Abstract
CooD, the minor subunit of CS1 pili of enterotoxigenic Escherichia coli, is essential for the assembly of stable, functional pili. We previously proposed that CooD is a rate-limiting initiator of CS1 pilus assembly and predicted that the level of CooD expression should therefore determine the number of CS1 pili assembled on the cell surface. In this study, we confirm that CooD is required for the initiation of pilus assembly rather than for the stabilization of pili after they are assembled by demonstrating that specific modulation of cooD expression also modulates the number of CS1 pili on bacterial cells.
CS1 pili represent a family of adhesins associated with enterotoxigenic Escherichia coli (ETEC) that is pathogenic for humans and associated with Burkholderia cepacia, a pathogen associated with cystic fibrosis (23). Pili belonging to this family, including CS1, CFA/I, CS2, and CS14, mediate the binding of ETEC to human enterocytes in vitro (4, 11, 28) and are therefore thought to be required for colonization of the host intestine. This has been confirmed for CFA/I pili, which have been shown to be necessary for the maintenance of ETEC in the intestines of human volunteers (6) and for the CS1-related Cbl type II pili of B. cepacia which have been implicated in binding to respiratory mucins (20, 21).
The CS1 family is one of three major classes of pili associated with gram-negative bacterial pathogens. It is distinguished from the other two major families, the type IV and Pap (pyelonephrititis-associated pilus)-related pilus families, primarily by a lack of sequence similarity with any of the proteins involved in pilus assembly (23). Both the type IV pilus systems, which require up to 14 genes for the assembly of pili (16, 26, 27), and many Pap-related pilus systems, which may require up to 9 pilus assembly genes (9), are relatively complex. In contrast, for assembly, CS1 pili require only the four cotranscribed genes cooB, -A, -C, and -D (7, 15, 17, 25).
CS1 pili are composed almost entirely of the major pilin subunit, CooA, and contain on their tips CooD, which is estimated to contribute only one subunit per pilus (22). The assembly of CooA and CooD subunits into pili depends on the presence of two other proteins encoded by the coo operon: CooC, a large outer membrane protein that is probably involved in the transport of pilins across the outer membrane (22, 29), and CooB, a periplasmic chaperone-like protein that forms intermolecular complexes with each of the pilins in the periplasm and with CooC in the outer membrane and stabilizes these proteins (22, 29). CS1 and Pap-related pili share important morphogenetic features, including the transport of pilins via the periplasm, a requirement for a periplasmic chaperone, and a large outer membrane assembly protein. Since the proteins of CS1 and Pap-related pili are unrelated, it seems likely that these similarities result from convergent evolution (22, 29).
Although CooD is a minor pilin, it is also essential for the assembly of functional pili (7). If CS1 pili are assembled like type I pili by incorporation of subunits at the base of the pilus (14), the location of CooD at the pilus tip (22) suggests that it is probably the first subunit incorporated into the pilus. The absolute requirement for CooD in pilus assembly, the location of CooD at the pilus tip, and the very low level of CooD expression in wild-type cells (22) suggest that CooD may be a rate-limiting initiator for the assembly of CooA subunits into pili. In this model, CooD is essential for the initiation of pilus assembly and so the level of CooD expression should determine the number of pili on the cell surface (22).
Because pili are not detectable in the absence of CooD on the tip (7), the alternative model is that CooD is added last but is needed to stabilize the pilus structure. This model predicts that in the absence of CooD, CooA is secreted to the cell exterior but does not remain polymerized in a pilus structure. To test these models, we have investigated whether CooA is secreted to the cell exterior in the absence of CooD and whether modulating the expression of CooD influences the number of pili assembled on the cell surface.
CooD is necessary for the extracellular transport of CooA.
To investigate the extracellular secretion of CooA in a cooD mutant, the coo genes were expressed in the E. coli ara deletion mutant LMG194 (8) from plasmid pEU1290 which carries cooB, cooA, and cooC expressed under the pTrc99A promoter regulated by isopropyl-β-d-thiogalactopyranoside (IPTG) (1). For the construction of pEU1290, an insert carrying cooB, cooA, and cooC was amplified by PCR from the template plasmid pEU494 (7), using Pfu DNA polymerase (Stratagene) and the oligonucleotide primers BACUP (5′AAAAGGTACCGCCAAGTGTTAGGAGGGGG3′) and BACDOWN (5′AAAATAAGCTTCTTTTTCATTCAGTATCCTGATTG3′). The 4.1-kb insert was digested with HindIII-Acc65I and cloned into corresponding restriction sites in pTrc99A to place cooB, -A, and -C under the control of the Ptrc promoter.
