Abstract
Background
High bone mass (HBM, BMD Z-score ≥ + 3.2) and cam morphology (bulging of lateral femoral head) are associated with greater odds of prevalent radiographic hip osteoarthritis (rHOA). As cam morphology is itself a manifestation of increased bone deposition around the femoral head, it is conceivable that cam morphology may mediate the relationship between HBM and rHOA. We therefore aimed to determine if individuals with HBM have increased odds of prevalent cam morphology. In addition, we investigated whether the relationship between cam and prevalent and incident osteoarthritis was preserved in a HBM population.
Methods
In the HBM study, a UK based cohort of adults with unexplained HBM and their relatives and spouses (controls), we determined the presence of cam morphology using semi-automatic methods of alpha angle derivation from pelvic radiographs. Associations between HBM status and presence of cam morphology, and between cam morphology and presence of rHOA (or its subphenotypes: osteophytes, joint space narrowing, cysts, and subchondral sclerosis) were determined using multivariable logistic regression, adjusting for age, sex, height, weight, and adolescent physical activity levels. The association between cam at baseline and incidence of rHOA after an average of 8 years was determined. Generalised estimating equations accounted for individual-level clustering.
Results
The study included 352 individuals, of whom 235 (66.7%) were female and 234 (66.5%) had HBM. Included individuals contributed 694 hips, of which 143 had a cam deformity (20.6%). There was no evidence of an association between HBM and cam morphology (OR = 0.97 [95% CI: 0.63–1.51], p = 0.90) but a strong relationship was observed between cam morphology and rHOA (OR = 3.96 [2.63–5.98], p = 5.46 × 10–11) and rHOA subphenotypes joint space narrowing (OR = 3.70 [2.48–5.54], p = 1.76 × 10–10), subchondral sclerosis (OR = 3.28 [1.60–6.60], p = 9.57 × 10–4) and osteophytes (OR = 3.01 [1.87–4.87], p = 6.37 × 10–6). Cam morphology was not associated with incident osteoarthritis (OR = 0.76 [0.16–3.49], p = 0.72).
Conclusions
The relationship between cam morphology and rHOA seen in other studies is preserved in a HBM population. This study suggests that the risk of OA conferred by high BMD and by cam morphology are mediated via distinct pathways.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-022-05603-3.
Keywords: Cam morphology, Bone mineral density, Osteoarthritis, High Bone Mass
Background
Osteoarthritis (OA) is the most common joint disease, with between 10–12% of the adult population having symptomatic OA [1]. This carries a substantial economic burden, largely attributed to disability and the costs of joint replacement [2]. Hip osteoarthritis (hOA) is one of the most common manifestations of OA with a rising incidence and prevalence globally [3, 4]. The lack of preventative treatments means that increasing emphasis is placed on understanding and mitigating risk factors for its development.
One such risk factor is cam morphology, a bulging of the lateral aspect of the femoral head/neck junction, which has long been associated with hOA, total hip replacement (THR, a proxy for end stage disease) and, more recently, specific subphenotypes of hip OA [5–7]. It has been hypothesized that hip movement, in particular flexion, compresses the acetabular labrum leading to joint degeneration over time [8]. Randomised controlled trials (RCTs) have started to explore surgical options, such as arthroscopic cam resection, as a means of treating pain and possibly preventing progression of OA [9]. Importantly, adolescent activity has been associated with the development of cam morphology and is a potential confounder because it also predisposes individuals to hOA [10–12].
Cam morphology is often defined by the alpha angle (AA), a measure of hip sphericity, which can be derived from 2-dimensional (2D) and 3-dimensional (3D) imaging, with a higher AA used to define cam morphology (most commonly ≥ 60°) [13]. Methods for deriving AA vary but most involve time-consuming manual measurement using different software packages [14–16]. These methods have been found to differ in consistency, with a wide range in inter-rater reliability statistics reported across studies [16, 17]. Recently, semi-automatic methods have been developed with the potential to reduce inter-operator variation, increase reliability and reduce measurement time [18].
High bone mass (HBM), defined as an extreme elevation in bone mineral density (BMD) [19], is a risk factor for OA [20]. HBM individuals have been shown to have increased incidence and progression of radiographic features of OA (i.e. osteophytes and joint space narrowing [JSN]) at load bearing joints (knee and hip), as well as an increased prevalence of radiographic OA in non-load-bearing joints (hands) [21–24]. It is thought that these individuals may have a predisposition to a bone-forming phenotype that may be, at least partially, genetically determined [25]. This genetic component may be a result of rare monogenic mutations of large effect or multiple small-effect polygenic variants, or an interaction of the two [26]. Cam morphology itself is a manifestation of increased bone deposition around the femoral head, which may occur after childhood hip diseases or after high levels of physical activity during skeletal development [27–29]. Though cam morphology does not arise through osteophyte or enthesophyte formation, which is increased in HBM individuals [25], to the extent that cam morphology is also more common in this context, this might contribute to the relationship between HBM individuals and hOA.
