Abstract
Background
To contribute to the mission of the National Malaria Control Programme (NMCP) and guide future interventions in Cameroon in general, and in Makenene in particular, this study assessed the knowledge, attitudes and practices of the population of Makenene towards the fight against malaria.
Methods
Using a semi-structured questionnaire, a descriptive cross-sectional household community survey was carried out in randomly selected households in Makenene, a locality situated between forest and savannah ecotypes.
Results
Out of the 413 households surveyed, all (100%) claimed to have heard of malaria with over 94% (n = 391) associating disease transmission with mosquito bites. The main mosquito control tools used in the area were mosquito nets (92.25%). The majority of participants had good knowledge (55.93%; n = 231), good practices (71.67%, n = 296) but moderate attitudes (47.94%; n = 198) towards malaria control and fight. Good knowledge and practices were recorded mostly in educated persons including public servants and students. Good attitudes were adopted mostly by public servants and students of secondary and higher levels of education.
Conclusion
In Makenene, the population exhibits good knowledge and practices towards malaria and its control. However, despite high LLINs ownership and use, people still complain about malaria in the area. Control tools should be monitored, repaired or replaced when necessary to support the achievement of the NMCP mission.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-022-04253-z.
Keywords: Knowledge, Attitudes, Practices, Malaria, Households, Makenene
Background
Malaria still remains a real public health problem in the world especially in Africa. In 2020, 241 million cases and 627,000 death were reported worldwide [1]. In Cameroon, malaria affects the whole country with different levels of endemicity according to ecological settings, ranging from hypo-endemic to hyper-endemic areas [2]. With more than 11 million cases per year, malaria is one of the main reasons for consultation in many health centres in Cameroon, especially for children under 5 years and pregnant women [3]. Vector control operations through the use of long-lasting insecticidal nets (LLINs), and rapid patient care based on an early diagnosis and administration of effective artemisinin-based combinations drugs, are among measures employed by the Government to limit the progress of the disease [3].
However, several factors contribute to the underperformance of the current control methods leading to malaria persistence. These include the rapid expansion of vector resistance to insecticides, changes in biting and resting behaviour and the bad utilization of preventive methods [4–7]. Added to this, the social consequences linked to the COVID 19 pandemic are being noted [8, 9]. In fact, during this period, efforts in the health services mostly oriented towards the COVID 19, retort activities in detriments to malaria prevention and treatment. In addition to disruptions in malaria diagnosis and treatment, the fear to catch COVID 19 by population in hospital reduced significantly their visit in case of suspected malaria cases or other diseases during COVID 19 pandemic [10–13], increasing auto medication [14]. Furthermore, the lack of knowledge about the disease is sometimes responsible for an approximate treatment which might lead to other types of resistance such as the emergence of anti-malarial drugs resistance, and results in repeated cases of malaria within the same household [15, 16].
In order to better understand the limits of LLINs deployed so far by public authorities, factors related to human behaviour must be taken into account for an effective behaviour change towards the elimination of the disease [17]. Previous studies in some localities have shown that, people's adherence to malaria control measures depends in part on their intellectual and cultural background [18, 19]. However, this background differs from one locality to another [5, 17–22]. In fact, the level of malaria endemicity in a locality (entomological inoculation rate and malaria infection rate) depends upon environmental factors. This latter vary from a locality to another, and characterize the different facies, which are widely spreaded in Cameroon. The country therefore displays from Southern part to the Northern zone: the rainy equatorial forest with hyperendemic malaria [23, 24], the savannah zone with hyperendemic malaria and the sahelian zone in the North with hypoendemic malaria [2]. Within each of these zones, some microfacies can be defined, where malaria endemicity is modified either by the nature or the human activities such as: highland areas [25, 26], damps and other industrial infrastructures [27], urbanizations, and forest-savannah intermediate localities which is the case of Makenene. The locality of Makenene is prone to imported malaria, because it is a crossroads for travellers from different parts of the country. Moreover, the locality has not benefited from a third mass distribution campaign of LLINs planned for 2019 due to the fact that, the Centre region to which Makenene belongs was devoted to the government for LLINs coverage. Unfortunately, government is been facing financial crisis which delayed the activity contrary to the other regions attributed to the Global Fund where this third campaign was effectively conducted.
Little is known about the transmission of malaria in Makenene, a locality located in a forest-savannah limit in Cameroon, and which has been hosting internal displaced people (IDPs) due to the socio-political crisis in the country for four years now. Based on previous studies carried out in the central region of Cameroon in general and in Yaoundé in particular, transmission in Makenene could be holo-endemic and seasonal with the main vector Anopheles gambiae sensu lato [28] and an annual prevalence around 35% [29]. This study was designed to assess the knowledge, attitudes and practices (KAP) of populations towards the fight against malaria in Makenene in order to shed light into aspects relevant to malaria control programmes (LLINs, free treatment of children under 5 years with artemisinin-based combination therapy, intermittent preventive treatment of pregnant women with quarterly single dose of sulfadoxine-pyrimethamine [25]) and contribute to reduce the failure of the control measures.
