Abstract
Background
Using cohort data from the Japan Environment and Children’s Study (JECS), we previously reported that the risk of sleep deprivation in 1-year-old children was reduced with a higher maternal intake of fermented foods, particularly miso. The present study, which evaluates children from the same cohort at 3 years of age, is a continuation of that work.
Methods
After applying exclusion criteria to 104,062 records in the JECS dataset, we evaluated 64,200 mother-child pairs in which the child was 3 years old. We examined the association of the dietary intake of fermented foods during pregnancy with child sleep duration < 10 h at the age of 3 years.
Results
Multivariable logistic regression analysis with the lowest quartile used as a reference revealed adjusted odds ratios (95% confidence intervals) for the second through fourth quartiles of 0.98 (0.90–1.06), 0.93 (0.85–1.01), and 0.85 (0.78–0.94) for cheese intake.
Conclusions
The consumption of fermented foods during pregnancy is associated with reduced risk of sleep deprivation in 3-year-old children, albeit in a limited way.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-022-13805-6.
Keywords: Probiotics, Child, Sleep, Circadian rhythm, Cheese, Health
Background
Children need a sufficient amount of good-quality sleep for healthy development. From the neonatal period to infancy and then early childhood, sleep patterns change with the child’s development. Short sleep duration has been reported to negatively affect physical and neurological development, including obesity in infancy and childhood [1, 2] and hyperactivity at 6 years of age [3]. Therefore, it is important to investigate the risk factors for sleep deprivation in children.
One of the factors that affect children is the diet of their mothers during pregnancy, which is recognized as a lifestyle factor. For example, probiotic-containing and fermented foods are thought to influence the gut microbiota [4] and have received considerable interest because they are associated with maternal health [5, 6] or, conversely, the development of diseases [7, 8], depending on the amount consumed. It has also been reported that children born by cesarean section are at higher risk of mental and developmental disorders, and one possible reason for this is that they are not exposed to their mother's gut bacteria at birth. With respect to the reported association between the microbiota at 1 year of age and neurocognitive development at 1 and 2 years of age [9, 10], maternal intake of fermented foods has been suggested to influence the normal development of children, especially sleep duration. In particular, the intestinal microbiota of children changes significantly from the neonatal period through infancy and weaning and stabilizes at around 3 years of year, reaching a composition similar to that of adults [11, 12]. In other words, vertical transmission of intestinal bacteria of maternal origin and maternal diet are predicted to affect the intestinal microbiota of children, but the association between maternal intake of fermented foods and children's sleep duration has not been examined on a large scale in epidemiological studies.
Against this background, we previously examined the association of maternal food intake preferences during pregnancy with infant sleep duration [13]. Specifically, using data from approximately 70,000 mother–infant pairs from a large cohort study, the Japan Environment and Children’s Study (JECS), we investigated the association between fermented food intake during pregnancy and infant sleep during the first postpartum year. We found that the higher the intake of fermented foods, especially miso soup, the more likely it is for the infant to sleep for at least 11 h. However, because the child’s brain grows exponentially until 2 years of age [14], it is important to clarify whether this association with fermented food persists beyond that point.
Therefore, to expand on our recent findings [13], we investigated whether maternal fermented food intake during pregnancy was associated with the sleep deprivation of children in the same cohort at 3 years of age.
Methods
Study population
The JECS protocol has been described elsewhere [15, 16]. In short, the JECS is a nationwide government-funded birth cohort study that aims to determine the associations of various environmental factors with child health and development. JECS participants are women residing in 15 regions of Japan who were enrolled during the first trimester of pregnancy between January 2011 and March 2014 [15, 16]. Follow-ups were conducted during the second or third trimester, at childbirth, and at 1 month postpartum during scheduled in-hospital checkups. Subsequent follow-ups were conducted at 12 and 36 months postpartum by mail.
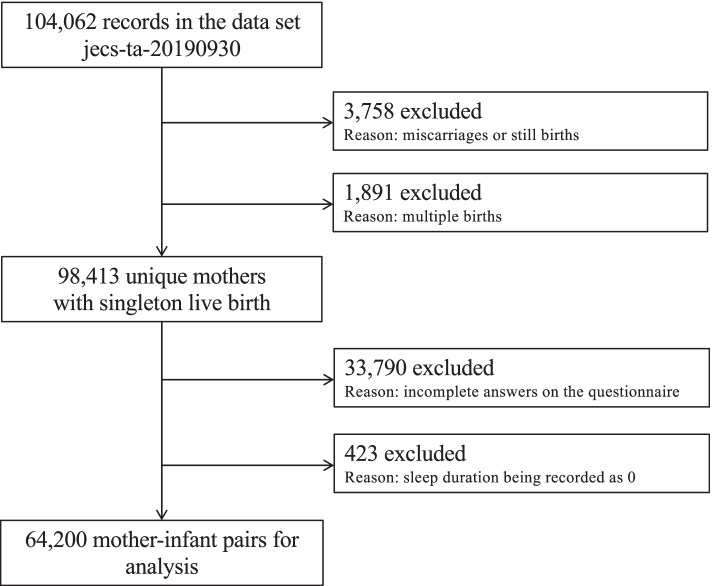
The present study analyzed the jecs-ta-20190930 dataset released in October 2019, which comprises 104,062 records obtained from a questionnaire-based survey of the participants. We excluded 3,758 cases that resulted in miscarriage or stillbirth and 1,891 cases of multiple births to focus on typical pregnancies (Fig. 1). Additionally, we also excluded 33,790 records because of incomplete responses to the questionnaire and 423 records for children whose sleep duration was recorded as 0, leaving 64,200 questionnaires with all data available for the final analysis.