In this study, we aimed to deploy the latest semi-automatic methods to measure AA on radiographs from the HBM Study and assess the cross-sectional relationships between HBM and cam morphology. In addition, we aimed to assess the relationships between cam morphology and the presence and progression of rHOA and rHOA subphenotypes.
Methods
The HBM population
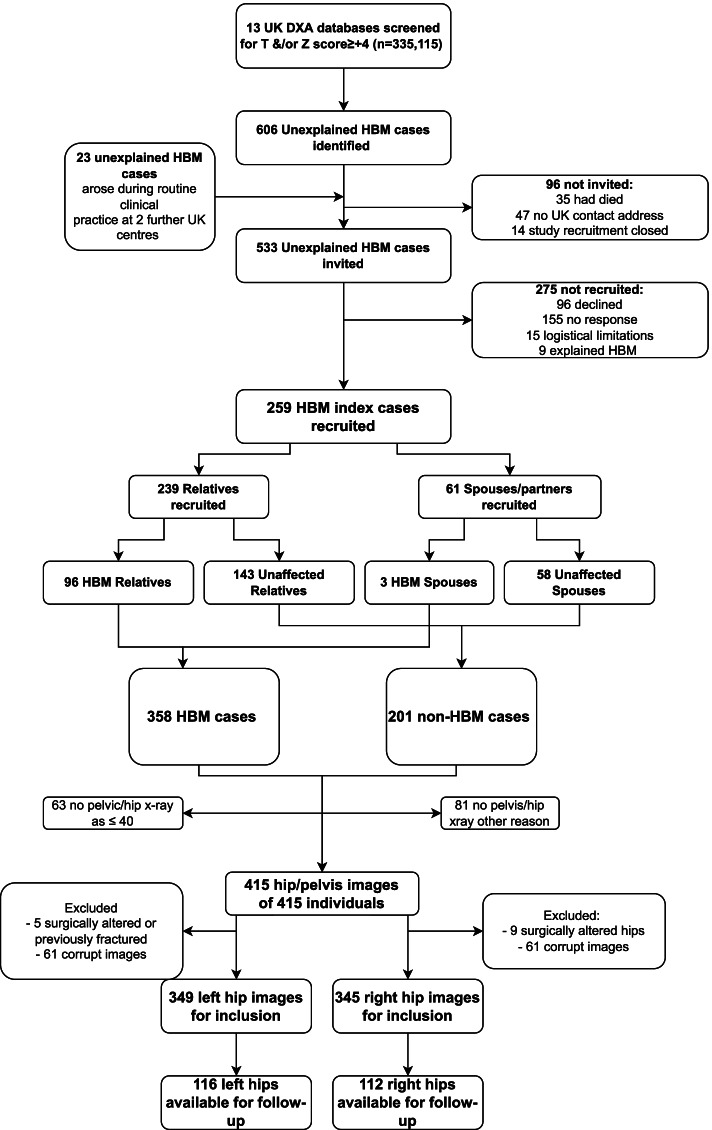
The study population was derived from the HBM study, a UK based cohort of adults with unexplained HBM. Full details of baseline recruitment, which occurred between 2005–2010, have been published previously [19]. Briefly, index HBM cases were identified by screening routine National Health Service (NHS) DXA databases (335,115 DXA scans from 13 UK DXA databases) for individuals who had T and/or Z-scores ≥ + 4 at the lumbar spine or hip. All DXA images were screened to exclude scans with artefactually raised BMD (e.g. degenerative disease). Though differing in extent, a generalised HBM trait would be expected to affect both spine and hip BMD. L1 Z-score was used as it was not associated with the presence of spinal OA [30]. HBM cases were defined as having a L1 or total hip (TH) BMD Z-score ≥ + 3.2 with a Z-score ≥ + 1.2 at the other skeletal site. This threshold was consistent with the published precedent for identifying HBM using DXA [31]. Recruited index cases were asked to invite relatives and spouses to undergo DXA screening. Relatives and spouses both with and without HBM were included (Fig. 1). All individuals received assessment including clinical examination and structured interview. Values for age, sex, weight and height were obtained from baseline structured interview and clinical examination. Historical physical activity levels (hours per week of sport between ages 14–21) were determined by postal questionnaire. Informed written consent was obtained from all participants. Participants were re-contacted in 2016 and asked to complete a postal questionnaire and then to attend for follow-up hip radiographs between 2017 and 2018.
Fig. 1.
Identification and recruitment of the study population and derivation of the study population. Abbreviations: DXA: Dual-energy X-ray absorptiometry, HBM: high bone mass
Assessment of bone mineral density
BMD was primarily assessed as continuous variables using maximum (of left and right) TH BMD in g/cm2 and L1 BMD in g/cm2. These measures were used as the exposures of interest in the relationship between HBM and cam morphology. HBM status (defined above) was used as a binary exposure of interest in the relationship between HBM and cam morphology.