Methods
Study sites
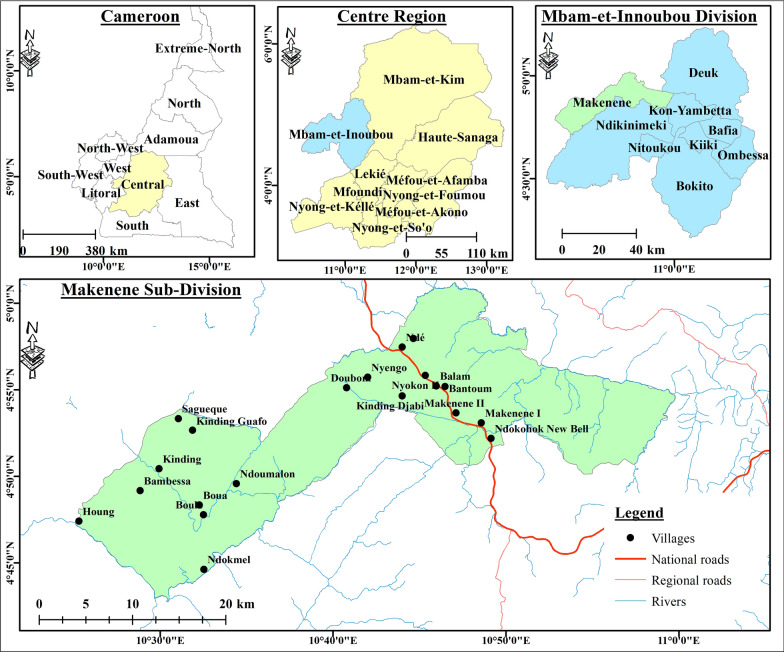
The study was conducted in the locality of Makenene, Mbam and Inoubou Division, Centre Region of Cameroon (Fig. 1). Located at 580 m of altitude, the locality of Makenene covers an area of 885 km2 and is distant from Bafia, the capital of the Division by about 100 km. It is bordered to the north by the Noun River, to the south by the coastal region forest (between 3° 20′ N and 6° N) and to the west and east by the rivers Makenene and Nde (between 9° 40′ E and 13° E) [30]. The Climate in Makenene is equatorial humid and characterized by two dry seasons and two rainy seasons of unequal length (with rainfall up to 721 mm/year): a long dry season (mid-October to March), a small dry season (June to mid-August), a long rainy season (from mid-August to October), and a short rainy season (from March to June) [31]. The population of Makenene is estimated at 35,000 people. The main activity is agriculture to which are added small business, animal husbandry and urban transport by motorbikes among others. The city is full of some basic infrastructures, including health centres (the District Medical Centre of Makenene, the Catholic health centre, the Baptist Church health centre and the Nyokon Integrated health care) schools and public services [32]. The population of Makenene has not benefited from the 3rd mass distribution campaign of mosquito nets scheduled and effective in other regions/localities of the country since 2019.
Fig. 1.
Map of the study site
Study design and data collection
This was a cross-sectional and descriptive study carried out between 28th July and 20th September 2021. In the absence of the total number of households which unfortunately is lacking in the last census statistical document [33] and no data on the prevalence of malaria in the locality and its surroundings, the sample size was calculated using the Yamane formula [34] as follows: ɲ = N/(1 + N(ε)2); where ɲ is the size of sampling sought; N the population size, and ε the sampling error (5%). According to the last census of DIPAMAK [32], the locality has 35,000 inhabitants. The minimum sample size for this study was estimated at 395 participants but 413 HoH participated to this study.
After obtaining administrative authorizations and ethical clearance, pre-tested questionnaires (a well-designed questionnaire was first randomly pretested on 30 volunteers) were submitted to the HoHs (from randomly selected houses) after obtaining their free and informed consents. In the absence of the HoH, their spouses or adult children (aged above 18 years old) answered the questionnaires. The questionnaire (see Additional file 1), adapted from a copy used for a community survey in Cameroon [5, 19] consisted of a set of 45 questions grouped into 4 levels: (i) socio-demographic characteristics of the studied population; (ii) knowledge of the population about malaria and their mode of transmission; (iii) control/preventive methods applied by the population and, (iv) nocturnal activities of the studied population. Other variables such as the presence or absence of grass and mosquito’s breeding sites around houses and the number of malaria episodes were also recorded.
Household surveys were carried out by a team of trained students and researchers from VBID-URBEA with experience in surveys assisted by trained local community health workers (CHWs). CHWs facilitated the integration in households and helped in the translation of the questionnaire into the local language during the interview/questionnaire survey to people who did not speak neither French nor English.
Data analysis
After questionnaires administration, responses collected were processed in Microsoft Office Excel version 2016 (see Additional file 2), and analysed using R software version 4.0.4 (R version 4.0.4, 2021-02-15). Frequencies of the different categories of variables were calculated for descriptive statistics and compared using the Chi-square test. Logistic Regression was used to assess the effect of predictive variables (level of education, gender, religion and occupation of the respondent) on good knowledge, good attitudes and good practices (independent variables). Results were considered significant when p < 0.05.
To assess respondents' level of knowledge about malaria, responses to questions on how malaria is transmitted to humans and symptoms of the disease were considered. Participants who answered those two questions correctly (knowledge of the vector and at least two symptoms of the disease) were considered to have good knowledge while those who did not answer those two questions correctly were considered to have poor/bad knowledge. Participants with good attitudes were those who had knowledge about the disease and went to the hospital when they suspected it, whereas those who possessed LLINs, had LLNIs on all beds and used them regularly were considered to have good practices.
Results
Sociodemographic characteristics of the study participants
A total of 413 households were surveyed and the socio-demographic characteristics of the population are presented in Table 1. The majority of respondents/participants were males (70.94%) of over 40 years old (56.89%). They were predominantly Christians (91.77%) with a secondary school level (68.28%). The heads of households were mostly of small-scale business profession (42.61%) and farmers (33.41%). Different types of houses were found in the locality (Fig. 2) with the majority built in earth brick (76.76%) and without ceiling (68.04%) (Table 1).
Table 1.