Bacteria were grown in Luria-Bertani (LB) medium (24) with aeration at 37°C in the presence of ampicillin (100 μg/ml) and 0.1 mM IPTG to induce expression of cooB, -A, and -C, and extracts were assayed by immunoblotting with anti-CooA antiserum. Although no CooA was found in extracts of cells plus supernatant of LMG194, as expected, CooA was readily detectable (data not shown) in extracts of the LMG194/pEU1290 culture containing both bacteria and supernatant. A comparison between the dilutions of this extract and an extract from undiluted supernatant of this strain from which the bacterial cells had been removed showed that the supernatant contained between 1 and 10% of the total CooA present (data not shown). Immunoblots showed that the fraction of CooA in the culture supernatant was not greater than that of MalE, a periplasmically located protein that would be present in the supernatant only if the cells lysed (data not shown).
The stability of CooA subunits was tested with monomeric CooA isolated from purified CS1 pili by boiling the pili for 20 min in sterile distilled water, as previously described for CFA/I pilins (2). These subunits were added to a culture of LMG194 and incubated at 37°C for 2 h. Immunoblots showed that the concentration of subunits did not change (data not shown), so if CooA had been secreted from the cooBAC strain, it should have been detectable. We conclude that CooA does not appear to be secreted to the cell exterior in the absence of CooD.
System to modulate cooD and cooBAC expression independently.
To investigate whether the level of cooD expression influences the number of CS1 pili assembled on the cell surface, we used a two-plasmid system which allowed us to specifically modulate the expression of cooD while keeping the expression of cooB, -A, and -C constant. Plasmid pEU1290 carries cooB, -A, and -C under control of the IPTG-inducible Ptrc promoter, and plasmid pEU1206 carries cooD under the transcriptional control of the arabinose-inducible ParaBAD promoter (8). To create pEU1206, the HindIII-BglII cooD fragment from pEU493 (23) was blunt ended, passed through an intermediate vector to provide appropriate flanking restriction sites, and cloned into pBAD30 (8) to which the 1.9-kb spectinomycin resistance cassette of pHP45 (18) had been added at the FspI site.
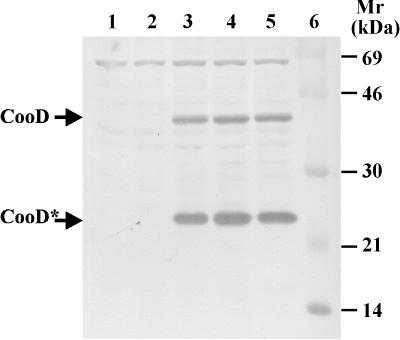
For full repression of the ParaBAD promoter on multicopy plasmids, glucose-mediated catabolite repression is usually needed (8). To confirm that glucose represses and arabinose induces cooD expression in E. coli LMG194/pEU1290/pEU1206, immunoblots of whole-cell extracts were probed with anti-CooD. As expected, no CooD was detectable in the negative-control strain LMG194/pEU1290 (cooBAC+) (Fig. 1, lane 1). When grown in the presence of glucose, LMG194/pEU1290/pEU1206 (cooBAC+ cooD+) did not produce enough CooD to be detected either (Fig. 1, lane 2). However, in the presence of arabinose, CooD was easily detected in this strain as a 38-kDa protein and a 25-kDa truncated product (Fig. 1, lanes 3 to 5) which has been described previously (22, 29). Using immunoblots, we have also confirmed that neither glucose nor arabinose affects the expression of CooA from LMG194/pEU1290 (cooBAC+) (data not shown).
FIG. 1.
Regulated expression of CooD from the ParaBAD promoter. Protein extracts were prepared from E. coli LMG194/pEU1290/pEU1206 grown in the presence of 0.2% glucose (wt/vol) (lane 2) or in the presence of arabinose at 0.002% (lane 3), 0.01% (lane 4), and 0.05% (lane 5) and were analyzed by immunoblotting with anti-CooD antiserum. Lane 1 contains a negative-control extract from LMG194/pEU1290. Lane 6 contains protein markers with molecular masses indicated in kilodaltons. The positions of the full-length, 38-kDa form of CooD and the 25-kDa truncated form (CooD*) are indicated by arrows.
