Key Points
Question
Is population-based atrial fibrillation (AF) screening using wearable devices cost-effective?
Findings
In this economic evaluation of 30 million simulated individuals with an age, sex, and comorbidity profile matching the US population aged 65 years or older, AF screening using wearable devices was cost-effective, with the overall preferred strategy identified as wearable photoplethysmography, followed conditionally by wearable electrocardiography with patch monitor confirmation (incremental cost-effectiveness ratio, $57 894 per quality-adjusted life-year). The cost-effectiveness of screening was consistent across multiple scenarios, including strata of sex, screening at earlier ages, and with variation in the association of anticoagulation with risk of stroke associated with screening-detected AF.
Meaning
This study suggests that contemporary AF screening using wearable devices may be cost-effective.
Abstract
Importance
Undiagnosed atrial fibrillation (AF) is an important cause of stroke. Screening for AF using wrist-worn wearable devices may prevent strokes, but their cost-effectiveness is unknown.
Objective
To evaluate the cost-effectiveness of contemporary AF screening strategies, particularly wrist-worn wearable devices.
Design, Setting, and Participants
This economic evaluation used a microsimulation decision-analytic model and was conducted from September 8, 2020, to May 23, 2022, comprising 30 million simulated individuals with an age, sex, and comorbidity profile matching the US population aged 65 years or older.
Interventions
Eight AF screening strategies, with 6 using wrist-worn wearable devices (watch or band photoplethysmography, with or without watch or band electrocardiography) and 2 using traditional modalities (ie, pulse palpation and 12-lead electrocardiogram) vs no screening.
Main Outcomes and Measures
The primary outcome was the incremental cost-effectiveness ratio, defined as US dollars per quality-adjusted life-year (QALY). Secondary measures included rates of stroke and major bleeding.
Results
In the base case analysis of this model, the mean (SD) age was 72.5 (7.5) years, and 50% of the individuals were women. All 6 screening strategies using wrist-worn wearable devices were estimated to be more effective than no screening (range of QALYs gained vs no screening, 226-957 per 100 000 individuals) and were associated with greater relative benefit than screening using traditional modalities (range of QALYs gained vs no screening, −116 to 93 per 100 000 individuals). Compared with no screening, screening using wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100 000 person-years but an increase in major bleeding by 20 to 44 per 100 000 person-years. The overall preferred strategy was wearable photoplethysmography, followed conditionally by wearable electrocardiography with patch monitor confirmation, which had an incremental cost-effectiveness ratio of $57 894 per QALY, meeting the acceptability threshold of $100 000 per QALY. The cost-effectiveness of screening was consistent across multiple scenarios, including strata of sex, screening at earlier ages (eg, ≥50 years), and with variation in the association of anticoagulation with risk of stroke in the setting of screening-detected AF.
Conclusions and Relevance
This economic evaluation of AF screening using a microsimulation decision-analytic model suggests that screening using wearable devices is cost-effective compared with either no screening or AF screening using traditional methods.
This economic evaluation uses a microsimulation decision-analytic model to assess the cost-effectiveness of contemporary atrial fibrillation screening strategies, particularly wrist-worn wearable devices.
Introduction
Undiagnosed atrial fibrillation (AF) is an important cause of stroke.1 Oral anticoagulation (OAC) can prevent AF-related strokes.2 Population-based AF screening may facilitate earlier initiation of OAC treatment to prevent stroke, but improved outcomes have not been clearly demonstrated.3,4,5,6 Most conventional AF screening strategies studied to date have used single time-point screening,7,8,9 which may be limited in the detection of paroxysmal AF. More recently, it has become possible to perform screening using wrist-worn wearable devices, which may facilitate repeated screening with greater sampling density and over longer durations to detect less-frequent AF episodes. However, longer screening durations may lead to increased costs and harms associated with downstream testing and false positives.10,11 False positives may be amplified by disproportionate screening of younger individuals, who are more likely to use wearable devices and less likely to have AF.12 The association between very infrequent AF episodes and stroke risk is less clear.10
Given the uncertainty regarding the effectiveness of AF screening, current guidelines offer conflicting endorsements. Cardiology societies from Europe13 and Australia or New Zealand14 provide a class I recommendation for opportunistic screening using pulse palpation or electrocardiography (ECG) rhythm strip for individuals 65 years of age or older. In contrast, the US Preventive Services Task Force has concluded that there is insufficient evidence for or against AF screening using ECG.15 Logistical constraints may render randomized clinical trials comparing the myriad potential AF screening strategies impractical. For example, many consumers are increasingly adopting wearable devices,16 potentially contaminating any randomization approach, and trials assessing hard end points, such as stroke, require exceptionally large samples for adequate power.
We therefore developed a simulation model to flexibly compare multiple AF screening strategies, including both traditional modalities (eg, 12-lead ECG [12L ECG]) and wrist-worn wearable devices. A previous study using a related model demonstrated that screening via wearable devices may decrease AF-related stroke on a population level.11 Because mass AF screening may lead to increased health care use and associated costs, however, it is imperative to assess whether the benefits of screening come at a cost acceptable to potential payers.
In the present study, the cost-effectiveness of 8 AF screening strategies, including 6 using wrist-worn wearable devices, was compared with no screening. The model adopted a health care system perspective and a screening regimen under the hypothetical scenario that a clinician would prescribe the wearable device.