Socio-demographic characteristics of households surveyed in the city of Makenene
| Categories | Characteristics | N | % |
|---|---|---|---|
| Gender | Male | 293 | 70.94 |
| Female | 120 | 29.06 | |
| Age (in years) | ≤ 40 | 175 | 43.10 |
| ≥ 41 | 231 | 56.89 | |
| Education level | Primary level | 64 | 15.49 |
| Secondary level | 282 | 68.28 | |
| University level | 59 | 14.29 | |
| None | 8 | 1.93 | |
| Religion | Christian | 379 | 91.77 |
| Muslim | 20 | 4.84 | |
| Other | 14 | 3.39 | |
| Occupation of the head of household | Farmer | 138 | 33.41 |
| Small business | 176 | 42.61 | |
| Civil servant | 60 | 14.53 | |
| Housewife | 31 | 7.51 | |
| Student | 8 | 1.94 | |
| Number of people in a household | 1 to 5 | 267 | 64.65 |
| 6 to 10 | 130 | 31.48 | |
| > 10 | 16 | 3.87 | |
| House construction material | Cement blocks | 56 | 13.56 |
| Mud and plank | 38 | 9.20 | |
| Earth brick | 317 | 76.76 | |
| Plank | 2 | 0.48 | |
| Ceiling | Present | 132 | 31.96 |
| Absent | 281 | 68.04 | |
| Openings between roof and walls | Open | 114 | 27.60 |
| Close | 299 | 72.4 | |
| Grass around house | Present | 161 | 38.98 |
| Absent | 252 | 61.02 | |
| Stagnant water around house | Present | 134 | 32.45 |
| Absent | 279 | 67.55 | |
| Domestic animals | Present | 181 | 43.83 |
| Absent | 232 | 56.17 | |
| Resting places for domestic animals | Inside house | 44 | 22 |
| Outside house | 156 | 78 |
General knowledge and behaviour of the study participants about malaria
Fig. 2.

Types of houses in Makenene. Four types were found consisting of A cement blocks, B Mud and plank, C earth brick and D plank
General knowledge and behaviour of the study participants about malaria
General knowledge about malaria (the mode of transmission of the disease and preventive measures) of the study participants is shown in Table 2. All respondents declared to have heard about malaria before. The majority of respondents (94.67%) attributed the cause of malaria to mosquito bites and more than 93% complained about mosquito bites in their home. Over 90% of respondent participants (92.25%) stated to use bed nets as a tool of protection against mosquito bites and malaria and most of them declared to use them regularly (77.86%). However, the presence of holes in 68.15% of all mosquito nets independently of the year of acquisition was noted (Table 2). Other protective measures such as sprays and spirals were also used (20.34%).
Table 2.
Population knowledge and behaviour about malaria in Makenene
| Variables | Answers | N | % |
|---|---|---|---|
| Heard about malaria | Yes | 413 | 100 |
| No | 0 | 0 | |
| Mode of transmission of malaria | Mosquito bites | 391 | 94.67 |
| Dirt | 13 | 3.15 | |
| Other | 6 | 1.45 | |
| No idea | 3 | 0.73 | |
| Mosquito bites at home | Yes | 387 | 93.70 |
| No | 26 | 6.30 | |
| Preventive measuresa | Using mosquito nets | 381 | 92.25 |
| Using insecticides spray/coil | 84 | 20.34 | |
| Using nets on windows | 20 | 4.84 | |
| Nothing | 11 | 2.66 | |
| Period of use of mosquito nets | Rainy season | 62 | 16.15 |
| Dry season | 15 | 3.91 | |
| Regularly | 299 | 77.86 | |
| Rarely | 8 | 2.08 | |
| Origin of bed nets used | Bought | 73 | 18.91 |
| Freely acquired by MoHb | 281 | 72.80 | |
| Donation other than MoHc | 32 | 9.29 | |
| Reasons for not using mosquito nets regularly | Forgetfulness | 62 | 14.19 |
| Heat | 72 | 16.47 | |
| Absence of mosquito nets | 286 | 65.45 | |
| Otherd | 17 | 3.89 | |
| Age of bed nets used | < 6 months | 36 | 9.33 |
| [6 to 12 months] | 41 | 10.62 | |
| [1 to 2 years] | 77 | 19.95 | |
| > 2 years | 232 | 60.10 | |
| Presence of holes on bed nets | No | 122 | 31.85 |
| Yes | 261 | 68.15 |
N number of responses
a Percentages do not add up to 100 because these results are from multiple response questions
b Ministry of Public Health
c Donation by family or hospital
d Other (Insufficient, Muffle, Torn, Crowding)
Home management of malaria cases by the study participants of Makenene
Out the 413 households surveyed, the majority of participants (51.09%) said not to go to local health centres/hospitals in case of suspected malaria infection. About 30% declared to practice self-medication with conventional drugs bought from roadside sellers, in hospitals or pharmacy while 22.04% used traditional pharmacopoeia. On the other hand, 67.07% of participants said to have suffered from several suspected episodes of malaria from January to August 2021 (Table 3).
Table 3.
Home management and episodes of malaria cases in households in Makenene from January to August 2021
| Items | Characteristics | N | % | p-value |
|---|---|---|---|---|
| Perceived malaria infections during the year | < 2 times | 132 | 31.96 | – |
| > 2 times | 277 | 67.07 | < 0.001 | |
| No idea | 4 | 0.97 | – | |
| Management of malaria cases | Hospital | 202 | 48.91 | – |
| Traditional | 91 | 22.04 | < 0.001 | |
| Self-medication | 120 | 29.05 | < 0.001 |
Ownership and usage of LLINs in households
Ownership and usage of LLINs in households
A very high LLINs coverage was observed in Makenene (Table 4). In fact, 92.25% of the households surveyed had at least one LLIN, and 51.57% (n = 213) had 1 LLINs for at least 2 people. The LLIN use rate (proportion of people who slept under LLINs the night before the day of the survey) was 86.70%. However, when considering one LLIN per room in households, only 42.85% of houses had all their bed covered.
Table 4.
LLINs ownership and usage in households in Makenene
| Variables | N | % |
|---|---|---|
| HHs owning ≥ 1 LLINs | 381 | 92.25 |
| HoHs owning ≥ 1 LLINs for 2 people | 2&” | 51.57 |
| Persons with access to a LLIN within their own HH | 174 | 42.13 |
| Persons that used LLIN the previous night | 358 | 86.70 |
N number of responses, HoH household, LLIN long last insecticides treated net
Factors associated with good knowledge about malaria in Makenene
Knowledge of the disease, the mode of transmission and the symptoms were necessary to assign a participant the qualification of good (56.20%) or bad (43.8%) knowledge. All participants claimed to know malaria and heard about it before. The majority (94.67%) incriminated mosquito bites as the mode of transmission of malaria. Subsequently, univariate and multivariate analyses were carried out in order to determine associations between population good knowledge and some sociodemographic characteristics.
