Abstract
Introduction
Life-space and frailty are closely linked to health-related quality of life and understanding their inter-relationship could indicate potential intervention targets for improving quality of life. We set out to examine the relationship between frailty and life-space and their relative impact on quality of life measures.
Methods
Using cross-sectional data from a population-representative cohort of people aged ≥ 70 years, we assessed quality of life with the EuroQol Health Index tool (5-levels) (EQ-5D-5L). We also undertook a life-space assessment and derived a frailty index. Linear regression models estimated EQ-5D-5L scores (dependent variable) using life-space assessment, frailty index and interactions between them. All models were adjusted by age, sex, lifestyle, and social care factors.
Results
A higher EQ-5D Index was associated with higher life-space (0.02 per life-space assessment score, 95%CI: 0.01 to 0.03, p < 0.01) and decreasing frailty (-0.1 per SD, 95%CI: -0.1 to -0.1, p < 0.01). There was evidence of an interaction between life-space and frailty, where the steepest gradient for life-space and EQ-5D was in those with the highest frailty (interaction term = 0.02 per SD of frailty, 95%CI: 0.01 to 0.03, p < 0.01).
Conclusion
Individuals with the highest frailty were twice as likely to have higher quality of life in association with a larger life-space. Interventions designed to improve quality of life in frail older people could focus on increasing a person’s life-space.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-03355-2.
Keywords: Life-space, Frailty, Quality of life, Epidemiology
Introduction
Maintaining health-related quality of life (HRQoL) into older age is a key ambition for multidisciplinary healthcare teams [1, 2]. Quality of life has several dimensions, covering the physical, psychological, and social aspects of an individual’s well-being and function [3]. Other multidimensional quantities relevant to older people include frailty and life-space [4, 5]. Frailty results from cumulative decline across multiple physiological systems. Life-space assessments integrate several aspects of functional mobility. Each measure is closely linked, and understanding their inter-relationship could indicate potential intervention targets for improving quality of life [2, 6].
Life-space is a rich and informative assessment of mobility with good construct validity, yet it is not yet well-established as a clinical tool [7]. Higher scores reflect greater degrees of function, which can be multiplied to give a single measure [8]. An individual’s total life-space is dynamic and may change as a result of acute (e.g., after stroke or surgery) and chronic (e.g., dementia or osteoarthritis) health conditions [9, 10]. Smaller life-space is associated with a lower quality of life [11], though how changes in life-space impact quality of life across the spectrum of frailty has not been described [12]. Frailty is an important contextual factor given that frail and pre-frail individuals have greater life-space decline than non-frail individuals [13].
We set out to examine the relationship between life-space, frailty, and their relative impact on overall quality of life in a population-representative cohort. We investigated variables related to quality of life and life-space, hypothesising that increasing life-space would be associated with improving quality of life to varying degrees between frail and robust older adults.
Methods
We used data collected between 2018 and 2019 from the Delirium and Population Health Informatics Cohort (DELPHIC). Details have already been published [14–16]. In brief, DELPHIC is a population-based prospective study of residents aged ≥ 70 years in the London Borough of Camden. The sample was mainly enrolled from primary care lists and is representative of the borough in terms of age distribution and income deprivation indices. Here, we used data from the first 1,510 individuals recruited. This is a cross-sectional analysis of the baseline assessment.
Outcome
Quality of life was defined by the EuroQol Health Index tool (5-levels) (EQ-5D-5L) [17], which includes a visual analogue scale (VAS) summarising a self-rating for quality of life from 0 to 100 (100 = ‘best health’). EQ-5D-5L also has domains on mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Using empirical value sets for an English population, a score in each domain generates an overall EQ-5D index where 0 is equivalent to dead (negative values mean ‘worse than dead’) and 1 refers to ‘full health’ (values above 1 indicate even higher health utility).
Exposure
The life-space assessment is a self-reported measure of an individual’s independent mobility [18]. It relates to the dimensions of geographical space in which a person’s life takes place. Life-space has three components: distance travelled (5 levels, from bedroom only to beyond the neighbourhood); frequency of travel (4 levels, from daily to < 1/week); need for assistance (3 levels, none / with equipment / with personal assistance). Responses refer to the previous four weeks’ activity. Multiplying these scores indicates an individual’s functional mobility (range 0 to 120). Higher scores indicate greater mobility.
