Abstract
As an emerging pollutant in the life cycle of plastic products, micro/nanoplastics (M/NPs) are increasingly being released into the natural environment. Substantial concerns have been raised regarding the environmental and health impacts of M/NPs. Although diverse M/NPs have been detected in natural environment, most of them display two similar features, i.e.,high surface area and strong binding affinity, which enable extensive interactions between M/NPs and surrounding substances. This results in the formation of coronas, including eco-coronas and bio-coronas, on the plastic surface in different media. In real exposure scenarios, corona formation on M/NPs is inevitable and often displays variable and complex structures. The surface coronas have been found to impact the transportation, uptake, distribution, biotransformation and toxicity of particulates. Different from conventional toxins, packages on M/NPs rather than bare particles are more dangerous. We, therefore, recommend seriously consideration of the role of surface coronas in safety assessments. This review summarizes recent progress on the eco–coronas and bio-coronas of M/NPs, and further discusses the analytical methods to interpret corona structures, highlights the impacts of the corona on toxicity and provides future perspectives.
Keywords: Micro/nanoplastics, Nanotoxicity, Corona, Structure–activity relationship, Biotransformation
Introduction
Plastic is one of the most important engineered materials discovered in all aspects of life [1]. It is an ideal substance that is used to manufacture household, commercial and industrial products [2, 3]. Plastic products are widely present in our daily life, and a major portion of them are disposable packaging or single–use items that are discarded within a short period of time [4]. The global production of plastic is growing rapidly and reached 368 million metric tons in 2019. It has been estimated that 33 billion tons of plastics will be produced by 2050 [5], whereas global plastic waste is expected to grow to 155–265 million metric tons by 2060 [6]. Currently, the end–of–life fate of 74–94% of discarded plastics is landfills, incinerators, and/or the natural environment, leading to the accumulation of plastic waste within the environment [7]. Once released into the environment, plastics persist for many years and gradually breakdown into small debris, i.e.,microplastic (< 5 mm) and nanoplastic (< 100 nm) debris [8, 9], via natural degradation (e.g., hydrolysis, biodegradation, photodegradation, wind, water erosion). In addition, some micro/nanoplastics (M/NPs) are deliberately engineered for commercial and domestic use in cosmetics [10], personal care products [3] and pigments [11] and may be directly released into the natural environment. Due to their small sizes, M/NPs may spread into diverse media, including air, lakes, rivers, oceans and soil, interacting with organisms in the ecosystem and further causing adverse outcomes [12].
Although over 30,000 types of plastic polymers have been manufactured and applied in real scenarios, the resulting M/NPs have two similar features, i.e.,high surface area and strong binding affinity, which enable extensive interactions between M/NPs and other substances such as metal cations, inorganic anions, organic chemicals and biomolecules [13]. These substances may be adsorbed on the surfaces by hydrophobic interactions with the C–C/C = C skeletons or electrostatic forces with the functionalities (e.g., –OH, –COOH, –HSO3, –NH3) of M/NPs. The adsorbed substances on fine particulates are defined as “corona”, consisting of a “hard” and a “soft” layer [14]. The “hard” corona often consists of a monolayer of bound molecules that tightly associate on the particle surface and form in the initial transient period (seconds to minutes) of interaction with surrounding media [15]. This layer is often long lived and relatively stable because the constituents in this layer are difficult to replace with new molecules when the particles transfer to a new environment. The “hard” corona is then covered by loosely bound molecules, which are termed the “soft” corona. In contrast to the “hard” corona, the constituents of the soft corona are unstable and may rapidly exchange with environs [16]. The coronas on M/NPs could be classified into two categories, eco-corona and bio-corona, in terms of the settling circumstances (i.e.,abiotic environmental media and living organisms) of M/NPs [17]. The difference in corona structures of M/NPs in culture media and physiological fluids [18] (e.g., blood, gastric, intestinal and bronchoalveolar lavage fluids) may result in distinct hazard effects, but this has rarely been explored. While metal ions, inorganic anions and organic chemicals mainly account for the compositions of eco–corona [19], bio-coronas are composed of proteins, lipids, nucleic acids, amino acids, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) [20].
In risk assessments of fine particulates, coronas play a critical role in adverse outcome pathways, such as hemolysis [21], thrombocyte activation [22], biodistribution [23], immune response [24], reactive oxygen species (ROS) production [25], cellular uptake [26], and cell death [22]. For instance, the formation of a serum–derived corona on some nanoparticles (i.e.,silica [27], iron oxide [28], carbon [29], polystyrene [30]) could reduce their cellular internalizations. Deng et al. [31], reported that negatively charged poly (acrylic acid)–coated gold nanoparticles could enrich unfolded plasma fibrinogen, which enables strong binding to the membrane Mac–1 receptor, resulting in an inflammatory response by NF–κB activation. In terms of the real exposure scenarios, there are limited accesses for bare M/NPs to interact with biological systems. However, previous toxicity studies on M/NPs ignored or failed to consider the impacts of surface coronas.
Recently, substantial research interest has been directed to the environmental and health impacts of M/NPs [32, 33]. The number of studies has increased at 30–50% annually in the past five years [34, 35]. It could be expected that more research will be directed towards the subject area. Before considerable efforts are put forward in behavior investigation, safety assessment and treatment of M/NPs, it is important to highlight the key factors that determine their fates in the natural environment. Micro/nanoplastics themselves are typically considered inert and stable materials because their basal skeletons are hard to break [36], suggesting that these materials have limited access to directly interact with key mediators in living organisms. In contrast, corona formation on M/NPs is inevitable and often displays variable and complex structures, which may alter the physicochemical properties of M/NPs as well as their environmental/biological behaviors [37]. From this perspective, we reviewed recent progress on eco- and bio-coronas of M/NPs, discussed corona impacts on toxicity, summarized the analytical methods to interpret corona structures, and provided future perspectives on M/NPs coronas.
Matrices and physicochemical properties of environmental micro/nanoplastics
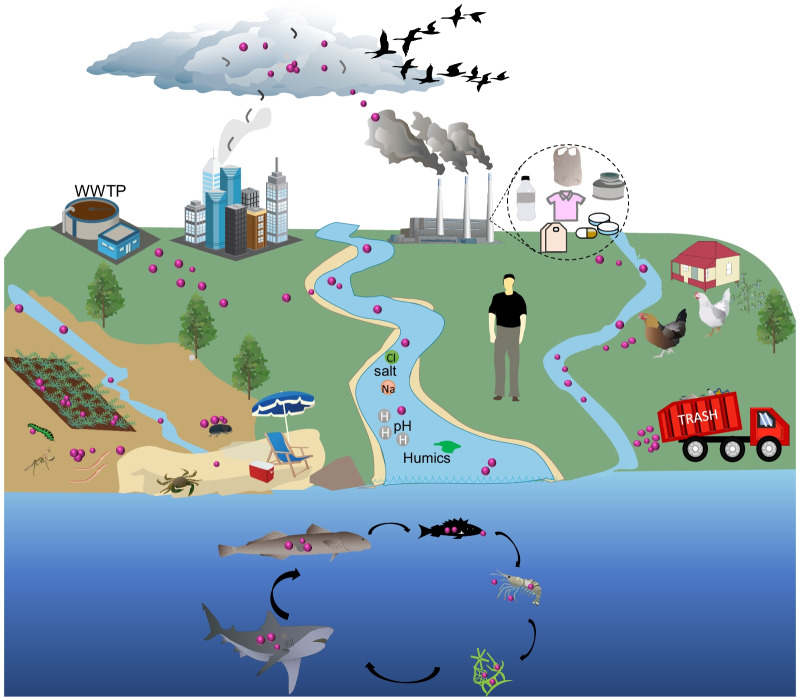
Global production and mismanaged plastic waste lead to a magnitude of M/NP contamination in the environment [38]. Owing to their small size, M/NPs can easily diffuse elsewhere through water and/or air circulation [39], as shown in Fig. 1. They have been found to be widespread in water [40], soil [41] and air [42], where they can be taken up by plants and animal organisms, with serious detrimental effects [43]. Since the first plastics were made from fossil fuels, a large number of plastic types have been developed for various applications [44]. According to the data collected by PlasticsEurope, more than 30,000 different plastic polymers are registered in the European Union [45]. As a result, the chemical composition, size, shape and surface properties of observed plastics in the environment usually vary significantly.
Fig. 1.
The production, migration and distribution of M/NPs in the environment. Plastics are released to the environment during manufacture, use and disposal. The environmental plastics can be gradually broken down into M/NPs. M/NPs can easily diffuse elsewhere through water and/or air circulation. M/NPs have been found to be widespread in air, lakes, rivers, oceans and soil, where they can be taken up by various organisms.
Matrices contaminated by micro/nanoplastics
Waters
Micro/nanoplastics are ubiquitous in the water environment, especially in oceans. From 4.8 to 12.7 million metric tons of mismanaged plastic waste is released to the ocean every year [46]. According to Wayman et al. [47],the number of plastics released into the ocean will grow by 2.6–fold between year 2016 to 2040. In addition, freshwater has been considered as one of the origins and transport pathways of ocean plastic waste [48]. Since the first reported microplastics (MPs) in a UK freshwater system by Horton et al. [49], MPs have been detected in various freshwaters, such as Danube River [50], Ottawa River [51], Yangtze River [52], Erie Lake [53] and Taihu Lake [54]. In addition, plastic particles have been found in drinking water from Changsha, China at range abundances of 2173–3998 (mean = 2753.0), 338–400 (mean = 351.9), and 267–404 (mean = 343.5) particles per liter in freshwater, treated water, and tap water, respectively [55]. The M/NPs released in waters may be taken up by aquatic organisms, such as Echinodermata [56], mollusks [57], crustaceans [58] and fish [59].
Soil
Although less attention has been received, soil is regarded as a larger reservoir for M/NPs than the ocean [60]. Microplastic contamination on land was estimated to be 4–23–fold larger than that of oceans [61]. Soils may be contaminated by M/NPs from wastewater irrigation [62], plastic mulching [63], sludge utilization [64], atmospheric deposition [65], road runoff [66]. Indeed, M/NPs have been detected in floodplains, coastal beaches, and agricultural soils. For instance, MPs are found in 90% of Swiss floodplain soils, with a range of 5 mg/kg to 55.5 mg/kg [67]. Zhou et al. [68] reported 1.3–14,712.5 particles/kg of MPs in coastal beach soils from Shandong, China. In another report, 78.00 ± 12.91 and 62.50 ± 12.97 particles/kg of MPs were detected in shallow and deep farmland soils from 20 vegetable fields around the suburbs of Shanghai, China [69]. In addition, accidental loss or improper handling of plastic debris in landfills and urban and industrial centers may result in the input of M/NPs in land soil [70]. Accumulated M/NPs have also been found in plants [71] and worms [72].
Air
Atmospheric M/NPs have aroused substantial concerns in recent years because they can be quickly transported over long distances by wind. Airborne plastics mainly originate from synthetic clothing, synthetic fibers in building materials, plastic waste incineration and road dust [73]. Researchers have found atmospheric M/NPs both indoors and outdoors [74]. Most of the detected atmospheric M/NPs are fibers, and the concentrations of fibers suspended in indoor environments are significantly higher than those outdoor areas [75]. Although the reported sizes of atmospheric plastics are 2–9555 µm, nanoplastics (NPs) may account for a large portion because they are more stable in airborne environs and can be suspended in air for long periods. This, therefore, highlights that reliable and sensitive detection techniques for airborne NPs are urgently needed. These fine particulates have high respiratory exposure risks and may reach the lower airways and pass through the air–blood barrier of mammals and birds [76], resulting in severe pulmonary diseases such as lung fibrosis and pleural granuloma formation [77].
Diet
Currently, it is well known that plastic particles can be taken up by aquatic and terrestrial organisms and thus enter the food chain. The impact of plastic debris on marine life has been extensively studied [78]. It has been revealed that over 1 million marine animals die each year due to plastic debris in the ocean [79]. Recently, a meta-analysis estimated the level of microplastic contamination in seafood and the amount of microplastic people may ingest each year, for example, microplastic abundances in mollusks, crustaceans, fish, and echinodermata were 10.5, 8.6, 2.9 and 1 particles/g, respectively [80], with a maximum human uptake of MPs from seafood of ~ 55,000 particles annually. Although most of the ingested M/NPs are excreted by the peristalsis of GI tract [81, 82], some M/NPs may cross the intestinal barrier and spread into other organs (e.g., liver, spleen, blood) [83]. For instance, nylon MPs could be completely excreted from rats by feces in 48 h [83]. Forty-five percent of fluorescent polypropylene NPs were excreted from zebrafish after 24 h depuration [82], which is similar to the behavior of polystyrene NPs in scallops with half-life of elimination in 1.4 days [84]. The excretion rates may closely relate to the composition, size and shape of M/NPs. For example, Hoang et al. [81] found that particles with round smooth shape are excreted faster than pointy shaped particles or fibers in Pimephales promelas. In addition, microplastic contamination has also been detected in other foods such as beverages, honey, and salt [85]. Moreover, food or drink containers may release substantial amounts of M/NPs during their use [86]. Recently, Su et al. [87] detected numerous flake– or oil–film–shaped M/NPs (0.6–332 μm) during the sterilization of feeding bottles. Interestingly, one–year bottle feeding will result in an ingestion of ca. 660,000 MPs by infants.
Physicochemical properties of micro/nanoplastics
Compositions
Despite the large number of plastic polymers manufactured, there are six commonly used namely polyethylene (PE), polypropylene (PP), poly (vinyl chloride) (PVC), polyurethane (PUR), poly (ethylene terephthalate) (PET) and polystyrene (PS) which account for approximately 81.2% of the global plastic demand in 2019 [88]. The building blocks of all six plastic materials are polymers with long chains of covalent–bonded atoms. The backbones of PE, PP, PVC and PS are carbon chains, which are nonbiodegradable [89]. The PE is merely made up of an ethyl chain, while PP, PVC and PS have methyl, chloride and phenyl side groups on the carbon skeleton, respectively. The PE can be further divided into high–density PE (HDPE), low–density PE (LDPE) and linear low–density PE (LLDPE) according to the degree of branching in the polymer [90]. Due to its wide application and poor biodegradability, PE has been reported to be the most abundant plastic accumulated in the environment [91]. The fourth and fifth most–produced polymers PUR and PET contain carbamate (–COONH–) linkage and ester groups, respectively, which are supposedly to be more susceptible to degradation by natural microorganisms. However, PUR and PET degradation in the natural environment is slower than the expected level, and substantial amounts of PUR and PET M/NPs still accumulate in the environment [92]. Besides the raw polymers, plasticizers, such as phthalates, trimellitates, citrates are commonly added into the polymers to improve the performances of plastic products. During the degradation or treatment of plastics, these additives may be released into the environment and co-exist with M/NPs. Since the addition of plasticizers could significantly enhance the physicochemical properties (e.g., stability and flexibility) of plastic products [93], these additives may alter the surface chemistry of M/NPs, and consequently impact the formation of surface coronas as well as the interactions with biological systems [94].
Shapes
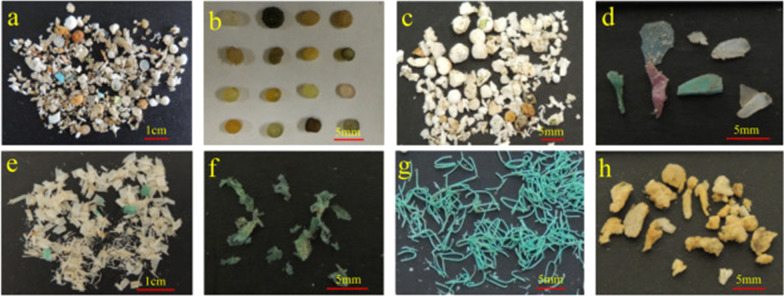
Environmental M/NPs can be classified by their shape into beads, fragment fibers, films, flakes, sponges, foam (Fig. 2) [68]. Generally, microplastic pellets are hard, regular, discoid– or cylindrical–shaped, while fragments are described as hard, irregular and jagged particles. Micro/nanoparticle fibers are described as long, thin, and having a smooth linear morphology. The films are very thin, transparent and soft, the flakes are flat sheets, and the foam and sponges are usually lightweight and porous plastics [68]. However, the definition of the actual shapes of each category of M/NPs is still highly diverse. In addition, these descriptive shape categories are often meaningless for studying particle fate. In view of this situation, Kooi et al. [95] recently proposed using length:width:height (L:W:H) ratios as a more generalized approach for different shape categories. According to this approach, the W:H ratio for fragments, foams and films should be ≤ 1, the L: W = H ratios for fibers vary between 0.50 and 0.001, and the L:W:H ratios range from 1 to 0.36 for perfect spheres or ellipsoidal particles.
Fig. 2.
Typical shapes of environmental M/NPs. a mixed M/NPs, b pellets, c foams, d fragments, e flakes, f films, g fibers (fishing lines), h sponges. Reprinted with permission from [68]
The shape of environmental plastic debris is related to its origin and history of degradation in the environment. For example, fibrous MPs are reported to be the most encountered shape in atmospheric particulates, as synthetic fibers in clothes and building material are the main source for airborne plastics [96]. On the basis of their category approach, Kooi et al. [95] estimated that the most abundant M/NPs in water and sediment are fibers (48.5%), followed by fragments (31%), beads (6.5%), films (5.5%), and foam (3.5%). Several studies on coastal soils also reported that foams or fibers were the predominant microplastic types [97]. However, Zhou et al. [68] revealed that the most abundant shape category of microplastics in coastal soils of the Bohai Sea and the Yellow Sea is flakes (69.0%), followed by foams (27.8%), fragments (1.1%), fibers (1.0%), sponges (0.8%), films (0.2%) and pellets (0.1%). Therefore, the shape distributions of M/NPs may vary greatly among different environmental matrices and regions.