Univariate analyzes showed that Muslims had much better knowledge about malaria than Christians (non adjusted odd ratio [naOR] 3.34; 95% CI: 1.15–11.59; p = 0.03), and participants with a university education level had significantly higher knowledge compared to illiterates (naOR: 7.29; 95% CI 1.37–58.27; p = 0.03) (Table 5). Furthermore, civil servants had better knowledge compared to farmers (naOR: 2.27; 95% CI: 1.12–4.79; p = 0.02). Same results were obtained after multivariate analyses; after adjusting other variables (student, small business, housewife), civil servants still had significantly good knowledge compared to farmers (adjusted odds ratio [aOR] 2.31, 95% CI: 1.14–4.88, p = 0.02). Apart from this, no other significant association between good knowledge and population sociodemographic characteristics was observed after multivariate analyses (Table 5).
Table 5.
Factors associated with good knowledge about malaria in the study site
| Categories | Good knowledge (%) | Bad knowledge (%) | Univariate analysis | Multivariate analysis |
|---|---|---|---|---|
| [naOR] (95% CI; p-value) | [aOR] (95% CI p-value) | |||
| Gender (Female = Ref) | 64 (54.32) | 54 (45.77) | – | – |
| Male | 167 (57) | 126 (43) | 1.08 (0.65–1.79, p = 0.75) | 1.07 (0.65–1.7, p = 0.76) |
| Religion (Christian = Ref) | 209 (55.14) | 168 (44.32) | – | – |
| Muslim | 15 (75) | 5 (25) | 3.34 (1.15–11.59, p = 0.03) | 4.22 (0.39–47.74, p = 0.21) |
| Others | 5 (35.71) | 9 (64.29) | 0.99 (0.25–3.86, p = 0.098) | 1.23 (0.10–14.99, p = 0.86) |
| Level of education (Illiterate = Ref) | 2 (25) | 6 (75) | – | – |
| Primary level | 33 (53.23) | 29 (46.77) | 3.95 (0.78–30.39, p = 0.12) | 0.24 (0.03–1.24, p = 0.11) |
| Secondary level | 154 (54.6) | 128 (45.4) | 4.42 (0.90–32.25, p = 0.93) | 1.04 (0.58–4.88, p = 0.87) |
| University level | 42 (71.19) | 17 (28.81) | 7.29 (1.37–58.27, p = 0.03) | 1.77 (0.79–4.01, p = 0.16) |
| Occupation (Farmer = Ref) | 74 (54.01) | 63 (45.99) | – | – |
| Public servant | 46 (76.67) | 14 (23.33) | 2.27 (1.12–4.79, p = 0.02) | 2.31 (1.14–4.88, p = 0.02) |
| Student | 6 (75) | 2 (25) | 2.45 (0.54–17.25, p = 0.28) | 2.43 (0.53–17.13, p = 0.28) |
| Small business | 88 (50.29) | 87 (49.71) | 0.81 (0.51–1.31, p = 0.52) | 0.78 (0.48–1.26, p = 0.32) |
| Housewife | 17 (54.84) | 14 (45.16) | 1.07 (0.44–2.61, p = 0.88) | 1.04 (0.43–2.56, p = 0.91) |
[naOR]: Non Adjusted OR; [aOR]: Adjusted OR; OR: Odd ratio; CI: Confidence interval
Factors associated with good practices about malaria in Makenene
As in the case of population knowledge about malaria, good (71.67%) and bad (28.33%) practices were recorded, and analyses (univariate and multivariate) were carried out with the aim of assessing factors related to good practices of households’ head of Makenene towards malaria prevention and control. No factor was found significantly associated with good practices following univariate analyses except occupation after multivariate analyses (Table 6); public servants had significantly higher good malaria control practices compared to farmers (aOR: 2.31; 95% CI: 1.14 to 4.88; p = 0.02) after adjusting student, small business and housewife.
Table 6.
Factors associated with practices about malaria in the study sites
| Categories | Good practices (%) | Bad practices (%) | Univariate analysis | Multivariate analysis |
|---|---|---|---|---|
| [naOR] (95% CI; p-value) | [aOR] (95% CI p-value) | |||
| Gender (Female = Ref) | 89 (74.17) | 31 (25.83) | – | – |
| Male | 207 (70.65) | 86 (29.35) | 0.80 (0.45–1.39, p = 0.43) | 1.07 (0.65–1.7, p = 0.76) |
| Religion (Christian = Ref) | 274 (72.30) | 105 (27.70) | – | – |
| Muslim | 14 (0.70) | 6 (0.30) | 0.75 (0.28–2.25, p = 0.05) | 4.22 (0.39–47.74, p = 0.21) |
| Others | 5 (50) | 5 (50) | 0.38 (0.10–1.47, p = 0.15) | 1.23 (0.10–14.99, p = 0.86) |
| Level of education (Illiterate = Ref) | 5 (62.5) | 3 (37.5) | – | – |
| Primary level | 45 (70.31) | 19 (29.69) | 1.54 (0.28–7.27, p = 0.59) | 0.24 (0.03–1.24, p = 0.11) |
| Secondary level | 199 (70.57) | 83 (29.43) | 1.31 (0.25–5.80, p = 0.72) | 1.04 (0.58–1.86, p = 0.87) |
| University level | 47 (69.66) | 12 (20.34) | 2.29 (0.39–11.56, p = 0.32) | 1.77 (0.79–4.01, p = 0.16) |
| Occupation (Farmer = Ref) | 91 (65.94) | 47 (34.06) | – | – |
| Public servant | 43 (71.67) | 17 (28.33) | 1.06 (0.52–2.19, p = 0.88) | 2.31 (1.14–4.88, p = 0.02) |
| Student | 6 (75) | 2 (25) | 1.49 (0.32–10.49, p = 0.63) | 2.43 (0.53–17.13, p = 0.28) |
| Small business | 136 (77.28) | 40 (22.72) | 1.68 (1.00–2.84, p = 0.05) | 2.43 (0.48–1.26, p = 0.32) |
| Housewife | 20 (64.52) | 11 (35.48) | 0.75 (0.29–1.95, p = 0.54) | 1.04 (0.43–2.56, p = 0.91) |
naOR non adjusted OR, aOR adjusted OR, OR odd ratio, CI confidence interval
Factors associated with good attitudes about malaria in Makenene
Analyses were also made to assess any association between good (48.41%) or bad (51.59%) attitudes concerning malaria treatment and participants’ sociodemographic characteristics. Univariate analyses revealed that public servants (naOR: 2.24; 95% CI: 1.12–4.59; p = 0.02) had good attitudes towards malaria compared to farmers and that housewives were less likely to adopt good attitudes towards malaria than farmers (naOR: 0.35; 95% CI: 0.13–0.88; p = 0.03). Same results were obtained with multivariate analyses; after adjusting other variables, public servants (aOR: 2.08; 95% CI: 1.05 to 4.24; p = 0.03) were still more likely to adopt good attitudes in case of malaria infection than farmers, while housewives were less likely to adopt good attitudes than farmers (aOR: 0.33; 95% CI: 0.12 to 0.85; p = 0.02). Apart from occupation (profession), no other factor significantly influenced population good attitudes towards malaria treatment (Table 7).