Methods
Model Overview
The general structure of our AF screening simulation model has been described previously.11 In brief, the clinical course of AF was modeled using a patient-level state-transition approach, implemented using C++. The model simulated a 30 million–person cohort matched on age and comorbidity distribution with the 2019 US population aged 65 years or older—the age at which AF screening is recommended by guidelines.13,14 A 50%-50% distribution of sexes was assumed, approximating the US sex distribution. Only individuals at sufficient risk of stroke to merit OAC treatment based on the CHA2DS2-VASC (cardiac failure or dysfunction, hypertension, age 65-74 [1 point] or ≥75 years [2 points], diabetes, and stroke, transient ischemic attack, or thromboembolism [2 points]–vascular disease, and sex category [female]) risk score17,18 in the presence of an AF diagnosis were screened. The time between state transitions was 1 month, and the simulation continued until death or 100 years of age. Given our intent to compare very different AF screening strategies, including those using wearable devices (which have not been assessed in randomized clinical trials, to our knowledge), the association of screening with outcomes was estimated using population-based estimates of event rates and treatment effects, as well as the test characteristics of the screening modalities used, while accounting for uncertainty in model estimates. Model development and reporting were performed in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline. The analysis was conducted from September 8, 2020, to May 23, 2022. The study was exempt from institutional review board review because it used only publicly available data and was not human participants research. Informed consent was not necessary because simulated individuals were used.
Screening Strategies
The simulation included no screening in addition to 8 AF screening strategies. Six strategies using wrist-worn wearable devices were modeled, with strategies chosen based on evidence of clinical effectiveness in a prior analysis,11 as well as 2 traditional strategies previously studied in randomized clinical trials of AF screening8 as a comparison (ie, pulse palpation with confirmatory 12L ECG and 12L ECG alone). An overview of all strategies is shown in Figure 1.
Figure 1. Simulated Atrial Fibrillation Screening Strategies.
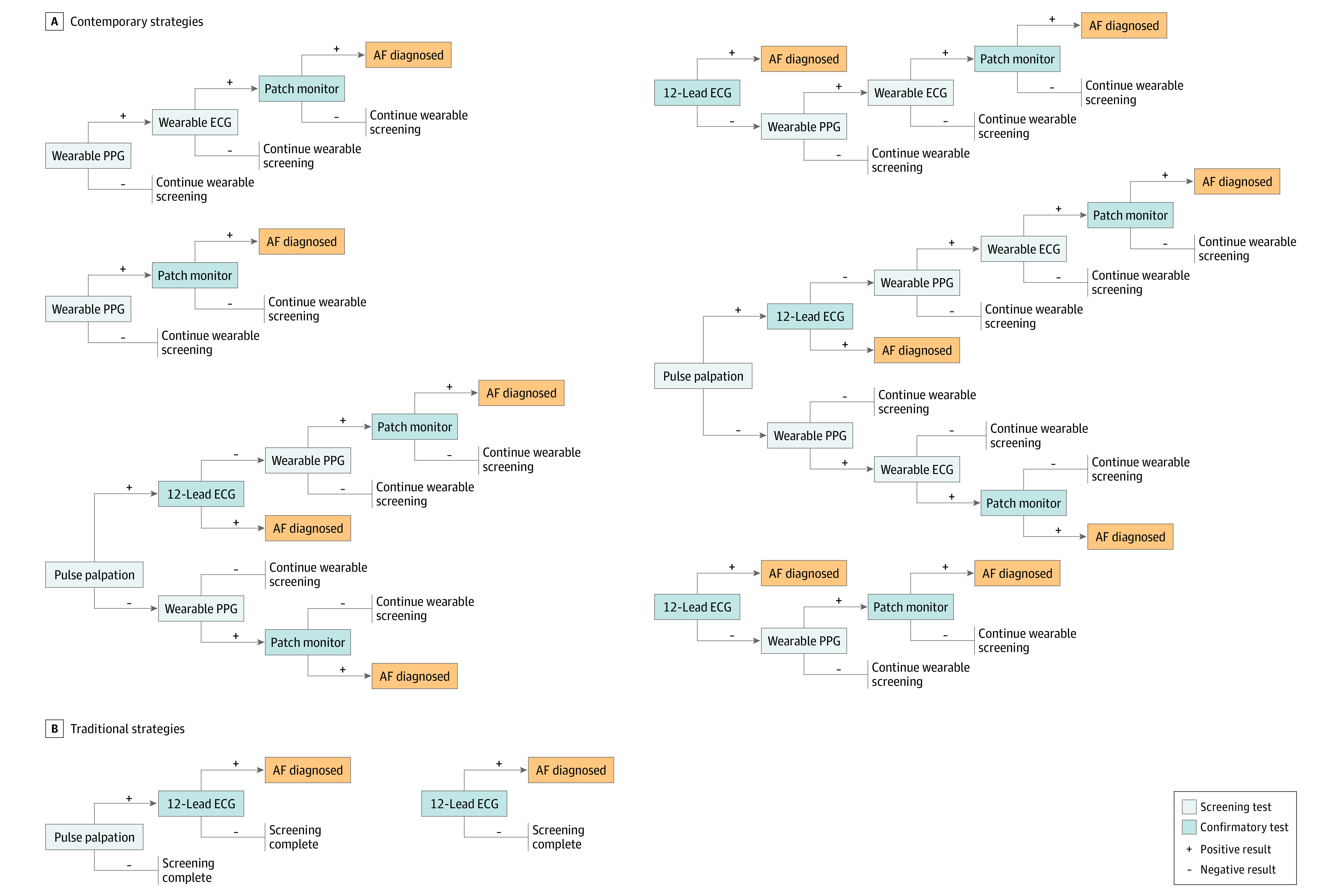
Eight atrial fibrillation (AF) screening strategies evaluated in our cost-effectiveness model are shown. Six contemporary strategies use a wearable device in the screening pathway. Two established traditional screening strategies were included for comparison. ECG indicates electrocardiography; PPG, photoplethysmography.