Sizes
Environment plastics are available in a wide range of sizes and are usually classified by their sizes into macroplastics (> 25 mm), meso–plastics (5–25 mm) and MPs (< 5 mm) [98]. Microplastics can be further divided into large MPs (1–5 mm) and small MPs (< 1 mm) according to the Guidance on Monitoring of Marine Litter in European Seas of the EU Marine Strategy Framework Directive (MSFD). From an analytical point of view, Hidalgo–Ruz et al. [99] suggested differentiating MPs of 500 μm–5 mm and < 500 μm since the first fraction is suitable for visual sorting and spectroscopic techniques are required to differentiate the second category. Numerous studies have demonstrated the ubiquitous presence of the two size fractions of microplastics in the environment, including water [100], soil [60] and air [101]. The measurement of MPs with different size ranges has also been summarized by a series of review articles [102].
Recently, NPs have emerged as a hot topic, as the weathering of macroplastics and MPs may lead to a considerable burden of NPs in the environment. Unlike MPs, the size definition of NPs is still under debate [103]. Although nanoplastics are generally defined as particle sizes < 100 nm, which follows the threshold commonly used in engineered nanomaterials [104], Gigault et al. propose a definition of nanoplastics with the size range from 1 to 1000 nm because nanoplastics at this range present a colloidal behavior [105]. This debate on the definition of NPs may continue for years, even decades. Regardless of the conflict on size definition, the hazard and exposure risks of NPs have attracted more attention as NPs are more extensively distributed and detrimental than MPs [106].
Surface charges
The surface of plastics can be engineered with different moieties to improve their stability [107], adhesion [108] and biocompatibility [109]. Debris of these functionalized plastics may preserve the engineered functionalities and display different surface charges [110]. Moreover, diverse chemical groups may arise on the surfaces of M/NPs during their degradation and migration in the environment. Currently, reactive functional groups, including carboxylic, phenoxy hydroxyls, alcoholic hydroxyls, carbonyl, hydroxides, amine, and sulfonic groups [111], have been widely identified in environmental polymers. These functionalities of M/NPs result in different surface charges, impacting their corona formation, environmental and biological fates and toxicity.
Corona formation on micro/nanoplastics
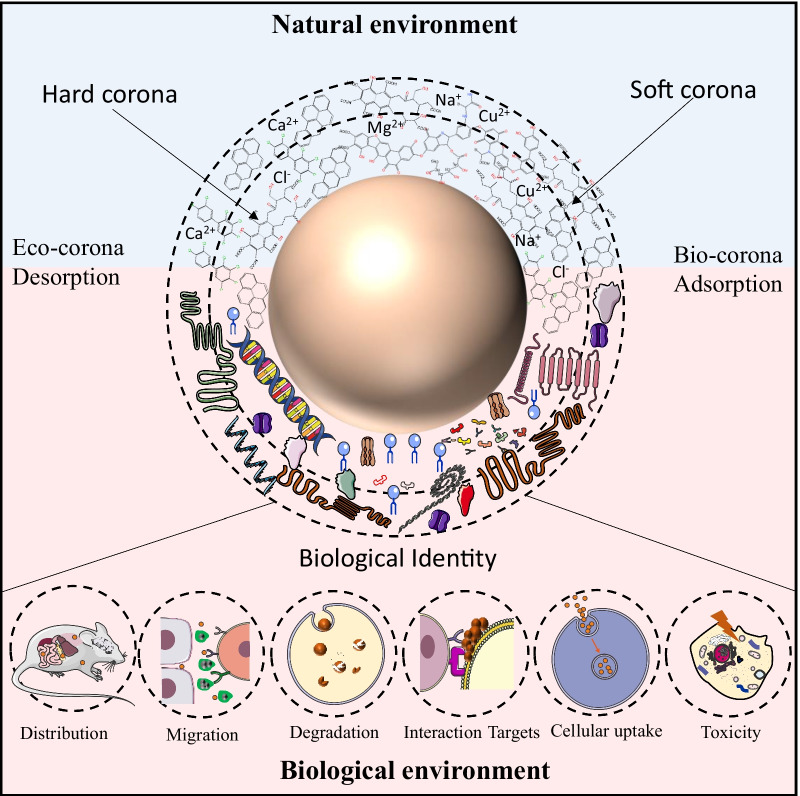
In addition to the primary properties, M/NPs may display secondary properties in media, such as agglomeration and corona formation. Both the primary and secondary physicochemical properties of particulates have been considered to play a decisive role in their biological effects [112]. Alternations of the physicochemical properties in media, i.e.,biotransformation often results in molecular initiating events that further elicit adverse outcomes [113, 114]. Given that bare M/NPs are extremely stable and inert, corona formation is the key event that may impact the transportation, cellular internalization, biodistribution and elimination of M/NPs in biological systems [115]. In the migration paths of M/NPs, they mainly encounter two types of media, including environmental media (e.g., rivers, lakes, ocean, soil) and biological fluids (e.g., saliva, gastric, intestinal and lung lining / alveolar fluids, blood), and form eco–coronas and bio-coronas, respectively (Fig. 3). The corona structures offer new environmental or biological identities for M/NPs, resulting in different distributions [116], migration [117], degradation [118], cellular internalization [115], interaction targets [118]. In biological systems, corona formation on M/NP surfaces may result in three types of adverse effects, i.e.,antagonism, synergism and independent action [119]. If the combined effect of corona constituents and M/NPs is greater than their individual toxicity, it is defined as a synergistic effect. Otherwise, the antagonistic effect is regarded. Independent action refers to the effect that M/NPs and corona constituents interact with their own targets by independent signaling pathways without crosstalk.
Fig. 3.
Eco-coronas (top) and bio-coronas (bottom) on M/NP. The natural components in the environment can be adsorbed on the surface of M/NP and form eco-coronas, which consist of metal ions (Cu2+, Mg2+, Pd2+, etc.), inorganic salts (Na+, Cl−, etc.), natural organic matters (HAs, FAs, etc.), and persistent organic pollutants (PCBs, PAHs, etc.). When this M/NP encounters biological systems, it may interact with abundant biomolecules, including proteins, lipids, and nucleic acids to replace some of the constituents in eco-coronas and form bio-coronas on surface. The corona structures offer new environmental or biological identities for M/NP and impact its distribution, migration, degradation, interaction targets, cellular internalization and toxicity in organisms. Adapted and reprinted with permission from [120].
Eco–coronas
Inorganic salts and humic acids (HAs) are the major natural components in environmental media [121]. In addition, due to increases in human activities, some engineered chemicals, especially persistent organic pollutants, have been extensively released into the environment [122]. These species may be adsorbed on the surfaces of M/NPs and form an “eco–corona”. Several studies have indicated that the eco–corona is involved in the degradation, migration and toxicity of M/NPs [123]. For instance, studies have shown that environmentally exposed microplastic particles were internalized significantly more often than pristine microplastic particles into macrophages [115]. Meanwhile, the formation of eco–corona on M/NPs may alter the behaviors of environmental pollutants, such as heavy metals and persistent organic pollutants (POPs). A study on the amphipod Talitrus saltator demonstrated that ingestion of contaminated MPs (polybrominated diphenyl ether) transferred organic pollutants to its tissues. In contrast, pristine MPs ingested by an amphipod contaminated by organic pollutants removed the pollutants from the tissues [124].
Metal cations
Since most M/NPs display a negative surface charge, electrostatic binding is the major mechanism for the interactions between M/NPs and heavy metal cations. Metal ions often bind with polar surface functionalities, such as –COOH, –NH2, and phenyl–OH. Holmes et al. [125] studied M/NPs in estuarine conditions and observed greater metal adsorption (at least an order of magnitude greater) on beached pellets vs. virgin pellets because the former M/NPs had more surface functional groups. The adsorption of metals to polymers increases with material aging, and its polarity, surface area and porosity increase [126]. In addition, M/NPs may first adsorb a layer consisting of biomolecules or natural organic matter (NOM), resulting in charged surfaces for electrostatic binding with metal ions [127]. Rochman et al. [128] found that M/NPs suspended in seawater for a year enriched similar levels of metals irrespective of different compositions, indicating that the accumulation of metals on M/NPs may be mediated by the organic corona layer. A recent study indicated that the corona layer not only intensified the vector role of MPs in the migration of heavy metals in freshwater but also enhanced their combined toxicity [129]. Moreover, the surfaces of M/NPs provide a suitable solid phase to concentrate metal ions in aqueous solutions for the precipitation or crystallization of metal salts and oxyhydroxides [130]. In real scenarios, two or three of the above mechanisms might be involved in the interactions of M/NPs with metal ions.
Metal ions in the eco–corona of M/NPs may alter the physicochemical properties of M/NPs and further impact their adverse outcome pathways (AOPs), for example, cellular uptake [131, 132], distribution [131], interaction targets [132], and metabolism [133]. A report by Ramirezet al. [134] indicated that cation concentrations affect the surface properties and aggregation dynamics of PS NPs. Consistently, Abe et al. [135] discovered that divalent cations, in particular, Ca2+ and Mg2+ ions, can neutralize M/NP electrostatic stabilization that is enhanced by NOMs and induce aggregation through cation bridging and charge neutralization mechanisms.
In aquatic environments, M/NPs can be initially ingested by various aquatic organisms such as zooplankton [136], mussels [137], bivalve mollusks [138] and phytoplankton algae [139], and can accumulate in high aquatic organisms (e.g., fish) through the food chain. Interestingly, the formation of coronas on M/NPs may antagonize the hazardous effects of heavy metals in algae [140]. For instance, after binding to polyacrylonitrile polymer (PAN), the toxicity of Cu2+ in Chlorella pyrenoidosa was significantly ameliorated [141]. Fu et al. [142] found that the combination of Cu2+ (0.5 mg/L) and aged PVC (10 mg/L) even promotes the growth of Chlorella vulgaris. Coexposure of Cd2+, Cu2+ and Ni2+ with M/NPs showed negative impacts in earthworms. However, the combined toxicity of M/NPs and metal ions is still debatable, probably due to the differences in the tested M/NPs (e.g., sizes, compositions), metal species and animal models in different studies. For instance, small PS (0.5 μm) binding with metal ions shows a greater growth inhibition effect on microalgae than bare PS [143]; Cd2+–bearing MPs (2–4 μm) are more toxic in the cladoceran Moina monogolica Dadaya [144]. In addition, both synergistic and antagonistic effects on mercury adsorbed by M/NPs were reported. Fernández et al. [145] found that HDPE facilitated the ingestion of Hg2+ in mussels, but negligible bioaccumulation was detected in clams exposed to Hg2+–contaminated PE. Combined exposure to PS MPs and Cu2+ led to oxidative damage and inflammation in fish tissues and embryos, but the combination of Cd2+ and MPs had few toxic effects [146]. In mammalian systems, while free ions mainly pass through plasma membranes via ion channels or passive distributions and react with cytoplastic proteins [147], metal ions in the corona are internalized into lysosomes by endocytosis and interact with lysosomal proteins [148]. The persistent feature of M/NPs restricted the elimination of metal ions from the organism [149], resulting in prolonged bioavailability.
Natural organic matters
There is increasing evidence showing that M/NPs can sorb NOMs, which are mixtures of slightly water–soluble organic components consisting of humic substances (e.g., HA, fulvic acids (FA) and humins) and nonhumic fractions, such as amino acids, carbohydrates, and proteins [135]. The NOMs are widely present in aquatic and soil environments, with concentrations ranging from a few to hundreds of mg/L [150]. The HAs are the most studied NOMs and often display strong binding affinity with M/NPs by π–π and hydrophobic interactions [151]. In addition, since HA consists of sufficient carboxyl, hydroxyl and amine groups, the polar surface groups (e.g., –OH, –COOH, –NH2, –HSO3) on M/NPs may interact with HA by electrostatic interactions [152]. The intense hydrophobic and electrostatic forces may result in the formation of a hard corona of HA on the M/NP surfaces. However, there is no clear evidence supporting this speculation because the differentiation of hard and soft coronas is challenging.
The formation of NOM coronas on M/NPs may affect particle transportation, retention, and toxicity in the environment [153, 154]. Particles coated with NOM were generally observed to have lower attachment efficiencies than bare particles. Chen et al. [155] showed that HA–coated nanoparticles display reduced retention in saturated sand columns. The stability of positively charged PS increased significantly as NOM concentration increased from 5 to 20 ppm, but a reversal trend was examined in negatively charged NPs [156]. This is not surprising, as humic substances are commonly negatively charged at environmentally relevant pH values, and the adsorption of NOM onto positively charged M/NPs will reverse the surface charge. Formation of NOM coronas may slow the degradation of M/NPs. Wu et al. [157] recently found that photoaging of PP MPs was significantly inhibited in lake water compared with ultrapure water due to the coating of HA and FA on microplastic surfaces. This is because the humic/fulvic acid layer could act as a scavenger of oxidative radicals and optical light filters. In addition, the NOM corona may facilitate the transportation of M/NPs in porous media [158], seawater [159] and sands [160] and stabilize M/NPs by reducing aggregation and sedimentation [161]. In addition to environmental migration, NOM coronas showed differential effects in safety assessments of M/NPs. Liu et al. [162] systematically investigated the toxicological effects of three PS NPs with different functional groups (bare, amino and carboxyl) in the absence and presence of FA and HA. The formation of NOM coronas on PS NPs increased oxidative stress and membrane disruption in S. obliquus cells. The hazard effects were dependent on the concentrations and types of both M/NPs and NOM. Fadare et al. [163] investigated the impacts of NOM coronas on the toxicity of FA–coated NPs in Daphnia magna. The presence of HA resulted in the mitigation of gene expression, whereas significantly higher upregulation of all the genes was observed in Daphnia magna exposed to FA–coated NPs. Short–term exposure to HA–coated PS NPs elicited an immunomodulatory response, with activation of steroidogenic stress–related pathways in European seabass [164], but no notable alteration in inflammatory markers was found, indicating a protective anti–inflammatory effect of HA. Consistently, Wu et al. [165] showed an amelioration effect of HA in a toxicity test of PS NPs. Four PS NPs with different functional groups and charges were collected to investigate the effect of HA on particle aggregation behavior and toxicity. The results showed that HA exerted a stabilizing effect on three negatively charged NPs. The presence of HA effectively reduced the toxicity of PS and positively charged p–PS–NH2, as the survival rates of Daphnia magna increased from 15 to 45–95% and 100%, respectively [165].
Persistent organic pollutants
Persistent organic pollutants (POPs) are a class of organic compounds that are resistant to environmental degradation and have detrimental effects on the environment and human health [166]. Given that POPs and M/NPs are all from the wastes of engineered products, they may coincidentally exist in some specific areas, such as farmland, fishpond, and raw sewage [167]. M/NPs may stably adsorb hydrophobic POPs such as polycyclic aromatic hydrocarbons (PAHs) [168], polychlorinated biphenyls (PCBs) [169] and dichlorodiphenyltrichloroethane (DDT) [170] on their surfaces to form surface coronas. Hydrophobic interactions, halogen bonding, electrostatic interactions, and hydrogen bonding may be responsible for the binding of POPs to M/NPs [171]. For this reason, it is important not only to assess the occurrence and fate of M/NP binding with POPs in the environment but also to study the role of M/NPs as carriers of POPs [172]. Calculation results suggested that the delivery effect of M/NPs toward organic chemicals is limited in biological systems. Gouin et al. [173] used a thermodynamic approach to decipher the relationships between the physicochemical properties of organic chemicals and MPs. It was found that chemicals with log K(OW) > 5 could partition > 1% to PE. Foodweb model results suggested that the body burden concentrations may decline for nonpolar organic chemicals with log K(OW) between 5.5 and 6.5 after binding with MPs. Consistently, the bioavailability of phenanthrene (Phe) and 17α–ethinylestradiol (EE2) was reduced by 33% and 48%, respectively, after association with MPs [174]. Cormier et al. [175] investigated the adsorption processes of perfluorooctanesulfonate (PFOS) on four PE MPs with different size ranges. The adsorption capacity of PFOS increased with decreasing particle size and continuous binding of PFOS on particle surfaces over six months. Ma et al. [176] selected triclosan (TCS) as a model pollutant to investigate its interactions with small and large PVC MPs. Small PVC particles showed higher distribution coefficient values of TCS (1.35 L/g v.s. 1.05 L/g) and stronger adsorption capacity (12.7 mg/g v.s. 8.98 mg/g) than large PVC MPs. This is not surprising, as small M/NPs often have a higher specific surface area, better suspension, and stronger hydrophobicity. The initial pH value and salinity of the solution are critical factors dictating the adsorption process. Yeo et al. [177] found that the sorption of TCS on PS is higher within the pH range of 3.0–6.0 but is not affected by temperature at 288–318 K. Sørensen et al. [178] studied the sorption kinetics of two model PAHs (fluoranthene and phenanthrene) to PE and PS MP surfaces in natural seawater at 10 and 20 ℃. Temperature–dependent sorption was profiled. In terms of the sorption mechanism, PAH sorption behavior could be fitted by a monolayer sorption model at low PAH concentrations, whereas multilayer sorption was detected at higher concentrations [179].