Covariates
We included health, social and lifestyle factors, such as frailty, frequency of contact with next of kin, and receiving a care package. We selected these based on previously reported associations with quality of life [19, 20] and life-space [7]. These items were self-reported and collected by an interviewer who could corroborate data through real-time access to health and social care records. Distance from next of kin was documented in miles and living alone was recorded as ‘yes’ or ‘no’. Contact with a next of kin was categorised as in-person or by phone, recorded as daily, weekly, monthly, yearly, or less. Monthly or less frequent contact with a next of kin was used to define isolation. Care package receipt was recorded by frequency (from hired cleaners only up to 24-h care). For health behaviours, cigarette smoking status was asked directly and classified as: current smoker, ex-smoker and non-smoker, and alcohol consumption was recorded as daily, weekly, monthly or less frequently. Frailty was quantified using a Frailty Index, representing the proportion of accumulated health deficits, including comorbidities. The Frailty Index ranges from 0 to 1, with higher values indicating more frailty. We derived it using 35 items from the baseline assessment, covering general health, comorbidities, medications, health behaviours, hearing, vision, dental health, continence, falls, depression, personal and instrumental activities of daily living, and calculated according to standard procedures [21] (Supplementary table 1). Tertiles of frailty were: low frailty up to 0.08; medium frailty 0.09 to 0.24; high frailty 0.25 or greater [22]. Socioeconomic position (SEP) was operationalised using highest educational attainment (primary/secondary/tertiary), the Office for National Statistics occupational skill classification (4 levels) and Index of Multiple Deprivation (IMD) score, an ecological measure where higher scores indicate neighbourhood disadvantage [23, 24].
Statistical analysis
We used a series of linear regression models to estimate the associations between our continuous outcomes (EQ-5D index, visual analogue scale) and covariates. We used median imputation for any data missing within the life-space assessment dimensions and multiple imputation (20 imputations) for other missing covariate data. We assessed interactions between life-space and frailty index scores by creating a parameter multiplying the two continuous measures, testing the model with and without this interaction term. To improve comparability and ease of interpretation, we transformed these into standardised z-scores (score-mean)/standard deviation). We used Stata version 16.1 for all analyses (StataCorp LLC, College Station, Texas).
Results
The mean age of the full sample was 78 (SD 6.2), and 41% were men, and most individuals were educated to degree, or postgraduate level and had high-skill occupations (Table 1). Outcome scores were missing in 24% of participants, with 5% missing both EQ-5D Index and the Visual Analogue Scale score. Missing outcome scores were more likely in people with higher frailty (FI 0.19 versus 0.15) and with more multimorbidity (1.4 versus 1.6 conditions) (Table 1). The remainder of the analyses were on participants with available EQ-5D Index and Visual Analogue Scale data (n = 1152). Individuals in the middle tertile of EQ-5D Index (between 0.7 and 0.8) had a Visual Analogue Scale score of 79/100. Participants reported an average life-space of 66/120, broadly equivalent to someone who is able to leave their neighbourhood several times a week with the assistance of equipment. The mean frailty index was 0.15 (Table 1). Table 2 describes typical clinical presentations of different levels of life-space by degree of frailty.
Table 1.