As performed previously,11 we assessed wearable devices with photoplethysmography (PPG) with or without reflexive single-lead ECG (1L ECG) capability. For wearable devices with both PPG and 1L ECG capability, PPG was the default function and operated passively throughout the wear time. Single-lead ECG would be triggered only after abnormal PPG signals. Discrete modalities were those capable only of instantaneous AF detection (eg, pulse palpation, 12L ECG), and confirmatory tests were those performed conditionally after an abnormal result on a previous test. Lifetime screening was modeled for wearable devices, with adjustment for clinical attrition rates and incomplete wear time. All positive wearable signals were assumed to require confirmation with 2-week ECG patch monitor (Figure 1) prior to an AF diagnosis being made. Individuals were assumed to adhere to the screening strategy recommended by a clinician, but with consideration of wearable device attrition. There was no crossover among strategies and no background wearable use in the no-screening strategy. Key model parameters are shown in Table 1.19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45
Table 1. Key Clinical Parameters.
| Parameter | Base case (lower-upper estimates) | References |
|---|---|---|
| AF | ||
| Proportion of AF that is undiagnosed | 0.24 (0.22-0.28) | 19,20 |
| Proportion of undiagnosed AF that is persistent | 0.41 (0.04-0.66) | 20,21,22,23 |
| Mean AF burden in individuals with paroxysmal AF, % | 4.5 (1.1-17.0) | 20,24,25 |
| Relative risk of ischemic stroke for paroxysmal screening-detected AF (vs persistent AF)a | 1.00 (0.75-1.00) | 26 (Assumption) |
| Patient factors | ||
| Proportion of OAC that is NOAC (vs warfarin) | 0.33 (0.10-0.50) | 27,28 |
| Yearly probability of warfarin discontinuation | 0.10 (0.08-0.40) | 29,30 |
| Relative risk of NOAC discontinuation (vs warfarin) | 0.69 (0.57-0.84) | 29 |
| Initial uptake of follow-up patch monitoring, % | 100 (62-100) | 31,32 (Assumption) |
| Ischemic stroke | ||
| Proportion of strokes that are ischemic | 0.87 (0.83-0.88) | 33 |
| Relative risk of ischemic stroke | ||
| Aspirin vs placebo, AF | 0.78 (0.65-0.94) | 34 |
| Warfarin vs placebo, AF | 0.33 (0.23-0.46) | 34 |
| OAC vs placebo, no AF | 0.58 (0.44-0.76) | 35 |
| NOAC vs warfarin | 1 (0.83-1.02) | 36 |
| OAC plus aspirin vs OAC alone | 1 (0.44-2.22) | 37 |
| Screening methods | ||
| Sensitivity (single time point), % | ||
| Pulse palpation | 89.0 (16-100) | 8,38,39 |
| Single-lead handheld ECG | 96.9 (36.8-100) | 40,41 |
| Patch monitor | 100 (90-100) | 42 |
| 12-Lead ECG | 90.0 (52-100) | 43 |
| Smartwatch or band | ||
| PPGb | 95.3 (92-97.4) | 31,44 |
| ECG | 85.2 (76.7-98.3) | 45 |
| Specificity (single time point), % | ||
| Pulse palpation | 81.0 (65-91) | 8,38,39 |
| Single-lead handheld ECG | 89.6 (71-100) | 40 |
| Patch monitor | 96.6 (86.9-100) | 42 |
| 12-Lead ECG | 98.3 (55-100) | 43 |
| Smartwatch or band | ||
| PPGb | 99.7 (98.1-99.9) | 31,44 |
| ECGb | 99.6 (89.6-100) | 45 |
Abbreviations: AF, atrial fibrillation; ECG, electrocardiography; NOAC, novel oral anticoagulant; OAC, oral anticoagulation; PPG, photoplethysmography.
Range, 1% to 100% additionally assessed in dedicated 1-way sensitivity analyses.
Values of 85% and 80% additionally assessed in dedicated scenario analyses.
Events and Utilities
Rates of AF, stroke, and bleeding were obtained from the published literature.11 Associations of antithrombotic therapy with AF-related stroke were similarly estimated. In the primary model, paroxysmal and persistent AF conferred similar stroke risk, but because the association between AF burden and stroke risk remains incompletely characterized, we performed sensitivity analyses, varying the relative risk of stroke associated with paroxysmal AF between 1% and 100% of that observed with persistent AF.24,46,47 Adherence to OAC treatment was 100% at AF diagnosis, but discontinuation was modeled using clinical OAC discontinuation rates.29,30 Sensitivity analyses were performed to assess the association with outcomes of incomplete OAC uptake (60%-100%). Utilities were estimated for all major events, including stroke, intracranial hemorrhage, and major bleeding, stratified by severity. Treatment-associated utilities were also modeled. All utilities were derived from the published literature.
Costs
Costs were modeled from the health care system perspective. One-time event costs were applied for ischemic stroke, intracranial hemorrhage, and bleeding events, stratified by severity. Estimates were obtained from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project reflecting US hospitalization data from 2016.48 Long-term event costs were obtained from the published literature and stratified by severity. Treatment-related costs included primary drug costs and costs associated with regular international normalized ratio testing for individuals taking warfarin and annual physician visits for all individuals receiving OAC. Screening-related costs included direct costs associated with the modalities used (eg, cost of patch monitor), nurse visit costs at screening administration, and physician visit costs after each new AF diagnosis (ie, true positive or false positive). For strategies using wrist-worn wearable devices, the manufacturer-suggested retail price of the device was applied as the direct cost. Devices were assumed to require replacement after every 5 years of consecutive screening. Physician and nurse professional fees were obtained from the Centers for Medicare & Medicaid Services.49 All other costs were extracted from the published literature. Cost estimates were adjusted for inflation and converted to US dollars as of June 2020. All event rate, utility, and cost parameters are listed in eTables 1 to 7 in the Supplement.