Effect on CS1 piliation of modulating cooD expression.
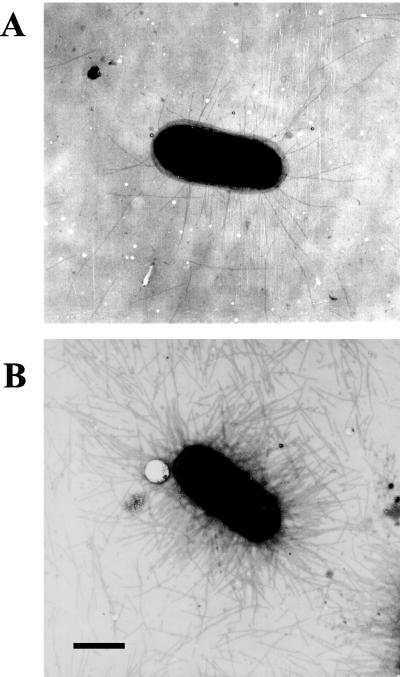
To examine the effect of cooD expression on CS1 piliation, cultures of E. coli LMG194/pEU1290/pEU1206 were grown in LB broth containing ampicillin (100 μg/ml) and spectinomycin (100 μg/ml) in the presence of 0.1 mM IPTG to derepress cooB, -A, and -C. The number of pili on bacteria from cultures in which cooD was repressed by growth in glucose (0.2% [wt/vol]) or induced by addition of arabinose (0.002, 0.01, or 0.05% [wt/vol]) was determined by electron microscopy of broth-grown cells collected by centrifugation (as described previously [22]). In the negative-control strain LMG194/pEU1290, no pili were detected, as expected (data not shown). When glucose was used to repress cooD expression, some pili (average of 28 pili/cell) were detectable on the bacterial surface (Fig. 2 and Table 1). Since CooD is required for the production of pili, it appears that there is some expression of cooD even in the presence of glucose. However, when cooD expression was induced with arabinose, the number of CS1 pili per cell increased dramatically, with most cells producing >150 pili per cell (Fig. 2 and Table 1). Therefore, the level of cooD expression determines the number of CS1 pili assembled on the cell surface.
FIG. 2.
Electron micrographs showing the effect of CooD expression on CS1 piliation. E. coli LMG194/pEU1290/pEU1206 grown in 0.2% glucose (A) or in 0.002% arabinose (B) is shown.
TABLE 1.
Effect of modulating CooD expression on the number of CS1 pili produced per cell in E. coli LMG194/pEU1206
| Cell no. | No. of pili/cella
|
|||
|---|---|---|---|---|
| Glucoseb (0.20%) | Arabinose
|
|||
| 0.002% | 0.01% | 0.05% | ||
| 1 | 11 | 4 | 0 | 12 |
| 2 | 11 | 12 | 3 | 47 |
| 3 | 14 | 21 | 4 | 60 |
| 4 | 16 | 23 | 6 | 64 |
| 5 | 16 | 129 | 20 | 75 |
| 6 | 19 | 134 | 64 | 85 |
| 7 | 20 | >150 | 97 | 100 |
| 8 | 21 | >150 | 110 | 105 |
| 9 | 22 | >150 | 126 | >150 |
| 10 | 23 | >150 | >150 | >150 |
| 11 | 26 | >150 | >150 | >150 |
| 12 | 26 | >150 | >150 | >150 |
| 13 | 26 | >150 | >150 | >150 |
| 14 | 26 | >150 | >150 | >150 |
| 15 | 26 | >150 | >150 | >150 |
| 16 | 27 | >150 | >150 | >150 |
| 17 | 27 | >150 | >150 | >150 |
| 18 | 28 | >150 | >150 | >150 |
| 19 | 28 | >150 | >150 | >150 |
| 20 | 34 | >150 | >150 | >150 |
| 21 | 39 | >150 | >150 | >150 |
| 22 | 41 | >150 | >150 | >150 |
| 23 | 45 | >150 | >150 | >150 |
| 24 | 53 | >150 | >150 | >150 |
| 25 | 82 | >150 | >150 | >150 |
The number of pili per cell was determined for 25 bacteria grown in the presence of glucose or arabinose.