These calculation or simulation results were confirmed in M/NPs collected from real environments. Zhang et al. [180] analyzed the MPs in sediment samples from deep–sea locations (4601–5732 m) of the western Pacific Ocean by micro-Fourier transform infrared spectroscopy (FTIR) and found a strong correlation (p = 0.016) between the concentrations of PCBs and MP distributions in sediments. This finding confirms that POPs exist in the surface corona of M/NPs even in deep–sea ecosystems. Both PCBs and PBDEs were observed in buoyant MPs collected in surface water at 27 locations in the Pacific Ocean around the coast of Japan, and their concentrations were 0.04–124 ng/g and 0–2158 ng/g, respectively [177]. The PCBs and organochlorine pesticides (OCPs) were detected in microplastic samples collected in six “clean” Italian minor islands and two “polluted” areas near the mouths of two major Italian rivers [181]. These findings indicated that POPs broadly coexist with M/NPs in the environment and become an important constituent of M/NP coronas.
Most toxicity studies showed that M/NP adsorption significantly reduced the bioavailability of POPs and adverse effects. Besseling et al. [182] provided the first study of plastic effects on benthic organisms, including the transfer of 19 PCBs in Arenicola marina. PAHs and M/NPs were co-exposed to represent the environmentally relevant exposure scenarios. M/NP sorption resulted in a corresponding reduction in free PAHs and amelioration of lethality and bioaccumulation, indicating that only freely dissolved PAHs are available to copepods under co-exposure conditions [178]. Gerdes et al. [183] evaluated the effect of microplastics on PCB removal in the cladoceran Daphnia magna exposed to a high body burden of PCBs 18, 40, 128 and 209. In the Daphnias fed microplastics, PCB 209 was removed efficiently, while no differences were detected for other PCBs. Grigorakis et al. [184] studied the diet assimilation efficiencies (AEs) of PCBs absorbed to MPs in goldfish (Carassius auratus). Compared to PCBs directly mixed in food, microplastic–bound PCBs had fewer AEs (13.36% v.s. 51.64%). Batel et al. [185] studied the fate, transfer and accumulation of microplastic–associated POPs, including PAHs and benzotalpyrene (BaP), in gills and embryos by fluorophore labeling. The biotransfer of BaP was visualized by fluorescence microscopy. A significant BaP signal was detected in gill filaments and arches as well as zebrafish embryos after incubation. While MPs did not release sufficient BaP to induce morphological changes in fish embryos, free waterborne BaP did induce effects in embryos. Heinrich et al. [186] investigated this in RTL–W1 cells exposed to 7–ethoxyresorufin–O–deethylase (EROD) adsorbed on three PE MPs. As a result, the bioavailability of EROD was reduced up to 79%; the activity was also significantly reduced in the presence of MPs. Scopetani et al.’s study showed that M/NPs could act both as carriers and scavengers for the bioaccumulation of organic pollutants (i.e.,PBDEs), suggesting that chemical leaching from MPs has a limited impact on biota [124]. However, this antagonizing effect may depend on the properties and doses of M/NPs. Jeong et al. observed enhanced toxicity of 2,2',4,4'–tetrabromodiphenyl ether and triclosan in B. koreanus during coexposure to nanosized plastics, probably because NPs had higher accumulation and more oxidative stress–induced damage than MPs [187].
Bio-coronas
The M/NPs often initially interact with natural substances in environments to form eco–corona. However, once these particulates encounter biological systems, they may interact with abundant biomolecules, including proteins, lipids, and nucleic acids to replace some of the constituents in eco–coronas and form bio-coronas on surfaces [24]. These biomolecules are either primarily extracellular polymeric substances (EPS) from organism metabolism or intracellular components. The driving forces of bio-corona formation are also hydrophobic and electrostatic interactions [163]. In most cases, environmental matter and biomolecules may coexist in the surface corona to confer a new biological identity for M/NPs. During the past decade, bio-corona formation has attracted substantial research interest in the field of nanomedicine because the corona structure may significantly affect the pharmacological/toxicological profiles of some engineered nanosized drug carriers [188], imaging agents [189] and/or transplants [190]. The bioavailability, immune recognition, cellular internalization, cellular distributions, and cytotoxicity are largely dependent on the constituents of bio-coronas [191]. To comprehensively assess the biosafety of M/NPs for health protection, the formation of bio-corona as a critical determinant needs more exploration. Three types of biomolecules, including proteins, lipids and nucleic acids, have been identified in surface coronas (Table 1).
Table 1.
The identified bio-coronas on M/NPs
| Bio-corona composition | Source | M/NPs | References |
|---|---|---|---|
| Proteins | Physiological fluid of Daphnia magna | PS–NH2 | [163] |
| Proteins | Cell culture medium for 24 h | PS | [190] |
| Mucin | Mucous layer in lung epithelial | PS@Bap NPs | [194] |
| MgC1q6 protein | Serum soluble components | PS–NH2 | [191] |
| Carbohydrates and Proteins | EPS from marine diatom Phaeodactylum tricornutum | PS–COOH | [192] |
| Lipids and Proteins | Alveolar fluid | PS PET PP PE PVC | [195] |
| Biofilm consist of nitrogen– and sulfur–related substances | Staphylococcus aureus | Nontreated and amino acid–treated plastic traces | [196] |
| Nucleic acids | Coral microbiome | PE PP PS PVC | [197] |
| Toxins and ARGs | Bacteria | PE PP | [198–200] |
Proteins
Each physiological compartment in biological systems has its own distinct set of proteins that interact in a unique way with M/NPs. The formula of NOM, metal ions or POPs in the eco–corona of M/NPs may significantly impact the bio-corona constitution. For instance, the adsorption of proteins on NPs was increased in the presence of FA in D. magna culture medium, while the presence of HA led to a decline in protein adsorption [163]. Grassi et al. [192] employed EPS from the marine diatom Phaeodactylum tricornutum to study the changes in eco–corona on PS–COOH nanoplastic surfaces. EPS significantly reduced the PS–COOH aggregation rate and caused the formation of a complex bio-corona consisting of carbohydrates and proteins. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of proteins adsorbed on PS–COOH showed abundant proteins in the molecular weight range of 30–100 kDa.
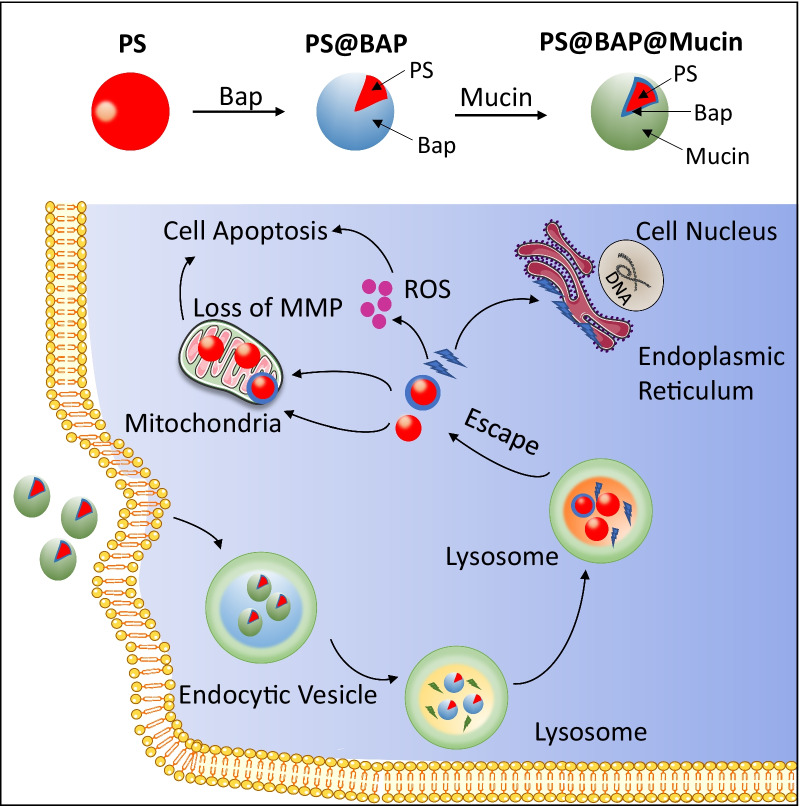
In mammals, ingestion is the major entry route for most M/NPs. However, NPs may be inhaled into the respiratory system since they may form stable aerosols in air [193]. Taking PS–benzopyrene (PS@Bap) nanoparticles as an example, Ji et al. found that the PS@Bap particles could be inhaled by the respiratory system, interacted with the mucous layer in the lung epithelium and formed a mucin corona on the surfaces. Further study showed that although the mucin corona is stable at the early stages of PS@Bap endocytosis in A549 cells, it degrades during the maturation of endosomes into lysosomes [194]. In addition, the mucin corona enhances the cellular uptake of PS@Bap but delays the intracellular trafficking of PS@Bap as well as the release of Bap from PS, resulting in reduced ROS generation, mitochondrial impairment and apoptotic cell death (Fig. 4).
Fig. 4.
The formation of a protein corona (mucin) on nanoplastics alters the intracellular fate and final destinations of the particles. PS@Bap with mucin corona was internalized into lysosomes in A549 cells via endocytosis. The mucin corona is stable at the early stages and degrades in the maturation of lysosomes, altering the intracellular trafficking of PS@Bap and the release of Bap from PS. Adapted and reprinted with permission from [194].
Lipids
Lipids are an essential component of biomembranes (e.g., vesicles), which are involved in a variety of physiological processes, including carbon storage, tissue protection, and resistance to exogenous substances [201]. Compared to proteins, the biological functions and structures of lipids are poorly understood. There are diverse lipid derivatives in biological systems, such as waxes, triacyl–glycerides, phospholipids, sphingolipids and glycolipids [202], which have differences in hydrophobic tails and hydrophilic heads. By interacting with M/NPs, lipids, especially unsaturated lipids, may be oxidized, with the subsequent generation of –OOH, –OH, –O–, and hypochlorite moieties on the hydrophobic tails of lipids. These functionalized lipids often take part in many biological processes. For instance, lipid peroxides are important hazard signals that activate ferroptosis, which was recently identified in iron–dependent programmed cell death [203, 204]. Lipid oxides have different biological functions due to their distinct structures, sites and quantities of oxidation [205]. Therefore, the analysis and identification of the composition and structure information of functionalized lipids is of great significance for the in–depth analysis of their biological functions.
Digestion and absorption of lipids in the intestinal tract are the key processes for energy and fat biosynthesis, which play key roles in the early growth of organisms [206]. After ingestion, M/NPs were primarily distributed in the intestine and could induce intestinal dysfunction. A few studies revealed that M/NPs disturbed lipid metabolism in aquatic organisms by causing intestinal microbiota dysbiosis or affecting the relevant enzymatic activities [207]. Recently, an in vitro study found that PS MPs inhibited lipase activities in a simulated human gastrointestinal system, reducing lipid digestion and bioavailability of dietary lipids [208]. However, the impact of MPs on the whole lipid metabolism process (e.g., intestinal digestion, bile acid secretion, triglyceride resynthesis and transport) in vivo remains largely unknown. In terms of respiratory exposure, M/NPs may interact with a thin layer of lipids and lung surfactants in alveoli. Theodorou et al. [209] showed that the formation of phospholipid coronas on ZnO nanowires preincubated with Curosurf® (a natural porcine pulmonary surfactant) could significantly increase the cellular uptake of the nanowires within human alveolar epithelial type 1–like cells (TT1) while decreasing intracellular dissolution and thus impacting cell death. However, few attempts have been made to investigate the detailed interactions between M/NPs and lipids, and critical data on the types and quantities of lipids associated with M/NPs are lacking.
Nucleic acids
Nucleic acids, including DNA and RNA, are the primary information–carrying molecules in organisms. A recent study demonstrated the presence of fish pathogens and human–associated bacteria on marine plastics from Norway [198]. Genes encoding toxins, hemolysins and adhesion factors as well as new variants of antibiotic resistance genes (ARGs) were identified in a salmonicida isolate obtained from marine plastics. Their study strengthens the notion that plastic debris may serve as vectors for the transport of fish pathogens as well as other opportunistic human pathogens in the marine environment. In aquatic systems, M/NPs provide a suitable solid support for the growth of microorganisms to generate biofilms. For example, PE and PP polymers (335 mm) from the Jiaxing River, China, were shown to have selective enrichment of 25 ARGs, most of which were linked with sulfonamide resistance gene (SA), aminoglycoside resistance genes (AMG), tetracycline resistance genes (TC), macrolide–lincosamide–streptogramin resistance genes (ML), and abundance of florfenicol resistance genes [199]. On PE films (5 mm) incubated (28 d) in waters from Taihu Lake, China, 8 ARGs (sul1, sulA/folP, ermE, ermF, tetA, tetC, tetW, tetX) were identified. This study concluded that MPs in the river had more ARGs than those in the estuary and seawater. The adsorption of antibiotic resistance elements (AREs), including ARGs and mobile genetic elements (MGEs), on M/NP surfaces has induced substantial concerns about the spread of antimicrobial resistance [200].
Analytical methods for coronas on M/NPs
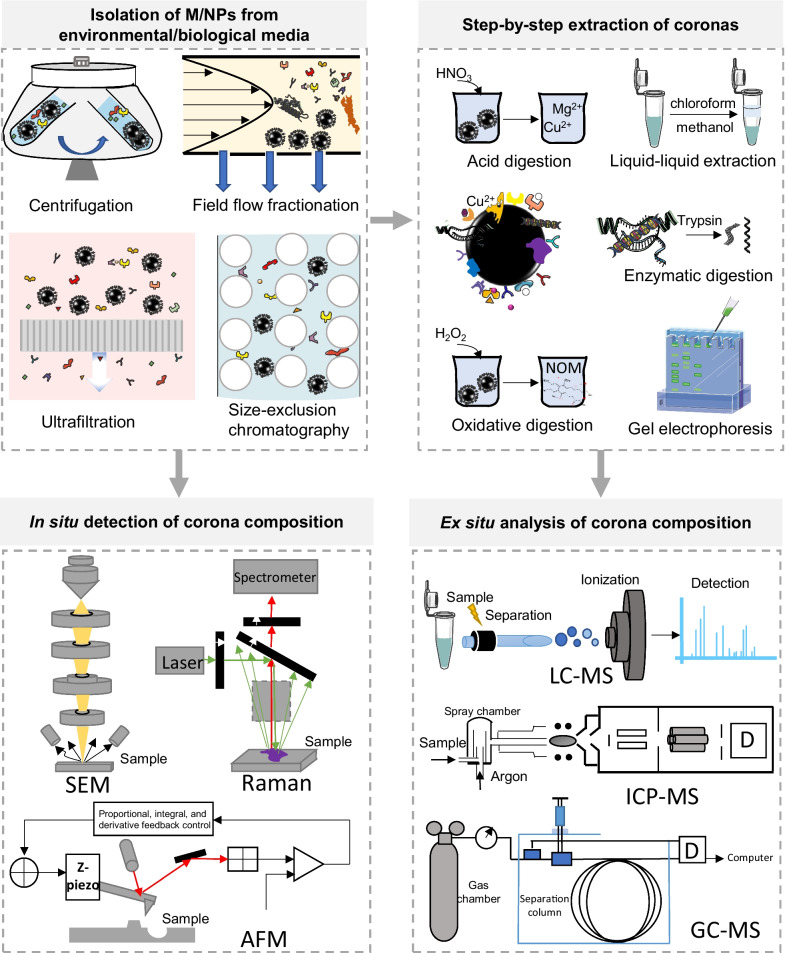
To date, it remains largely unexplored how corona formation on M/NPs determines their biological effects in organisms. Gaining insight into the biological impacts of M/NPs requires identification and quantification of the corona constitutions, raising challenges for analytical methods. In situ analysis was initially proposed to interpret the corona structures by material characterization techniques, such as ultraviolet-visible spectroscopy (UV-vis) [210], Raman spectroscopy [211], FTIR [212], X-ray photoelectron spectroscopy (XPS) [213], Auger electron spectroscopy (AES) [214], atomic force microscopy (AFM) [215], time of flight secondary ion mass spectrometry (TOF-SIMS) [216], or energy–dispersive X-ray (EDX) [217] analysis. However, most of these methods are not suitable to identify all molecular constituents in coronas. For complex M/NP samples, an ex–situ systematic analysis strategy is required for a deep quantitative analysis of the corona structures (Fig. 5), including isolation of M/NPs from environmental/biological media, layer–by–layer extraction of coronas, identification and quantification of the constituents.
Fig. 5.
Systematic analysis strategy for a deep quantitative analysis of the corona structure on M/NPs. M/NPs can be isolated from environmental/biological media by centrifugation, ultrafiltration, field flow fractionation, etc. The coronas on the M/NPs can be detected both by in situ and ex situ methods. For in situ analysis, the corona structures are directly interpreted by some microscopic and spectroscopic techniques, such as SEM, AFM, and Raman spectroscopy. For ex situ analysis, the coronas need to be separated from the M/NPs by layer–by–layer extraction methods and detected by various MS–based methods, including LC–MS, ICP–MS, and GC–MS.