Cost-effectiveness
For each simulated strategy, total quality-adjusted life-years (QALYs), total costs, and incremental cost-effectiveness ratios (ICERs) were calculated. All future QALYs and costs were discounted at 3% per year. To identify the optimal cost-effective strategy, a willingness-to-pay threshold of $100 000 per QALY was applied.50,51
Scenario Analyses
Multiple scenario analyses were performed to assess the cost-effectiveness of screening across subgroups of clinical interest. Specifically, 3 scenarios for the age distribution of the cohort (≥50, ≥55, and ≥60 years) were assessed, as a surrogate for the age at which AF screening begins. In addition, screening was modeled among only women and separately among only men. Three scenarios for the mean daily wear time of the wrist-worn wearable devices (6, 12, and 24 hours) and 4 scenarios for the cost of the wrist-worn wearable devices ($150, $200, $250, and $300) were also assessed.
Uncertainty Analyses
The association of parameter uncertainty of the strategies with the cost-effectiveness frontier and their corresponding ICERs was assessed using sensitivity analysis. In 1-way sensitivity analyses, a subset of all model parameters chosen based on economic importance, clinical salience, or demonstration of influence in previously published models (eTable 8 in the Supplement) was varied across evidence-based ranges.52,53 In probabilistic sensitivity analyses, all model parameters were varied according to their corresponding random distributions across the same ranges (eTable 8 in the Supplement). Because uncertainty was modeled using probabilistic analyses, formal statistical testing was not used.
Results
Base Case Analyses
In the base case analysis, the mean (SD) age was 72.5 (7.5) years, and 50% of the individuals were women. No screening resulted in 7.092 QALYs per individual. In total, 7 of 8 strategies (87.5%) resulted in additional QALYs compared with no screening (93-957 QALYs gained per 100 000 persons). The single strategy that provided lower QALYs vs no screening was 1-time 12L ECG screening (7.090 QALYs per person). The strategy providing the greatest QALYs was a sequence of wrist-worn wearable PPG followed conditionally by wrist-worn wearable 1L ECG and confirmatory patch monitor (7.101 QALYs per person). All 6 screening strategies involving wrist-worn wearable devices were estimated to be more effective than no screening (226-957 QALYs gained per 100 000 persons) and were associated with greater relative benefit than screening using traditional modalities vs no screening (−116 to 93 QALYs gained per 100 000 persons). Strategies using wrist-worn wearable devices were associated with reduction in stroke incidence by 20 to 23 stroke events per 100 000 person-years but an increase in major bleeding by 20 to 44 major bleeding events per 100 000 person-years.
Compared with no screening, screening using pulse palpation and 12L ECG was cost saving ($43 per person). All other strategies incurred additional costs ($61-$603 per person), with strategies using wrist-worn wearable devices being more costly ($458-$603 per person) than 12L ECG alone ($61 per person screened). Excess costs were primarily associated with direct screening costs (eg, cost of the wrist-worn wearable device) and use of OAC (eTable 9 in the Supplement).
In terms of cost-effectiveness, pulse palpation and 12L ECG dominated no screening ($43 saved per person; 93 QALYs gained per 100 000 persons). The overall cost-effective strategy was wrist-worn wearable PPG followed conditionally by wrist-worn wearable 1L ECG and confirmatory patch monitor (ICER, $57 894 per QALY) (Table 119,20,21,22,23,25,26,27,28,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 and Figure 2), which had a favorable false-positive rate of 0.4% (eTable 10 in the Supplement).
Figure 2. Cost-effectiveness Frontier of Simulated Atrial Fibrillation Screening Strategies.

Each point indicates the combination of effectiveness and cost of a given strategy. The slope connecting 2 points indicates the incremental cost-effectiveness ratio (ICER), a summary measure of cost-effectiveness, representing the ratio of the incremental cost vs the incremental effectiveness associated with going from 1 strategy to another. The diagonal line indicates the ICER of the most cost-effective strategy (wrist-worn wearable photoplethysmography [PPG] followed conditionally by wrist-worn wearable single-lead electrocardiography [ECG] and confirmatory patch monitor; ICER, $57 894). Every row in the table to the right represents a given strategy. For each row, an X indicates that a given modality was included in the screening strategy. Absence of an X indicates that a given modality was not included.
Scenario Analyses
The relative cost-effectiveness of the AF screening strategies modeled was generally consistent across the 12 clinical scenarios considered. Specifically, 1-time screening using pulse palpation and 12L ECG consistently dominated no screening, and the most cost-effective strategy was consistently wrist-worn wearable PPG followed conditionally by wrist-worn wearable 1L ECG and confirmatory patch monitor (ICER range, $34 583-$90 909 per QALY).
Across scenarios of target age distribution (Table 2), the ICER of the most cost-effective strategy increased as the minimum age of the cohort decreased starting with individuals aged 60 years or older (individuals aged ≥60 years: $59 143 per QALY; individuals aged ≥50 years: $90 909 per QALY), although screening remained cost-effective even at the lowest target age evaluated (≥50 years). Similarly, the ICER of the most cost-effective strategy increased as the cost of the wrist-worn wearable increased from $150 to $300 ($150: $34 583 per QALY; $300: $76 956 per QALY) and the mean daily wear time decreased from 24 hours to 6 hours (24 hours: $59 288 per QALY; 6 hours: $56 314 per QALY), but increases were generally modest (eTable 11 in the Supplement). Screening with the preferred strategy also remained cost-effective when targeting only women (ICER, $57 340 per QALY) and separately only men (ICER, $60 375 per QALY). Cost-effectiveness also persisted when values as low as 80% were assumed for wearable sensitivity and specificity (ICER: 80% with PPG, $57 874 per QALY; 80% with ECG, $69 891 per QALY). Details of scenario analyses are shown in eTable 11 in the Supplement.