Table 7.
Factors associated with good attitudes toward malaria control in the study sites
| Categories | Good attitudes (%) | Bad attitudes (%) | Univariate analysis | Multivariate analysis |
|---|---|---|---|---|
| [naOR] (95% CI; p-value) | [aOR] (95% CI p-value) | |||
| Gender (Female = Ref) | 57 (48.31) | 61 (51.69) | – | – |
| Male | 141 (48.45) | 150 (51.55) | 0.77 (0.46–1.27, p = 0.30) | 0.76 (0.45–1.26, p = 0.29) |
| Religion (Christian = Ref) | 185 (49.33) | 190 (50.67) | – | – |
| Muslim | 10 (50) | 10 (50) | 1.38 (0.52–3.76, p = 0.51) | 4.44 (0.43–104.04, p = 0.24) |
| Others | 2 (20) | 8 (80) | 0.36 (0.05–1.61, p = 0.22) | 1.16 (0.77–32.49, p = 0.91) |
| Level of education (None = Ref) | 1 (12.5) | 8 (87.5) | – | – |
| Primary level | 24 (37.5) | 40 (62.5) | 3.34 (0.63–76.94, p = 0.21) | 0.24 (0.01–1.50, p = 0.19) |
| Secondary level | 145 (51.79) | 135 (48.21) | 6.90 (1.16–131.63, p = 0.07) | 1.62 (0.91–2.94, p = 0.10) |
| University level | 28 (49.12) | 29 (50.88) | 4.59 (0.71–90.82, p = 0.17) | 1.16 (0.52–2.58, p = 0.77) |
| Occupation (Farmer = Ref) | 67 (48.55) | 71 (51.45) | – | – |
| Public servant | 39 (67.24) | 19 (32.76) | 2.24 (1.12–4.59, p = 0.02) | 2.08 (1.05–4.24, p = 0.03) |
| Student | 7 (87.5) | 1 (12.5) | 6.62 (1.12–126.07, p = 0.82) | 6.51 (1.10–124.06, p = 0.08) |
| Small business | 76 (43.68) | 98 (56.32) | 0.79 (0.49–1.28, p = 0.33) | 0.76 (0.47–1.22, p = 0.27) |
| Housewife | 9 (29.03) | 22 (70.97) | 0.35 (0.13–0.88, p = 0.03) | 0.33 (0.12–0.85, p = 0.02) |
naOR non adjusted OR, aOR adjusted OR, OR odd ratio, CI confidence interval
Discussion
The level of knowledge of a population on a given disease is an indicator to guide efficient strategies to its control [35]. In order to understand factors that can hinder people adherence to control tools against malaria and for a better understanding of behaviours likely to promote the transmission of the disease in Makenene, this study assessed knowledge, attitudes and practices of population of Makenene towards the fight against malaria.
Overall, good knowledge, attitudes and practices towards malaria control were observed in Makenene. Almost all heads of households declared to have heard about malaria with over 94% of respondents associating the disease transmission with mosquito bites. This could be linked both to the level of education of the study participants where more than 97% had at least primary level and to the fact that they live in an endemic region. In addition, this endemicity of malaria in the country favors the communication about the disease through different channels such as TV, social media, advertising campaigns, interpersonal discussions and in hospitals through posters/pictograms or discussions with hospital care workers and also sensitization of community health workers [5, 19, 36].
More than half of participants (57.87%) clearly identified at least two symptoms of malaria. Knowledge of symptoms can also be linked to the different sources of information on malaria. Fever appeared to be the most well-known symptom, but it is worth to mention that fever is not necessarily synonymous with malaria. These results are in line with the World Health Organization (WHO) knowledge guidelines [1] and are similar to previous studies [18, 19, 21].
Despite the affordable cost of care in public health hospitals such as in the District Medical Centre of Makenene declare by the government, a high proportion of respondents declared to practice self-medication using commercial drugs and traditional medicines in case of suspected malaria. In fact, there is a schism between the national policy fixing the cost of treatment of malaria and what actually families have to pay in hospitals, which is more higher. Population avoid hospital to limit the cost of treatment from consultations, laboratory testing and drugs. In addition, they believe that going to health service may reduce the time needed for their various activities. This observation was found previously in population living in Yaoundé, Cameroon [5], Tanzania [37] and Bangladesh [38].
With the advance of traditional medicine, people used plants, such as Artemisia (Artemisia annua), Quinqueliba (Combretum micranthum) and other plants for malaria treatment. Similar results have been found in previous studies [5, 37, 38]. This result could be explained by the fact that, more than half of participants knowing already the main symptoms of the disease (fever, headache, vomiting) have become aware of the routine treatment to take, hence practice self-medication. The over practice of self-medication, the use of traditional drugs coupled with no visit in hospitals may be also due to the fear the population has of visiting health centres in this time of COVID-19 [10, 11].