Assessment of cam morphology
Anteroposterior (AP) pelvic radiographs were performed on the day of structured interview and clinical examination for participants aged over 40 according to local protocols [19]. Both hips were examined. Publicly available BoneFinder software (University of Manchester) was used to automatically place 65 points around the outline of the proximal femur (Fig. 2) [32]. BoneFinder is a machine learning trained algorithm which applies a Random Forest approach to automatically outline the bone with a number of point positions [33]. All points on all images were manually checked and, if necessary, corrected by two raters (BEZ, orthopaedic trainee surgeon and then BGF, rheumatology trainee physician) (Fig. 2). Osteophytes were deliberately excluded as part of the point plotting process. A third rater (MRW, consultant orthopaedic surgeon) was consulted in cases of uncertain optimal point placement (31 hips). Ninety-five left hips had points corrected, 105 right hips had points corrected. The coordinates denoting the agreed upon contour of the bone were then used to determine the alpha angle using a freely available Python 3.0 software package developed on DXA scans (Fig. 2) [18]. Cam morphology was defined as an AA ≥ 60° [13].
Fig. 2.
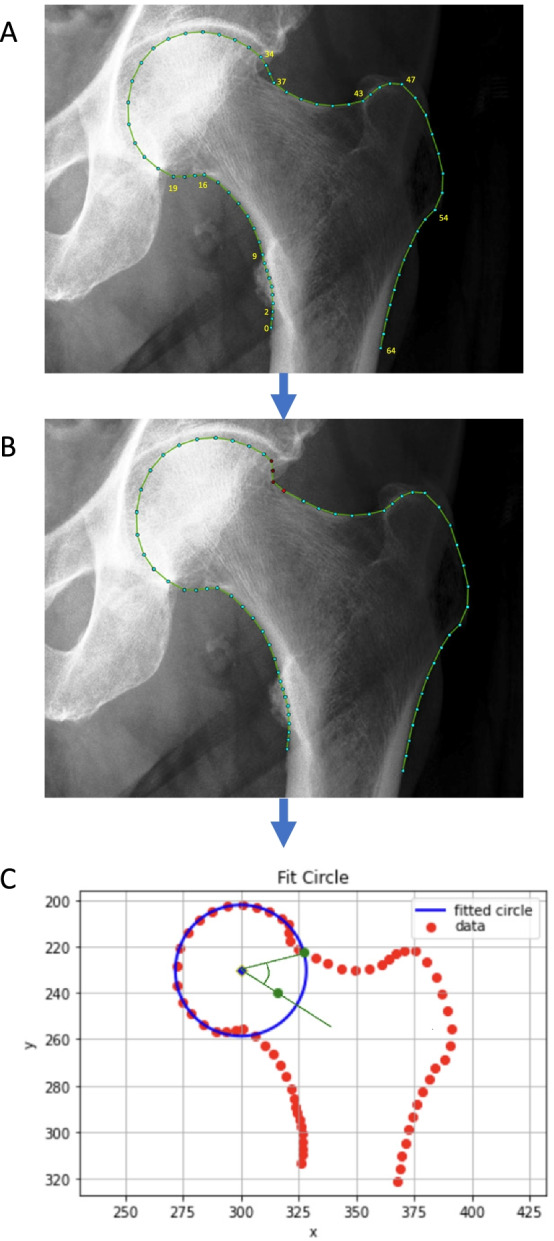
Conversion of plotted points of contour of femoral head and neck in BoneFinder to circle of best fit using custom Python script. Panel A: Automated points applied to onto contour of bone using BoneFinder. Anatomically guided points: 0, 2, 9, 16, 19, 34, 37, 43, 47, 55, 65. Panel B: Points manually adjusted. Panel C: Output of Python script where the red points denote the agreed upon contour of the bone. A circle of best fit around the femoral head is depicted in blue that is fitted to points 19–32. The alpha angle is calculated between a line from the centre point of the circle (blue diamond) to the midpoint of femoral neck (green point, the midpoint of the narrowest section between points 2–15 and 37–49) and a line from the centre of the circle to the location on the femoral head/neck junction where the femur leaves the circle of best fit (green point)
We used a random sample of 30 left hips to validate this DXA-derived method on hip radiographs. Blinded AA was derived from the corrected outline points and additionally by using a fully manual method, which has previously been described [18]. Kappas were > 0.80 when comparing the semi-automatic methods to manual gradings of binary cam morphology for both raters. The concordance correlation coefficient (CCC) was 0.72 for rater 1 and 0.98 for rater 2. The Pearson’s coefficient was > 0.85 for both raters. Agreement between raters on cam status was also high (kappa = 0.84), as was agreement on AA derivation (CCC = 0.76, Pearson’s R = 0.89).