Fig. 1.
Flow diagram of the recruitment and exclusion process for participants
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board on Epidemiological Studies of the Japanese Ministry of the Environment (authorization number: 100910001) and the ethics committees of all participating institutions. The JECS is conducted in accordance with the Declaration of Helsinki and all other national regulations, and written informed consent was obtained from parents/guardians of the participants whose age was below 16.
Data assessment
Exposure
Dietary intake of fermented foods during pregnancy (from the discovery of pregnancy to the second or third trimester) was assessed using a food frequency questionnaire (FFQ) [17]. Fermented foods were foods such as cheese and yogurt, the preparation of which involves fermentation of food ingredients by microorganisms. This FFQ is a semi-quantitative instrument that assesses the average consumption of 171 food and beverage items. The FFQ includes four fermented foods: miso soup (made with miso, a Japanese traditional fermented seasoning), yogurt, cheese, and natto (Japanese fermented soybeans). The FFQ has not been validated specifically for pregnant women but has been validated in a large epidemiological study of adults in the general population and has already been used in a number of the JECS studies [18–20]. In this FFQ, participants were asked how often they consumed each food type and how much of it they consumed from learning of the pregnancy to the present. For miso soup, six frequency categories were used to record overall consumption frequency (from almost never to every day), nine frequency categories were used to record the daily consumption frequency (from < 1 time to ≥ 10 times), and five categories were used to report the taste of the miso soup (from very bland to very strong), which was taken to indicate the amount of miso in the soup. The daily intake (g/day) of miso was then calculated by multiplying the overall consumption frequency by the daily consumption frequency by a factor based on the reported taste. For the other three fermented foods—yogurt, cheese, and natto—the standard portion size for each food type was categorized as small (50% smaller than standard), medium (same as standard), or large (50% larger than standard). Nine frequency categories for each item were used to record consumption frequency (< 1 time/month to ≥ 7 times/day).
The daily intake of each of these three fermented foods was calculated by multiplying the consumption frequency by the standard portion size. Then, participants were categorized by quartile of intake amount (g/day) for each of the four fermented foods.
Outcome
To measure child sleep duration at 3 years after childbirth, parents were instructed to indicate when their child slept on the previous day. Parents marked the times when their child was asleep by drawing lines through boxes, indicating 30-min intervals, for the 24-h period beginning from 12:00 am at the start of the previous day.
Sleep duration of 10–13 h in a 24-h period is recommended for 3-year-old children by the United States National Sleep Foundation [21]. Therefore, we selected 10 h as the lower limit of the appropriate sleep duration and defined children sleeping less than this amount as having sleep deprivation.
Covariates
The covariates adjusted for were energy intake during pregnancy as assessed using the FFQ [17], maternal age during pregnancy, previous childbirth, body mass index (BMI) at 1 month after childbirth, maternal education level, annual household income during pregnancy, marital status at 6 months after childbirth, alcohol intake at 1 month after childbirth, smoking status at 1 month after childbirth, employment status at 1 year after childbirth, sex of the child, child attendance at nursery at 1 year after childbirth, the location where the child slept at night at 1 year after childbirth, birth weight, gestational age, consumption of dairy products at 3 years after childbirth, presence of any disease up to 3 years after childbirth, and date (month) of birth. These variables were categorized as in our previous study [13].
Statistical analyses
Unless otherwise stated, data are expressed as the mean ± standard deviation or median. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for the risk of sleep deprivation according to each fermented food intake were calculated using logistic regression analysis, with each lowest quartile used as a reference. Adjusted ORs were calculated using all of the covariates described in the previous section, whereas crude ORs were calculated without adjustment for any covariates. In trend tests, categorical numbers were assigned to the quartile distributions for each fermented food intake and were treated as continuous variables. A two-sided p-value of < 0.05 was regarded as statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Additional analysis
To determine the association between overall fermented food intake during pregnancy and the sleep of their children at 3 years of age, we calculated the total score for quartiles of each of miso, yogurt, cheese, and natto during pregnancy, where the first quartile counted as 1 point, the second quartile as 2 points, and so forth. Thus, the score for the overall intake of fermented foods ranged from 4 to 16 points. The total score was also further categorized into quartiles. Analysis was likewise calculated using logistic regression analysis to obtain ORs and 95% CIs, setting the lowest quartile as the reference group.
Results
Table 1 shows maternal characteristics according to the quartile of cheese intake during pregnancy. Participants with higher cheese intake were more likely to have high energy intake, to be older, to be multiparous, to have a normal weight (BMI: 18.5–<25), to have a higher education level, to have a higher household income, to be a nonsmoker, to be unemployed, and to send their child to a nursery. Table 2 shows maternal characteristics according to the quartile of miso intake during pregnancy. Participants with higher miso intake were more likely to be multiparous and nonsmokers, and less likely to send their child to a nursery. Tables S1 and S2 show maternal characteristics according to quartiles of yogurt and natto intake during pregnancy, which were similar to those for cheese intake.
Table 1.