The lack of knowledge on the doses of traditional drugs, coupled with the unknown quality of drugs from street sellers which are generally stored in poor conditions could be sources of rapid growth in drug resistance [39]. A significant difference was observed when comparing the number of perceived malaria infections made by participants during the period from January to August 2021; about 70% of participants said to have suffered from several episodes of malaria during that period. This could be explained by the fact that malaria is endemic in the locality, patients are in permanent contact with the vector, the environment is appropriate to malaria vectors development, or to a phenomenon of drug resistance. Furthermore, people living in rural settlements usually lack sufficient access to health-care facilities and have in most cases poor housing conditions that expose children to malaria transmitting vectors [40, 41]. In addition, previous studies showed that, the likelihood of malaria episodes increases with the increasing size of the household [42]. The reason for this is that, if there are many individuals in the household as in Makenene where the majority of households had more than four people; when one of them is infected, he/she could serve as reservoir for others [40].
The use of mosquito bed nets was found to play an essential role in preventing malaria infection in African countries [43, 44]. In families where mosquito bed nets were not used when sleeping, were more vulnerable to malaria infection than in families where they were used [41]. In Makenene, more than 92% of respondents said they used it as a means of protection against malaria and 77.86% used it regularly. Similar results have been obtained in studies carried out in some localities in Cameroon [5, 19, 22, 45] and elsewhere [18]. This high possession and regular use of LLINs could be due to the massive distribution campaign of LLINs done by the Ministry of Public Health in 2016 in the locality and sensitization on the usage by community health workers.
Other means of protection, such as insecticide sprays, mosquito coils and screens on windows were also used by the population. Previous studies have shown that the use of insecticide sprays, especially in purely agricultural areas, would increase the rate of resistance in the mosquito population [4, 28, 46] and would, therefore, reduce the bio-efficacy of LLINs [47].
Although LLINs provide both chemical and physical barriers against mosquitoes by reducing their contact with humans, when damaged, they become less effective against mosquito bites. More than 68% of the mosquito nets used in the surveyed households had holes of different sizes. In contrary, results by Talipouo et al. [5] in Yaoundé (Cameroon) reported more than half of the LLINs in use in good condition. Diouf et al. [48] concluded that the rapid degradation of mosquito nets would largely depends on their improper use (washing, drying, threading), which also depends on the year of acquisition. Messages about LLIN washing procedures should be disseminated in the locality to help maintain their integrity, effectiveness and sustainability. This rapid degradation of mosquito nets could also come from the quality of the material used in their manufacture. The materials used in the manufacture of the mosquito nets include cotton, polyethylene, polypropylene, polyester and nylon. Polyester is the most popular because of its lightness and it also allows easy air circulation while, cotton is stronger but less comfortable.
In general, the study showed that more than half of the participants had good knowledge and good practices towards the prevention and control of malaria in Makénéné. Good knowledge and practices were recorded mostly in educated persons including public servants and students. The considerably high rate obtained would therefore constitute an asset for the control of the disease in the locality. Despite this, more than half of the population was found to adopt attitudes not recommended by the WHO when suspecting malaria infection. These results are similar to those obtained by Mbongue et al. [49]. Good attitudes were adopted by public servants and students mostly of secondary and higher levels of education. Similar results were found by others [5, 19, 45]. This testifies the ability of educated people to well practice recommendations received during sensitization campaigns.
The categorization of good knowledge based of only two questions and the use of only one collection tools (questionnaire) are some limitations of this study. In future studies, reasons of answers received from field using in depth interview such as focal group discussion will be seek.
Conclusion
The level of knowledge and behaviour of populations about a disease is an indicator to guide efficient strategies to fight this disease. The majority of participants in this study claimed to know the mode of transmission of malaria as well as the symptoms of the disease. Good attitudes and practices were also recorded in general with the use of LLINs as the main preventive measure. However, considerable efforts should be made by the public authorities to further raise awareness about care of existing preventive tools and malaria treatment because more than half of the population was found to adopt bad attitudes such self-medication which could contribute to the persistence of the disease in the locality and drug resistance as well.
Supplementary Information
Acknowledgements
We are grateful to the inhabitants of Makenene for cooperation throughout the survey and to the community health worker of the Baptist health centre of Makenene for our integration in the locality.
Abbreviations
- LLINs
Long-lasting insecticidal nets
- KAP
Knowledge, Attitude and Practice
- NMCP
National Malaria Control Programme
- COVID-19
Corona virus 2019
- WHO
World Health Organization
- CHWs
Community health workers
- HoH
Household
Author contributions
Conceived and designed the experiments: DJ, TT, MMPA, RB. Performed survey: DJ, KCNA. Analyzed the data: DJ, MMPA, RB, contributed materials ANC, AAP, TR, AD. Drafted the paper: DJ, MMPA, BR. Revised critically the paper: all the authors. All authors read and approved the final manuscript.
Funding
This study did not received funding for any sources or agency.
Availability of data and materials
All relevant data are within the paper and its additional information files.
Declarations
Ethics approval and consent to participate
The study was conducted under the ethical clearance N° 2020/04/CE/CNERSH/SP delivered by the Centre Regional Ethics (CNE) Committee for Research on Human Health. Authorization to carry out the study in selected houses was obtained from the local administration and heads of households (HH) through an inform consent form.
Consent for publication.
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roland Bamou, Email: bamou2011@gmail.com.
Timoléon Tchuinkam, Email: timotchuinkam@yahoo.fr.
References
- 1.WHO. World malaria report 2021. Geneva: World Health Organization; 2021. https://www.who.int/publications-detail-redirect/9789240040496. Accessed 4 Feb 2022.
- 2.Antonio-Nkondjio C, Ndo C, Njiokou F, Bigoga JD, Awono-Ambene P, Etang J, et al. Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasit Vectors. 2019;12:501. doi: 10.1186/s13071-019-3753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PNLP. Plan Strategique National de lutte contre le paludisme au Cameroun 2019. Minsanté; 2019.