Assessment of prevalent radiographic hip osteoarthritis
Methods to assess the study participants for rHOA have been described [20]. Briefly, all available radiographs from both HBM and non-HBM individuals were pooled for assessment to limit observer bias; reasons for unavailability of individual radiographs were ascertained and recorded. Blinded radiographs were graded for Croft score and the presence of radiographic subphenotypes of osteoarthritis including JSN, osteophytes, sclerosis (either femoral or acetabular) and cysts were graded 0–3 using an established atlas [34, 35]. Croft grade ≥ 3 (defined as two of osteophytosis, JSN, subchondral sclerosis or cyst formation) determined the presence of OA. Hips with joint replacements or protheses were recorded and excluded from the main assessment.
Assessment of incident and/or progressive radiographic hip osteoarthritis
The method of assessment of incident and/or progressive OA has previously been described [22]. Baseline and follow-up pelvic radiographs were pooled for analysis and graded for OA subphenotypes [36], with the reader blinded to HBM status, demographics and timepoint. Endophenotypes were osteophytes, sclerosis, JSN and cysts. OA was graded using Croft score, as described for prevalent OA. Incident OA was defined as the presence of OA in a hip joint that had been free of OA at baseline. Progressive OA was defined as any increase in Croft score in a hip with OA (Croft ≥ 3) at baseline. Progression in osteophyte score and JSN score were defined as any increase in osteophyte score and JSN score between baseline and follow-up.
Statistical analysis
Demographic statistics for the different study populations were summarised as mean and standard deviation (SD) for continuous variables, and counts and frequencies for categorical variables. The chi-squared test was used to assess the association between binary variables, and the unpaired two-tailed t-test to compare mean values for continuous variables. Analysis of variance was conducted to test for differences in mean AA across investigation centres at which radiographs were taken. Multivariable logistic regression was used to examine associations between BMD variables and cam status, and between cam status and radiographic OA outcome variables. Generalised estimating equations (GEE) using a logistic link function were used to generate an odds ratio accounting for intra-individual clustering between left and right hips. Directed acyclic graphs were used a priori to choose potential confounders for adjustment; analyses were first performed unadjusted (Model 1), then a basic adjusted model included age and sex (Model 2), and then with the covariates weight, height and adolescent physical activity level for a further advanced adjusted model (Model 3). For the analysis of the association between cam morphology and rHOA, we additionally adjusted for HBM status (Model 3). Odds ratios (OR) before and after adjustment for confounders are presented with 95% confidence intervals (95% CI). A sex-stratified analysis was conducted to investigate for sex-differences in the relationship between HBM and cam morphology. A pre-planned sensitivity analysis included testing the association between cam morphology and hip OA defined by a Croft grade ≥ 2. The purpose of this was to increase understanding of the relationship between cam morphology and rHOA. All data were analysed using RStudio (version 1.4.1103).
Results
Characteristics of the study population
The HBM study recruited 559 individuals, of whom 352 had satisfactory radiographic data. Reasons for lack of satisfactory radiographic data included: no imaging, imaging files inaccessible, surgically altered hips, or high likelihood of previous hip fracture (Fig. 1). All 352 individuals with satisfactory radiographs were included in the study, 235 (66.7%) were female and 234 (66.5%) had HBM (index cases or relatives with HBM). The mean summed total hip and L1 Z-score in the HBM population was 6.9 (SD 2.1) compared to 1.1 (SD 1.8) in the non-HBM population. HBM individuals were shorter (166.7 cm [SD 8.5] vs. 169.7 cm [9.2]) and heavier (84.7 kg [15.5] vs. 80.8 kg [16.6]) compared to individuals without HBM (supplementary table 1). HBM individuals were slightly older than individuals without HBM (62.5 [11.1] vs. 59.8 [12.9] years). Nearly 20% of all hips had rHOA defined as Croft score ≥ 3 (Table 1). A total of 694 hips were examined for AA and measures of HBM. Of these, 143 were cam hips (20.6%) and the mean AA was 53.9° (15.7). Individuals with a cam deformity were more commonly male (76.4% vs. 39.8%) and were taller and heavier than those without a cam deformity (Table 1). There were no significant differences between mean AA at different investigation centres at which radiographs were taken (p = 0.62).
Table 1.