Isolation of M/NPs from environmental/biological media
The isolation of M/NPs usually depends on the sample matrix. For M/NPs in clean aquatic or gas environments, this step can simply be achieved by filtration or centrifugation [218]. Nevertheless, more sophisticated techniques, such as ultracentrifugation [219], ultrafiltration [220], cloud point extraction (CPE) [221], and continuous flow centrifugation (CFC) [222], may be needed for the separation of M/NPs from complex matrices. Ultracentrifugation is a widening of benchtop centrifugation, which spins samples at exceptionally high speeds and separates small particles in a solution by their size, shape and density. Since the first use of ultracentrifugation in the separation of gold nanoparticles, it has been one of the most popular methods in nanoscience [223]. Recently, several studies reported the application of ultracentrifugation in the separation of plastic particles from aqueous matrix [224] or sediments [225, 226]. Before separation, matrix digestion with acid or alkali is usually considered a useful step to remove unbonded organic matter and improve the separation efficiency in ultracentrifugation; however, it may lead to the loss of organic eco–corona and bio-corona [227, 228]. Enzymatic digestion is also a commonly used method to remove macrobiomolecules, such as protein and DNA, in organic matrices. Enzymatic digestion can be performed for the measurement of small molecules in eco– and bio-coronas but will cause the loss of proteins [229]. Attempts have been made to facilitate the isolation of M/NPs by additives to tune solution density and control centrifugation force and times. Löder et al. [230] and Thompson et al. [8] demonstrated that the introduction of an appropriate salt (> 1.4 g/cm3) in MP aqueous samples could be used to retrieve M/NPs because most M/NPs have a density ranging from 0.8 to 1.4 g/cm3. Cai et al.’s [224] study indicated that the theoretical centrifugal time at a speed of 20,000 rpm for 2 h could completely assure sediment of 600–1000 nm spherical PS from environmental water samples. However, the presence of other particulates in M/NP suspensions may significantly affect the purity of isolated M/NPs by ultracentrifugation. Therefore, more separation methods, e.g., magnetic separation [231], field flow fractionation [232], and size–exclusion chromatography (SEC) [233], have been developed for the efficient isolation of M/NPs. For the efficient collection of M/NPs in real environmental/biological samples, combined separation methods are recommended.
Extraction of coronas from M/NPs
The composition of coronas on M/NPs can be extremely complex due to the high diversity of the matrix in the environment. The coronas are usually complexes of metal ions, inorganic salts, proteins, DNAs, lipids, HA and other small organic compounds. Obviously, it is impossible to measure both the amount and composition of all substances in the corona structure by a single determination. A more feasible way is to extract each constituent of coronas step by step and analyze the compounds by different methods. Separation of the coronas from M/NPs can be achieved by a rational design of sample pretreatment methods according to the chemical properties of the target compounds. For instance, the absorbed small organic substance could be decoupled from M/NPs by solvent extraction, such as H2O2, Fenton’s reagent, and alkaline digestion with NaOH and KOH [234], while inorganic salts and metal cations could be directly eluted in aqueous solvent after digestion of the particles by 68% HNO3 (Aristar grade) or HCl–HNO3–HF–HClO4 acids [235, 236]. Many of the approaches for elution of small molecules from biofluids or tissues could be applied in the extraction of corona constituents. One of the successive ways is to wash the particles by solvents ranging from polar (such as water) to less polar solvents (such as methanol, hexane and chloroform) [237]. The choice of solvents mainly depends on the type of molecules being extracted. Water or ethanol are more suitable for polar molecules, whereas hexane or chloroform are more suitable for nonpolar compounds. Biphasic extraction, such as the Bligh–Dyer, Folch and Matyash methods, is suitable in cases where both polar and nonpolar coronas need to be collected [238]. The pH values of the extraction solvent could be adjusted to alter the charge status of coronas or promote the hydrolysis of bonds between M/NPs and coronas [239]. Despite these general rules for corona extraction, explorations are still desired to optimize the extraction method for analysis of the whole corona rather than a small subset of compounds bound with M/NPs.
In addition to small molecules, large biomolecules, including proteins, polysaccharides and nucleic acids, are another major component of coronas. Of them, the protein corona is the most studied and can be extracted by buffer containing sodium dodecyl sulfate and other ionic detergents that have the ability to solubilize proteins [240]. The total protein content in the extraction buffers can be quantified by Bradford [241], BCA assays [242] or UV–vis [243]. Other separation methods, such as western blotting [244], gel electrophoresis [245], and SEC [246], can also be applied to isolate the proteins for further detection. Recently, Grassi et al. studied coronas on carboxylated PS NPs, which were incubated with EPS. A separation of the coronas by SEC revealed four main distinct groups of peaks, containing high (> 100 kDa) to low molecular weight (20 kDa) species with high chemical heterogeneity [192]. Another classical approach for characterization of the protein coronas on NPs is enzymatic digestion of the attached proteins and then quantification of the peptides in digests by mass spectrometry, which is subsequently submitted to a protein database for protein identification. To date, high–resolution mass spectrometry has been the most recommended state–of–the–art method for the identification of protein coronas. There are a series of review articles that can be referenced to obtain deeper knowledge on this topic [22, 247–249].
Identification and quantification of the coronas
Since the first report on nanocoronas, many analytical techniques have been implemented in the characterization of the coronas composition on various engineered nanomaterials. In recent years, some of these techniques, including microscopic, spectroscopic, and mass spectrometry–based methods, have been explored for identifying the coronas on plastic debris. Table 2 lists the utilities of these techniques to determine the corona information of M/NPs, their strengths, and limitations. Obviously, no single technique is sufficient to address all the questions about coronas on M/NPs on its own. In most cases, combined detection methods are recommended. The methods for corona analysis can be mainly divided into two categories.
Table 2.
Potential analytical techniques for the characterization of corona formation on M/NPs, their strengths and limitations
| Techniques | Corona Information | Sample states | Strengths | Limitations |
|---|---|---|---|---|
| UV–vis | Binding of protein corona to M/NPs | Particles | High reproducible and precision. nondestructive | Not suitable for qualification, susceptible to sample matrix, low sensitivity |
| DLS | Binding of coronas to M/NPs | Particles | Rapid and reproducible measurement of many nanoparticles, nondestructive | Not suitable for qualification and quantification, not suitable for heterogeneous particles |
| Fluorescence microscopy | Binding of coronas to M/NPs | Particles | High sensitive and good for real–time detection | Requirements for fluorescence labeling |
| TEM | Interactions between M/NPs and corona | Particles | High spatial resolution | Not suitable for qualification and quantification |
| SEM | Interactions between M/NPs and corona | Particles | High spatial resolution | Low particle population, poor qualification and quantification |
| AFM | Interactions between M/NPs and corona | Particles | High spatial resolution, possibility for 3D imaging | Poor efficiency |
| EDX | Elemental, especially metal elemental distribution | Particles | High efficiency | Not suitable for organic elements |
| Raman | Structures of organic substances, such as natural organic matters, lipids, persistent organic pollutants | Particles | Broad coverage of both organic and inorganic species | Low spatial resolution |
| FTIR | Structures of organic substances, such as natural organic matters, lipids, persistent organic pollutants | Particles | Good repeatability, high flexibility | Low accuracy and sensitivity, not available for inorganic species |
| ICP–MS | Compositions of metal or metal ions | Acid digested solution | High sensitivity and accuracy, quantification | Not suitable for organic compounds |
| TOF–SIMS | Structures of organic substances, such as natural organic matters, lipids, persistent organic pollutants, proteins | Particles | 3D profile with high mass resolution and spatial resolution | Poor quantification, not suitable for unknown compounds, requirements for samples |
| GC–MS | Compositions of hydrophobic and volatile organic compounds, such as flame retardants, lubricants and plasticizers and persistent organic pollutants | Solvent extracted solution | High sensitivity and reproducibility | Not suitable for thermally labile compounds |
| LC–MS | Compositions of thermally labile coronas, such as proteins, lipids, peptides | Enzyme digested or solvent extracted solution | Broad coverage of compounds, high sensitivity and accuracy | Requirements for multistep sample pretreatment, ex situ analysis |
| CE–MS | Compositions of thermally labile coronas, such as proteins and metabolites | Enzyme digested or solvent extracted solution | High resolution and selectivity | Not suitable for direct analysis of high molecular proteins, limited loading capacity |
Microscopic and spectroscopic methods
Microscopic and spectroscopic methods are integral parts of techniques for nanoparticle characterization. Some of these methods, such as UV–vis, DLS, TEM, SEM, EDX, AFM, Raman or FTIR, are also commonly used for in situ deciphering corona formation on particle surfaces. For example, UV–vis can be used to evaluate the binding of proteins to particles by assessing the absorption peak changes before and after binding [250, 251]. Corona formation on M/NPs could also be determined by measuring the changes in hydrodynamic size of M/NPs using DLS [252], fluorescence microscopy [253]. However, such measurements are not suitable when particles are colloidally unstable and exhibit a wide size distribution. TEM, SEM and AFM can provide high–resolution images of M/NPs for visualizing the interactions between plastics and corona by morphology changes [254, 255]. By coupling with EDS, TEM and SEM can reveal the elemental distribution on plastic particle surfaces, which is a convincing technique to analyze metal elements in the corona [255]. Although phosphorus, oxygen and carbon elements are also detectable, this technique has limitations in differentiating adsorbed organic substances from plastic surfaces. Instead, FTIR and Raman spectroscopy are more suitable to detect the organic substances in corona structures. For instance, FA, lipids and sodium dodecyl sulfate were successfully detected on nano–PS by surface–enhanced Raman scattering in the study of Zhang et al. [256]. However, both FTIR and Raman spectroscopy are incapable of directly visualizing M/NPs. Recent advances in characterization techniques, e.g., AFM–IR, combine the advantages of microscopic and spectroscopic methods and may offer opportunities for the identification of organic substances in the corona structure of a single NP [257]. Among other optical microscopy techniques, hyperspectral microscopy in dark-field [258] has recently been introduced as an effective method to detect and identify nanoplastics down to 100 nm [259, 260]. Supplemented by artificial intelligence [261] and machine learning [262–264], this technique may hold promise in spectral characterization of adlayers deposited onto microplastics. Future work is needed to evaluate the feasibility of this technology in characterization of coronas on microplastics. However, all these microscopic and spectroscopic techniques are not suitable for a comprehensive interpretation of each constituent in corona structures. To acquire the fingerprints of M/NP coronas, mass spectrometry–based methods should be considered.
Mass spectrometry–based methods
MS has been predominantly used as a sensitive and high–resolution technique to examine the chemical composition of coronas. It can be used to detect molecular weights or structures via the mass–to–charge ratio of fragments of metal ions, small chemicals and biomolecules, covering almost all known constituents in corona structures. Currently, various MS–based procedures, including inductively coupled plasma–mass spectrometry (ICP–MS), TOF–SIMS, gas chromatography–mass spectrometry (GC–MS), capillary electrophoresis–mass spectrometry (CE–MS) and liquid chromatography–mass spectrometry (LC–MS), have been explored for the examination of coronas on M/NPs. ICP–MS has been extensively used for the measurement of inorganic elements, particularly trace metal contaminants, in M/NP coronas. For instance, Qiao et al. [265] used ICP–MS to detect Cu uptake in zebrafish and found that small–sized MPs deliver more Cu. This technique has also been successfully exploited to examine the transport of adsorbed heavy metals (Pb, Cd, and Zn) by PET MPs in wheat [132]. Although trace levels (~ 0.3 ppb) of metal elements could be well quantified by ICP–MS analysis, they could merely reflect the average metal levels of the bulk particle population. While each M/NP in nature has its own individual physicochemical properties and may form distinct coronas, the interpretation of coronas on a single plastic particle is meaningful. In 2003, Degueldre developed single particle (sp)ICP–MS, which measures the mass of recorded elements in individual particles and the total particle number concentration [266]. Recently, Bolea–Fernandez et al. [267] successfully detected 13C–labeled and lanthanide–doped PS beads by single particle–ICP–MS. However, neither ICP–MS nor SP–ICP–MS can characterize the molecular structure of organic coronas. Compared to ICP–MS, TOF–SIMS is more suitable for the characterization of organic compounds. TOF–SIMS can determine the element and molecular fragments by the SIMS peak, which is produced by measuring the exact mass and intensity of secondary ions and ion clusters emitted from a solid surface with a finely focused ion beam. Basically, TOF–SIMS is a surface analysis technique that functions in a manner analogous to SEM/EDS. However, the depth of analysis for TOF–SIMS is less than 2 nm, which is better suited for the analysis of ultrathin layers and nanoscale samples when compared to SEM/EDS. In addition, TOF–SIMS can provide a unique 3D profile with high mass resolution and high spatial resolution and is an ideal technique for directly imaging organic constituents in coronas. Neunzehn et al. [268] exploited this technique to detect different nanoscale protein coatings on gold nanoparticles, but few attempts have been made on M/NPs. Notably, TOF–SIMS is not suitable for unknown compounds. In addition, this technique is sensitive to sample preparation and time–consuming in data interpretation and has poor performance in quantification analysis.
MS can also be coupled with various separation techniques, such as GC, LC and CE, to acquire massive information of a complex corona. GC–MS is one of the most useful techniques for monitoring highly hydrophobic and volatile organic compounds and has been extensively used for monitoring plastic additives (flame retardants, lubricants and plasticizers) and microplastic–adsorbed organic contaminants, such as OCPs, PAHs, PCBs, and organophosphorous pesticides (OPPs) [269]. León et al. [270] detected 91 organic pollutants, including 17 PAHs, 7 PCBs, 54 pesticides, and 5 plastic additives, by GC–MS in plastic debris from the Mar Menor coastal lagoon in southeastern Spain. The GC–MS allows the analysis and detection of tiny amounts of organic substances with high sensitivity and reproducibility, whereas it is not suitable for the analysis of thermally labile compounds. Alternatively, LC–MS is more commonly used to investigate the thermally labile constituents in coronas, including proteins, lipids, peptides. LC–MS can provide qualitative and quantitative information regarding the complex mixture of coronas and has been considered the first choice for protein corona studies. Fadare et al. [163] exploited this technique to analyze the coronas on M/NPs after interacting with humic substances and identified a total of 1004 proteins, with 281 and 723 unique proteins for eco–corona and bio-corona, respectively. In addition, CE–MS has recently been introduced as a new technique for the characterization of protein and metabolite coronas on nanomaterials [271]. Nevertheless, full interpretation of coronas on M/NPs is still a major challenge, which may require a rational combination of several analytical techniques.
Summary and Perspectives
Spreading and accumulation of M/NPs have led to increasing interaction opportunities of M/NPs with substances in environmental and biological media. Given the hydrophobic, inert and persistent features of M/NPs, corona formation is a prominent process that is highly related to the migration, uptake, distribution, metabolism, clearance and toxicity of M/NPs. Although substantial evidence has demonstrated the coexistence of environmental/biological species and M/NPs, most studies merely assess the impacts of one or two substances on M/NP toxicity rather than the whole integrates. While concerns have been raised about the environmental and health risks of M/NPs and substantial efforts have been made in this research area, we recommend serious consideration of the role of surface coronas in safety assessments. Different from conventional toxins, packages on M/NPs rather than bare particles are more dangerous. The adsorption process on M/NPs may impact the bioavailability, entry routes, distribution and clearance of corona constituents in biological systems. Given that environmental and biological transport may significantly alter the physicochemical properties of M/NPs, each M/NP particle may have a distinct corona formula that generates a unique identity. Full interpretation of a corona structure is an extreme challenge and may require a rational combination of different analytical methods. All considered, corona formation in M/NP safety assessment is a new research field that faces challenges and opportunities.
Rational combinations of analytical methods or exploration of new analytical technologies are extremely desired for a comprehensive interpretation of corona compositions.
In addition to chemical composition, the advanced space structures of coronas, especially for bio-coronas, have rarely been explored.
The transportation of M/NPs in a biological system may lead to a dynamic evolution of corona structures, which may further elicit molecular initiating events and adverse outcomes. The connections among these events are largely unknown.
In situ detection of coronas on M/NPs in biological systems is a major challenge. Attempts should be made to use multiple independent labeling strategies (e.g., fluorescent and radiological labeling) for differentiation of the corona institutes and M/NPs in cells or animals.
Big data analysis or machine learning is recommended to decipher the connections between corona structures and toxic effects.
Overcoming these challenges may require the exploration of interdisciplinary approaches in toxicology, materials science and analytical chemistry. A full understanding of the impacts of the corona on toxicity may facilitate the establishment of a predictive toxicology paradigm for risk assessments of M/NPs.
Acknowledgements
Not applicable.
Author contributions
All authors contributed to the design and concept of this article. JC drafted the manuscript. QY, JJ, TD, AK, JS, RF and FW made revisions. XC and RL critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (No. 2020YFA0710700), the National Natural Science Foundation of China (No. 21976126), and the Natural Science Foundation of Jiangsu Province (BK20211545). TD acknowledges funding from the National Research Foundation (No. 138206). Rawil Fakhrullin acknowledges the support by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaoming Cai, Email: xmcai@suda.edu.cn.
Ruibin Li, Email: liruibin@suda.edu.cn.
