., Huber J., Bonn F., Dotsch V., Rogov V.V., Bremm A. Oxygen-dependent asparagine hydroxylation of the ubiquitin-associated (UBA) domain in Cezanne regulates ubiquitin binding. J. Biol. Chem. 2020;295:2160–2174. doi: 10.1074/jbc.RA119.010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Udeshi N.D., Mani D.C., Satpathy S., Fereshetian S., Gasser J.A., Svinkina T., et al. Rapid and deep-scale ubiquitylation profiling for biology and translational research. Nat. Commun. 2020;11:359. doi: 10.1038/s41467-019-14175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim W., Bennett E.J., Huttlin E.L., Guo A., Li J., Possemato A., et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Udeshi N.D., Svinkina T., Mertins P., Kuhn E., Mani D.R., Qiao J.W., et al. Refined preparation and use of anti-diglycine remnant (K-epsilon-GG) antibody enables routine quantification of 10,000s of ubiquitination sites in single proteomics experiments. Mol. Cell Proteomics. 2013;12:825–831. doi: 10.1074/mcp.O112.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jung E.S., Hong H., Kim C., Mook-Jung I. Acute ER stress regulates amyloid precursor protein processing through ubiquitin-dependent degradation. Sci. Rep. 2015;5:8805. doi: 10.1038/srep08805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zheng Q., Li G., Wang S., Zhou Y., Liu K., Gao Y., et al. Trisomy 21-induced dysregulation of microglial homeostasis in Alzheimer's brains is mediated by USP25. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abe1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang P., Joberty G., Buist A., Vanoosthuyse A., Stancu I.C., Vasconcelos B., et al. Tau interactome mapping based identification of Otub1 as Tau deubiquitinase involved in accumulation of pathological Tau forms in vitro and in vivo. Acta Neuropathol. 2017;133:731–749. doi: 10.1007/s00401-016-1663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi J., Levey A.I., Weintraub S.T., Rees H.D., Gearing M., Chin L.S., et al. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J. Biol. Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 180.Zhao Z.B., Wu L., Xiong R., Wang L.L., Zhang B., Wang C., et al. MicroRNA-922 promotes tau phosphorylation by downregulating ubiquitin carboxy-terminal hydrolase L1 (UCHL1) expression in the pathogenesis of Alzheimer's disease. Neuroscience. 2014;275:232–237. doi: 10.1016/j.neuroscience.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 181.Albrecht M., Golatta M., Wullner U., Lengauer T. Structural and functional analysis of ataxin-2 and ataxin-3. Eur. J. Biochem. 2004;271:3155–3170. doi: 10.1111/j.1432-1033.2004.04245.x. [DOI] [PubMed] [Google Scholar]
- 182.Li X., Stevens P.D., Yang H., Gulhati P., Wang W., Evers B.M., et al. The deubiquitination enzyme USP46 functions as a tumor suppressor by controlling PHLPP-dependent attenuation of Akt signaling in colon cancer. Oncogene. 2013;32:471–478. doi: 10.1038/onc.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bott M., Brevet M., Taylor B.S., Shimizu S., Ito T., Wang L., et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Diefenbacher M.E., Popov N., Blake S.M., Schulein-Volk C., Nye E., Spencer-Dene B., et al. The deubiquitinase USP28 controls intestinal homeostasis and promotes colorectal cancer. J. Clin. Invest. 2014;124:3407–3418. doi: 10.1172/JCI73733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lv J., Zhang S., Wu H., Lu J., Lu Y., Wang F., et al. Deubiquitinase PSMD14 enhances hepatocellular carcinoma growth and metastasis by stabilizing GRB2. Cancer Lett. 2020;469:22–34. doi: 10.1016/j.canlet.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 186.Strobel P., Zettl A., Ren Z., Starostik P., Riedmiller H., Storkel S., et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am. J. Surg. Pathol. 