Demographics of the total study population and prevalence of radiographic hip osteoarthritis features by cam morphology status
| With Cam | Without Cam | Total | |
|---|---|---|---|
| N (%) of individuals | N (%) of individuals | N (%) of individuals | |
| Female sex | 37 (39.8) | 198 (76.4) | 235 (66.7) |
| Sport aged 14–21 | |||
| 0–1 h per week | 5 (7.9) | 21 (12.1) | 26 (11.0) |
| 2–3 h per week | 18 (28.6) | 51 (29.3) | 69 (29.1) |
| 4–7 h per week | 15 (23.8) | 42 (24.1) | 57 (24.0) |
| Over 7 h per week | 25 (39.7) | 60 (34.5) | 85 (35.9) |
| At recruitment | Mean (SD) | Mean (SD) | Mean (SD) |
| Age in years | 62.2 (13.6) | 61.4 (11.1) | 61.6 (11.8) |
| Height in cm | 171.6 (9.0) | 166.3 (8.4) | 167.7 (8.8) |
| Weight in kg | 88.4 (15.5) | 81.5 (15.8) | 83.4 (16.0) |
| Summed total hip and L1 Z-score | 4.7 (3.5) | 5.0 (3.3) | 4.9 (3.4) |
| Max total hip Z-score | 1.2 (0.2) | 1.1 (0.2) | 1.2 (0.2) |
| L1 lumbar spine BMD in g/cm2 | 1.3 (0.2) | 1.3 (0.2) | 1.4 (0.2) |
| N (%) of hips | N (%) of hips | N (%) of hips | |
| OA (Croft > 3) | 57 (40.1) | 79 (14.5) | 136 (19.8) |
| Osteophyte | 119 (83.8) | 345 (63.2) | 464 (67.4) |
| JSN | 60 (42.3) | 90 (16.5) | 150 (21.8) |
| Sclerosis | 15 (10.6) | 19 (3.5) | 34 (4.9) |
| HBM | 81 (56.6) | 379 (68.8) | 460 (66.3) |
Abbreviations: BMD Bone mineral density, OA Osteoarthritis, JSN Joint space narrowing, HBM High bone mass, SD Standard deviation
HBM and Cam morphology
Although, a protective association between HBM status and cam status was seen in the unadjusted model (OR 0.59, 95% CI 0.41–0.86, p = 6.5 × 10–3), this was attenuated by adjustment for sex and age (OR 0.97, 95% CI 0.63–1.51, p = 0.90) (Table 2). No association was seen between HBM status and cam status in further sex stratified analysis (Table 3). Furthermore, there was no association between L1 or maximum TH BMD (using continuous measures of BMD) and cam status (Model 3: OR 0.86, 95% CI 0.67–1.09, p = 0.21 and OR 0.88, 95% CI 0.68–1.13, p = 0.31, OR per SD unit change in BMD).
Table 2.
Relationships between bone mass and cam morphology, determined by generalised estimating equations
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Exposure | Outcome | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| Max total hip BMD in g/cm2 | Cam | 1.12 (0.92–1.36) | 0.25 | 1.09 (0.89–1.33) | 0.40 | 0.88 (0.68–1.13) | 0.31 |
| L1 BMD in g/cm2 | Cam | 1.03 (0.84–1.25) | 0.79 | 0.98 (0.80–1.20) | 0.84 | 0.86 (0.67–1.09) | 0.21 |
| HBM status | Cam | 0.59 (0.41–0.86) | 6.5 × 10–3 | 0.97 (0.63–1.51) | 0.90 | 0.66 (0.38–1.13) | 0.13 |
Model 1 = unadjusted; Model 2 = age and sex adjusted; Model 3 = adjusted for sex, age, weight, height and adolescent activity
Odds ratios are per SD increase in BMD or the odds in HBM individuals compared to non-HBM individuals
Abbreviations: HBM High bone mass, BMD Bone mineral density, OR Odds ratio, CI 95% confidence interval
Table 3.
Relationships between bone mass and cam morphology, determined by generalised estimating equations, stratified by sex
| Model 1 | Model 2 | ||||
| Male sex | |||||
| Exposure | Outcome | OR (95% CI) | p value | OR (95% CI) | p value |
| Max total hip BMD in g/cm2 | Cam | 1.11 (0.85–1.47) | 0.44 | 0.90 (0.65–1.25) | 0.54 |
| L1 BMD in g/cm2 | Cam | 1.04 (0.80–1.36) | 0.75 | 0.92 (0.65–1.30) | 0.62 |
| HBM status | Cam | 1.09 (0.65–1.84) | 0.74 | 0.75 (0.38–1.46) | 0.40 |
| Female sex | |||||
| Exposure | Outcome | OR (95% CI) | p value | OR (95% CI) | p value |
| Max total hip BMD in g/cm2 | Cam | 1.00 (0.75–1.34) | 0.98 | 0.84 (0.57–1.24) | 0.38 |