Characteristics according to quartile of maternal cheese intake during pregnancy (n = 64,200)
| Quartile of cheese intake | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 (high) | Total | |||||||
| Median intake of cheese, g/day | 0.0 | 1.3 | 4.3 | 10.0 | |||||||
| Mean intake of energy, cal | 1,534 | 1,619 | 1,758 | 2,034 | 1,736 | ||||||
| Age at childbirth, years | 30.9 | 31.4 | 31.9 | 32.3 | 31.6 | ||||||
| Previous childbirth | Nulliparous | 7,090 | (46.0) | 7,187 | (43.9) | 6,748 | (39.9) | 5,993 | (38.6) | 27,018 | (42.1) |
| Multiparous | 8,314 | (54.0) | 9,170 | (56.1) | 10,164 | (60.1) | 9,534 | (61.4) | 37182 | (57.9) | |
| BMI (kg/m2) | <18.5 | 783 | (5.1) | 779 | (4.8) | 859 | (5.1) | 797 | (5.1) | 3,218 | (5.0) |
| 18.5–<25 | 11,979 | (77.8) | 13,068 | (79.9) | 13,752 | (81.3) | 12,635 | (81.4) | 51,434 | (80.1) | |
| ≥25 | 2,642 | (17.2) | 2,510 | (15.4) | 2,301 | (13.6) | 2,095 | (13.5) | 9,548 | (14.9) | |
| Education level | Junior high school or high school | 6,175 | (40.1) | 5,591 | (34.2) | 4,852 | (28.7) | 3,972 | (25.6) | 20,590 | (32.1) |
| Technical junior college, technical/vocational college or associate degree | 6,443 | (41.8) | 7,085 | (43.3) | 7,554 | (44.7) | 6,936 | (44.7) | 28,018 | (43.6) | |
| Bachelor’s degree, postgraduate degree | 2,786 | (18.1) | 3,681 | (22.5) | 4,506 | (26.6) | 4,619 | (29.8) | 15,592 | (24.3) | |
| Annual household income, JPY | <4 million | 6,861 | (44.5) | 6,330 | (38.7) | 6,076 | (35.9) | 5,230 | (33.7) | 24,497 | (38.2) |
| 4–<6 million | 4,844 | (31.5) | 5,577 | (34.1) | 5,878 | (34.8) | 5,487 | (35.3) | 21,786 | (33.9) | |
| ≥6 million | 3,699 | (24.0) | 4,450 | (27.2) | 4,958 | (29.3) | 4,810 | (31.0) | 17,917 | (27.9) | |
| Marital status | Married (including common law marriage) | 15,107 | (98.1) | 16,142 | (98.7) | 16,728 | (98.9) | 15,375 | (99.0) | 63,352 | (98.7) |
| Divorced or widowed | 130 | (0.8) | 112 | (0.7) | 90 | (0.5) | 73 | (0.5) | 405 | (0.6) | |
| Others | 167 | (1.1) | 103 | (0.6) | 94 | (0.6) | 79 | (0.5) | 443 | (0.7) | |
| Alcohol intake | Never | 14,220 | (92.3) | 15,021 | (91.8) | 15,523 | (91.8) | 14,190 | (91.4) | 58,954 | (91.8) |
| Ex-drinker | 618 | (4.0) | 692 | (4.2) | 788 | (4.7) | 703 | (4.5) | 2,801 | (4.4) | |
| One to three times/month | 382 | (2.5) | 438 | (2.7) | 426 | (2.5) | 442 | (2.9) | 1,688 | (2.6) | |
| Once a week or more | 184 | (1.2) | 206 | (1.3) | 175 | (1.0) | 192 | (1.2) | 757 | (1.2) | |
| Smoking status | Never | 8,803 | (57.2) | 9,909 | (60.6) | 10,810 | (63.9) | 9,985 | (64.3) | 39,507 | (61.5) |
| Ex-drinker | 3,472 | (22.5) | 3,786 | (23.2) | 3,871 | (22.9) | 3,728 | (24.0) | 14,857 | (23.1) | |
| One to three times/month | 2,413 | (15.7) | 2,130 | (13.0) | 1,827 | (10.8) | 1,464 | (9.4) | 7,834 | (12.2) | |
| Once a week or more | 716 | (4.7) | 532 | (3.3) | 404 | (2.4) | 350 | (2.3) | 2,002 | (3.1) | |
| Employed | No | 7,752 | (50.3) | 8,395 | (51.3) | 8,953 | (52.9) | 8,471 | (54.6) | 33,571 | (52.3) |
| Yes | 7,652 | (49.7) | 7,962 | (48.7) | 7,959 | (47.1) | 7,056 | (45.4) | 30,629 | (47.7) | |
| Sex of the child | Boy | 7,504 | (48.7) | 8,124 | (49.7) | 8,171 | (48.3) | 7,667 | (49.4) | 31,466 | (49.0) |
| Girl | 7,900 | (51.3) | 8,233 | (50.3) | 8,741 | (51.7) | 7,860 | (50.6) | 32,734 | (51.0) | |
| Nursery | No | 5,354 | (34.8) | 5,887 | (36.0) | 6,248 | (36.9) | 5,927 | (38.2) | 23,416 | (36.5) |
| Yes | 10,050 | (65.2) | 10,470 | (64.0) | 10,664 | (63.1) | 9,600 | (61.8) | 40,784 | (63.5) | |
| Location where the baby sleeps at night | In the parent's bed | 12,933 | (84.0) | 13,719 | (83.9) | 14,294 | (84.5) | 13,019 | (83.9) | 53,965 | (84.1) |