Table 2. Cost-effectiveness Results in the Base Case and Across Varying Age Thresholdsa.
| Strategy | QALYs | Cost, $ | Incremental cost-effectiveness ratio, $/QALY | |||||
|---|---|---|---|---|---|---|---|---|
| PP | 12L ECG | PPG | 1L ECG | PM | Frequency | |||
| Aged ≥65 y (base case) | ||||||||
| X | X | Once | 7.09 | 30 182 | [Reference] | |||
| X | X | X | Life | 7.10 | 30 683 | 57 894 | ||
| No screening | 7.09 | 30 225 | Strongly dominated | |||||
| X | Once | 7.09 | 30 286 | Strongly dominated | ||||
| X | X | X | Life | 7.09 | 30 828 | Strongly dominated | ||
| X | X | X | X | Life | 7.10 | 30 735 | Strongly dominated | |
| X | X | Life | 7.10 | 30 730 | Strongly dominated | |||
| X | X | X | X | Life | 7.10 | 30 772 | Strongly dominated | |
| X | X | X | X | X | Life | 7.10 | 30 698 | Strongly dominated |
| Aged ≥60 y | ||||||||
| X | X | Once | 8.26 | 31 396 | [Reference] | |||
| X | X | X | Life | 8.27 | 31 927 | 59 143 | ||
| No screening | 8.26 | 31 410 | Strongly dominated | |||||
| X | Once | 8.25 | 31 500 | Strongly dominated | ||||
| X | X | X | Life | 8.26 | 32 118 | Strongly dominated | ||
| X | X | X | X | Life | 8.26 | 31 991 | Strongly dominated | |
| X | X | Life | 8.26 | 32 009 | Strongly dominated | |||
| X | X | X | X | Life | 8.26 | 32 035 | Strongly dominated | |
| X | X | X | X | X | Life | 8.27 | 31 914 | Weakly dominated |
| Aged ≥55 y | ||||||||
| X | X | Once | 9.35 | 29 813 | [Reference] | |||
| X | X | X | Life | 9.36 | 30 448 | 76 889 | ||
| No screening | 9.35 | 29 855 | Strongly dominated | |||||
| X | X | 9.35 | 29 960 | Strongly dominated | ||||
| X | X | X | 9.35 | 30 651 | Strongly dominated | |||
| X | X | X | X | X | 9.35 | 30 537 | Strongly dominated | |
| X | 9.36 | 30 527 | Strongly dominated | |||||
| X | X | X | X | 9.35 | 30 603 | Strongly dominated | ||
| X | X | X | X | X | X | 9.36 | 30 458 | Strongly dominated |
| Aged ≥50 y | ||||||||
| X | X | Once | 10.31 | 28 538 | [Reference] | |||
| No screening | 10.31 | 28 555 | 57 333 | |||||
| X | X | X | Life | 10.32 | 29 255 | 90 909 | ||
| X | Once | 10.30 | 28 683 | Strongly dominated | ||||
| X | X | X | Life | 10.30 | 29 433 | Strongly dominated | ||
| X | X | X | X | Life | 10.31 | 29 285 | Strongly dominated | |
| X | X | Life | 10.31 | 29 318 | Strongly dominated | |||
| X | X | X | X | Life | 10.31 | 29 389 | Strongly dominated | |
| X | X | X | X | X | Life | 10.32 | 29 261 | Strongly dominated |
Abbreviations: PM, patch monitor; PP, pulse palpation; PPG, photoplethysmography; QALYs, quality-adjusted life-years; 1L ECG, single-lead electrocardiogram; 12L ECG, 12-lead electrocardiogram.
Every row represents a given strategy. For each row, an X indicates that a given modality was included in the screening strategy. Absence of an X indicates that a given modality was not included.
Sensitivity Analyses
One-way sensitivity analyses were broadly consistent with base case results. Specifically, wrist-worn wearable PPG followed conditionally by wrist-worn wearable 1L ECG and confirmatory patch monitor remained the most cost-effective strategy in 94% of scenarios (ICER range, $47 822-$71 576 per QALY) (eTable 12 in the Supplement). Modality specificity was particularly associated with cost-effectiveness. For example, at lower 12L ECG specificity (76%), 1-time screening using pulse palpation and 12L ECG was no longer cost-effective. At a higher wrist-worn PPG specificity (100%), wrist-worn wearable PPG followed conditionally by patch monitor alone (ie, without reflexive 1L ECG) was preferred (ICER, $56 610 per QALY). The relative risk of stroke given paroxysmal vs persistent AF was also associated with cost-effectiveness, whereby wearable screening remained the preferred strategy until stroke risk with paroxysmal AF decreased less than 5% of that observed with persistent AF, at which point traditional screening with pulse palpation followed by 12L ECG became the preferred strategy. Wearable screening remained cost-effective even as initial OAC uptake varied between 60% and 100%. All 1-way sensitivity analyses are presented in eTable 12 in the Supplement.
Probabilistic sensitivity analyses demonstrated moderate changes in cost-effectiveness associated with parameter uncertainty. The preferred strategy in the base case remained cost-effective in the plurality (33%) of simulations (Figure 3). Two strategies emerged as cost-effective in a considerable proportion of simulations, specifically pulse palpation and 12L ECG followed conditionally by wrist-worn wearable PPG with confirmatory patch monitor (25% of simulations) and 12L ECG followed conditionally by wrist-worn wearable PPG and confirmatory patch monitor (20% of simulations). Cost-effectiveness acceptability curves are shown in eFigure in the Supplement.
Figure 3. Probabilistic Sensitivity Analysis.
Each bar indicates the probability that a given strategy is cost-effective, when accounting for parameter uncertainty. Strategies are displayed in order of decreasing probability of cost-effectiveness, with the strategy most likely to be cost-effective at the top. Every row in the table to the left represents a given strategy. For each row, an X indicates that a given modality was included in the screening strategy. Absence of an X indicates that a given modality was not included. ECG indicates electrocardiography; PPG, photoplethysmography.