| L1 BMD in g/cm2 | Cam | 0.92 (0.66–1.28) | 0.64 | 0.74 (0.52–1.05) | 0.09 |
| HBM status | Cam | 0.87 (0.43–1.74) | 0.69 | 0.55 (0.22–1.37) | 0.20 |
Model 1 = unadjusted; Model 2 = adjusted for age, weight, height, and adolescent activity
Odds ratios are per SD increase in BMD or the odds in HBM individuals compared to non-HBM individuals
Abbreviations: HBM High bone mass, BMD bone mineral density, OR Odds ratio, CI 95% confidence interval
Cam morphology and prevalent radiographic hip OA
Croft score was available for 668 hips (142 hips with cam morphology and 546 without). In unadjusted analyses, there was a strong association between cam morphology and the presence of rHOA (OR 3.96, 95% CI 2.63–5.98, p = 5.5 × 10–11). Furthermore, cam was associated with radiographic subphenotypes of OA including JSN (OR 3.7, 95% CI 2.48–5.54, p = 11.76 × 10–10), subchondral sclerosis (OR 3.28, 95% CI 1.62–6.62, p = 9.57 × 10–4), the presence of osteophytes overall (OR 3.01, 95% CI 1.87–4.87, p = 6.37 × 10–6), as well as the presence of osteophytes at the acetabulum (OR 2.31, 95% CI 1.50–3.56, p = 1.56 × 10–4), the lateral femoral head (OR 3.66, 95% CI 2.37–5.66, p = 5.01 × 10–9) and the infero-medial femoral head (OR 4.49, 95% CI 1.94–10.40, p = 4.6 × 10–4). The associations between cam morphology and rHOA, JSN and osteophytes persisted after adjustment for age, sex, weight, height, adolescent physical activity level and HBM status (Model 3, Table 4). The sensitivity analysis revealed a strong relationship between the presence of cam morphology and rHOA defined by a Croft score ≥ 2 (OR 3.65, 95% CI 2.45–5.42, p = 1.65 × 10–10).
Table 4.
Associations between cam and prevalent radiographic hip osteoarthritis, determined by generalised estimating equations
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Exposure | Outcome | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| Cam | Croft score ≥ 3 | 3.96 (2.63–5.98) | 5.46 × 10–11 | 3.27 (2.10–5.09) | 1.60 × 10–7 | 1.95 (1.11–3.43) | 0.02 |
| Cam | Osteophyte (any) | 3.01 (1.87–4.87) | 6.37 × 10–6 | 2.39 (1.41–4.04) | 1.21 × 10–3 | 2.31 (1.28–4.20) | 5.74 × 10–3 |
| Cam | JSN (any) | 3.70 (2.48–5.54) | 1.76 × 10–10 | 3.19 (2.06–4.96) | 2.19 × 10–7 | 2.25 (1.30–3.89) | 3.80 × 10–3 |
| Cam | Sclerosis | 3.28 (1.60–6.60) | 9.57 × 10–4 | 2.63 (1.22–5.65) | 0.01 | 1.46 (0.51–4.13) | 0.48 |
Model 1: unadjusted; Model 2: age and sex adjusted; Model 3: adjusted for sex, age, weight and height, adolescent activity level and HBM status
Odds in hips with cam morphology compared to hips without cam morphology
Abbreviations: JSN Joint space narrowing, OR Odds ratio, CI 95% confidence interval
Cam morphology and incident/progressive radiographic hip OA
Follow-up data for rHOA were only available for 228 hips from 116 individuals, a mean of 8.3 (SD 0.66) years after their first radiograph. The odds of cam hips having rHOA at either baseline or follow-up visit was 2.83 (95% CI 1.00–8.06, p = 0.051, Model 3). Incident OA was detected in 17 hips. Cam morphology was not associated with incident OA (Model 3: OR 2.94, 95% CI 0.61–14.27, p = 0.18) or any increase in Croft score between baseline and follow-up (Model 1: OR 1.17, 95% CI 0.32–4.27, p = 0.81) (Table 5). Furthermore, no evidence was detected for a relationship between cam and change in osteophyte score between baseline and follow-up (Model 1: OR 1.00, 95% CI 0.67–1.49, p = 0.99). Similarly, no evidence was detected for a relationship between cam and change in JSN score (Model 1: OR 1.00, 95% CI 0.87–1.15, p = 0.99).
Table 5.