Descriptive characteristics of the sample (n = 1510, missing EQ-5D-5L Index = 358 (24%))
| EQ-5D-5L Index | |||||||
|---|---|---|---|---|---|---|---|
| Total sample (n = 1510) |
Missing Quality of life (Both measures) |
P | < 0.70 (n = 324) |
0.70 to 0.80 (n = 428) |
> 0.80 (n = 400) |
P | |
| n or mean | n or mean | n or mean | n or mean | n or mean | |||
| Men | 625 (41%) | 27 (2%) | 0.305 | 133 (43%) | 178 (43%) | 172 (44%) | 0.875 |
| EQ-Visual Analogue Scale (SD) | 78.6 (15.6) | 78.6 (16.4) | 79.1 (15.5) | 78.7 (15.4) | 0.928 | ||
| Life-space (score, SD) | 65.7 (17.5) | 82 (53.7) | 0.186 | 64.7 (18.5) | 66.3 (17.4) | 65.5 (17.6) | 0.585 |
| Frailty index (SD) | 0.15 (0.13) | 0.19 (0.15) | 0.005 | 0.15 (0.12) | 0.14 (0.12) | 0.16 (0.14) | 0.447 |
| Age (years, SD) | 78 (6.2) | 78 (6.0) | 0.488 | 78 (6.4) | 78 (5.9) | 78 (6.2) | 0.774 |
| Multimorbidity (count, SD) | 1.4 (1.2) | 1.6 (1.3) | 0.039 | 1.3 (1.1) | 1.4 (1.3) | 1.4 (1.1) | 0.488 |
| IMD (deprivation score, SD) | 16.6 (9.1) | 17.9 (8.9) | 0.226 | 15.8 (8.5) | 17.1 (9.6) | 17.0 (9.1) | 0.125 |
| Education | |||||||
| Up to primary | 213 (14%) | 12 (1%) | 0.44 | 50 (16%) | 52 (12%) | 58 (15%) | 0.679 |
| Up to secondary | 313 (21%) | 21 (1%) | 62 (19%) | 89 (21%) | 83 (21%) | ||
| Degree level | 968 (64%) | 47 (3%) | 210 (65%) | 285 (67%) | 251 (64%) | ||
| Occupational skill level | |||||||
| Level 1 | 81 (5%) | 6 (0.4%) | 0.068 | 21 (7%) | 21 (5%) | 22 (6%) | 0.446 |
| Level 2 | 237 (16%) | 13 (1%) | 46 (14%) | 59 (14%) | 68 (17%) | ||
| Level 3 | 246 (16%) | 20 (1%) | 41 (13%) | 70 (16%) | 67 (17%) | ||
| Level 4 | 935 (62%) | 39 (3%) | 215 (67%) | 276 (65%) | 242 (61%) | ||
| NOK contact (in person) | |||||||
| Daily or weekly | 1099 (73%) | 63 (4%) | 0.411 | 237 (75%) | 325 (76%) | 286 (72%) | 0.485 |
| Monthly | 211 (14%) | 8 (1%) | 44 (14%) | 57 (14%) | 61 (15%) | ||
| Yearly or less | 167 (11%) | 7 (0.5%) | 35 (11%) | 37 (9%) | 48 (12%) | ||
| NOK contact (by phone) | |||||||
| Daily or weekly | 1026 (68%) | 53 (5%) | 0.231 | 222 (88%) | 299 (91%) | 267 (86%) | 0.372 |
| Monthly | 97 (6%) | 3 (0.3%) | 21 (8%) | 22 (7%) | 34 (11%) | ||
| Yearly or less | 40 (3%) | 0 (0%) | 9 (4%) | 9 (3%) | 11 (4%) | ||
| Care package | |||||||
| None | 1413 (94%) | 73 (5%) | 0.632 | 303 (94%) | 405 (95%) | 370 (93%) | 0.706 |
| Weekly | 26 (2%) | 2 (0.1%) | 4 (1%) | 7 (2%) | 8 (2%) | ||
| Daily or more | 68 (5%) | 5 (0.3%) | 16 (5%) | 16 (4%) | 22 (6%) | ||
| Smoking status | |||||||
| Never | 640 (42%) | 30 (2%) | 0.447 | 144 (45%) | 179 (42%) | 170 (43%) | 0.907 |
| Ex-smoker | 774 (51%) | 43 (3%) | 160 (50%) | 226 (53%) | 203 (51%) | ||
| Current | 91 (6%) | 7 (0.5%) | 19 (6%) | 23 (5%) | 25 (6%) | ||
| Alcohol intake | |||||||
| Daily | 534 (35%) | 30 (2%) | 0.12 | 110 (39%) | 140 (41%) | 149 (44%) | 0.754 |
| Weekly | 458 (30%) | 16 (1%) | 112 (40%) | 134 (39%) | 120 (35%) | ||
| Monthly or less | 267 (18%) | 18 (1%) | 60 (21%) | 71 (21%) | 71 (21%) | ||
| Distance from NOK (miles, SD) | 201.9 (1299.2) | 299 (1242.9) | 0.507 | 120.2 (595.3) | 151.1 (1088.2) | 207.1 (1074.2) | 0.478 |
Items all assessed by interview or self-reported
IMD Index of Multiple Deprivation, NOK Next of kin
Table 2.