| In a baby bed located in the parent's room | 2,262 | (14.7) | 2,425 | (14.8) | 2,403 | (14.2) | 2,282 | (14.7) | 9,372 | (14.6) | |
| In a baby bed located in a room other than the bedroom of his/her parents | 152 | (1.0) | 145 | (0.9) | 161 | (1.0) | 173 | (1.1) | 631 | (1.0) | |
| Other | 57 | (0.4) | 68 | (0.4) | 54 | (0.3) | 53 | (0.3) | 232 | (0.4) | |
| Birth weight, g | 3,018 | 3,028 | 3,034 | 3,035 | 3,029 | ||||||
| Gestational weeks | 39.3 | 39.3 | 39.3 | 39.3 | 39.3 | ||||||
| Eating dairy products | Yes | 14,971 | (97.2) | 15,953 | (97.5) | 16,502 | (97.6) | 15,164 | (97.7) | 62,590 | (97.5) |
| No | 433 | (2.8) | 404 | (2.5) | 410 | (2.4) | 363 | (2.3) | 1,610 | (2.5) | |
| Disease | No | 9,111 | (59.2) | 9,728 | (59.5) | 10,024 | (59.3) | 9,193 | (59.2) | 38,056 | (59.3) |
| Yes | 6,293 | (40.9) | 6,629 | (40.5) | 6,888 | (40.7) | 6,334 | (40.8) | 26,144 | (40.7) | |
| Birth month | 1 | 1,259 | (8.2) | 1,214 | (7.4) | 1,285 | (7.6) | 1,169 | (7.5) | 4,927 | (7.7) |
| 2 | 1,063 | (6.9) | 1,126 | (6.9) | 1,187 | (7.0) | 1,056 | (6.8) | 4,432 | (6.9) | |
| 3 | 1,178 | (7.7) | 1,285 | (7.9) | 1,259 | (7.4) | 1,188 | (7.7) | 4,910 | (7.6) | |
| 4 | 1,223 | (7.9) | 1,254 | (7.7) | 1,297 | (7.7) | 1,179 | (7.6) | 4,953 | (7.7) | |
| 5 | 1,202 | (7.8) | 1,320 | (8.1) | 1,400 | (8.3) | 1,258 | (8.1) | 5,180 | (8.1) | |
| 6 | 1,202 | (7.8) | 1,258 | (7.7) | 1,368 | (8.1) | 1,243 | (8.0) | 5,071 | (7.9) | |
| 7 | 1,382 | (9.0) | 1,477 | (9.0) | 1,484 | (8.8) | 1,414 | (9.1) | 5,757 | (9.0) | |
| 8 | 1,555 | (10.1) | 1,660 | (10.2) | 1,669 | (9.9) | 1,618 | (10.4) | 6,502 | (10.1) | |
| 9 | 1,568 | (10.2) | 1,689 | (10.3) | 1,683 | (10.0) | 1,627 | (10.5) | 6,567 | (10.2) | |
| 10 | 1,433 | (9.3) | 1,567 | (9.6) | 1,631 | (9.6) | 1,416 | (9.1) | 6,047 | (9.4) | |
| 11 | 1,178 | (7.7) | 1,251 | (7.7) | 1,350 | (8.0) | 1,177 | (7.6) | 4,956 | (7.7) | |
| 12 | 1,161 | (7.5) | 1,256 | (7.7) | 1,299 | (7.7) | 1,182 | (7.6) | 4,898 | (7.6) | |
Table 2.
Characteristics according to quartile of maternal miso intake during pregnancy (n = 64,200)
| Quartile of miso intake | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 (high) | Total | |||||||
| Median intake of miso, g/day | 10.0 | 32.1 | 88.4 | 225.0 | |||||||
| Mean intake of energy, cal | 1,620 | 1,697 | 1,752 | 1,868 | 1,736 | ||||||
| Age at childbirth, years | 31.4 | 31.4 | 31.9 | 31.7 | 31.6 | ||||||
| Previous childbirth | Nulliparous | 7,798 | (47.6) | 5,902 | (43.0) | 7,250 | (40.3) | 6,068 | (37.7) | 27,018 | (42.1) |
| Multiparous | 8,603 | (52.5) | 7,818 | (57.0) | 10,738 | (59.7) | 10,023 | (62.3) | 37182 | (57.9) | |
| BMI (kg/m2) | <18.5 | 829 | (5.1) | 684 | (5.0) | 952 | (5.3) | 753 | (4.7) | 3,218 | (5.0) |
| 18.5–<25 | 12,940 | (78.9) | 10,989 | (80.1) | 14,653 | (81.5) | 12,852 | (79.9) | 51,434 | (80.1) | |
| ≥25 | 2,632 | (16.1) | 2,047 | (14.9) | 2,383 | (13.3) | 2,486 | (15.5) | 9,548 | (14.9) | |
| Education level | Junior high school or high school | 5,491 | (33.5) | 4,310 | (31.4) | 5,412 | (30.1) | 5,377 | (33.4) | 20,590 | (32.1) |
| Technical junior college, technical/vocational college or associate degree | 7,062 | (43.1) | 6,041 | (44.0) | 7,921 | (44.0) | 6,994 | (43.5) | 28,018 | (43.6) | |
| Bachelor’s degree, postgraduate degree | 3,848 | (23.5) | 3,369 | (24.6) | 4,655 | (25.9) | 3,720 | (23.1) | 15,592 | (24.3) | |
| Annual household income, JPY | <4 million | 6,579 | (40.1) | 5,360 | (39.1) | 6,511 | (36.2) | 6,047 | (37.6) | 24,497 | (38.2) |
| 4–<6 million | 5,374 | (32.8) | 4,624 | (33.7) | 6,233 | (34.7) | 5,555 | (34.5) | 21,786 | (33.9) | |
| ≥6 million | 4,448 | (27.1) | 3,736 | (27.2) | 5,244 | (29.2) | 4,489 | (27.9) | 17,917 | (27.9) | |