- 4.Antonio-Nkondjio C, Sonhafouo-Chiana N, Ngadjeu CS, Doumbe-Belisse P, Talipouo A, Djamouko-Djonkam L, et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit Vectors. 2017;10:472. doi: 10.1186/s13071-017-2417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talipouo A, Ngadjeu CS, Doumbe-Belisse P, Djamouko-Djonkam L, Sonhafouo-Chiana N, Kopya E, et al. Malaria prevention in the city of Yaoundé: knowledge and practices of urban dwellers. Malar J. 2019;18:167. doi: 10.1186/s12936-019-2799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamou R, Kopya E, Djamouko-Djonkam L, Awono-Ambene P, Tchuinkam T, Njiokou F, et al. Assessment of the anophelinae blood seeking bionomic and pyrethroids resistance of local malaria vectors in the forest region of southern Cameroon. JEZS. 2020;8:1054–1062. [Google Scholar]
- 7.Bamou R, Rono M, Degefa T, Midega J, Mbogo C, Ingosi P, et al. Entomological and Anthropological factors contributing to persistent malaria transmission in Kenya, Ethiopia, and Cameroon. J Infect Dis. 2021;223:S155–S170. doi: 10.1093/infdis/jiaa774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burt JF, Ouma J, Lubyayi L, Amone A, Aol L, Sekikubo M, et al. Indirect effects of COVID-19 on maternal, neonatal, child, sexual and reproductive health services in Kampala Uganda. BMJ Global Health. 2021;6:e006102. doi: 10.1136/bmjgh-2021-006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed T, Rahman AE, Amole TG, Galadanci H, Matjila M, Soma-Pillay P, et al. The effect of COVID-19 on maternal newborn and child health (MNCH) services in Bangladesh, Nigeria and South Africa: call for a contextualised pandemic response in LMICs. Int J Equity Health. 2021;20:77. doi: 10.1186/s12939-021-01414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindzeka ME. COVID-19 frightens malaria patients in Cameroon. VOA. [cited 2022 May 29]. https://www.voanews.com/a/science-health_covid-19-frightens-malaria-patients-cameroon/6188194.html. Accessed 29 May 2022.
- 11.Holtz L. COVID-19’s impact on overall health care services in Africa. Brookings. 2021. https://www.brookings.edu/blog/africa-in-focus/2021/10/12/covid-19s-impact-on-overall-health-care-services-in-africa/. Accessed 29 May 2022.
- 12.The impact of COVID-19 on HIV, TB and malaria services and systems for health. https://www.theglobalfund.org/en/updates/2021/2021-04-13-the-impact-of-covid-19-on-hiv-tb-and-malaria-services-and-systems-for-health/. Accessed 29 May 2022.
- 13.Heuschen A-K, Lu G, Razum O, Abdul-Mumin A, Sankoh O, von Seidlein L, et al. Public health-relevant consequences of the COVID-19 pandemic on malaria in sub-Saharan Africa: a scoping review. Malar J. 2021;20:339. doi: 10.1186/s12936-021-03872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ataba E, Dorkenoo AM, Nguepou CT, Bakai T, Tchadjobo T, Kadzahlo KD, et al. Potential emergence of Plasmodium resistance to artemisinin induced by the use of Artemisia annua for malaria and COVID-19 prevention in sub-Saharan African Region. Acta Parasitol. 2022;67:55–60. doi: 10.1007/s11686-021-00489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watts C, Atieli H, Alacapa J, Lee M-C, Zhou G, Githeko A, et al. Rethinking the economic costs of hospitalization for malaria: accounting for the comorbidities of malaria patients in western Kenya. Malar J. 2021;20:429. doi: 10.1186/s12936-021-03958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gansané A, Moriarty LF, Ménard D, Yerbanga I, Ouedraogo E, Sondo P, et al. Anti-malarial efficacy and resistance monitoring of artemether-lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso, 2017–2018. Malar J. 2021;20:48. doi: 10.1186/s12936-021-03585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha A, Sarker M, Kabir M, Lu G, Müller O. Knowledge, attitudes, and practices regarding malaria control among the slash and burn cultivators in Rangamati Hill tracts of Bangladesh. Malar J. 2019;18:216. doi: 10.1186/s12936-019-2849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DePina AJ, Dia AK, Martins A, Ferreira MC, Moreira AL, Leal SV, et al. Knowledge, attitudes and practices about malaria in Cabo Verde: a country in the pre-elimination context. BMC Public Health. 2019;19:850. doi: 10.1186/s12889-019-7130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kala NA, Ngingahi LG, Bamou R, Talipouo A, Ngadjeu CS, Mayi MPA, et al. Knowledge, attitude, and practices (KAP) of human populations towards malaria control in four ecoepidemiological settings in Cameroon. J Trop Med; 2021;2021:9925135. [DOI] [PMC free article] [PubMed]
- 20.Mbongue RS, Akono PN, Offono LE. Connaissances, attitudes et pratiques des chefs de ménages sur le paludisme dans les zones urbaine et rurale de Kribi Sud-Cameroun. Camer J Biol Biochem Sci. 2020;28:38–45. [Google Scholar]
- 21.Manana PN, Kuonza L, Musekiwa A, Mpangane HD, Koekemoer LL. Knowledge, attitudes and practices on malaria transmission in Mamfene, KwaZulu-Natal Province, South Africa 2015. BMC Pub Health. 2018;18:41. doi: 10.1186/s12889-017-4583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enama MLO, Ntonga PA, Mbida AM, Takap NN, Mbiada B, Hondt OEN, et al. Le paludisme : connaissances, attitudes et pratiques des chefs de ménage de la region de l’Ouest Cameroun. J Appl Biosci. 2020;147:15117–15124. doi: 10.35759/JABs.147.5. [DOI] [Google Scholar]
- 23.Bamou R, Mbakop LR, Kopya E, Ndo C, Awono-Ambene P, Tchuinkam T, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasit Vectors. 2018;11:464. doi: 10.1186/s13071-018-3049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonio-Nkondjio C, Simard F, Awono-Ambene P, Ngassam P, Toto J-C, Tchuinkam T, et al. Malaria vectors and urbanization in the equatorial forest region of south Cameroon. Trans R Soc Trop Med Hyg. 2005;99:347–354. doi: 10.1016/j.trstmh.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Tchuinkam T, Nyih-Kong B, Fopa F, Simard F, Antonio-Nkondjio C, Awono-Ambene H-P, et al. Distribution of Plasmodium falciparum gametocytes and malaria-attributable fraction of fever episodes along an altitudinal transect in Western Cameroon. Malar J. 2015;14:96. doi: 10.1186/s12936-015-0594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchuinkam T, Simard F, Lélé-Defo E, Téné-Fossog B, Tateng-Ngouateu A, Antonio-Nkondjio C, et al. Bionomics of Anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. BMC Infect Dis. 2010;10:119. doi: 10.1186/1471-2334-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabue RN, Awono-Ambene P, Etang J, Atangana J, Antonio-Nkondjio C, Toto JC, et al. Role of Anopheles (Cellia) rufipes (Gough, 1910) and other local anophelines in human malaria transmission in the northern savannah of Cameroon: a cross-sectional survey. Parasit Vectors. 2017;10:22. doi: 10.1186/s13071-016-1933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonio-Nkondjio C, Fossog BT, Ndo C, Djantio BM, Togouet SZ, Awono-Ambene P, et al. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): influence of urban agriculture and pollution. Malar J. 2011;10:154. doi: 10.1186/1475-2875-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World malaria report 2019. https://www.who.int/publications-detail-redirect/9789241565721
- 30.Letouzey R. Note of the phytogeographical map of Cameroon with 1/500 000. Always green dense forest wetland domains Institute of the International Vegetation map, Toulouse, France. 1985;63–142.