References
- 1.Lodge TP. Celebrating 50 Years of macromolecules. Macromolecules. 2017;50(24):9525–9527. doi: 10.1021/acs.macromol.7b02507. [DOI] [Google Scholar]
- 2.Heng N, Ungul LU, Mehrdadi N. Recycling and reuse of household plastics. Int J Environ Res. 2008;2(1):27–36. [Google Scholar]
- 3.Hernandez LM, Yousefi N, Tufenkji N. Are there nanoplastics in your personal care products? Environ Sci Tech Let. 2017;4(7):280–285. doi: 10.1021/acs.estlett.7b00187. [DOI] [Google Scholar]
- 4.Hopewell J, Dvorak R, Kosior E. Plastics recycling: challenges and opportunities. Philos T R Soc B. 2009;364(1526):2115–2126. doi: 10.1098/rstb.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma MD, Elanjickal AI, Mankar JS, Krupadam RJ. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J Hazard Mater. 2020;10.1016/j.jhazmat.2020.122994. [DOI] [PubMed]
- 6.Lebreton L, Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019; 10.1057/s41599-018-0212-7.
- 7.Alimi OS, Budarz JF, Hernandez LM, Tufenkji N. Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol. 2018;52(4):1704–1724. doi: 10.1021/acs.est.7b05559. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, et al. Lost at sea: where is all the plastic? Science. 2004;304(5672):838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- 9.Sobhani Z, Zhang X, Gibson C, Naidu R, Megharaj M, Fang C. Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm. Water Res. 2020;174:115658. doi: 10.1016/j.watres.2020.115658. [DOI] [PubMed] [Google Scholar]
- 10.Guerranti C, Martellini T, Perra G, Scopetani C, Cincinelli A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ Toxicol Pharmacol. 2019;68:75–79. doi: 10.1016/j.etap.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Turner A. Paint particles in the marine environment: An overlooked component of microplastics. Water Res X. 2021; 10.1016/j.wroa.2021.100110. [DOI] [PMC free article] [PubMed]
- 12.Zhao K, Wei Y, Dong J, Zhao P, Wang Y, Pan X, et al. Separation and characterization of microplastic and nanoplastic particles in marine environment. Environ Pollut (Barking, Essex : 1987). 2022;297:118773. 10.1016/j.envpol.2021.118773. [DOI] [PubMed]
- 13.Zhang X, Ma GH, Wei W. Simulation of nanoparticles interacting with a cell membrane: probing the structural basis and potential biomedical application. Npg Asia Mater. 2021;13(1):52. doi: 10.1038/s41427-021-00320-0. [DOI] [Google Scholar]
- 14.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano. 2010;4(7):3623–3632. doi: 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- 15.Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3:40–47. doi: 10.1016/S1748-0132(08)70014-8. [DOI] [Google Scholar]
- 16.Milani S, Bombelli FB, Pitek AS, Dawson KA, Radler J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: soft and hard corona. ACS Nano. 2012;6(3):2532–2541. doi: 10.1021/nn204951s. [DOI] [PubMed] [Google Scholar]
- 17.Galloway TS, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol. 2017;1(5):116. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- 18.Strojan K, Leonardi A, Bregar VB, Krizaj I, Svete J, Pavlin M. Dispersion of Nanoparticles in Different Media Importantly Determines the Composition of Their Protein Corona. Plos One. 2017;12(1):169. 10.1371/journal.pone.0169552. [DOI] [PMC free article] [PubMed]
- 19.Chakraborty D, Giri S, Natarajan L, Chandrasekaran N, Mukherjee A. Recent Advances in Understanding the Facets of Eco-corona on Engineered Nanomaterials. J. Indian Inst. Sci. 10.1007/s41745-021-00266-w.
- 20.Kurepa J, Shull TE, Smalle JA. Metabolomic analyses of the bio-corona formed on TiO2 nanoparticles incubated with plant leaf tissues. J Nanobiotechnol. 2020;18(1):10. doi: 10.1186/s12951-020-00592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saha K, Moyano DF, Rotello VM. Protein coronas suppress the hemolytic activity of hydrophilic and hydrophobic nanoparticles. Mater Horiz. 2014;1(1):102–105. doi: 10.1039/c3mh00075c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–U1000. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 23.Chinen AB, Guan CXM, Ko CH, Mirkin CA. The Impact of Protein Corona Formation on the Macrophage Cellular Uptake and Biodistribution of Spherical Nucleic Acids. Small. 2017;13(16):1603847. doi: 10.1002/smll.201603847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbero F, Russo L, Vitali M, Piella J, Salvo I, Borrajo ML, et al. Formation of the protein Corona: The interface between nanoparticles and the immune system. Semin Immunol. 2017; 10.1016/j.smim.2017.10.001. [DOI] [PubMed]
- 25.Ma ZF, Bai J, Jiang XE. Monitoring of the enzymatic degradation of protein corona and evaluating the accompanying cytotoxicity of nanoparticles. Acs Appl Mater Inter. 2015;7(32):17614–17622. doi: 10.1021/acsami.5b05744. [DOI] [PubMed] [Google Scholar]
- 26.Lesniak A, Campbell A, Monopoli MP, Lynch I, Salvati A, Dawson KA. Serum heat inactivation affects protein corona composition and nanoparticle uptake. Biomaterials. 2010;31(36):9511–9518. doi: 10.1016/j.biomaterials.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 27.Choi JW, Kim IY, Kwak M, Kim J, Yoon S, Jang HJ, et al. Effect of serum protein on cell internalization of silica nanoparticles. Micro Nano Lett. 2022 doi: 10.1049/mna2.12105. [DOI] [Google Scholar]
- 28.Zou YJ, Ito S, Fujiwara M, Komatsu N. Probing the role of charged functional groups on nanoparticles grafted with polyglycerol in protein adsorption and cellular uptake. Adv Funct Mater. 2022: 10.1002/adfm.202111077.
- 29.Yan HJ, Cacioppo M, Megahed S, Arcudi F, Dordevic L, Zhu DC, et al. Influence of the chirality of carbon nanodots on their interaction with proteins and cells. Nat Commun. 2021;12(1):14. doi: 10.1038/s41467-021-27406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritz S, Schottler S, Kotman N, Baier G, Musyanovych A, Kuharev J, et al. Protein Corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromol. 2015;16(4):1311–1321. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 31.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/NNANO.2010.250. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed Nor NH, Kooi M, Diepens NJ, Koelmans AA. Lifetime accumulation of microplastic in children and adults. Environ Sci Technol. 2021;55:5084–5096. doi: 10.1021/ACS.EST.0C07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trevisan R, Ranasinghe P, Jayasundara N, Di Giulio RT. Nanoplastics in aquatic environments: impacts on aquatic species and interactions with environmental factors and pollutants. Toxics. 2022;10(6):326. 10.3390/toxics10060326. [DOI] [PMC free article] [PubMed]
- 34.Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol. 2019;53(4):1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- 35.Hanif MA, Ibrahim N, Dahalan FA, Ali UFM, Hasan M, Jalil AA. Microplastics and nanoplastics: Recent literature studies and patents on their removal from aqueous environment. Sci Total Environ. 2022; 10.1016/j.scitotenv.2021.152115. [DOI] [PubMed]
- 36.Guan XY, Li H, Ma YC, Xue M, Fang QR, Yan YS, et al. Chemically stable polyarylether-based covalent organic frameworks. Nat Chem. 2019;11(6):587–594. doi: 10.1038/s41557-019-0238-5. [DOI] [PubMed] [Google Scholar]
- 37.Kihara S, Ghosh S, McDougall DR, Whitten AE, Mata JP, Koper I, et al. Structure of soft and hard protein corona around polystyrene nanoplastics-Particle size and protein types. Biointerphases. 2020;15(5). 0510021116/6.0000404 [DOI] [PubMed]
- 38.Rai PK, Lee J, Brown RJC, Kim KH. Micro- and nano-plastic pollution: Behavior, microbial ecology, and remediation technologies. J Clean Prod. 2021;291:125240. doi: 10.1016/j.jclepro.2020.125240. [DOI] [Google Scholar]
- 39.Enfrin M, Dumee LF, Lee J. Nano/microplastics in water and wastewater treatment processes - origin, impact and potential solutions. Water Res. 2019;161:621–638. doi: 10.1016/j.watres.2019.06.049. [DOI] [PubMed] [Google Scholar]
- 40.Piccardo M, Renzi M, Terlizzi A. Nanoplastics in the oceans: Theory, experimental evidence and real world. Mar Pollut Bull. 2020;157:111317. doi: 10.1016/j.marpolbul.2020.111317. [DOI] [PubMed] [Google Scholar]
- 41.Wahl A, Le Juge C, Davranche M, El Hadri H, Grassl B, Reynaud S, et al. Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere. 2021;262:127784. doi: 10.1016/j.chemosphere.2020.127784. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Zhao Y, Du F, Cai H, Wang G, Shi H. Microplastic fallout in different indoor environments. Environ Sci Technol. 2020;54(11):6530–6539. doi: 10.1021/acs.est.0c00087. [DOI] [PubMed] [Google Scholar]
- 43.Toussaint B, Raffael B, Angers-Loustau A, Gilliland D, Kestens V, Petrillo M, et al. Review of micro- and nanoplastic contamination in the food chain. Food Addit Contam A. 2019;36(5):639–673. doi: 10.1080/19440049.2019.1583381. [DOI] [PubMed] [Google Scholar]
- 44.Making plastics sustainable isn't the whole solution Nature. 2021;590(7846):363–364. doi: 10.1038/d41586-021-00391-7. [DOI] [PubMed] [Google Scholar]
- 45.Strungaru S-A, Jijie R, Nicoara M, Plavan G, Faggio C. Micro- (nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. Trac-Trend Anal Chem. 2019;110:116–128. doi: 10.1016/j.trac.2018.10.025. [DOI] [Google Scholar]
- 46.Abelouah MR, Ben-Haddad M, Rangel-Buitrago N, Hajji S, El Alem N, Ait AA. Microplastics pollution along the central Atlantic coastline of Morocco. Mar Pollut Bull. 2022;174:113190. doi: 10.1016/j.marpolbul.2021.113190. [DOI] [PubMed] [Google Scholar]
- 47.Wayman C, Niemann H. The fate of plastic in the ocean environment - a minireview. Environ Sci Process Impacts. 2021;23(2):198–212. doi: 10.1039/d0em00446d. [DOI] [PubMed] [Google Scholar]
- 48.Wagner M, Scherer C, Alvarez-Munoz D, Brennholt N, Bourrain X, Buchinger S, et al. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur. 2014;26(1):12–12. doi: 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horton AA, Svendsen C, Williams RJ, Spurgeon DJ, Lahive E. Large microplastic particles in sediments of tributaries of the River Thames, UK - Abundance, sources and methods for effective quantification. Mar Pollut Bull. 2017;114(1):218–226. doi: 10.1016/j.marpolbul.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Pojar I, Stanica A, Stock F, Kochleus C, Schultz M, Bradley C. Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea. Sci Rep-Uk. 2021; 10.1038/s41598-021-81724-4. [DOI] [PMC free article] [PubMed]
- 51.Forrest SA, Holman L, Murphy M, Vermaire JC. Citizen science sampling programs as a technique for monitoring microplastic pollution: results lessons learned and recommendations for working with volunteers for monitoring plastic pollution in freshwater ecosystems. Environ Monitor Assess. 2019; 10.1007/s10661-019-7297-3. [DOI] [PubMed]
- 52.Li W, Duo J, Wufuer R, Wang S, Pan X. Characteristics and distribution of microplastics in shoreline sediments of the Yangtze River, main tributaries and lakes in China-from upper reaches to the estuary. Environ Sci Pollut Res Int. 2022 doi: 10.1007/s11356-021-18284-7. [DOI] [PubMed] [Google Scholar]
- 53.Bowman KL, Lamborg CH, Agather AM, Hammerschmidt CR. The role of plastic debris in the biogeochemical cycle of mercury in Lake Erie and San Francisco Bay. Marine Pollut Bull. 2021; 10.1016/j.marpolbul.2021.112768. [DOI] [PubMed]
- 54.Zhang Q, Liu T, Liu L, Fan Y, Rao W, Zheng J, et al. Distribution and sedimentation of microplastics in Taihu Lake. Sci Total Environ. 2021; 10.1016/j.scitotenv.2021.148745. [DOI] [PubMed]
- 55.Shen M, Zeng Z, Wen X, Ren X, Zeng G, Zhang Y, et al. Presence of microplastics in drinking water from freshwater sources: the investigation in Changsha. China Environ Sci Pollut R. 2021;28:42313–42324. doi: 10.1007/S11356-021-13769-X. [DOI] [PubMed] [Google Scholar]
- 56.Botterell ZLR, Beaumont N, Dorrington T, Steinke M, Thompson RC, Lindeque PK. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ Pollut. 2019;245:98–110. doi: 10.1016/j.envpol.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Zhu X, Teng J, Zhao J, Li C, Shan E, et al. Pollution characteristics of Microplastics in Mollusks from the coastal area of Yantai. China Bull Environ Contam Toxicol. 2021;107(4):693–699. doi: 10.1007/s00128-021-03276-7. [DOI] [PubMed] [Google Scholar]
- 58.D'Costa AH. Microplastics in decapod crustaceans: Accumulation toxicity and impacts a review. Sci Total Environ. 2022:10.1016/j.scitotenv.2022.154963. [DOI] [PubMed]
- 59.Smith M, Love DC, Rochman CM, Neff RA. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018;5(3):375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boots B, Russell CW, Green DS. Effects of microplastics in soil ecosystems: Above and below ground. Environ Sci Technol. 2019;53:11496–11506. doi: 10.1021/ACS.EST.9B03304. [DOI] [PubMed] [Google Scholar]
- 61.Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- 62.Perez-Reveron R, Gonzalez-Salamo J, Hernandez-Sanchez C, Gonzalez-Pleiter M, Hernandez-Borges J, Diaz-Pena FJ. Recycled wastewater as a potential source of microplastics in irrigated soils from an arid-insular territory (Fuerteventura, Spain). Sci Total Environ. 2022; 10.1016/j.scitotenv.2021.152830. [DOI] [PubMed]
- 63.Steinmetz Z, Wollmann C, Schaefer M, Buchmann C, David J, Troeger J, et al. Plastic mulching in agriculture. trading short-term agronomic benefits for long-term soil degradation? Sci Total Environ. 2016; 10.1016/j.scitotenv.2016.01.153. [DOI] [PubMed]
- 64.Ragoobur D, Huerta-Lwanga E, Somaroo GD. Microplastics in agricultural soils, wastewater effluents and sewage sludge in Mauritius. Sci Total Environ. 2021;798:149326. doi: 10.1016/j.scitotenv.2021.149326. [DOI] [PubMed] [Google Scholar]
- 65.He DF, Luo YM, Lu SB, Liu MT, Song Y, Lei LL. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. Trac-Trend Anal Chem. 2018;109:163–172. doi: 10.1016/j.trac.2018.10.006. [DOI] [Google Scholar]
- 66.Baensch-Baltruschat B, Kocher B, Kochleus C, Stock F, Reifferscheid G. Tyre and road wear particles - A calculation of generation, transport and release to water and soil with special regard to German roads. Sci Total Environ. 2021;752:141939. doi: 10.1016/j.scitotenv.2020.141939. [DOI] [PubMed] [Google Scholar]
- 67.Scheurer M, Bigalke M. Microplastics in swiss floodplain soils. Environ Sci Technol. 2018;52(6):3591–3598. doi: 10.1021/acs.est.7b06003. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Q, Zhang H, Fu C, Zhou Y, Dai Z, Li Y, et al. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma. 2018;322:201–208. doi: 10.1016/J.GEODERMA.2018.02.015. [DOI] [Google Scholar]
- 69.Liu M, Lu S, Song Y, Lei L, Hu J, Lv W, et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai. China Environ Pollut. 2018;242:855–862. doi: 10.1016/J.ENVPOL.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 70.Nematollahi MJ, Keshavarzi B, Mohit F, Moore F, Busquets R. Microplastic occurrence in urban and industrial soils of Ahvaz metropolis: A city with a sustained record of air pollution. Sci Total Environ. 2021; 10.1016/j.scitotenv.2021.152051. [DOI] [PubMed]
- 71.Rillig MC, Lehmann A, de Souza Machado AA, Yang G. Microplastic effects on plants. New Phytol. 2019;223(3):1066–1070. doi: 10.1111/nph.15794. [DOI] [PubMed] [Google Scholar]
- 72.Browne MA, Niven SJ, Galloway TS, Rowland SJ, Thompson RC. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr Biol. 2013;23(23):2388–2392. doi: 10.1016/j.cub.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 73.Zhu X, Huang W, Fang M, Liao Z, Wang Y, Xu L, et al. Airborne microplastic concentrations in five megacities of northern and Southeast China. Environ Sci Technol. 2021;55:12871–12881. doi: 10.1021/ACS.EST.1C03618. [DOI] [PubMed] [Google Scholar]
- 74.Amato-Lourenco LF, Dos Santos Galvao L, Wiebeck H, Carvalho-Oliveira R, Mauad T. Atmospheric microplastic fallout in outdoor and indoor environments in Sao Paulo megacity. Sci Total Environ. 2022: 10.1016/j.scitotenv.2022.153450. [DOI] [PubMed]
- 75.Zhang Q, Zhao Y, Du F, Cai H, Wang G, Shi H. Microplastic fallout in different indoor environments. Environ Sci Technol. 2020;54:6530–6539. doi: 10.1021/ACS.EST.0C00087. [DOI] [PubMed] [Google Scholar]
- 76.Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25(12):563–570. doi: 10.1016/j.tibtech.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34(3):559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- 78.Collard F, Gasperi J, Gabrielsen GW, Tassin B. Plastic particle ingestion by wild freshwater fish: A critical review. Environ Sci Technol. 2019 doi: 10.1021/ACS.EST.9B03083. [DOI] [PubMed] [Google Scholar]
- 79.Barnes DK. Biodiversity: invasions by marine life on plastic debris. Nature. 2002;416(6883):808–809. doi: 10.1038/416808a. [DOI] [PubMed] [Google Scholar]
- 80.Danopoulos E, Jenner LC, Twiddy M, Rotchell JM. Microplastic Contamination of seafood intended for human consumption: A systematic review and meta-analysis. Environ Health Persp. 2020; 10.1289/EHP7171. [DOI] [PMC free article] [PubMed]
- 81.Hoang TC, Felix-Kim M. Microplastic consumption and excretion by fathead minnows (Pimephales promelas): Influence of particles size and body shape of fish. Sci Total Environ. 2020;704:135433. doi: 10.1016/j.scitotenv.2019.135433. [DOI] [PubMed] [Google Scholar]
- 82.Lee WS, Kim H, Sim Y, Kang T, Jeong J. Fluorescent polypropylene nanoplastics for studying uptake, biodistribution, and excretion in Zebrafish Embryos. ACS Omega. 2022;7(2):2467–2473. doi: 10.1021/acsomega.1c06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng C, He N, Wu Y, Lu Y, Sun H, Wang L. Excretion characteristics of nylon microplastics and absorption risk of nanoplastics in rats. Ecotoxicol Environ Saf. 2022;238:113586. doi: 10.1016/j.ecoenv.2022.113586. [DOI] [PubMed] [Google Scholar]
- 84.Al-Sid-Cheikh M, Rowland SJ, Stevenson K, Rouleau C, Henry TB, Thompson RC. Uptake, whole-body distribution, and depuration of nanoplastics by the scallop pecten maximus at environmentally realistic concentrations. Environ Sci Technol. 2018;52(24):14480–14486. doi: 10.1021/acs.est.8b05266. [DOI] [PubMed] [Google Scholar]
- 85.Yang D, Shi H, Li L, Li J, Jabeen K, Kolandhasamy P. Microplastic pollution in table salts from China. Environ Sci Technol. 2015;49:13622–13627. doi: 10.1021/ACS.EST.5B03163. [DOI] [PubMed] [Google Scholar]
- 86.Shi Y, Li D, Xiao L, Sheerin ED, Mullarkey D, Yang L, et al. The influence of drinking water constituents on the level of microplastic release from plastic kettles. J Hazard Mater. 2022; 10.1016/j.jhazmat.2021.127997. [DOI] [PubMed]
- 87.Su Y, Hu X, Tang HJ, Lu K, Li HM, Liu SJ, et al. Steam disinfection releases micro(nano)plastics from silicone-rubber baby teats as examined by optical photothermal infrared microspectroscopy. Nat Nanotechnol. 2022;17(1):76 10.1038/s41565-021-00998-x. [DOI] [PubMed]
- 88.Wei R, Zimmermann W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb Biotechnol. 2017;10(6):1308–1322. doi: 10.1111/1751-7915.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rahman MH, Bhoi PR. An overview of non-biodegradable bioplastics. J Clean Prod. 2021;294:126218. doi: 10.1016/j.jclepro.2021.126218. [DOI] [Google Scholar]
- 90.Arndt J-H, Bruell R, Macko T, Perez F, Panitzky J. Solubility of polyethylene in n-hexane and cyclohexane: experimental determination and comparison with solid-liquid equilibria-based predictions. Ind Eng Chem Res. 2021;60(41):14968–14976. doi: 10.1021/acs.iecr.1c02406. [DOI] [Google Scholar]
- 91.Montazer Z, Habibi Najafi MB, Levin DB. Challenges with verifying microbial degradation of polyethylene. Polymers. 2020;12(1):123. doi: 10.3390/polym12010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qi XH, Ma Y, Chang HC, Li BZ, Ding MZ, Yuan YJ. Evaluation of PET degradation using artificial microbial consortia. Front Microbiol. 2021;12:778828. doi: 10.3389/fmicb.2021.778828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Csernica J, Brown A. Effect of plasticisers on the properties of polystyrene films. J Chem Educ. 1999;76(11):1526–1528. doi: 10.1021/ed076p1526. [DOI] [Google Scholar]
- 94.Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Kooi M, Koelmans AA. Simplifying Microplastic via Continuous Probability Distributions for Size, Shape, and Density. 2019. 10.1021/acs.estlett.9b00379.