| Marital status | Married (including common law marriage) | 16,092 | (98.1) | 13,536 | (98.7) | 17,808 | (99.0) | 15,916 | (98.9) | 63,352 | (98.7) |
| Divorced or widowed | 136 | (0.8) | 89 | (0.7) | 93 | (0.5) | 87 | (0.5) | 405 | (0.6) | |
| Others | 173 | (1.1) | 95 | (0.7) | 87 | (0.5) | 88 | (0.6) | 443 | (0.7) | |
| Alcohol intake | Never | 14,963 | (91.2) | 12,574 | (91.7) | 16,586 | (92.2) | 14,831 | (92.2) | 58,954 | (91.8) |
| Ex-drinker | 731 | (4.5) | 608 | (4.4) | 770 | (4.3) | 692 | (4.3) | 2,801 | (4.4) | |
| One to three times/month | 476 | (2.9) | 380 | (2.8) | 449 | (2.5) | 383 | (2.4) | 1,688 | (2.6) | |
| Once a week or more | 231 | (1.4) | 158 | (1.2) | 183 | (1.0) | 185 | (1.2) | 757 | (1.2) | |
| Smoking status | Never | 9,851 | (60.1) | 8,525 | (62.1) | 11,221 | (62.4) | 9,910 | (61.6) | 39,507 | (61.5) |
| Ex-drinker | 3,755 | (22.9) | 3,150 | (23.0) | 4,195 | (23.3) | 3,757 | (23.4) | 14,857 | (23.1) | |
| One to three times/month | 2,157 | (13.2) | 1,611 | (11.7) | 2,096 | (11.7) | 1,970 | (12.2) | 7,834 | (12.2) | |
| Once a week or more | 638 | (3.9) | 434 | (3.2) | 476 | (2.7) | 454 | (2.8) | 2,002 | (3.1) | |
| Employed | No | 8,507 | (51.9) | 7,361 | (53.7) | 9,562 | (53.2) | 8,141 | (50.6) | 33,571 | (52.3) |
| Yes | 7,894 | (48.1) | 6,359 | (46.4) | 8,426 | (46.8) | 7,950 | (49.4) | 30,629 | (47.7) | |
| Sex of the child | Boy | 8,059 | (49.1) | 6,668 | (48.6) | 8,778 | (48.8) | 7,961 | (49.5) | 31,466 | (49.0) |
| Girl | 8,342 | (50.9) | 7,052 | (51.4) | 9,210 | (51.2) | 8,130 | (50.5) | 32,734 | (51.0) | |
| Nursery | No | 5,810 | (35.4) | 4,961 | (36.2) | 6,624 | (36.8) | 6,021 | (37.4) | 23,416 | (36.5) |
| Yes | 10,591 | (64.6) | 8,759 | (63.8) | 11,364 | (63.2) | 10,070 | (62.6) | 40,784 | (63.5) | |
| Location where the baby sleeps at night | In the parent's bed | 13,666 | (83.3) | 11,581 | (84.4) | 15,103 | (84.0) | 13,615 | (84.6) | 53,965 | (84.1) |
| In a baby bed located in the parent's room | 2,489 | (15.2) | 1,960 | (14.3) | 2,662 | (14.8) | 2,261 | (14.1) | 9,372 | (14.6) | |
| In a baby bed located in a room other than the bedroom of his/her parents | 188 | (1.2) | 132 | (1.0) | 167 | (0.9) | 144 | (0.9) | 631 | (1.0) | |
| Other | 58 | (0.4) | 47 | (0.3) | 56 | (0.3) | 71 | (0.4) | 232 | (0.4) | |
| Birth weight, g | 3,027 | 3,025 | 3,027 | 3,036 | 3,029 | ||||||
| Gestational weeks | 39.3 | 39.3 | 39.3 | 39.2 | 39.3 | ||||||
| Eating dairy products | Yes | 15,979 | (97.4) | 13,366 | (97.4) | 17,546 | (97.5) | 15,699 | (97.6) | 62,590 | (97.5) |
| No | 422 | (2.6) | 354 | (2.6) | 442 | (2.5) | 392 | (2.4) | 1,610 | (2.5) | |
| Disease | No | 9,676 | (59.0) | 8,074 | (58.9) | 10,740 | (59.7) | 9,566 | (59.5) | 38,056 | (59.3) |
| Yes | 6,725 | (41.0) | 5,646 | (41.2) | 7,248 | (40.3) | 6,525 | (40.6) | 26,144 | (40.7) | |
| Birth month | 1 | 1,140 | (7.0) | 1,030 | (7.5) | 1,418 | (7.9) | 1,339 | (8.3) | 4,927 | (7.7) |
| 2 | 970 | (5.9) | 974 | (7.1) | 1,329 | (7.4) | 1,159 | (7.2) | 4,432 | (6.9) | |
| 3 | 1,059 | (6.5) | 993 | (7.2) | 1,491 | (8.3) | 1,367 | (8.5) | 4,910 | (7.7) | |
| 4 | 1,047 | (6.4) | 1,045 | (7.6) | 1,504 | (8.4) | 1,357 | (8.4) | 4,953 | (7.7) | |
| 5 | 1,138 | (6.9) | 1,094 | (8.0) | 1,551 | (8.6) | 1,397 | (8.7) | 5,180 | (8.1) | |
| 6 | 1,168 | (7.1) | 1,058 | (7.7) | 1,473 | (8.2) | 1,372 | (8.5) | 5,071 | (7.9) | |
| 7 | 1,450 | (8.8) | 1,207 | (8.8) | 1,595 | (8.9) | 1,505 | (9.4) | 5,757 | (9.0) | |
| 8 | 1,697 | (10.4) | 1,380 | (10.1) | 1,811 | (10.1) | 1,614 | (10.0) | 6,502 | (10.1) | |
| 9 | 1,877 | (11.4) | 1,433 | (10.4) | 1,694 | (9.4) | 1,563 | (9.7) | 6,567 | (10.2) | |
| 10 | 1,915 | (11.7) | 1,307 | (9.5) | 1,538 | (8.6) | 1,287 | (8.0) | 6,047 | (9.4) | |