Discussion
With the use of a comprehensive economic model to simulate 30 million individuals matching the US population with a guideline-based indication for AF screening (ie, aged ≥65 years),13,14 AF screening using wearable devices was estimated to be cost-effective. Specifically, of 8 strategies evaluated in addition to no screening, the economically preferred strategy was wrist-worn wearable PPG followed conditionally by wrist-worn wearable 1L ECG with patch monitor confirmation, having an ICER just below $60 000 per QALY. In scenario analyses, wearable screening remained cost-effective across multiple conditions, including targeting individuals at younger ages than currently endorsed by guidelines (eg, ≥50 years).13,14 On balance, our findings suggest that AF screening using wearable devices may be cost-effective.
Our study extends previous findings by comparing the cost-effectiveness of AF screening using both traditional modalities and wearable devices. Aronsson et al47 previously estimated that AF screening with handheld 1L ECG twice daily over 2 weeks is cost-effective among individuals aged 75 years or older (ICER, €7855 per QALY) as well as among individuals aged 65 years or older (ICER, €24 500 per QALY).54 Given our focus on wearable devices, we did not directly evaluate handheld 1L ECG, although we observed that a similar combination of pulse palpation followed by 12L ECG was on the cost-effectiveness frontier with comparable ICERs. More recently, Oguz et al55 found that 1-time screening using 12L ECG alone followed by 14-day screening with patch monitor at 75 years of age was also cost-effective. In general, our findings support previous observations suggesting that AF screening can be cost-effective, and they provide new evidence that strategies using wearable devices may be economically favorable.
Our results provide justification for the potential use of wrist-worn wearable devices integrated into composite AF screening pathways. The use of wearable devices for AF screening is expanding,16 but their optimal role within an AF screening program is unclear. The most cost-effective strategy that we identified was wrist-worn wearable PPG followed conditionally by wrist-worn wearable 1L ECG and confirmatory patch monitor, providing a net benefit at $57 894 per QALY. The use of wrist-worn wearable devices over years may facilitate an extended duration of screening, which may lead to detection of infrequent paroxysmal AF that may be otherwise difficult to ascertain. At the same time, the use of patch monitor confirmation of abnormal signals from wrist-worn wearable devices reduced false positives. Wearable screening remained cost-effective even as analyzable wear time decreased to 6 hours per day,56 suggesting that wearable devices may remain effective even if wear time is incomplete or limited to certain periods (eg, sleep). Assessment of contemporary screening pathways, including wearable devices applied over many years, may account for differences between our results and the results of recent trials demonstrating little or no effect of discrete screening on short-term AF diagnosis.4,7,9 The preferred screening pathway began directly with wearable devices, suggesting that screening initiated at the level of the consumer device may be economically favorable. Randomized clinical trials are warranted to better quantify potential benefits of AF screening using wearable devices, and our findings may be useful to inform the design of such trials to optimize clinical and economic efficiency.
The cost-effectiveness of AF screening pathways may be maximized by using scalable, highly sensitive screening methods followed by confirmatory testing. Both screening strategies appearing on the cost-effectiveness frontier included a highly sensitive modality upfront (eg, pulse palpation, wearable PPG), followed by a confirmatory test (eg, 12L ECG, patch monitor). Use of a sensitive modality likely functions to rule out most individuals without AF with relatively modest resource use. At the same time, confirmatory testing prevents excess costs and harm associated with false positives. Our results suggest that the optimal combination of modalities may depend on the resources available to the health care system, with wrist-worn wearable screening and confirmatory patch monitor identified as the preferred strategy at the $100 000 per QALY threshold, whereas pulse palpation and 12L ECG may be preferable in lower-resource settings. Because health care costs are relatively high in the US and vary across settings,57 future analyses of other health care systems may be useful to quantify the cost-effectiveness of AF screening using wearable devices in alternative settings.
Our analyses suggest that contemporary AF screening remains cost-effective across a range of scenarios and may even extend to populations not currently considered for AF screening. Wearable screening remained cost-effective across strata of sex and substantial variations in wearable device–related costs and mean daily wear time. Cost-effectiveness of wearable screening also persisted when assuming values as low as 80% for the sensitivity and specificity of wearable modalities, likely owing to the use of patch monitor confirmation. In analyses varying the target age for screening, we observed continued cost-effectiveness even as screening was extended to individuals aged 50 years or older with risk factors for stroke. On balance, such findings suggest that AF screening may be effective and economically acceptable even among individuals below the guideline-based age threshold of 65 years who have risk factors for AF-related stroke.13,14
Limitations
This study has some limitations. First, this is a simulation study using population-based estimates to infer the cost-effectiveness of several AF screening strategies, some of which have not been assessed in randomized clinical trials. Nevertheless, our results are consistent with previous analyses of traditional screening modalities14,47 and remained robust across wide variation in model estimates to account for uncertainty. Second, the test characteristics of contemporary AF screening modalities are less well known than those for more established modalities. As a result, we varied all test characteristics over plausible ranges in sensitivity analyses. Third, to quantify the relative associations of each AF screening strategy with outcomes, we assumed no background wearable technology use in the no-screening strategy. Current data on the use of wearable devices, particularly among the older age groups being simulated, are too limited to accurately model.12 Fourth, we assumed in our base model that paroxysmal AF and persistent AF were associated with similar stroke risk, although recent evidence suggests that AF burden may be significantly associated with stroke.10 Nevertheless, we found that wearable screening remained cost-effective even when the relative risk of stroke with paroxysmal AF was only 5% that of persistent AF (ie, assuming a larger association between AF burden and stroke than previously reported).10,58 Fifth, a large simulation size and longer screening duration allowed us to detect potential associations of long-term AF screening with stroke, which, although clinically significant on a population scale, may be challenging to demonstrate in a traditional clinical trial.6,7,59 Therefore, although our results cannot be considered definitive, they prioritize specific wearable screening strategies as likely to be cost-effective, thereby meriting dedicated prospective study.