Relationship between cam and incident radiographic OA progression, determined by generalised estimating equations
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Exposure | Outcome | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| Cam | Change in Osteophyte score | 1.00 (0.67–1.49) | 0.99 | 1.10 (0.79–1.65) | 0.65 | 1.13 (0.70–1.80) | 0.62 |
| Cam | Change in JSN score | 1.00 (0.87–1.15) | 0.99 | 0.99 (0.86–1.13) | 0.87 | 1.00 (0.85–1.18) | 0.99 |
| Cam | Incident OA | 0.76 (0.16–3.49) | 0.72 | 1.28 (0.26–6.37) | 0.76 | 2.94 (0.61–14.27) | 0.18 |
| Cam | Increase in Croft score | 1.17 (0.32–4.27) | 0.81 | 1.33 (0.34–5.14) | 0.68 | 2.24 (0.48–10.47) | 0.31 |
Incident OA: Any OA at follow-up in a hip with Croft < 3 at baseline
Model 1: unadjusted; Model 2: age and sex adjusted; Model 3: adjusted for sex, age, weight and height, adolescent activity level and HBM status
Odds in hips with cam morphology compared to hips without cam morphology
Abbreviations: JSN Joint space narrowing, OA Osteoarthritis, OR Odds ratio, CI 95% confidence interval
Discussion
We present results from the first observational study to examine the relationship between cam morphology and BMD using a cohort of extreme HBM cases. We found there was no association between BMD, whether measured at the hip or lumbar spine, and the prevalence of cam morphology. Furthermore, there was no association between HBM and the prevalence of cam morphology. We explored the relationship between cam morphology and rHOA in the same population and found that cam morphology was strongly associated with prevalent rHOA. To further understand this relationship, we examined the associations between cam morphology and the subphenotypes of rHOA, which have been investigated less frequently [7]. Similar to the relationship between cam and rHOA, there was a strong association between the presence of cam morphology and the prevalence of osteophytes, JSN and sclerosis, with the strongest associations seen between cam morphology and JSN, and cam morphology and osteophytes, particularly those at the inferomedial femoral head, replicating recent findings from a large DXA based study [7]. The proposed biological mechanism by which cam causes OA describes the bony deformity entering the hip joint on flexion, causing separation of the acetabular cartilage from the labrum [8, 37]. Therefore, one might expect the focus of osteophytes to be around the impinging surfaces, such as the lateral acetabulum and superomedial femoral head. Future research is justified to understand these relationships and their underlying mechanism.
The relationship between cam morphology and rHOA has been observed in a number of studies [5, 38, 39]. However, this is the first study to examine the relationship between cam morphology and BMD. Previous studies have established relationships between HBM and BMD and OA incidence and progression at various joints [40]. In particular, those studies which included this cohort have shown HBM individuals have an increased risk of prevalent hOA (OR 1.52 [CI 1.09, 2.11]) and of progression in OA subphenotypes [20–23]. As cam morphology itself is a manifestation of increased bone deposition around the femoral head it was hypothesised that cam morphology may mediate the relationship between HBM and hOA [27, 29]. However, the results of our study do not support this hypothesis and instead provide evidence that the relationship between cam morphology and rHOA is independent of BMD, acting via separate pathways. There is growing evidence that the HBM phenotype is, at least partially, genetically determined and a result of rare monogenic mutations of large effect and/or multiple small-effect polygenic variants [26]. There is additional evidence that hip shape may be partially genetically determined, and that these genetic influences may overlap with genetic risk factors for osteoarthritis [38]. Whether the genetic influences predisposing to HBM overlap with those influencing hip shape is not yet known. Further research is required to understand these genetic contributions to disease aetiology. In contrast to large population-based studies, our study found no association between the presence of cam at baseline and the subsequent development of rHOA [14, 15, 39]. This finding could be explained by our smaller sample size at follow-up with only 116 individuals (32.9%) having available data. Furthermore, the majority of studies reporting longitudinal associations between cam morphology and incident rHOA have much longer follow-up periods [14, 15, 39].
This is the first study to use novel machine learning based methods to measure AA on radiographs. AA measures were validated against manual methods, and as further validation our results replicate expected relationships in keeping with previously published studies. For example, the male predominance of cam morphology shown by the sex differences in AA are consistent with previous reports [7, 10, 41]. The prevalence of cam identified in our study was slightly higher than previous larger studies [7, 39], although there is a large range in the reported prevalence of cam morphology in the literature with no agreed cam prevalence estimate, largely attributable to the varying AA thresholds and methods used [42]. A large cadaveric study found a prevalence of cam morphology of 26.5%, suggesting our cam prevalence (20.6%) is plausible [43]. This is further substantiated by results from Frank et al., who found a cam prevalence of > 30% in asymptomatic individuals [44]. In terms of our findings between cam morphology and rHOA these are also consistent with results from previous studies which report ORs 1.05—3.67 [14, 39].
Strengths and limitations
This study is the first to investigate the relationship between BMD and cam morphology, and between cam morphology and rHOA, in a population of individuals with extreme elevations of BMD [19]. Another strength of this study is our use of novel semi-automatic methods of deriving AA which increase the inter-rater reliability of derivation and improve comparisons between studies. However, a limitation of our study stemmed from the multi-centre nature of recruitment. Positioning is thought to be important when investigating hip radiographs [45], as such the lack of a standardised positioning protocol across centres may predispose to high inter-centre variability in AA derivation. Despite this, no meaningful differences in AA were noted across sites. Furthermore, AP radiographs may be limited in their ability to detect anterior cam lesions compared to other 2-D views, 3-D imaging or cadaveric studies [10, 43, 46]. This may have resulted in under-identification of cam morphology in this study. However, it is unlikely that this measurement error was differential between those with and without hip OA; thus any bias would be towards the null. As similar associations between cam morphology and hOA were seen in previous studies, this suggests our methods were sufficient to assess cam morphology on a population level [5, 38, 39].