| 11 | 1,510 | (9.2) | 1,142 | (8.3) | 1,264 | (7.0) | 1,040 | (6.5) | 4,956 | (7.7) | |
| 12 | 1,430 | (8.7) | 1,057 | (7.7) | 1,320 | (7.3) | 1,091 | (6.8) | 4,898 | (7.6) | |
Compared with the excluded participants (n = 30,613), the included mothers (n = 64,200) were more likely to eat yogurt, cheese, and natto; to be older; to be married; to be a nonsmoker; to have a higher education level; to have a higher income; to be within the normal range of BMI; to be primiparas; and to have female infants with a heavier birth weight and longer gestational age.
ORs for children not meeting the 10-h sleep duration target were evaluated based on intake of miso, yogurt, cheese, and natto. In the cheese evaluation, ORs for inadequate sleep duration were significantly lower for children with mothers in the highest quartile of intake, and these associations were significant according to a trend test (Table 3).
Table 3.
Odds ratios (95% confidence intervals) for risk of 3-year-old children sleeping less than 10 h per night according to quartile of maternal fermented food intake during pregnancy (n = 64,200)
| Quartile of fermented food intake | p-value | ||||
|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 (high) | for trend | |
| Median intake of miso, g/day | 10.0 | 32.1 | 88.4 | 225.0 | |
| Total, n | 16,401 | 13,720 | 17,988 | 16,091 | |
| Cases, n | 1,238 | 943 | 1,279 | 1,219 | |
| Crude odds ratio | 1.00 (Ref.) | 0.90 (0.83–0.99) | 0.94 (0.86–1.02) | 1.00 (0.93–1.09) | 0.809 |
| Adjusted odds ratio | 1.00 (Ref.) | 0.91 (0.84–1.00) | 0.95 (0.87–1.03) | 1.00 (0.92–1.09) | 0.863 |
| Median intake of yogurt, g/day | 8.0 | 25.7 | 60.0 | 120.0 | |
| Total, n | 16,933 | 14,471 | 14,210 | 18,586 | |
| Cases, n | 1,236 | 1,064 | 1,042 | 1,337 | |
| Crude odds ratio | 1.00 (Ref.) | 1.01 (0.93–1.10) | 1.01 (0.92–1.10) | 0.98 (0.91–1.07) | 0.689 |
| Adjusted odds ratio | 1.00 (Ref.) | 1.04 (0.95–1.13) | 1.04 (0.95–1.14) | 1.02 (0.94–1.11) | 0.674 |
| Median intake of cheese, g/day | 0.0 | 1.3 | 4.3 | 10.0 | |
| Total, n | 15,404 | 16,357 | 16,912 | 15,527 | |
| Cases, n | 1,190 | 1,231 | 1,212 | 1,046 | |
| Crude odds ratio | 1.00 (Ref.) | 0.97 (0.90–1.06) | 0.92 (0.85–1.00) | 0.86 (0.79–0.94) | <0.001 |
| Adjusted odds ratio | 1.00 (Ref.) | 0.98 (0.90–1.06) | 0.93 (0.85–1.01) | 0.85 (0.78–0.94) | <0.001 |
| Median intake of natto, g/day | 0.0 | 3.3 | 10.7 | 25.0 | |
| Total, n | 11,477 | 15,293 | 22,114 | 15,316 | |
| Cases, n | 869 | 1,151 | 1,557 | 1,102 | |
| Crude odds ratio | 1.00 (Ref.) | 0.99 (0.91–1.09) | 0.93 (0.85–1.01) | 0.95 (0.86–1.04) | 0.089 |
| Adjusted odds ratio | 1.00 (Ref.) | 1.00 (0.91–1.10) | 0.93 (0.86–1.02) | 0.95 (0.86–1.05) | 0.133 |
Adjusted for energy intake, maternal age during pregnancy, previous childbirth, body mass index (BMI) at 1 month after childbirth, maternal education level, annual household income, marital status at 6 months after childbirth, alcohol intake at 1 month after childbirth, smoking status at 1 month after childbirth, employment status at 1 year after childbirth, sex of the child, attendance at nursery (at 1 year of age), the location where the child slept at night (at 1 year of age), birth weight, gestational age, eating dairy products at 3 years of age, presence of any disease (up to 3 years of age), and date (month) of birth
In additional analyses, ORs were calculated for overall fermented food intake and children's sleep duration. The results showed that the OR for inadequate sleep duration was significantly lower for children whose mothers were in the highest quartile (adjusted OR 0.90, 95% CI 0.82–0.99), but not in the second (adjusted OR 0.99, 95% CI 0.91–1.08) or third (adjusted OR 0.94, 95% CI 0.87–1.03) quartile.