Life-space scores and profiles according to frailty
| Low | Medium | High | |
|---|---|---|---|
| Life-space: mean (SD) | 74.3 (11.5) | 66.9 (14.0) | 41.4 (20.0) |
| Description of typical individual | Independently travels outside city on a weekly basis | Mobilises outdoors independently but rarely beyond their neighbourhood | Leaves house daily or able to but needs rollator frame; leaves neighbourhood rarely and would need personal assistance to do so |
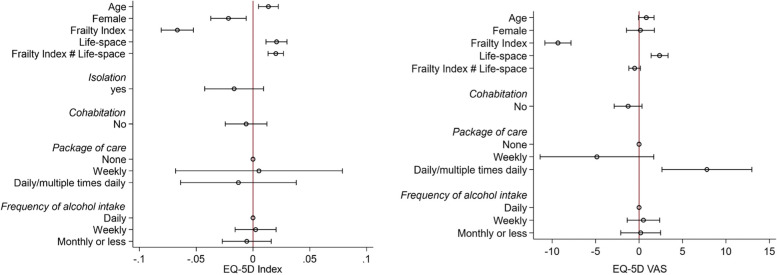
A higher EQ-5D Index was associated with older age (0.01 per year, 95%CI: 0.005 to 0.02, p < 0.01), higher life-space (0.02 per life-space assessment score, 95%CI: 0.01 to 0.03, p < 0.01) and decreasing frailty (-0.1 per SD, 95%CI: -0.1 to -0.1, p < 0.01) (Table 3, Fig. 1). Women had a lower EQ-5D Index (-0.02, 95%CI: -0.04 to -0.01, p < 0.01). Similar patterns were evident for the Visual Analogue Scale scores. Neither self-reported isolation, frequency of contact, nor distance from next of kin were associated with quality of life.
Table 3.
Variables associated with quality of life (n = 1,152)
| EQ-5D Index | Visual Analogue Scale | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Multivariate Linear Regression of 943 individuals | Unadjusted | Multivariate Linear Regression of 1,152 individuals | |||||||||||||
| Coef | [95% Conf. Interval] | P | Coef | [95% Conf. Interval] | P | Coef | [95% Conf. Interval] | P | Coef | [95% Conf. Interval] | P | |||||
| Age | -0.03 | -0.04 | -0.02 | < 0.001 | 0.01 | 0.005 | 0.02 | 0.002 | -3.6 | -4.5 | -2.7 | < 0.001 | 0.8 | -0.1 | 1.7 | 0.068 |
| Women (cf.) Men | -0.03 | -0.04 | -0.01 | 0.003 | -0.02 | -0.04 | -0.01 | 0.007 | 0.1 | -1.7 | 2.0 | 0.873 | 0.2 | -1.5 | 1.8 | 0.851 |
| Frailty index | -0.1 | -0.1 | -0.1 | < 0.001 | -0.1 | -0.1 | -0.1 | < 0.001 | -9.5 | -10.4 | -8.6 | < 0.001 | -9.3 | -10.8 | -7.8 | < 0.001 |
| Life-space | 0.1 | 0.1 | 0.1 | < 0.001 | 0.02 | 0.01 | 0.03 | < 0.001 | 6.0 | 5.2 | 6.7 | < 0.001 | 2.4 | 1.4 | 3.3 | < 0.001 |
| Frailty Index # Life-space | 0.02 | 0.01 | 0.03 | < 0.001 | -0.5 | -1.2 | 0.2 | 0.134 | ||||||||
| NOK contact (in person) | ||||||||||||||||
| Daily/weekly | [ref] | 0.047 | [ref] | 0.174 | ||||||||||||
| Monthly | -0.01 | -0.03 | 0.02 | -0.9 | -3.5 | 1.7 | ||||||||||
| Yearly or less | -0.04 | -0.1 | -0.01 | -2.6 | -5.4 | 0.2 | ||||||||||
| NOK contact (by phone) | ||||||||||||||||
| Daily/weekly | [ref] | 0.051 | [ref] | 0.219 | ||||||||||||
| Monthly | -0.04 | -0.1 | -0.005 | 0.2 | -3.3 | 3.8 | ||||||||||
| Yearly or less | -0.03 | -0.1 | 0.02 | -4.6 | -9.8 | 0.6 | ||||||||||
| Isolation | -0.04 | -0.1 | -0.01 | 0.010 | -0.02 | -0.04 | 0.01 | 0.210 | -1.8 | -4.8 | 1.3 | 0.252 | ||||
| Lives alone | -0.03 | -0.05 | -0.01 | 0.001 | -0.01 | -0.02 | 0.01 | 0.518 | -3.0 | -4.8 | -1.2 | 0.001 | -1.3 | -2.9 | 0.3 | 0.123 |