When the cells were grown with glucose, the mean number of pili per cell was 28.3, with a standard deviation of 15.1 and standard error of 3 (n = 25).
Conclusion.
From the work presented here, we conclude that CooD is essential for the extracellular transport of CooA, the major pilus subunit, and that the level of CooD expression controls the number of CS1 pili assembled. This is consistent with the model we previously described (22, 29) in which CooD is the rate-limiting initiator of pilus assembly. In this model, the major pilin subunit CooA cannot be transported through the outer membrane and polymerized into pili until CooD has first associated with CooC in the outer membrane. When this occurs, CooA molecules are then able to be added to the CooD-CooC complex and CooA can continue to polymerize and be transported through the outer membrane to form the pilus structure. Because it is required to initiate pilus assembly and because pili grow by addition of subunits proximal to the cell, a CooD molecule will be located on the distal tip of the pilus structure.
Pili often serve as attachment structures for bacteria. Because many bacterial pathogens are enclosed in capsules or other types of surface molecules, it has been suggested that the long flexible structure of the pilus serves to present the adhesin, often located at its tip (3, 10, 12), to its receptor in an environment free of other bacterial surface molecules (5). The production of a pilus structure with a distally located adhesin would be assured if the polar extrusion of the pilus depended on the presence of the minor tip protein.
As for some pili, some filamentous phages, like f1, are assembled at the outer cell membrane and use a minor component to initiate polar extrusion from the cell (13, 19). For f1, the two minor coat proteins, pVII and pIX, are required for initiation of the growing phage structure. However, because phage is completely released from the producing cell before it adheres to its target cell, it is possible for the phage adhesin protein that recognizes the bacterial host to be located at the proximal tip, i.e., on the other end from the initiator protein. Pili, on the other hand, remain attached to the producing cell when they adhere to their target and so have only the distal end free. It is presumably for this reason that the minor pilin protein(s) located on the pilus tip may sometimes serve both as the initiator for assembly and as the adhesin (3). We have presented evidence above that CooD, the minor CS1 pilus protein located at the distal tip of the structure, serves as the initiator for assembly. Work in progress suggests that it also serves as the specific adhesin which attaches to receptors on the host.
REFERENCES
- 1.Amann E, Ochs B, Abel K J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 2.Buhler T, Hoschutzky H, Jann K. Analysis of colonization factor antigen I, an adhesin of enterotoxigenic Escherichia coli O78:H11: fimbrial morphology and location of the receptor-binding site. Infect Immun. 1991;59:3876–3882. doi: 10.1128/iai.59.11.3876-3882.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao J, Khan A S, Bayer M E, Schifferli D M. Ordered translocation of 987P fimbrial subunits through the outer membrane of Escherichia coli. J Bacteriol. 1995;177:3704–3713. doi: 10.1128/jb.177.13.3704-3713.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheney C P, Boedeker E C. Adherence of an enterotoxigenic Escherichia coli strain, serotype O78:H11, to purified human intestinal brush borders. Infect Immun. 1983;39:1280–1284. doi: 10.1128/iai.39.3.1280-1284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton J W, Irvin R T, Cheng K J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8:303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- 6.Evans D G, Satterwhite T K, Evans D J, Jr, DuPont H L. Differences in serological responses and excretion patterns of volunteers challenged with enterotoxigenic Escherichia coli with and without the colonization factor antigen. Infect Immun. 1978;19:883–888. doi: 10.1128/iai.19.3.883-888.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froehlich B J, Karakashian A, Melsen L R, Wakefield J C, Scott J R. CooC and CooD are required for assembly of CS1 pili. Mol Microbiol. 1994;12:387–401. doi: 10.1111/j.1365-2958.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 8.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hultgren S J, Normark S, Abraham S N. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- 10.