Discussion
This study used data from 64,200 mother-child pairs from the JECS to determine the association of the dietary intake of fermented foods during pregnancy with less than 10 h of sleep among 3-year-old children. The results showed that cheese intake during pregnancy was associated with a significantly lower risk of sleep deprivation (< 10 h) among children of mothers in the fourth quartile compared with children of mothers in the first quartile. Miso intake was found to be associated with sleep duration in 1-year-old children [13] but not in 3-year-old children. These findings suggest that the effect of mothers’ consumption of fermented foods during pregnancy on their children’s sleep can continue to at least at 3 years of age.
The current results on the association between the maternal consumption of fermented foods during pregnancy and sleep duration in 3-year-old children are consistent with those from previous study [13]. It has already been reported that fermented foods positively affect the intestinal bacterial activity and growth [22].
In a randomized controlled trial with human participants, a group that consumed fermented foods such as yogurt and kimchi for 10 weeks had a greater variety of intestinal bacteria 4 weeks after the end of the study [23]. Animal experiments have shown that the gut microbiota, in addition to changing sleep-wake patterns and sleep quality, significantly alters gut metabolism, that the gut microbiota has a circadian rhythm, and that the intestinal bacteria exhibit circadian rhythms in composition and activity [24, 25]. It was also shown that mice without gut microbes have disrupted circadian rhythms compared with those with gut microbes [26]. In addition, maternal melatonin affects the fetus through the placenta [27, 28], and the gut microbiota is transferred to the infant at birth, causing changes in the infant’s gut microbiota [8]. The intestinal microbiota also reflects significant metabolic changes in the intestinal tract as well as changes in sleep-wake patterns and sleep quality [29]. Intestinal bacteria and hormones are thus expected to be closely related to sleep. Accordingly, fermented foods, intestinal flora, and hormones are closely related to sleep and the mother’s gut microbiota may have long-term effects on the child after birth.
The main strength of our study was the large sample size of over 60,000 mother–child pairs and the fact that the sample can be considered representative of mothers and toddlers in Japan, given that the JECS covers a wide geographic range across 15 regions. However, this study also has some limitations. Similar to the previous study [13], we did not directly investigate changes in intestinal microbiota. Another limitation is the reliance on maternal reports of child sleep duration. We observed that pregnant women who were well-educated and employed, and had higher income tended to have higher fermented food intake. To explain this, we speculated that these women likely recognized factors contributing to health and therefore tended to choose nutrient-rich options, such as fermented foods, more frequently than nutritionally unbalanced and/or nutrient-deficient options, such as junk food. The women’s health consciousness might affect the sleep duration of their children. In fact, the study found that cheese intake was associated with “health consciousness” factors such as BMI, education level, household income, and smoking status. Although we adjusted for these factors, “health consciousness” remained as a hidden factor independent of these other factors.
Conclusions
In this study, 64,200 pairs of mothers and their children were surveyed to determine the association between the mothers' intake of fermented foods during pregnancy and their children's sleep duration at 3 years of age. The results showed that mothers who consumed more cheese during pregnancy had a reduced risk of their children sleeping less than 10 h per night.
Supplementary Information
Acknowledgements
We are grateful to all participants of the JECS and to all individuals involved in data collection.
List of JECS Group members and their affiliations
Michihiro Kamijima4 (Principal Investigator), Shin Yamazaki5, Yukihiro Ohya6, Reiko Kishi7, Nobuo Yaegashi8, Koichi Hashimoto9, Chisato Mori10, Shuichi Ito11, Zentaro Yamagata12, Takeo Nakayama13, Hiroyasu Iso14, Masayuki Shima15, Hiroshige Nakamura16, Narufumi Suganuma17, Koichi Kusuhara18, Takahiko Katoh19
4 Nagoya City University, Nagoya, Japan
5 National Institute for Environmental Studies, Tsukuba, Japan
6 National Center for Child Health and Development, Tokyo, Japan
7 Hokkaido University, Sapporo, Japan
8 Tohoku University, Sendai, Japan
9 Fukushima Medical University, Fukushima, Japan
10 Chiba University, Chiba, Japan
11 Yokohama City University, Yokohama, Japan
12 University of Yamanashi, Chuo, Japan
13 Kyoto University, Kyoto, Japan
14 Osaka University, Suita, Japan
15 Hyogo College of Medicine, Nishinomiya, Japan
16 Tottori University, Yonago, Japan
17 Kochi University, Nankoku, Japan
18 University of Occupational and Environmental Health, Kitakyushu, Japan
19 Kumamoto University, Kumamoto, Japan
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- JECS
Japan Environment and Children’s Study
- JPY
Japanese Yen
- OR
odds ratio
Authors’ contributions
Conceptualization, M.I. and N.S.; methodology, K.M.; formal analysis, K.M..; data curation, the JECS Group; writing—original draft preparation, M.I. and N.S.; writing—review and editing, K.H., K.M., A.T., H.I., and the JECS Group. All authors have read and agreed to the published version of the manuscript.