- 31.PCD. Plan Communal de Développement de la ville de Makénéné; 2011.
- 32.DIPAMAK. Dynamique Participative de Makénéné pour le développement de Makénéné, rapport d’activité journées médicales de Makénéné les 15 et 16 juin 2018 à Makénéné; 2018.
- 33.BUCREP - Population en chiffre. http://www.bucrep.cm/index.php/fr/recensements/3eme-rgph/20-3eme-rgph/presentation/57-population-en-chiffre
- 34.Tepping BJ. Elementary Sampling Theory, Taro Yamane. Englewood Cliffs, New Jersey: Prentice-Hall, Inc., 1967. Pp. x–405. J Am Stat Assoc. 1968;63:728–30.
- 35.WHO. World malaria report 2014. https://www.who.int/publications-detail-redirect/9789241564830
- 36.Bowen HL. Impact of a mass media campaign on bed net use in Cameroon. Malar J. 2013;12:36. doi: 10.1186/1475-2875-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazigo HD, Obasy E, Mauka W, Manyiri P, Zinga M, Kweka EJ, et al. Knowledge, Attitudes, and Practices about Malaria and Its Control in Rural Northwest Tanzania. Malar Res Treat. 2010;2010:794261. doi: 10.4061/2010/794261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed SM, Haque R, Haque U, Hossain A. Knowledge on the transmission, prevention and treatment of malaria among two endemic populations of Bangladesh and their health-seeking behaviour. Malar J. 2009;8:173. doi: 10.1186/1475-2875-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. World malaria report 2015. Geneva: World Health Organization; 2015. www.who.int/malaria
- 40.Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246. doi: 10.1186/s12936-016-1290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Habyarimana F, Ramroop S. Prevalence and risk factors associated with malaria among children aged six months to 14 years old in Rwanda: evidence from 2017 Rwanda Malaria Indicator Survey. Int J Environ Res Public Health. 2020;17:7975. doi: 10.3390/ijerph17217975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ugwu CLJ, Zewotir TT. Using mixed effects logistic regression models for complex survey data on malaria rapid diagnostic test results. Malar J. 2018;17:453. doi: 10.1186/s12936-018-2604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winskill P, Rowland M, Mtove G, Malima RC, Kirby MJ. Malaria risk factors in north-east Tanzania. Malar J. 2011;10:98. doi: 10.1186/1475-2875-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wotodjo AN, Diagne N, Gaudart J, Richard V, Raoult D, Sokhna C. Malaria risk factors in Dielmo, a Senegalese malaria-endemic village, between October and November of 2013: a case-control study. Am J Trop Med Hyg. 2015;92:565–568. doi: 10.4269/ajtmh.14-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nsagha DS, Njunda AL, Kamga HLF, Assob JCN, Wiysonge CS, Nsagha SM, et al. Knowledge and practices relating to malaria in Ndu community of Cameroon: Signs and symptoms, causes and prevention. J Public Health Epidemiol. 2011;3:294–300. [Google Scholar]
- 46.Chouaïbou M, Etang J, Brévault T, Nwane P, Hinzoumbé CK, Mimpfoundi R, et al. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae s.l. from an area of extensive cotton cultivation in Northern Cameroon: dynamics of insecticide resistance. Trop Med Int Health. 2008;13:476–86. doi: 10.1111/j.1365-3156.2008.02025.x. [DOI] [PubMed] [Google Scholar]
- 47.Bamou R, Kopya E, Nkahe LD, Menze BD, Awono-Ambene P, Tchuinkam T, et al. Increased prevalence of insecticide resistance in Anopheles coluzzii populations in the city of Yaoundé, Cameroon and influence on pyrethroid-only treated bed net efficacy. Parasite. 2021;28:8. doi: 10.1051/parasite/2021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diouf M, Faye BT, Diouf EH, Dia AK, Konate A, Fall FB, et al. Survival of eight LLIN brands 6, 12, 24 and 36 months after a mass distribution campaign in rural and urban settings in Senegal. BMC Pub Health. 2022;22:1–12. doi: 10.1186/s12889-022-13051-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mbongue RS, Akono PN, Offono LE. Connaissances, attitudes et pratiques des chefs de ménages sur le paludisme dans les zones urbaine et rurale de Kribi Sud-Cameroun. Cameroon J Biol Biochem Sci. 2020;28:38–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its additional information files.