- 96.Li YW, Shao LY, Wang WH, Zhang MY, Feng XL, Li WJ, et al. Airborne fiber particles: Types, size and concentration observed in Beijing. Sci Total Environ. 2020;705:135967. doi: 10.1016/j.scitotenv.2019.135967. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Q, Tu C, Fu C, Li Y, Zhang H, Xiong K, et al. Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci Total Environ. 2020;703:134807. doi: 10.1016/j.scitotenv.2019.134807. [DOI] [PubMed] [Google Scholar]
- 98.Provencher JF, Bond AL, Avery-Gomm S, Borrelle SB, Rebolledo ELB, Hammer S, et al. Quantifying ingested debris in marine megafauna: a review and recommendations for standardization. Anal Methods-Uk. 2017;9(9):1454–1469. doi: 10.1039/c6ay02419j. [DOI] [Google Scholar]
- 99.Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol. 2012;46:3060–3075. doi: 10.1021/ES2031505. [DOI] [PubMed] [Google Scholar]
- 100.Fahrenfeld NL, Arbuckle-Keil G, Beni NN, Bartelt-Hunt SL. Source tracking microplastics in the freshwater environment. Trac-Trend Anal Chem. 2019;112:248–254. doi: 10.1016/j.trac.2018.11.030. [DOI] [Google Scholar]
- 101.Tao DY, Zhang K, Xu SP, Lin HJ, Liu Y, Kang JL, et al. p Microfibers released into the air from a household tumble dryer. Environ Sci Tech Let. 2022;9(2):120–126. doi: 10.1021/acs.estlett.1c00911. [DOI] [Google Scholar]
- 102.Zhang YL, Kang SC, Allen S, Allen D, Gao TG, Sillanpaa M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci Rev. 2020;203:103118. doi: 10.1016/j.earscirev.2020.103118. [DOI] [Google Scholar]
- 103.Gigault J, ter Halle A, Baudrimont M, Pascal PY, Gauffre F, Phi TL, et al. Current opinion: What is a nanoplastic? Environ Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 104.Guisbiers G, Mejia-Rosales S, Deepak FL. Nanomaterial properties: Size and shape dependencies. J Nanomater. 2012 doi: 10.1155/2012/180976. [DOI] [Google Scholar]
- 105.Gigault J, El Hadri H, Nguyen B, Grassl B, Rowenczyk L, Tufenkji N, et al. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat Nanotechnol. 2021;16(5):501–507. doi: 10.1038/s41565-021-00886-4. [DOI] [PubMed] [Google Scholar]
- 106.Shupe HJ, Boenisch KM, Harper BJ, Brander SM, Harper SL. Effect of nanoplastic type and surface chemistry on particle agglomeration over a salinity gradient. Environ Toxicol Chem. 2021;40(7):1822–1828. doi: 10.1002/etc.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Udupa A, Sugihara T, Viswanathan K, Chandrasekar S. Altering the stability of surface plastic flow via mechanochemical effects. Phys Rev Appl. 2019;11(1):014021. doi: 10.1103/PhysRevApplied.11.014021. [DOI] [Google Scholar]
- 108.Sabreen SR. Solving the problems of plastics adhesion. Plast Eng. 2011;67(4):6–8. doi: 10.1002/j.1941-9635.2011.tb01927.x. [DOI] [Google Scholar]
- 109.Hansjosten I, Takamiya M, Rapp J, Reiner L, Fritsch-Decker S, Mattern D, et al. Surface functionalisation-dependent adverse effects of metal nanoparticles and nanoplastics in zebrafish embryos. Environ Sci-Nano. 2022;9(1):375–392. doi: 10.1039/d1en00299f. [DOI] [Google Scholar]
- 110.Li M, He L, Zhang X, Rong H, Tong M. Different surface charged plastic particles have different cotransport behaviors with kaolinite particles in porous media. Environ Pollut. 2020 doi: 10.1016/j.envpol.2020.115534. [DOI] [PubMed] [Google Scholar]
- 111.Ramasamy BSS, Palanisamy S. A review on occurrence, characteristics, toxicology and treatment of nanoplastic waste in the environment. Environ Sci Pollut R. 2021;28(32):43258–43273. doi: 10.1007/s11356-021-14883-6. [DOI] [PubMed] [Google Scholar]
- 112.Cai X, Dong J, Liu J, Zheng H, Kaweeteerawat C, Wang F, et al. Multi-hierarchical profiling the structure-activity relationships of engineered nanomaterials at nano-bio interfaces. Nat Commun. 2018 doi: 10.1038/S41467-018-06869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosato A, Barone M, Negroni A, Brigidi P, Fava F, Xu P, et al. Microbial colonization of different microplastic types and biotransformation of sorbed PCBs by a marine anaerobic bacterial community. Sci Total Environ. 2020;705:135790. doi: 10.1016/j.scitotenv.2019.135790. [DOI] [PubMed] [Google Scholar]
- 114.Cai X, Liu X, Jiang J, Gao M, Wang W, Zheng H, et al. Molecular mechanisms, characterization methods, and utilities of nanoparticle biotransformation in nanosafety assessments. Small. 2020;16(36):e1907663. doi: 10.1002/smll.201907663. [DOI] [PubMed] [Google Scholar]
- 115.Ramsperger A, Narayana VKB, Gross W, Mohanraj J, Thelakkat M, Greiner A, et al. Environmental exposure enhances the internalization of microplastic particles into cells. Sci Adv. 2020;6(50):9. doi: 10.1126/sciadv.abd1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fadare OO, Wan B, Guo L-H, Xin Y, Qin W, Yang Y. Humic acid alleviates the toxicity of polystyrene nanoplastic particles to Daphnia magna. Environ Sci-Nano. 2019;6(5):1466–1477. doi: 10.1039/c8en01457d. [DOI] [Google Scholar]
- 117.Dey TK, Uddin ME, Jamal M. Detection and removal of microplastics in wastewater: evolution and impact. Environ Sci Pollut R. 2021;28(14):16925–16947. doi: 10.1007/s11356-021-12943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galloway TS, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol. 2017;1(5):0116. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- 119.Cao Y, Zhao M, Ma X, Song Y, Zuo S, Li H, et al. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci Total Environ. 2021;788:147620. doi: 10.1016/J.SCITOTENV.2021.147620. [DOI] [PubMed] [Google Scholar]
- 120.Pulido-Reyes G, Leganes F, Fernandez-Pinas F, Rosal R. Bio-nano interface and environment: A critical review. Environ Toxicol Chem. 2017;36(12):3181–3193. doi: 10.1002/etc.3924. [DOI] [PubMed] [Google Scholar]
- 121.Mudunkotuwa IA, Grassian VH. Biological and environmental media control oxide nanoparticle surface composition: the roles of biological components (proteins and amino acids), inorganic oxyanions and humic acid. Environ Sci-Nano. 2015;2(5):429–439. doi: 10.1039/c4en00215f. [DOI] [Google Scholar]
- 122.Rios LM, Moore C, Jones PR. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar Pollut Bull. 2007;54(8):1230–1237. doi: 10.1016/j.marpolbul.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 123.Junaid M, Wang J. Interaction of micro(nano)plastics with extracellular and intracellular biomolecules in the freshwater environment. Crit Rev Environ Sci Technol. 2021 doi: 10.1080/10643389.2021.2002078. [DOI] [Google Scholar]
- 124.Scopetani C, Cincinelli A, Martellini T, Lombardini E, Ciofini A, Fortunati A, et al. Ingested microplastic as a two-way transporter for PBDEs in Talitrus saltator. Environ Res. 2018;167:411–417. doi: 10.1016/J.ENVRES.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 125.Holmes LA, Turner A, Thompson RC. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar Chem. 2014;167:25–32. doi: 10.1016/J.MARCHEM.2014.06.001. [DOI] [Google Scholar]
- 126.Mao R, Lang M, Yu X, Wu R, Yang X, Guo X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J Hazard Mater. 2020;393:122515. doi: 10.1016/j.jhazmat.2020.122515. [DOI] [PubMed] [Google Scholar]
- 127.Zhou HX, Pang XD. Electrostatic interactions in protein structure, folding, binding, and condensation. Chem Rev. 2018;118(4):1691–1741. doi: 10.1021/acs.chemrev.7b00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rochman CM, Hentschel BT, The SJ. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE. 2014;9:e85433. doi: 10.1371/JOURNAL.PONE.0085433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qi K, Lu N, Zhang S, Wang W, Wang Z, Guan J. Uptake of Pb(II) onto microplastic-associated biofilms in freshwater: Adsorption and combined toxicity in comparison to natural solid substrates. J Hazard Mater. 2021 doi: 10.1016/J.JHAZMAT.2021.125115. [DOI] [PubMed] [Google Scholar]
- 130.Ma Y, Svard M, Xiao X, Gardner JM, Olsson RT, Forsberg K. Precipitation and crystallization used in the production of metal salts for Li-Ion battery materials: A review. Metals. 2020;10(12):1609. doi: 10.3390/met10121609. [DOI] [Google Scholar]
- 131.Khan FR, Syberg K, Shashoua Y, Bury NR. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio) Environ Pollut. 2015;206:73–79. doi: 10.1016/j.envpol.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 132.Abbasi S, Moore F, Keshavarzi B, Hopke PK, Naidu R, Rahman MM, et al. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci Total Environ. 2020;744:140984. doi: 10.1016/j.scitotenv.2020.140984. [DOI] [PubMed] [Google Scholar]
- 133.Antao Barboza LG, Vieira LR, Branco V, Figueiredo N, Carvalho F, Carvalho C, et al. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758) Aquat Toxicol. 2018;195:49–57. doi: 10.1016/j.aquatox.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 134.Ramirez L, Gentile SR, Zimmermann S, Stoll S. Behavior of TiO2 and CeO2 nanoparticles and polystyrene nanoplastics in bottled mineral drinking and lake geneva waters. Impact Water Hardness Nat Organic Matter Nanopart Surf Properties Aggregation Water. 2019 doi: 10.3390/W11040721. [DOI] [Google Scholar]
- 135.Abe T, Kobayashi S, Kobayashi M. Aggregation of colloidal silica particles in the presence of fulvic acid, humic acid, or alginate: Effects of ionic composition. Colloid Surface A. 2011;379(1–3):21–26. doi: 10.1016/j.colsurfa.2010.11.052. [DOI] [Google Scholar]
- 136.Wieczorek AM, Croot PL, Lombard F, Sheahan JN, Doyle TK. Microplastic Ingestion by gelatinous zooplankton may lower efficiency of the biological pump. Environ Sci Technol. 2019;53(9):5387–5395. doi: 10.1021/acs.est.8b07174. [DOI] [PubMed] [Google Scholar]
- 137.Wang SX, Hu MH, Zheng JH, Huang W, Shang YY, Fang JKH, et al. Ingestion of nano/micro plastic particles by the mussel Mytilus coruscus is size dependent. Chemosphere. 2021;263:127957. doi: 10.1016/j.chemosphere.2020.127957. [DOI] [PubMed] [Google Scholar]
- 138.Zha SJ, Rong JH, Guan XF, Tang Y, Han Y, Liu GX. Immunotoxicity of four nanoparticles to a marine bivalve species. Tegillarca granosa J Hazard Mater. 2019;377:237–248. doi: 10.1016/j.jhazmat.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 139.Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, et al. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar Chem. 2015;175:39–46. doi: 10.1016/j.marchem.2015.04.003. [DOI] [Google Scholar]
- 140.Pestana CJ, Moura DS, Capelo-Neto J, Edwards C, Dreisbach D, Spengler B, et al. Potentially poisonous plastic particles: microplastics as a vector for cyanobacterial toxins microcystin-LR and Microcystin-LF. Environ Sci Technol. 2021;55:15940–15949. doi: 10.1021/ACS.EST.1C05796. [DOI] [PubMed] [Google Scholar]
- 141.Lin W, Su F, Lin M, Jin M, Li Y, Ding K, et al. Effect of microplastics PAN polymer and/or Cu2+ pollution on the growth of Chlorella pyrenoidosa. Environ Pollut. 2020;265:114985. doi: 10.1016/J.ENVPOL.2020.114985. [DOI] [PubMed] [Google Scholar]
- 142.Fu D, Zhang Q, Fan Z, Qi H, Wang Z, Peng L. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat Toxicol. 2019;216:105319. doi: 10.1016/J.AQUATOX.2019.105319. [DOI] [PubMed] [Google Scholar]
- 143.Tunali M, Uzoefuna E, Tunali M, Yenigun O. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci Total Environ. 2020;743:140479. doi: 10.1016/j.scitotenv.2020.140479. [DOI] [PubMed] [Google Scholar]
- 144.Wang Z, Dong H, Wang Y, Ren R, Qin X, Wang S. Effects of microplastics and their adsorption of cadmium as vectors on the cladoceran Moina monogolica daday: Implications for plastic-ingesting organisms. J Hazard Mater. 2020;400:123239. doi: 10.1016/J.JHAZMAT.2020.123239. [DOI] [PubMed] [Google Scholar]
- 145.Fernández B, Santos-Echeandía J, Rivera-Hernández JR, Garrido S, Albentosa M. Mercury interactions with algal and plastic microparticles: Comparative role as vectors of metals for the mussel. Mytilus galloprovincialis J Hazard Mater. 2020;396:122739. doi: 10.1016/J.JHAZMAT.2020.122739. [DOI] [PubMed] [Google Scholar]
- 146.Zhang R, Wang M, Chen X, Yang C, Wu L. Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio) Sci Total Environ. 2020;743:140638. doi: 10.1016/J.SCITOTENV.2020.140638. [DOI] [PubMed] [Google Scholar]
- 147.Kulbacka J, Choromanska A, Rossowska J, Wezgowiec J, Saczko J, Rols MP. Cell membrane transport mechanisms: Ion channels and electrical properties of cell membranes. Adv Anat Embryol Cell Biol. 2017;227:39–58. doi: 10.1007/978-3-319-56895-9_3. [DOI] [PubMed] [Google Scholar]
- 148.Maity S, Biswas C, Banerjee S, Guchhait R, Adhikari M, Chatterjee A, et al. Interaction of plastic particles with heavy metals and the resulting toxicological impacts: a review. Environ Sci Pollut R. 2021;28(43):60291–60307. doi: 10.1007/s11356-021-16448-z. [DOI] [PubMed] [Google Scholar]
- 149.Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Sci Total Environ. 2020 doi: 10.1016/J.SCITOTENV.2019.134455. [DOI] [PubMed] [Google Scholar]
- 150.Yang H, Kim H, Tong M. Influence of humic acid on the transport behavior of bacteria in quartz sand. Coll Surf B-Biointerface. 2012;91:122–129. doi: 10.1016/j.colsurfb.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H, Wang J, Zhou B, Zhou Y, Dai Z, Zhou Q, et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ Pollut. 2018;243:1550–1557. doi: 10.1016/J.ENVPOL.2018.09.122. [DOI] [PubMed] [Google Scholar]
- 152.Li Y, Wang X, Fu W, Xia X, Liu C, Min J, et al. Interactions between nano/micro plastics and suspended sediment in water: Implications on aggregation and settling. Water Res. 2019;161:486–495. doi: 10.1016/j.watres.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 153.Hakim A, Kobayashi M. Aggregation and aggregate strength of microscale plastic particles in the presence of natural organic matter: Effects of ionic valence. J Polym Environ. 2021;29(6):1921–1929. doi: 10.1007/s10924-020-01985-4. [DOI] [Google Scholar]
- 154.Li M, Zhang X, Yi K, He L, Han P, Tong M. Transport and deposition of microplastic particles in saturated porous media: Co-effects of clay particles and natural organic matter. Environ Pollut. 2021 doi: 10.1016/j.envpol.2021.117585. [DOI] [PubMed] [Google Scholar]
- 155.Chen G, Liu X, Su C. Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ Sci Technol. 2012;46(13):7142–7150. doi: 10.1021/es204010g. [DOI] [PubMed] [Google Scholar]