Funding
The JECS is funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Availability of data and materials
This study’s data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare also restrict the open sharing of the epidemiological data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Declarations
Ethics approval and consent to participate
The JECS comprehensive protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (100910001) and the ethics committees of all participating institutions. This specific study was approved by the Ethics Committee of the University of Toyama (R2018032). The JECS is conducted in accordance with the Helsinki Declaration and other national regulations, and written informed consent was obtained from all participants/guardians of the participants whose age was below 16.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hidekuni Inadera, Email: inadera@med.u-toyama.ac.jp.
The Japan Environment and Children’s Study (JECS) Group:
Michihiro Kamijima, Shin Yamazaki, Yukihiro Ohya, Reiko Kishi, Nobuo Yaegashi, Koichi Hashimoto, Chisato Mori, Shuichi Ito, Zentaro Yamagata, Takeo Nakayama, Hiroyasu Iso, Masayuki Shima, Hiroshige Nakamura, Narufumi Suganuma, Koichi Kusuhara, and Takahiko Katoh
References
- 1.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekine M, Yamagami T, Handa K, Saito T, Nanri S, Kawaminami K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28(2):163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Touchette E, Côté SM, Petit D, Liu X, Boivin M, Falissard B, et al. Short nighttime sleep-duration and hyperactivity trajectories in early childhood. Pediatrics. 2009;124(5):e985–e993. doi: 10.1542/peds.2008-2005. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan A, Nord CE. Probiotics and gastrointestinal diseases. J Intern Med. 2005;257(1):78–92. doi: 10.1111/j.1365-2796.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 5.Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. 2014;44(1):1–8. doi: 10.1016/j.medmal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Reis D. The Effect of Probiotic Supplementation on Self-Reported Sleep Quality. University of Kansas; 2016. [Google Scholar]
- 7.Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Sanz Y. Gut microbiota and probiotics in maternal and infant health. Am J Clin Nutr. 2011;94(6 Suppl):2000s–2005s. doi: 10.3945/ajcn.110.001172. [DOI] [PubMed] [Google Scholar]
- 9.Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, et al. Infant Gut Microbiome Associated With Cognitive Development. Biol Psychiatry. 2018;83(2):148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs Res. 2016;65(1):76–88. doi: 10.1097/NNR.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuoka T. Establishment of intestinal bacteriology. Biosci Microbiota Food Health. 2014;33(3):99–116. doi: 10.12938/bmfh.33.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji H, Oozeer R, Matsuda K, Matsuki T, Ohta T, Nomoto K, et al. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef Microbes. 2012;3(2):113–125. doi: 10.3920/BM2011.0038. [DOI] [PubMed] [Google Scholar]
- 13.Sugimori N, Hamazaki K, Matsumura K, Kasamatsu H, Tsuchida A, Inadera H, et al. Association between maternal fermented food consumption and infant sleep duration: The Japan Environment and Children's Study. PLoS One. 2019;14(10):e0222792. doi: 10.1371/journal.pone.0222792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19(3):123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children's study (JECS) BMC Public Health. 2014;14:25. doi: 10.1186/1471-2458-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline Profile of Participants in the Japan Environment and Children's Study (JECS) J Epidemiol. 2018;28(2):99–104. doi: 10.2188/jea.JE20170018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama Y, Takachi R, Ishihara J, Ishii Y, Sasazuki S, Sawada N, et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J Epidemiol. 2016;26(8):420–432. doi: 10.2188/jea.JE20150064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura K, Hamazaki K, Tsuchida A, Inadera H. JECS Group: Omega-3 fatty acid intake during pregnancy and risk of infant maltreatment: a nationwide birth cohort - the Japan Environment and Children's Study. Psychol Med. 2021:1–10. [DOI] [PMC free article] [PubMed]
- 19.Nakamura M, Hamazaki K, Matsumura K, Kasamatsu H, Tsuchida A, Inadera H, et al. Infant dietary intake of yogurt and cheese and gastroenteritis at 1 year of age: The Japan Environment and Children's Study. PLoS One. 2019;14(10):e0223495. doi: 10.1371/journal.pone.0223495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimori N, Hamazaki K, Matsumura K, Kasamatsu H, Tsuchida A, Inadera H, et al. Association between mothers' fish intake during pregnancy and infants' sleep duration: a nationwide longitudinal study-The Japan Environment and Children's Study (JECS) Eur J Nutr. 2022;61(2):679–686. doi: 10.1007/s00394-021-02671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137–4153 e4114. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A. 2015;112(33):10479–10484. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17(5):681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. J Occup Environ Med. 1996;38(4):352–358. doi: 10.1097/00043764-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Mendez N, Abarzua-Catalan L, Vilches N, Galdames HA, Spichiger C, Richter HG, et al. Timed maternal melatonin treatment reverses circadian disruption of the fetal adrenal clock imposed by exposure to constant light. PLoS One. 2012;7(8):e42713. doi: 10.1371/journal.pone.0042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa Y, Miyoshi C, Obana N, Yajima K, Hotta-Hirashima N, Ikkyu A, et al. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci Rep. 2020;10(1):19554. doi: 10.1038/s41598-020-76562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study’s data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare also restrict the open sharing of the epidemiological data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.