2002;26:119–124. doi: 10.1097/00000478-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 187.Moll H.P., Lee A., Peterson C.R., Revuelta Cervantes J., Wojcik B.M., Parulkar A., et al. A20 haploinsufficiency aggravates transplant arteriosclerosis in mouse vascular allografts: implications for clinical transplantation. Transplantation. 2016;100:e106–e116. doi: 10.1097/TP.0000000000001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Patel V.I., Daniel S., Longo C.R., Shrikhande G.V., Scali S.T., Czismadia E., et al. A20, a modulator of smooth muscle cell proliferation and apoptosis, prevents and induces regression of neointimal hyperplasia. FASEB J. 2006;20:1418–1430. doi: 10.1096/fj.05-4981com. [DOI] [PubMed] [Google Scholar]
- 189.Palazon-Riquelme P., Worboys J.D., Green J., Valera A., Martin-Sanchez F., Pellegrini C., et al. USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep. 2018;19 doi: 10.15252/embr.201744766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou J.J., Li H., Li L., Li Y., Wang P.H., Meng X.M., et al. CYLD mediates human pulmonary artery smooth muscle cell dysfunction in congenital heart disease-associated pulmonary arterial hypertension. J. Cell Physiol. 2021;236:6297–6311. doi: 10.1002/jcp.30298. [DOI] [PubMed] [Google Scholar]
- 191.Kadariya Y., Cheung M., Xu J., Pei J., Sementino E., Menges C.W., et al. Bap1 is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res. 2016;76:2836–2844. doi: 10.1158/0008-5472.CAN-15-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hu X., Paul A., Wang B. Rap80 protein recruitment to DNA double-strand breaks requires binding to both small ubiquitin-like modifier (SUMO) and ubiquitin conjugates. J. Biol. Chem. 2012;287:25510–25519. doi: 10.1074/jbc.M112.374116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guzzo C.M., Berndsen C.E., Zhu J., Gupta V., Datta A., Greenberg R.A., et al. RNF4-dependent hybrid SUMO-ubiquitin chains are signals for RAP80 and thereby mediate the recruitment of BRCA1 to sites of DNA damage. Sci. Signal. 2012;5:ra88. doi: 10.1126/scisignal.2003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu J., Wang Y., Gong Y., Fu T., Hu S., Zhou Z., et al. Structural insights into SHARPIN-mediated activation of HOIP for the linear ubiquitin chain assembly. Cell Rep. 2017;21:27–36. doi: 10.1016/j.celrep.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 195.Hrdinka M., Fiil B.K., Zucca M., Leske D., Bagola K., Yabal M., et al. CYLD limits Lys63- and met1-linked ubiquitin at receptor complexes to regulate innate immune signaling. Cell Rep. 2016;14:2846–2858. doi: 10.1016/j.celrep.2016.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu D., Liu J., Fu T., Shan B., Qian L., Pan L., et al. USP25 regulates Wnt signaling by controlling the stability of tankyrases. Genes Dev. 2017;31:1024–1035. doi: 10.1101/gad.300889.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Goldbraikh D., Neufeld D., Eid-Mutlak Y., Lasry I., Gilda J.E., Parnis A., et al. USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 2020;21 doi: 10.15252/embr.201948791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Song H., Zhao C., Yu Z., Li Q., Yan R., Qin Y., et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat. Commun. 2020;11:6042. doi: 10.1038/s41467-020-19939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McClurg U.L., Summerscales E.E., Harle V.J., Gaughan L., Robson C.N. Deubiquitinating enzyme Usp12 regulates the interaction between the androgen receptor and the Akt pathway. Oncotarget. 2014;5:7081–7092. doi: 10.18632/oncotarget.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gangula N.R., Maddika S. WD repeat protein WDR48 in complex with deubiquitinase USP12 suppresses Akt-dependent cell survival signaling by stabilizing PH domain leucine-rich repeat protein phosphatase 1 (PHLPP1) J. Biol. Chem. 2013;288:34545–34554. doi: 10.1074/jbc.M113.503383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xiong Z., Xia P., Zhu X., Geng J., Wang S., Ye B., et al. Glutamylation of deubiquitinase BAP1 controls self-renewal of hematopoietic stem cells and hematopoiesis. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu Z., Meray R.K., Grammatopoulos T.N., Fredenburg R.A., Cookson M.R., Liu Y., et al. Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4635–4640. doi: 10.1073/pnas.0806474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Denuc A., Bosch-Comas A., Gonzalez-Duarte R., Marfany G. The UBA-UIM domains of the USP25 regulate the enzyme ubiquitination state and modulate substrate recognition. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim S., Lee D., Lee J., Song H., Kim H.J., Kim K.T. Vaccinia-related kinase 2 controls the stability of the eukaryotic chaperonin TRiC/CCT by inhibiting the deubiquitinating enzyme USP25. Mol. Cell Biol. 2015;35:1754–1762. doi: 10.1128/MCB.01325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cholay M., Reverdy C., Benarous R., Colland F., Daviet L. Functional interaction between the ubiquitin-specific protease 25 and the SYK tyrosine kinase. Exp. Cell Res. 2010;316:667–675. doi: 10.1016/j.yexcr.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 206.Kulathu Y., Garcia F.J., Mevissen T.E., Busch M., Arnaudo N., Carroll K.S., et al. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat. Commun. 2013;4:1569. doi: 10.1038/ncomms2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hutti J.E., Turk B.E., Asara J.M., Ma A., Cantley L.C., Abbott D.W. IkappaB kinase beta phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-kappaB pathway. Mol. Cell Biol. 2007;27:7451–7461. doi: 10.1128/MCB.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Coornaert B., Baens M., Heyninck K., Bekaert T., Haegman M., Staal J., et al. T cell antigen receptor stimulation induces MALT1 paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat. Immunol. 2008;9:263–271. doi: 10.1038/ni1561. [DOI] [PubMed] [Google Scholar]
- 209.Mauri P.L., Riva M., Ambu D., De Palma A., Secundo F., Benazzi L., et al. Ataxin-3 is subject to autolytic cleavage. FEBS J. 2006;273:4277–4286. doi: 10.1111/j.1742-4658.2006.05419.x. [DOI] [PubMed] [Google Scholar]
- 210.Pozzi C., Valtorta M., Tedeschi G., Galbusera E., Pastori V., Bigi A., et al. Study of subcellular localization and proteolysis of ataxin-3. Neurobiol. Dis. 2008;30:190–200. doi: 10.1016/j.nbd.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 211.Matos C.A., Nobrega C., Louros S.R., Almeida B., Ferreiro E., Valero J., et al. Ataxin-3 phosphorylation decreases neuronal defects in spinocerebellar ataxia type 3 models. J. Cell Biol. 2016;212:465–480. doi: 10.1083/jcb.201506025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Almeida B., Abreu I.A., Matos C.A., Fraga J.S., Fernandes S., Macedo M.G., et al. SUMOylation of the brain-predominant Ataxin-3 isoform modulates its interaction with p97. Biochim. Biophys. Acta. 2015;1852:1950–1959. doi: 10.1016/j.bbadis.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 213.Mao Y., Senic-Matuglia F., Di Fiore P.P., Polo S., Hodsdon M.E., De Camilli P. Deubiquitinating function of ataxin-3: insights from the solution structure of the josephin domain. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12700–12705. doi: 10.1073/pnas.0506344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mueller T., Breuer P., Schmitt I., Walter J., Evert B.O., Wullner U. CK2-dependent phosphorylation determines cellular localization and stability of ataxin-3. Hum. Mol. Genet. 2009;18:3334–3343. doi: 10.1093/hmg/ddp274. [DOI] [PubMed] [Google Scholar]
- 215.Pastori V., Sangalli E., Coccetti P., Pozzi C., Nonnis S., Tedeschi G., et al. CK2 and GSK3 phosphorylation on S29 controls wild-type ATXN3 nuclear uptake. Biochim. Biophys. Acta. 2010;1802:583–592. doi: 10.1016/j.bbadis.2010.03.007. [DOI] [PubMed] [Google Scholar]