- 156.Saavedra J, Stoll S, Slaveykova VI. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton. Environ Pollut. 2019;252:715–722. doi: 10.1016/j.envpol.2019.05.135. [DOI] [PubMed] [Google Scholar]
- 157.Wu X, Liu P, Gong Z, Wang H, Huang H, Shi Y, et al. Humic acid and Fulvic acid hinder long-term weathering of microplastics in lake water. Environ Sci Technol. 2021;55:15810–15820. doi: 10.1021/ACS.EST.1C04501. [DOI] [PubMed] [Google Scholar]
- 158.Rong H, Li M, He L, Zhang M, Hsieh L, Wang S, et al. Transport and deposition behaviors of microplastics in porous media: Co-impacts of N fertilizers and humic acid. J Hazard Mater. 2021 doi: 10.1016/J.JHAZMAT.2021.127787. [DOI] [PubMed] [Google Scholar]
- 159.Dong Z, Zhu L, Zhang W, Huang R, Lv XW, Jing X, et al. Role of surface functionalities of nanoplastics on their transport in seawater-saturated sea sand. Environ Pollut. 2019 doi: 10.1016/J.ENVPOL.2019.113177. [DOI] [PubMed] [Google Scholar]
- 160.Tan M, Liu L, Zhang M, Liu Y, Li C. Effects of solution chemistry and humic acid on the transport of polystyrene microplastics in manganese oxides coated sand. J Hazard Mater. 2021 doi: 10.1016/J.JHAZMAT.2021.125410. [DOI] [PubMed] [Google Scholar]
- 161.Wu J, Jiang R, Liu Q, Ouyang G. Impact of different modes of adsorption of natural organic matter on the environmental fate of nanoplastics. Chemosphere. 2021 doi: 10.1016/J.CHEMOSPHERE.2020.127967. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Wang Z, Wang S, Fang H, Ye N, Wang D. Ecotoxicological effects on Scenedesmus obliquus and Danio rerio Co-exposed to polystyrene nano-plastic particles and natural acidic organic polymer. Environ Toxicol Pharmacol. 2019;67:21–28. doi: 10.1016/J.ETAP.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 163.Fadare OO, Wan B, Liu K, Yang Y, Zhao L, Guo LH. Eco-Corona vs protein Corona: Effects of humic substances on corona formation and nanoplastic particle toxicity in daphnia magna. Environ Sci Technol. 2020;54:8001–8009. doi: 10.1021/acs.est.0c00615. [DOI] [PubMed] [Google Scholar]
- 164.Brandts I, Balasch JC, Gonçalves AP, Martins MA, Pereira ML, Tvarijonaviciute A, et al. Immuno-modulatory effects of nanoplastics and humic acids in the European seabass (Dicentrarchus labrax) J Hazard Mater. 2021 doi: 10.1016/J.JHAZMAT.2021.125562. [DOI] [PubMed] [Google Scholar]
- 165.Wu J, Jiang R, Lin W, Ouyang G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ Pollut. 2019;245:836–843. doi: 10.1016/J.ENVPOL.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 166.Ashraf MA. Persistent organic pollutants (POPs): A global issue, a global challenge. Environ Sci Pollut Res Int. 2017;24(5):4223–4227. doi: 10.1007/s11356-015-5225-9. [DOI] [PubMed] [Google Scholar]
- 167.Wang J, Coffin S, Schlenk D, Gan J. Accumulation of HOCs via precontaminated microplastics by earthworm eisenia fetida in soil. Environ Sci Technol. 2020;54:11220–11229. doi: 10.1021/ACS.EST.0C02922. [DOI] [PubMed] [Google Scholar]
- 168.Tan XL, Yu XB, Cai LQ, Wang JD, Peng JP. Microplastics and associated PAHs in surface water from the Feilaixia reservoir in the Beijiang river. China Chemosphere. 2019;221:834–840. doi: 10.1016/j.chemosphere.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 169.Devriese LI, De Witte B, Vethaak AD, Hostens K, Leslie HA. Bioaccumulation of PCBs from microplastics in Norway lobster (Nephrops norvegicus): An experimental study. Chemosphere. 2017;186:10–16. doi: 10.1016/j.chemosphere.2017.07.121. [DOI] [PubMed] [Google Scholar]
- 170.Liu SQ, Fang ST, Xiang ZM, Chen XT, Song YM, Chen C, et al. Combined effect of microplastics and DDT on microbial growth: A bacteriological and metabolomics investigation in Escherichia coli. J Hazard Mater. 2021;407:124849. doi: 10.1016/j.jhazmat.2020.124849. [DOI] [PubMed] [Google Scholar]
- 171.Joo SH, Liang Y, Kim M, Byun J, Choi H. Microplastics with adsorbed contaminants: Mechanisms and treatment. Environmental Challenges. 2021 doi: 10.1016/j.envc.2021.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Re V. Shedding light on the invisible: addressing the potential for groundwater contamination by plastic microfibers. Hydrogeol J. 2019;27:2719–2727. doi: 10.1007/S10040-019-01998-X. [DOI] [Google Scholar]
- 173.Gouin T, Roche N, Lohmann R, Hodges G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ Sci Technol. 2011;45:1466–1472. doi: 10.1021/ES1032025. [DOI] [PubMed] [Google Scholar]
- 174.Sleight VA, Bakir A, Thompson RC, Henry TB. Assessment of microplastic-sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar Pollut Bull. 2017;116:291–297. doi: 10.1016/J.MARPOLBUL.2016.12.055. [DOI] [PubMed] [Google Scholar]
- 175.Cormier B, Borchet F, Kärrman A, Szot M, Yeung LWY, Keiter SH. Sorption and desorption kinetics of PFOS to pristine microplastic. Environ Sci Pollut R. 2021 doi: 10.1007/S11356-021-15923-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma J, Zhao J, Zhu Z, Li L, Yu F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ Pollut. 2019 doi: 10.1016/J.ENVPOL.2019.113104. [DOI] [PubMed] [Google Scholar]
- 177.Yeo BG, Takada H, Yamashita R, Okazaki Y, Uchida K, Tokai T, et al. PCBs and PBDEs in microplastic particles and zooplankton in open water in the Pacific Ocean and around the coast of Japan. Marine Pollut Bull. 2020 doi: 10.1016/J.MARPOLBUL.2019.110806. [DOI] [PubMed] [Google Scholar]
- 178.Sørensen L, Rogers E, Altin D, Salaberria I, Booth AM. Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ Pollut. 2020 doi: 10.1016/J.ENVPOL.2019.113844. [DOI] [PubMed] [Google Scholar]
- 179.Wang L, Yang Z, Niu J. Temperature-dependent sorption of polycyclic aromatic hydrocarbons on natural and treated sediments. Chemosphere. 2011;82(6):895–900. doi: 10.1016/j.chemosphere.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 180.Zhang D, Liu X, Huang W, Li J, Wang C, Zhang D, et al. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ Pollut. 2020 doi: 10.1016/J.ENVPOL.2020.113948. [DOI] [PubMed] [Google Scholar]
- 181.de Lucia GA, Vianello A, Camedda A, Vani D, Tomassetti P, Coppa S, et al. Sea water contamination in the Vicinity of the Italian minor islands caused by microplastic pollution. Water (Switzerland). 2018 doi: 10.3390/W10081108. [DOI] [Google Scholar]
- 182.Besseling E, Wegner A, Foekema EM, Van Den Heuvel-Greve MJ, Koelmans AA. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.) Environ Sci Technol. 2013;47(1):593–600. doi: 10.1021/ES302763X. [DOI] [PubMed] [Google Scholar]
- 183.Gerdes Z, Ogonowski M, Nybom I, Ek C, Adolfsson-Erici M, Barth A, et al. Microplastic-mediated transport of PCBs? A depuration study with Daphnia magna. PLoS ONE. 2019 doi: 10.1371/JOURNAL.PONE.0205378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grigorakis S, Drouillard KG. Effect of microplastic amendment to food on diet assimilation efficiencies of PCBs by fish. Environ Sci Technol. 2018;52:10796–10802. doi: 10.1021/ACS.EST.8B02497. [DOI] [PubMed] [Google Scholar]
- 185.Batel A, Borchert F, Reinwald H, Erdinger L, Braunbeck T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ Pollut. 2018;235:918–930. doi: 10.1016/J.ENVPOL.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 186.Heinrich P, Braunbeck T. Microplastic particles reduce EROD-induction specifically by highly lipophilic compounds in RTL-W1 cells. Ecotox Environ Safe. 2020 doi: 10.1016/J.ECOENV.2019.110041. [DOI] [PubMed] [Google Scholar]
- 187.Jeong CB, Kang HM, Lee YH, Kim MS, Lee JS, Seo JS, et al. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer brachionus koreanus via multixenobiotic resistance (MXR) disruption. Environ Sci Technol. 2018;52:11411–11418. doi: 10.1021/ACS.EST.8B03211. [DOI] [PubMed] [Google Scholar]
- 188.Francia V, Yang K, Deville S, Reker-Smit C, Nelissen I, Salvati A. Corona Composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano. 2019;13(10):11107–11121. doi: 10.1021/acsnano.9b03824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lin CAJ, Chuang WK, Huang ZY, Kang ST, Chang CY, Chen CT, et al. Rapid transformation of protein-caged nanomaterials into microbubbles as bimodal imaging agents. ACS Nano. 2012;6(6):5111–5121. doi: 10.1021/nn300768d. [DOI] [PubMed] [Google Scholar]
- 190.Nagy A, Robbins NL. The hurdles of nanotoxicity in transplant nanomedicine. Nanomedicine-Uk. 2019;14(20):2749–2762. doi: 10.2217/nnm-2019-0192. [DOI] [PubMed] [Google Scholar]
- 191.Canesi L, Ciacci C, Fabbri R, Balbi T, Salis A, Damonte G, et al. Interactions of cationic polystyrene nanoparticles with marine bivalve hemocytes in a physiological environment: Role of soluble hemolymph proteins. Environ Res. 2016;150:73–81. doi: 10.1016/j.envres.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 192.Grassi G, Gabellieri E, Cioni P, Paccagnini E, Faleri C, Lupetti P, et al. Interplay between extracellular polymeric substances (EPS) from a marine diatom and model nanoplastic through eco-corona formation. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138457. [DOI] [PubMed] [Google Scholar]
- 193.Poh TY, Ali NABM, Mac Aogain M, Kathawala MH, Setyawati MI, Ng KW, et al. Inhaled nanomaterials and the respiratory microbiome: clinical, immunological and toxicological perspectives. Part Fibre Toxicol. 2018;15:46. doi: 10.1186/s12989-018-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ji YX, Wang YQ, Shen DH, Kang Q, Chen LX. Mucin corona delays intracellular trafficking and alleviates cytotoxicity of nanoplastic-benzopyrene combined contaminant. J Hazard Mater. 2021;406:124306. doi: 10.1016/j.jhazmat.2020.124306. [DOI] [PubMed] [Google Scholar]
- 195.Li L, Xu Y, Li S, Zhang X, Feng H, Dai Y, et al. Molecular modeling of nanoplastic transformations in alveolar fluid and impacts on the lung surfactant film. J Hazard Mater. 2022;427:127872. doi: 10.1016/j.jhazmat.2021.127872. [DOI] [PubMed] [Google Scholar]
- 196.Saygin H, Baysal A. Interaction of amino acids with nanoplastic traces and their effect on staphylococcus aureus. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2021;56(11):1253–1263. doi: 10.1080/10934529.2021.1980308. [DOI] [PubMed] [Google Scholar]
- 197.Soares MO, Matos E, Lucas C, Rizzo L, Allcock L, Rossi S. Microplastics in corals: An emergent threat. Mar Pollut Bull. 2020;161(Pt A):111810. doi: 10.1016/j.marpolbul.2020.111810. [DOI] [PubMed] [Google Scholar]
- 198.Radisic V, Nimje PS, Bienfait AM, Marathe NP. Marine plastics from norwegian west coast carry potentially virulent fish pathogens and opportunistic human pathogens harboring new variants of antibiotic resistance genes. Microorganisms. 2020;8(8):1200. doi: 10.3390/microorganisms8081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang J, Qin X, Guo JB, Jia WQ, Wang Q, Zhang MJ, et al. Evidence of selective enrichment of bacterial assemblages and antibiotic resistant genes by microplastics in urban rivers. Water Res. 2020;183:116113. doi: 10.1016/j.watres.2020.116113. [DOI] [PubMed] [Google Scholar]
- 200.Wang S, Xue N, Li W, Zhang D, Pan X, Luo Y. Selectively enrichment of antibiotics and ARGs by microplastics in river, estuary and marine waters. Sci Total Environ. 2020;708:134594. doi: 10.1016/j.scitotenv.2019.134594. [DOI] [PubMed] [Google Scholar]
- 201.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tayebati SK. Phospholipid and lipid derivatives as potential neuroprotective compounds. Molecules. 2018;23(9):2257. doi: 10.3390/molecules23092257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu SJ, Zheng HZ, Ma RL, Wu D, Pan YX, Yin CY, et al. Vacancies on 2D transition metal dichalcogenides elicit ferroptotic cell death. Nat Commun. 2020;11(1):3484. doi: 10.1038/s41467-020-17300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zheng HZ, Jiang J, Xu SJ, Liu W, Xie QQ, Cai XM, et al. Nanoparticle-induced ferroptosis: detection methods, mechanisms and applications. Nanoscale. 2021;13(4):2266–2285. doi: 10.1039/d0nr08478f. [DOI] [PubMed] [Google Scholar]
- 205.Yagi K. Lipid peroxides in hepatic, gastrointestinal, and pancreatic diseases. Adv Exp Med Biol. 1994;366:165–169. doi: 10.1007/978-1-4615-1833-4_12. [DOI] [PubMed] [Google Scholar]
- 206.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296(6):E1183–1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang X, Xia M, Zhao J, Cao Z, Zou W, Zhou Q. Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ Int. 2022;158:106922. doi: 10.1016/j.envint.2021.106922. [DOI] [PubMed] [Google Scholar]
- 208.Tan H, Yue T, Xu Y, Zhao J, Xing B. Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ Sci Technol. 2020;54(19):12285–12294. doi: 10.1021/acs.est.0c02608. [DOI] [PubMed] [Google Scholar]
- 209.Theodorou IG, Ruenraroengsak P, Gow A, Schwander S, Zhang JJ, Chung KF, et al. Effect of pulmonary surfactant on the dissolution, stability and uptake of zinc oxide nanowires by human respiratory epithelial cells. Nanotoxicology. 2016;10:1351–1362. doi: 10.1080/17435390.2016.1214762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sanchez-Valdes S, Munoz-Jimenez L, Francisco Ramos-deValle L, Vanesa Sanchez-Martinez Z, Flores-Gallardo S, Rene Ramirez-Vargas R, et al. Antibacterial silver nanoparticle coating on oxo-biodegradable polyethylene film surface using modified polyethylene and corona discharge. Polym Bull. 2018;75(9):3987–4002. doi: 10.1007/s00289-017-2247-0. [DOI] [Google Scholar]
- 211.Jin N, Song Y, Ma R, Li J, Li G, Zhang D. Characterization and identification of microplastics using Raman spectroscopy coupled with multivariate analysis. Anal Chim Acta. 2022 doi: 10.1016/j.aca.2022.339519. [DOI] [PubMed] [Google Scholar]
- 212.Eibes PM, Gabel F. Floating microplastic debris in a rural river in Germany: Distribution, types and potential sources and sinks. Sci Total Environ. 2022;816(1):151641–151641. doi: 10.1016/j.scitotenv.2021.151641. [DOI] [PubMed] [Google Scholar]
- 213.Zafar R, Park SY, Kim CG. Surface modification of polyethylene microplastic particles during the aqueous-phase ozonation process. Environ Eng Res. 2021;26(5):200412. doi: 10.4491/eer.2020.412. [DOI] [Google Scholar]
- 214.Grigorakis S, Drouillard KG. Effect of microplastic amendment to food on diet assimilation efficiencies of PCBs by fish. Environ Sci Technol. 2018;52(18):10796–10802. doi: 10.1021/acs.est.8b02497. [DOI] [PubMed] [Google Scholar]