| Distance from NOK (miles) | -0.001 | -0.01 | 0.01 | 0.809 | 0.001 | -0.9 | 0.9 | 0.999 | ||||||||
| Care package | ||||||||||||||||
| None | [ref] | < 0.001 | 0.853 | [ref] | < 0.001 | 0.001 | ||||||||||
| Weekly | -0.2 | -0.2 | -0.1 | 0.01 | -0.1 | 0.1 | -23.8 | -30.8 | -16.8 | -4.9 | -11.4 | 1.7 | ||||
| Daily/multiple times daily | -0.3 | -0.4 | -0.3 | -0.01 | -0.1 | 0.04 | -15.6 | -20.2 | -11.1 | 7.8 | 2.6 | 13.0 | ||||
| Smoking status | ||||||||||||||||
| Never | [ref] | 0.533 | [ref] | 0.168 | ||||||||||||
| Ex-smoker | -0.01 | -0.02 | 0.01 | -0.8 | -2.7 | 1.0 | ||||||||||
| Current | -0.02 | -0.1 | 0.02 | -3.7 | -7.6 | 0.3 | ||||||||||
| Alcohol intake | ||||||||||||||||
| Daily | [ref] | < 0.001 | 0.769 | [ref] | < 0.001 | 0.886 | ||||||||||
| Weekly | -0.004 | -0.03 | 0.02 | 0.002 | -0.02 | 0.02 | 0.1 | -2.1 | 2.3 | 0.5 | -1.4 | 2.4 | ||||
| Monthly or less | -0.06 | -0.08 | -0.04 | -0.01 | -0.03 | 0.02 | -4.7 | -7.3 | -2.2 | 0.2 | -2.1 | 2.5 | ||||
NOK Next of kin
Fig. 1.
Variables associated with HRQoL
Lower life-space was associated with older age (-2.5 per year (95%CI: -3.2 to -1.7, p < 0.01) and frailty (-6.6 per SD, 95%CI: -7.4 to -5.7, p < 0.01) (Supplementary Table 2, Supplementary Fig. 1). Women had lower life-space (-2.0, 95%CI: -3.4 to -0.5, p < 0.01). There was a graded association between educational attainment and life-space, such that those with no qualifications had the lowest life-space (-3.5, 95%CI: -6.3 to -0.7, p = 0.03).
We found evidence of an interaction between life-space and frailty and associated EQ-5D (Table 3, Fig. 2). The steepest gradient for life-space and EQ-5D was in those with the highest frailty (interaction term = 0.02 per SD of frailty, 95%CI: 0.01 to 0.03, p < 0.01). This coefficient was the same as the association between life-space and EQ-5D, which translates to a doubling of the effect size for each SD of increasing frailty. The highest effect was in those with a high level of frailty compared to those with a low and medium level of frailty. Improving indoor and outdoor mobility (and frequency of being outdoors) in the middle range of life-space (-1SD to + 1SD), would be associated with a 0.1 point (one tertile) improvement in quality of life (Table 2). This difference was comparable to an individual 10 years younger. Conversely, additional gains in life-space were not associated with better quality of life for those with low frailty, indeed these were slightly lower. The estimated coefficients for these relationships between life-space and quality of life was as follows: highest frailty (0.06, 95%CI: 0.03 to 0.08, p < 0.01); medium frailty (0.02, 96%CI: -0.002 to 0.04, p < 0.01), compared to low frailty (Supplementary Table 3).
Fig. 2.