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S N, Hultgren S J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutton S, Lloyd D R, Candy D C, McNeish A S. In vitro adhesion of enterotoxigenic Escherichia coli to human intestinal epithelial cells from mucosal biopsies. Infect Immun. 1984;44:514–518. doi: 10.1128/iai.44.2.514-518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuehn M J, Heuser J, Normark S, Hultgren S J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 13.Lopez J, Webster R E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983;127:177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- 14.Lowe M A, Holt S C, Eisenstein B I. Immunoelectron microscopic analysis of elongation of type 1 fimbriae in Escherichia coli. J Bacteriol. 1987;169:157–163. doi: 10.1128/jb.169.1.157-163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marron M B, Smyth C J. Molecular analysis of the cso operon of enterotoxigenic Escherichia coli reveals that CsoA is the adhesin of CS1 fimbriae and that the accessory genes are interchangeable with those of the cfa operon. Microbiology. 1995;141:2849–2859. doi: 10.1099/13500872-141-11-2849. [DOI] [PubMed] [Google Scholar]
- 16.Ogierman M A, Zabihi S, Mourtzios L, Manning P A. Genetic organization and sequence of the promoter-distal region of the tcp gene cluster of Vibrio cholerae. Gene. 1993;126:51–60. doi: 10.1016/0378-1119(93)90589-u. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Casal J, Swartley J S, Scott J R. Gene encoding the major subunit of CS1 pili of human enterotoxigenic Escherichia coli. Infect Immun. 1990;58:3594–3600. doi: 10.1128/iai.58.11.3594-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 19.Russel M. Filamentous phage assembly. Mol Microbiol. 1991;5:1607–1613. doi: 10.1111/j.1365-2958.1991.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 20.Sajjan S U, Forstner J F. Identification of the mucin-binding adhesin of Pseudomonas cepacia isolated from patients with cystic fibrosis. Infect Immun. 1992;60:1434–1440. doi: 10.1128/iai.60.4.1434-1440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sajjan U S, Sun L, Goldstein R, Forstner J F. Cable (Cbl) type II pili of cystic fibrosis-associated Burkholderia (Pseudomonas) cepacia: nucleotide sequence of the cblA major pilin subunit pilin gene and novel morphology of the assembled appendage fibers. J Bacteriol. 1995;177:1030–1038. doi: 10.1128/jb.177.4.1030-1038.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakellaris H, Balding D P, Scott J R. Assembly proteins of CS1 pili of enterotoxigenic Escherichia coli. Mol Microbiol. 1996;21:529–541. doi: 10.1111/j.1365-2958.1996.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 23.Sakellaris H, Scott J R. New tools in an old trade: CS1 pilus morphogenesis. Mol Microbiol. 1998;30:681–687. doi: 10.1046/j.1365-2958.1998.01088.x. [DOI] [PubMed] [Google Scholar]
- 24.Scott J R. A turbid plaque-forming mutant of phage P1 that cannot lysogenize Escherichia coli. Virology. 1974;62:344–349. doi: 10.1016/0042-6822(74)90397-3. [DOI] [PubMed] [Google Scholar]
- 25.Scott J R, Wakefield J C, Russell P W, Orndorff P E, Froehlich B J. CooB is required for assembly but not transport of CS1 pilin. Mol Microbiol. 1992;6:293–300. doi: 10.1111/j.1365-2958.1992.tb01471.x. [DOI] [PubMed] [Google Scholar]
- 26.Sohel I, Puente J L, Ramer S W, Bieber D, Wu C Y, Schoolnik G K. Enteropathogenic Escherichia coli: identification of a gene cluster coding for bundle-forming pilus morphogenesis. J Bacteriol. 1996;178:2613–2628. doi: 10.1128/jb.178.9.2613-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone K D, Zhang H Z, Carlson L K, Donnenberg M S. A cluster of fourteen genes from enteropathogenic Escherichia coli is sufficient for the biogenesis of a type IV pilus. Mol Microbiol. 1996;20:325–337. doi: 10.1111/j.1365-2958.1996.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 28.Viboud G I, McConnell M M, Helander A, Svennerholm A M. Binding of enterotoxigenic Escherichia coli expressing different colonization factors to tissue-cultured Caco-2 cells and to isolated human enterocytes. Microb Pathog. 1996;21:139–147. doi: 10.1006/mpat.1996.0049. [DOI] [PubMed] [Google Scholar]
- 29.Voegele K, Sakellaris H, Scott J R. CooB plays a chaperone-like role for the protein involved in formation of CS1 pili of enterotoxigenic Escherichia coli. Proc Natl Acad Sci USA. 1997;94:13257–13261. doi: 10.1073/pnas.94.24.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]