Conclusions
This modeling study of 8 contemporary AF screening strategies suggests that specific forms of AF screening using wearable devices may be cost-effective. The cost-effectiveness of AF screening enabled by wearable devices persisted across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke. When deployed within specific AF screening pathways, wearable devices are likely to be an important component of cost-effective AF screening.
eMethods. Supplemental Methods
eTable 1. Disease Incidence (per 1000 Person-Years)
eTable 2. Comorbidity Prevalence/Incidence (per 1000 Person-Years)
eTable 3. Disease Recurrence Rates (Monthly Probabilities)
eTable 4. Disease-Related Mortality (Monthly Probabilities)
eTable 5. Severity Measures
eTable 6. Utilities
eTable 7. Costs
eTable 8. Summary of Parameters Included in Sensitivity Analyses
eTable 9. Summary of Costs Associated With Each Screening Strategy
eTable 10. True and False Positive Rates by Strategy
eTable 11. Cost-effectiveness Results for Scenario Analyses
eTable 12. One-Way Sensitivity Analysis
eFigure. Cost-effectiveness Acceptability Curves
eReferences.
References
- 1.Lubitz SA, Yin X, McManus DD, et al. Stroke as the initial manifestation of atrial fibrillation: the Framingham Heart Study. Stroke. 2017;48(2):490-492. doi: 10.1161/STROKEAHA.116.015071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly: the Framingham Study. Arch Intern Med. 1987;147(9):1561-1564. doi: 10.1001/archinte.1987.00370090041008 [DOI] [PubMed] [Google Scholar]
- 3.Khurshid S, Healey JS, McIntyre WF, Lubitz SA. Population-based screening for atrial fibrillation. Circ Res. 2020;127(1):143-154. doi: 10.1161/CIRCRESAHA.120.316341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladstone DJ, Wachter R, Schmalstieg-Bahr K, et al. ; SCREEN-AF Investigators and Coordinators . Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol. 2021;6(5):558-567. doi: 10.1001/jamacardio.2021.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398(10310):1507-1516. doi: 10.1016/S0140-6736(21)01698-6 [DOI] [PubMed] [Google Scholar]
- 6.Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet. 2021;398(10310):1498-1506. doi: 10.1016/S0140-6736(21)01637-8 [DOI] [PubMed] [Google Scholar]
- 7.Uittenbogaart SB, Verbiest-van Gurp N, Lucassen WAM, et al. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ. 2020:m3208. doi: 10.1136/bmj.m3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs FDR, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over: the SAFE study. Health Technol Assess. 2005;9(40):iii-iv, ix-x, 1-74. doi: 10.3310/hta9400 [DOI] [PubMed] [Google Scholar]
- 9.Ashburner JM, Atlas SJ, McManus DD, et al. Design and rationale of a pragmatic trial integrating routine screening for atrial fibrillation at primary care visits: the VITAL-AF trial. Am Heart J. 2019;215:147-156. doi: 10.1016/j.ahj.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140(20):1639-1646. doi: 10.1161/CIRCULATIONAHA.119.041303 [DOI] [PubMed] [Google Scholar]
- 11.Khurshid S, Chen W, Singer DE, et al. Comparative clinical effectiveness of population-based atrial fibrillation screening using contemporary modalities: a decision-analytic model. J Am Heart Assoc. 2021;10(18):e020330. doi: 10.1161/JAHA.120.020330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekaran R, Katthula V, Moustakas E. Patterns of use and key predictors for the use of wearable health care devices by US adults: insights from a national survey. J Med Internet Res. 2020;22(10):e22443. doi: 10.2196/22443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hindricks G, Potpara T, Dagres N, et al. ; ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 14.Brieger D, Amerena J, Attia J, et al. ; NHFA CSANZ Atrial Fibrillation Guideline Working Group . National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018;27(10):1209-1266. doi: 10.1016/j.hlc.2018.06.1043 [DOI] [PubMed] [Google Scholar]
- 15.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327(4):360-367. doi: 10.1001/jama.2021.23732 [DOI] [PubMed] [Google Scholar]
- 16.Musil S. One in 10 American adults expected to have a smartwatch next year. CNet.com. Published December 13, 2018. Accessed January 1, 2020. https://www.cnet.com/news/one-in-10-american-adults-expected-to-have-a-smartwatch-next-year/
- 17.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Calkins H, et al. ; Writing Group Members . 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16(8):e66-e93. doi: 10.1016/j.hrthm.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 19.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131(25):2176-2184. doi: 10.1161/CIRCULATIONAHA.114.014343 [DOI] [PubMed] [Google Scholar]
- 20.Rooney MR, Soliman EZ, Lutsey PL, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC study. Circ Arrhythm Electrophysiol. 2019;12(10):e007390. doi: 10.1161/CIRCEP.119.007390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320(2):146-155. doi: 10.1001/jama.2018.8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136(19):1784-1794. doi: 10.1161/CIRCULATIONAHA.117.030583 [DOI] [PubMed] [Google Scholar]
- 23.Fitzmaurice DA, Hobbs FDR, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335(7616):383. doi: 10.1136/bmj.39280.660567.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Go AS, Reynolds K, Yang J, et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol. 2018;3(7):601-608. doi: 10.1001/jamacardio.2018.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015;8(5):1040-1047. doi: 10.1161/CIRCEP.114.003057 [DOI] [PubMed] [Google Scholar]
- 26.Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies: the SEARCH-AF study. Thromb Haemost. 2014;111(6):1167-1176. doi: 10.1160/TH14-03-0231 [DOI] [PubMed] [Google Scholar]
- 27.Lubitz SA, Khurshid S, Weng LC, et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J. 2018;200:24-31. doi: 10.1016/j.ahj.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69(20):2475-2484. doi: 10.1016/j.jacc.2017.03.540 [DOI] [PubMed] [Google Scholar]