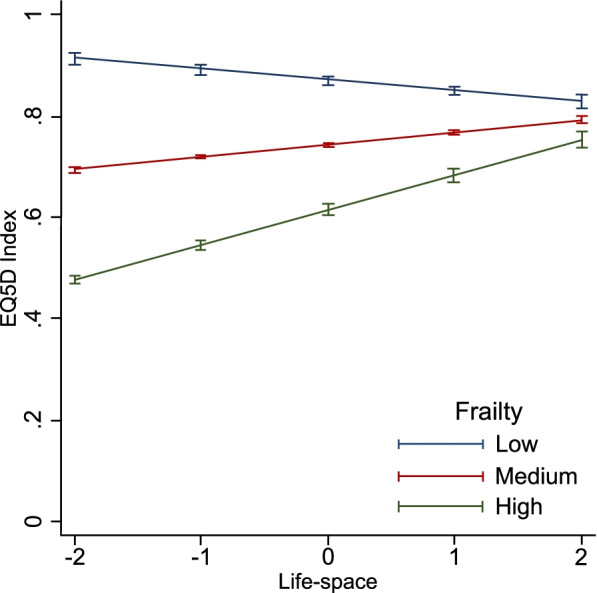
Interaction between life-space and frailty and their association with EQ-5D
Discussion
Health-related quality of life appears to depend on both life-space and frailty, even after adjustment for domestic contact, isolation and need for social care. While life-space and frailty are closely related, their associations with quality of life vary depending on the underlying level of frailty. Life-space and quality of life have a stronger association in those with high frailty. Taken together, these findings suggest that targeted improvements in life-space mobility may be most beneficial for quality of life in older adults with high frailty compared to those with a low and medium level of frailty.
Our results are consistent with studies separately demonstrating the two associations between frailty and quality of life [25, 26] and life-space and quality of life [27]. However, showing how changes in life-space could impact quality of life across the spectrum of frailty is novel. Our findings emphasise the importance of understanding the determinants of life-space and how interventions in this domain could improve quality of life. The nature of this interaction would suggest that interventions to improve life-space could have the largest impact in those already living with frailty. The degree to which life-space could be modified has not been extensively studied. In a study of post-acute patients recently discharged, inpatient rehabilitation did not appear to improve life-space [28]. Similarly, although a resistance and balance training programme decreased falls risk, it did not increase life-space in care home residents [29]. After knee arthroplasty, patients who were less frail (by selection) and receiving an extended walking intervention showed improved life-space [30]. However, a multidisciplinary team community rehabilitation intervention demonstrated greater life-space in frail patients, even after 12 months [31]. In this respect, it might be expected that such interventions could also lead to improvements in quality of life. Overall, we interpret the interaction between life-space and frailty as identifying a subpopulation of individuals, those with most frailty, for whom mobility-related goals might make the biggest difference to their quality of life.
The cross-sectional nature of the data limits our findings, so we cannot establish any temporal relationships. We had missing quality of life data for one quarter of the sample, with the likely effect that this under-estimated the associations with life-space and frailty. Simultaneously comparing data on life-space, frailty and quality of life required us to standardise and transform the independent variables. Though we could establish overall relationships, it is difficult to link the estimated models directly to absolute levels of frailty. As with other observational studies, our results are subject to residual confounding. It is also not possible to generalise our findings outside the sample’s predominantly white, well-educated urban setting. Nonetheless, population cohorts have the advantage of offering data on the full range of life-space and frailty states.
In a population-representative cohort of older people, we demonstrate that life-space has the strongest relationship with quality of life in frail older adults. Frail individuals were twice as likely to have higher quality of life in association with a larger life-space. Interventions designed to improve quality of life in frail older adults could focus on increasing a person’s life-space.
Supplementary Information
Additional file 1: Supplementary Table 1. Frailty Index creation.
Additional file 2: Supplementary Table 2. Variables associated with life-space (n=1,510).
Additional file 3: Supplementary Table 3. Variables associated with HRQoL (with frailty in levels)
Additional file 4: Supplementary Figure 1. Variables associated with life-space.
Acknowledgements
Not applicable.
Authors’ contributions
PC, AT, SDS, DD contributed to data curation and methodology. DD contributed to the conception, funding acquisition, resources, and supervision. Formal analysis and investigation were done by PC and DD. Project administration was done by AT and DD. PC wrote the original draft and all authors reviewed and edited the article. The author(s) read and approved the final manuscript.