- 215.Ramsperger AFRM, Jasinski J, Volkl M, Witzmann T, Meinhart M, Jerome V, et al. Supposedly identical microplastic particles substantially differ in their material properties influencing particle-cell interactions and cellular responses. J Hazard Mater. 2022 doi: 10.1016/j.jhazmat.2021.127961. [DOI] [PubMed] [Google Scholar]
- 216.Kundu A, Shetti NP, Basu S, Reddy KR, Nadagouda MN, Aminabhavi TM. Identification and removal of micro- and nano-plastics: Efficient and cost-effective methods. Chem Eng J. 2021 doi: 10.1016/j.cej.2021.129816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kabir A, Sekine M, Imai T, Yamamoto K, Kanno A, Higuchi T. Microplastics in the sediments of small-scale Japanese rivers: Abundance and distribution, characterization, sources-to-sink, and ecological risks. Sci Total Environ. 2022;812:15. doi: 10.1016/j.scitotenv.2021.152590. [DOI] [PubMed] [Google Scholar]
- 218.Li P, Li QC, Hao ZN, Yu SJ, Liu JF. Analytical methods and environmental processes of nanoplastics. J Environ Sci. 2020;94:88–99. doi: 10.1016/j.jes.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 219.Majedi SM, Lee HK. Recent advances in the separation and quantification of metallic nanoparticles and ions in the environment. Trac-Trend Anal Chem. 2016;75:183–196. doi: 10.1016/j.trac.2015.08.009. [DOI] [Google Scholar]
- 220.Pandiyan R, Ayyaru S, Ahn YH. Non-toxic properties of TiO2 and STiO2 nanocomposite PES ultrafiltration membranes for application in membrane-based environmental biotechnology. Ecotox Environ Safe. 2018;158:248–255. doi: 10.1016/j.ecoenv.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 221.Nazar MF, Shah SS, Eastoe J, Khan AM, Shah A. Separation and recycling of nanoparticles using cloud point extraction with non-ionic surfactant mixtures. J Colloid Interf Sci. 2011;363(2):490–496. doi: 10.1016/j.jcis.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 222.von der Kammer F, Legros S, Larsen EH, Loeschner K, Hofmann T. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. Trac-Trend Anal Chem. 2011;30(3):425–436. doi: 10.1016/j.trac.2010.11.012. [DOI] [Google Scholar]
- 223.Svedberg T, Rinde H. The ultra-centrifuge, a new instrument for the determination of size and distribution of size of particle in amicroscopic colloids. J Am Chem Soc. 2002;46:2677–2693. doi: 10.1021/JA01677A011. [DOI] [Google Scholar]
- 224.Cai HW, Chen MD, Du FN, Matthews S, Shi HH. Separation and enrichment of nanoplastics in environmental water samples via ultracentrifugation. Water Res. 2021;203:117509. doi: 10.1016/j.watres.2021.117509. [DOI] [PubMed] [Google Scholar]
- 225.von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, et al. Analysis of engineered nanomaterials in complex matrices (environment and biota): General considerations and conceptual case studies. Environ Toxicol Chem. 2012;31(1):32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- 226.Schwaferts C, Niessner R, Elsner M, Ivleva NP. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. Trac-Trend Anal Chem. 2019;112:52–65. doi: 10.1016/j.trac.2018.12.014. [DOI] [Google Scholar]
- 227.Prata JC, da Costa JP, Girao AV, Lopes I, Duarte AC, Rocha-Santos T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci Total Environ. 2019;686:131–139. doi: 10.1016/j.scitotenv.2019.05.456. [DOI] [PubMed] [Google Scholar]
- 228.Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci Rep-Uk. 2014;4:4528. doi: 10.1038/srep04528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Da Costa Filho PA, Andrey D, Eriksen B, Peixoto RP, Carreres BM, Ambuhl ME, et al. Detection and characterization of small-sized microplastics (>/= 5 microm) in milk products. Sci Rep. 2021;11(1):24046. doi: 10.1038/s41598-021-03458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Löder MGJ, Gerdts G. Methodology used for the detection and identification of microplastics—a critical appraisal. Marine Anthropogenic Litter. 2015: 10.1007/978-3-319-16510-3_8.
- 231.Mato T, Noguchi S. Microplastic collection with ultra-high magnetic field magnet by magnetic separation. Ieee Trans Appl Superconduct. 2022;32(4):1. doi: 10.1109/tasc.2021.3135796. [DOI] [Google Scholar]
- 232.Tirkey A, Upadhyay LSB. Microplastics: An overview on separation, identification and characterization of microplastics. Marine Pollut Bull. 2021 doi: 10.1016/j.marpolbul.2021.112604. [DOI] [PubMed] [Google Scholar]
- 233.Vega GC, Gross A, Birkved M. The impacts of plastic products on air pollution - A simulation study for advanced life cycle inventories of plastics covering secondary microplastic production. Sustain Product Consumption. 2021;28:848–865. doi: 10.1016/j.spc.2021.07.008. [DOI] [Google Scholar]
- 234.Hurley RR, Lusher AL, Olsen M, Nizzetto L. Validation of a method for extracting microplastics from complex, organic-rich. Environ Matrices Environ Sci Technol. 2018;52(13):7409–7417. doi: 10.1021/acs.est.8b01517. [DOI] [PubMed] [Google Scholar]
- 235.Pradhan S, Zhang J, Qu JG, Yun X. Characterization of geological certified reference materials by inductively coupled plasma-mass spectrometry. Anal Lett. 2015;48(13):2136–2158. doi: 10.1080/00032719.2015.1010123. [DOI] [Google Scholar]
- 236.Caldwell J, Taladriz-Blanco P, Lehner R, Lubskyy A, Ortuso RD, Rothen-Rutishauser B, et al. The micro-, submicron-, and nanoplastic hunt: A review of detection methods for plastic particles. Chemosphere. 2022;293:133514. doi: 10.1016/j.chemosphere.2022.133514. [DOI] [PubMed] [Google Scholar]
- 237.Patel K,Panchal N,Ingle P. Review of extraction techniques extraction methods: microwave ultrasonic pressurized fluid soxhlet extraction etc. Int J Adv Res Chem Sci 2019;6(3). 10.20431/2349-0403.0603002.
- 238.Ulmer CZ, Jones CM, Yost RA, Garrett TJ, Bowden JA. Optimization of folch, bligh-dyer, and matyash sample-to-extraction solvent ratios for human plasma-based lipidomics studies. Anal Chim Acta. 2018;1037:351–357. doi: 10.1016/j.aca.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cheok CY, Chin NL, Yusof YA, Talib RA, Law CL. Anthocyanin recovery from mangosteen (Garcinia mangostana L.) hull using lime juice acidified aqueous methanol solvent extraction. Food Sci Technol Res. 2013;19(6):971–978. doi: 10.3136/fstr.19.971. [DOI] [Google Scholar]
- 240.Tan SC, Yiap BC. DNA, RNA, and protein extraction: The past and the present. J Biomed Biotechnol. 2009;2009:574398. doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bonjoch NP, Tamayo PR. Protein content quantification by bradford method. handbook of plant ecophysiology techniques. 2003:283–295. 10.1007/0-306-48057-3_19.
- 242.Reichelt WN, Waldschitz D, Herwig C, Neutsch L. Bioprocess monitoring: minimizing sample matrix effects for total protein quantification with bicinchoninic acid assay. J Ind Microbiol Biot. 2016;43(9):1271–1280. doi: 10.1007/s10295-016-1796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pena-Pereira F, Costas-Mora I, Romero V, Lavilla I, Bendicho C. Advances in miniaturized UV-Vis spectrometric systems. Trac-Trend Anal Chem. 2011;30(10):1637–1648. doi: 10.1016/j.trac.2011.04.018. [DOI] [Google Scholar]
- 244.Mahmood T, Yang PC. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012;4(9):429–434. doi: 10.4103/1947-2714.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhu Z, Lu JJ, Liu S. Protein separation by capillary gel electrophoresis: a review. Anal Chim Acta. 2012;709:21–31. doi: 10.1016/j.aca.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hong P, Koza S, Bouvier ES. Size-exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. J Liq Chromatogr Relat Technol. 2012;35(20):2923–2950. doi: 10.1080/10826076.2012.743724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Raesch SS, Tenzer S, Storck W, Rurainski A, Selzer D, Ruge CA, et al. Proteomic and lipidomic analysis of nanoparticle corona upon contact with lung surfactant reveals differences in protein, but not lipid composition. ACS Nano. 2015;9(12):11872–11885. doi: 10.1021/acsnano.5b04215. [DOI] [PubMed] [Google Scholar]
- 248.Mohammad-Beigi H, Hayashi Y, Zeuthen CM, Eskandari H, Scavenius C, Juul-Madsen K, et al. Mapping and identification of soft corona proteins at nanoparticles and their impact on cellular association. Nat Commun. 2020;11(1):4535. doi: 10.1038/s41467-020-18237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Docter D, Distler U, Storck W, Kuharev J, Wunsch D, Hahlbrock A, et al. Quantitative profiling of the protein coronas that form around nanoparticles. Nat Protoc. 2014;9(9):2030–2044. doi: 10.1038/nprot.2014.139. [DOI] [PubMed] [Google Scholar]
- 250.Wang Y, Ni Y. Combination of UV-vis spectroscopy and chemometrics to understand protein-nanomaterial conjugate: a case study on human serum albumin and gold nanoparticles. Talanta. 2014;119:320–330. doi: 10.1016/j.talanta.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 251.Wang Y, Ni Y. New insight into protein-nanomaterial interactions with UV-visible spectroscopy and chemometrics: Human serum albumin and silver nanoparticles. Analyst. 2014;139(2):416–424. doi: 10.1039/c3an01818k. [DOI] [PubMed] [Google Scholar]
- 252.Kihara S, Ghosh S, McDougall DR, Whitten AE, Mata JP, Koper I, et al. Structure of soft and hard protein corona around polystyrene nanoplastics-Particle size and protein types. Biointerphases. 2020;15(5):051002. doi: 10.1116/6.0000404. [DOI] [PubMed] [Google Scholar]
- 253.Morgana S, Casentini B, Amalfitano S. Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J Hazard Mater. 2021;419:126507. doi: 10.1016/j.jhazmat.2021.126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gopinath PM, Saranya V, Vijayakumar S, Mythili Meera M, Ruprekha S, Kunal R, et al. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci Rep. 2019;9(1):8860. doi: 10.1038/s41598-019-45139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dey TK, Uddin ME, Jamal M. Detection and removal of microplastics in wastewater: evolution and impact. Environ Sci Pollut Res Int. 2021;28(14):16925–16947. doi: 10.1007/s11356-021-12943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang P, Wang Y, Zhao X, Ji Y, Mei R, Fu L, et al. Surface-enhanced Raman scattering labeled nanoplastic models for reliable bio-nano interaction investigations. J Hazard Mater. 2022;425:127959. doi: 10.1016/j.jhazmat.2021.127959. [DOI] [PubMed] [Google Scholar]
- 257.Mariano S, Tacconi S, Fidaleo M, Rossi M, Dini L. Micro and nanoplastics identification: classic methods and innovative detection techniques. Front Toxicol. 2021 doi: 10.3389/ftox.2021.636640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fakhrullin R, Nigamatzyanova L, Fakhrullina G. Dark-field/hyperspectral microscopy for detecting nanoscale particles in environmental nanotoxicology research. Sci Total Environ. 2021;772:145478. doi: 10.1016/j.scitotenv.2021.145478. [DOI] [PubMed] [Google Scholar]
- 259.Nigamatzyanova L, Fakhrullin R. Dark-field hyperspectral microscopy for label-free microplastics and nanoplastics detection and identification in vivo: A Caenorhabditis elegans study. Environ Pollut. 2021;271:116337. doi: 10.1016/j.envpol.2020.116337. [DOI] [PubMed] [Google Scholar]
- 260.Akhatova F, Ishmukhametov I, Fakhrullina G, Fakhrullin R. Nanomechanical atomic force microscopy to probe cellular microplastics uptake and distribution. Int J Mol Sci. 2022;23(2):806. doi: 10.3390/ijms23020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ishmukhametov I, Nigamatzyanova L, Fakhrullina G, Fakhrullin R. Label-free identification of microplastics in human cells: dark-field microscopy and deep learning study. Anal Bioanal Chem. 2022;414(3):1297–1312. doi: 10.1007/s00216-021-03749-y. [DOI] [PubMed] [Google Scholar]
- 262.Iakovlev IA, Deviatov AY, Lvov Y, Fakhrullina G, Fakhrullin RF, Mazurenko VV. Probing diffusive dynamics of natural tubule nanoclays with machine learning. ACS Nano. 2022 doi: 10.1021/acsnano.1c11025. [DOI] [PubMed] [Google Scholar]
- 263.Huang Y, Li X, Xu S, Zheng H, Zhang L, Chen J, et al. Quantitative structure-activity relationship models for predicting inflammatory potential of metal oxide nanoparticles. Environ Health Perspect. 2020;128(6):67010. doi: 10.1289/EHP6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang WL, Kong YF, Jiang J, Xie QQ, Huang Y, Li GN, et al. Engineering the protein corona structure on gold nanoclusters enables red-shifted emissions in the second near-infrared window for gastrointestinal imaging. Angew Chem Int Edit. 2020;59(50):22431–22435. doi: 10.1002/anie.202010089. [DOI] [PubMed] [Google Scholar]
- 265.Qiao R, Lu K, Deng Y, Ren H, Zhang Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci Total Environ. 2019;682:128–137. 10.1016/J.SCITOTENV.2019.05.163. [DOI] [PubMed]
- 266.Degueldre C, Favarger PY. Colloid analysis by single particle inductively coupled plasma-mass spectroscopy: A feasibility study. Colloid Surface A. 2003;217(1–3):137–142. doi: 10.1016/S0927-7757(02)00568-X. [DOI] [Google Scholar]
- 267.Bolea-Fernandez E, Rua-Ibarz A, Velimirovic M, Tirez K, Vanhaecke F. Detection of microplastics using inductively coupled plasma-mass spectrometry (ICP-MS) operated in single-event mode. J Anal At Spectrom. 2020;35:455–460. doi: 10.1039/C9JA00379G. [DOI] [Google Scholar]
- 268.Neunzehn J, Draude F, Golla-Schindler U, Arlinghaus HF, Wiesmann H-P. Detection of protein coatings on nanoparticles surfaces by ToF-SIMS and advanced electron microscopy. Surf Interface Anal. 2013;45(9):1340–1346. doi: 10.1002/sia.5287. [DOI] [Google Scholar]
- 269.Jiménez-Skrzypek G, Hernández-Sánchez C, Ortega-Zamora C, González-Sálamo J, González-Curbelo MÁ, Hernández-Borges J. Microplastic-adsorbed organic contaminants: Analytical methods and occurrence. TrAC - Trends Analyt Chem. 2021 doi: 10.1016/J.TRAC.2021.116186. [DOI] [Google Scholar]
- 270.León VM, García I, González E, Samper R, Fernández-González V, Muniategui-Lorenzo S. Potential transfer of organic pollutants from littoral plastics debris to the marine environment. Environ Pollut. 2018;236:442–453. doi: 10.1016/J.ENVPOL.2018.01.114. [DOI] [PubMed] [Google Scholar]
- 271.Chetwynd AJ, Zhang W, Faserl K, Thorn JA, Lynch I, Ramautar R, et al. Capillary electrophoresis mass spectrometry approaches for characterization of the protein and metabolite corona acquired by nanomaterials. J Visual Experiment. 2020 doi: 10.3791/61760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.