- 29.Lip GYH, Pan X, Kamble S, et al. Discontinuation risk comparison among “real-world” newly anticoagulated atrial fibrillation patients: apixaban, warfarin, dabigatran, or rivaroxaban. PLoS One. 2018;13(4):e0195950. doi: 10.1371/journal.pone.0195950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien EC, Simon DN, Allen LA, et al. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2014;168(4):487-494. doi: 10.1016/j.ahj.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Wang H, Zhang H, et al. ; MAFA II Investigators . Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74(19):2365-2375. doi: 10.1016/j.jacc.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 32.Perez MV, Mahaffey KW, Hedlin H, et al. ; Apple Heart Study Investigators . Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909-1917. doi: 10.1056/NEJMoa1901183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 34.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. doi: 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 35.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 36.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. doi: 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 37.Matsumura-Nakano Y, Shizuta S, Komasa A, et al. ; OAC-ALONE Study Investigators . Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139(5):604-616. doi: 10.1161/CIRCULATIONAHA.118.036768 [DOI] [PubMed] [Google Scholar]
- 38.Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract. 2002;52(478):373-374, 377-380. [PMC free article] [PubMed] [Google Scholar]
- 39.Sudlow M, Rodgers H, Kenny RA, Thomson R. Identification of patients with atrial fibrillation in general practice: a study of screening methods. BMJ. 1998;317(7154):327-328. doi: 10.1136/bmj.317.7154.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193-194. doi: 10.1016/j.ijcard.2013.01.220 [DOI] [PubMed] [Google Scholar]
- 41.Giebel GD, Gissel C. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth. 2019;7(6):e13641. doi: 10.2196/13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mena LJ, Félix VG, Ochoa A, et al. Mobile personal health monitoring for automated classification of electrocardiogram signals in elderly. Comput Math Methods Med. 2018;2018:9128054. doi: 10.1155/2018/9128054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taggar JS, Coleman T, Lewis S, Heneghan C, Jones M. Accuracy of methods for diagnosing atrial fibrillation using 12-lead ECG: a systematic review and meta-analysis. Int J Cardiol. 2015;184:175-183. doi: 10.1016/j.ijcard.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 44.Fan YY, Li YG, Li J, et al. Diagnostic performance of a smart device with photoplethysmography technology for atrial fibrillation detection: pilot study (pre-mAFA II registry). JMIR Mhealth Uhealth. 2019;7(3):e11437. doi: 10.2196/11437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apple Inc. Using apple watch for arrhythmia detection. Accessed July 1, 2022. https://www.apple.com/uk/healthcare/docs/site/Apple_Watch_Arrhythmia_Detection.pdf
- 46.Connolly S, Pogue J, Hart R, et al. ; ACTIVE Writing Group of the ACTIVE Investigators . Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention Of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367(9526):1903-1912. doi: 10.1016/S0140-6736(06)68845-4 [DOI] [PubMed] [Google Scholar]
- 47.Aronsson M, Svennberg E, Rosenqvist M, et al. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17(7):1023-1029. doi: 10.1093/europace/euv083 [DOI] [PubMed] [Google Scholar]
- 48.Agency for Healthcare Research and Quality . Accessed September 8, 2020. https://hcupnet.ahrq.gov/#setup
- 49.Centers for Medicare & Medicaid Services . Accessed September 8, 2020. https://www.cms.gov/medicare/physician-fee-schedule/search [PubMed]
- 50.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 51.Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann Intern Med. 2021;174(1):25-32. doi: 10.7326/M20-1392 [DOI] [PubMed] [Google Scholar]
- 52.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305(4):373-380. doi: 10.1001/jama.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115-1119. doi: 10.1161/01.STR.0000166053.83476.4a [DOI] [PubMed] [Google Scholar]
- 54.Aronsson M, Svennberg E, Rosenqvist M, et al. Designing an optimal screening program for unknown atrial fibrillation: a cost-effectiveness analysis. Europace. 2017;19(10):1650-1656. doi: 10.1093/europace/eux002 [DOI] [PubMed] [Google Scholar]
- 55.Oguz M, Lanitis T, Li X, et al. Cost-effectiveness of extended and one-time screening versus no screening for non-valvular atrial fibrillation in the USA. Appl Health Econ Health Policy. 2020;18(4):533-545. doi: 10.1007/s40258-019-00542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lubitz SA, Faranesh AZ, Atlas SJ, et al. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the Fitbit Heart Study. Am Heart J. 2021;238:16-26. doi: 10.1016/j.ahj.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 57.Tikkanen R, Abrams MK. U.S. health care from a global perspective 2019: higher spending, worse outcomes? Accessed May 25, 2022. https://www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
- 58.Noseworthy PA, Kaufman ES, Chen LY, et al. ; American Heart Association Council on Clinical Cardiology Electrocardiography and Arrhythmias Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council . Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140(25):e944-e963. doi: 10.1161/CIR.0000000000000740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lubitz SA, Atlas SJ, Ashburner JM, et al. Screening for atrial fibrillation in older adults at primary care visits: VITAL-AF randomized controlled trial. Circulation. 2022;145(13):946-954. doi: 10.1161/CIRCULATIONAHA.121.057014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eTable 1. Disease Incidence (per 1000 Person-Years)
eTable 2. Comorbidity Prevalence/Incidence (per 1000 Person-Years)
eTable 3. Disease Recurrence Rates (Monthly Probabilities)
eTable 4. Disease-Related Mortality (Monthly Probabilities)
eTable 5. Severity Measures
eTable 6. Utilities
eTable 7. Costs
eTable 8. Summary of Parameters Included in Sensitivity Analyses
eTable 9. Summary of Costs Associated With Each Screening Strategy
eTable 10. True and False Positive Rates by Strategy
eTable 11. Cost-effectiveness Results for Scenario Analyses
eTable 12. One-Way Sensitivity Analysis
eFigure. Cost-effectiveness Acceptability Curves
eReferences.