Funding
This report is independent research funded by the National Institute for Health Research ARC North Thames through a doctoral studentship awarded to PC. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care. DELPHIC is supported by the Wellcome Trust through a fellowship award to DD (WT107467). The MRC Unit for Lifelong Health and Ageing at UCL received core funding through the Medical Research Council (MC_UU_00019/1). AT is funded through an Alzheimer’s Society clinical research training fellowship. SDS receives funding from the Dalhousie Medical Research Foundation.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Dementias Platform UK (DPUK) repository, https://portal.dementiasplatform.uk/CohortDirectory/Item?fingerPrintID=DELPHIC.
Declarations
Ethics approval and consent to participate
DELPHIC received approval from an NHS Research Ethics Committee (16/LO/1217) and the Health Research Authority (IRAS 164446). All participants or their proxies gave informed consent before taking part. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vanleerberghe P, De Witte N, Claes C, Schalock RL, Verté D. The quality of life of older people aging in place: a literature review. Qual Life Res. 2017;26:2899–2907. doi: 10.1007/s11136-017-1651-0. [DOI] [PubMed] [Google Scholar]
- 2.Vanleerberghe P, De Witte N, Claes C, Verté D. The association between frailty and quality of life when aging in place. Arch Gerontol Geriatr. 2019;85:103915. doi: 10.1016/j.archger.2019.103915. [DOI] [PubMed] [Google Scholar]
- 3.Karimi M, Brazier J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? Pharmacoeconomics. 2016;34:645–649. doi: 10.1007/s40273-016-0389-9. [DOI] [PubMed] [Google Scholar]
- 4.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor JK, Buchan IE, van der Veer SN. Assessing life-space mobility for a more holistic view on wellbeing in geriatric research and clinical practice. Aging Clin Exp Res. 2019;31:439–445. doi: 10.1007/s40520-018-0999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley JP, Brown CJ, McGwin G, Sawyer P, Allman RM, Roth DL. Functional status, life-space mobility, and quality of life: A longitudinal mediation analysis. Qual Life Res. 2013;22:1621–1632. doi: 10.1007/s11136-012-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson J, Rodriguez MA, Snih S Al. Life-space mobility in the elderly: Current perspectives. Clin Interv Aging. 2020;15:1665–74. doi: 10.2147/CIA.S196944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker PS, Bodner EV, Allman RM. Measuring Life-Space Mobility in Community-Dwelling Older Adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyashita T, Tadaka E, Arimoto A. Cross-sectional study of individual and environmental factors associated with life-space mobility among community-dwelling independent older people. Environ Health Prev Med. 2021;26:9 [DOI] [PMC free article] [PubMed]
- 10.Lo AX, Brown CJ, Sawyer P, Kennedy RE, Allman RM. Life-space mobility declines associated with incident falls and fractures. J Am Geriatr Soc. 2014;62:919–923. doi: 10.1111/jgs.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rantanen T, Eronen J, Kauppinen M, Kokko K, Sanaslahti S, Kajan N, et al. Life-Space Mobility and Active Aging as Factors Underlying Quality of Life among Older People before and during COVID-19 Lockdown in Finland - A Longitudinal Study. J Gerontol - Ser A Biol Sci Med Sci. 2021;76:E60–E67. doi: 10.1093/gerona/glaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantakokko M, Portegijs E, Viljanen A, Iwarsson S, Kauppinen M, Rantanen T. Changes in life-space mobility and quality of life among community-dwelling older people: a 2-year follow-up study. Qual Life Res. 2016;25:1189–1197. doi: 10.1007/s11136-015-1137-x. [DOI] [PubMed] [Google Scholar]
- 13.Portegijs E, Rantakokko M, Viljanen A, Sipilä S, Rantanen T. Is frailty associated with life-space mobility and perceived autonomy in participation outdoors? A longitudinal study. Age Ageing. 2016;45:550–553. doi: 10.1093/ageing/afw072. [DOI] [PubMed] [Google Scholar]
- 14.Davis D, Richardson S, Hornby J, Bowden H, Hoffmann K, Weston-Clarke M, et al. The delirium and population health informatics cohort study protocol: ascertaining the determinants and outcomes from delirium in a whole population. BMC Geriatr. 2018;18:45. doi: 10.1186/s12877-018-0742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodyer E, Mah JC, Rangan A, Chitalu P, Andrew MK, Searle SD, et al. The relative impact of socioeconomic position and frailty varies by population setting. Aging Med. 2022;5:10–16. doi: 10.1002/agm2.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsui A, Searle SD, Bowden H, Hoffmann K, Hornby J, Goslett A, et al. The effect of baseline cognition and delirium on long-term cognitive impairment and mortality: a prospective population-based study. Lancet Heal Longev. 2022;3:e232–e241. doi: 10.1016/S2666-7568(22)00013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peel C, Baker PS, Roth DL, Brown CJ, Bodner EV, Allman RM. Assessing Mobility in Older Adults: The UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85:1008–1019. doi: 10.1093/ptj/85.10.1008. [DOI] [PubMed] [Google Scholar]
- 19.Jalenques I, Rondepierre F, Rachez C, Lauron S, Guiguet-Auclair C. Health-related quality of life among community-dwelling people aged 80 years and over: A cross-sectional study in France. Health Qual Life Outcomes. 2020;18:126. doi: 10.1186/s12955-020-01376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King J, Yourman L, Ahalt C, Eng C, Knight SJ, Pérez-Stable EJ, et al. Quality of life in late-life disability: “I Don’t Feel Bitter because i Am in a Wheelchair”. J Am Geriatr Soc. 2012;60:569–576. doi: 10.1111/j.1532-5415.2011.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:1–10. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song X, Mitnitski A, Rockwood K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). 2019;17. https://www.gov.uk/government/publications/english-indices-of-deprivation-2019-technical-report%0Ahttps://www.assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833959/IoD2019_Infographic.pdf. Accessed 21 Oct 2021.
- 24.Office for National Statistics . SOC 2020 Volume 1: structure and descriptions of unit groups. 2020. [Google Scholar]
- 25.Kim MJ, Park S, Jung Y-I, Kim S-H, Oh I-H. Exploring health-related quality of life and frailty in older adults based on the Korean Frailty and Aging Cohort Study. Qual Life Res. 2020;29:2911–2919. doi: 10.1007/s11136-020-02568-5. [DOI] [PubMed] [Google Scholar]
- 26.Hewston P, Grenier A, Burke E, Kennedy CC, Papaioannou A. Frailty and Life-Space Mobility: Implications for Clinical Practice and Research. Occup Ther Heal Care. 2020;35:16–24. doi: 10.1080/07380577.2020.1846235. [DOI] [PubMed] [Google Scholar]
- 27.Saraiva MD, Apolinario D, Avelino-Silva TJ, De AssisMoura Tavares C, Gattás-Vernaglia IF, Marques Fernandes C, et al. The Impact of Frailty on the Relationship between Life-Space Mobility and Quality of Life in Older Adults during the COVID-19 Pandemic. J Nutr Health Aging. 2021;25:440–7. doi: 10.1007/s12603-020-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown CJ, Foley KT, Lowman JD, MacLennan PA, Razjouyan J, Najafi B, et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients a randomized clinical trial. JAMA Intern Med. 2016;176:921–927. doi: 10.1001/jamainternmed.2016.1870. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt J, Goodall S, Clemson L, Henwood T, Refshauge K. Progressive Resistance and Balance Training for Falls Prevention in Long-Term Residential Aged Care: A Cluster Randomized Trial of the Sunbeam Program. J Am Med Dir Assoc. 2018;19:361–369. doi: 10.1016/j.jamda.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Hiyama Y, Kamitani T, Mori K. Effects of an Intervention to Improve Life-Space Mobility and Self-Efficacy in Patients following Total Knee Arthroplasty. J Knee Surg. 2019;32:966–971. doi: 10.1055/s-0038-1672199. [DOI] [PubMed] [Google Scholar]
- 31.Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Cameron ID. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMC Med. 2012;10:120 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Frailty Index creation.
Additional file 2: Supplementary Table 2. Variables associated with life-space (n=1,510).
Additional file 3: Supplementary Table 3. Variables associated with HRQoL (with frailty in levels)
Additional file 4: Supplementary Figure 1. Variables associated with life-space.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the Dementias Platform UK (DPUK) repository, https://portal.dementiasplatform.uk/CohortDirectory/Item?fingerPrintID=DELPHIC.