The study population suffered from a relatively high attrition rate between baseline and follow-up resulting in reduced statistical power in the detection of OA incidence and progression. This is largely due to the older age of the population at recruitment meaning follow-up was limited by death and poor health. This likely resulted in a follow-up population in which individuals were less likely to have significant OA, therefore introducing bias towards the null. Finally, the study of a population comprising individuals with extreme elevations of BMD limits the generalisability of these results. However, the use of this population is integral to the understanding of the mechanisms by which BMD influences OA. Further research is required to understand whether BMD is associated with cam morphology in less extreme populations.
Conclusions
This observational study, using novel methods of alpha angle derivation applied to plain hip radiographs, identified a strong relationship between cam morphology and prevalent rHOA in a population comprising individuals with extreme elevations in BMD. This study found no relationship between cam morphology and BMD, lending credence to our new hypothesis that their individual relationships with rHOA are mediated by distinct pathways. Further work is needed to understand whether the absence of a relationship between cam and BMD is replicated in individuals with less extreme elevations of BMD.
Supplementary Information
Additional file 1: Supplementary table 1. Demographics of the total study population by high bone mass status.
Acknowledgements
We would like to thank all High Bone Mass study participants and the staff at the University of Bristol and collaborating centres: The Wellcome Trust Clinical Research Facility in Birmingham, Royal National Hospital for Rheumatic Diseases in Bath, Cambridge NIHR Biomedical Research Centre and Addenbrooke's Wellcome Trust Clinical Research Facility, Bone Research Unit in Cardiff, Musculoskeletal Research Unit in Bristol, NIHR Bone Biomedical Research Unit in Sheffield and the Brocklehurst Centre for Metabolic Bone Disease in Hull.
Minors
No minor participants (< 16 yrs) were involved in this study.
Abbreviations
- 2D
2-Dimensional
- 3D
3-Dimensional
- AA
Alpha Angle
- AP
Anteroposterior
- BMD
Bone mineral density
- CI
95% Confidence interval
- CCC
Concordance correlation coefficient
- GEE
Generalised estimating equations
- HBM
High bone mass
- hOA
Hip osteoarthritis
- JSN
Joint space narrowing
- NHS
National Health Service
- OR
Odds ratios
- RCTs
Randomised contolled trials
- SD
Standard deviation
- THR
Total hip replacement
Authors’ contributions
BEZ conceived and completed the analysis, interpreted the results and drafted the manuscript. RE, CL, TC were involved in the acquisition of data, creation of software and interpretation of results. SH acquired data and interpreted results. JH, MW, CLG, BGF and AH were involved in study conception, analysis and interpretation of results. ALL authors helped revise the final manuscript, approve of its content and accept responsibility for this work.
Funding
The HBM study was supported by The Wellcome Trust (ref. 080280/Z/06/Z), the National Institute for Health Research Clinical Research Network (portfolio no. 5163) and Versus Arthritis (ref. 20000). AH was funded by the Wellcome Trust (ref. 20378/Z/16/Z). MRW was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. BGF is supported by a Medical Research Council (MRC) clinical research training fellowship (ref. MR/S021280/1). CL is funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (223267/Z/21/Z). This research was funded in whole, or in part, by the Wellcome Trust [Grant numbers 080280/Z/06/Z, 20378/Z/16/Z, 223267/Z/21/Z]. For the purpose of open access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Availability of data and materials
The data used in this study is available for all researchers. Please contact the corresponding author to arrange access.
Declarations
Ethics approval and consent to participate
Ethics approval and written informed consent was obtained in line with the Declaration of Helsinki. The study was approved by the Bath Multi-centre Research Ethics Committee (REC reference 05/Q2001/78) and each local NHS REC. Follow up data collection was approved by the Central Bristol REC and NHS Health Research Authority. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
All participants provided written informed consent at time of recruitment.
Competing interests
The authors have no competing interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
B. G. Faber and A. E. Hartley contributed equally.
Contributor Information
B. E. Zucker, Email: qo19163@bristol.ac.uk
B. G. Faber, Email: ben.faber@bristol.ac.uk
References
- 1.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(8 Suppl):S230–S235. [PubMed] [Google Scholar]
- 3.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 4.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 5.Saberi Hosnijeh F, Zuiderwijk ME, Versteeg M, Smeele HT, Hofman A, Uitterlinden AG, et al. Cam Deformity and Acetabular Dysplasia as Risk Factors for Hip Osteoarthritis. Arthritis Rheumatol. 2017;69(1):86–93. doi: 10.1002/art.39929. [DOI] [PubMed] [Google Scholar]
- 6.Griffin DR, Dickenson EJ, O'Donnell J, Agricola R, Awan T, Beck M, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50(19):1169–1176. doi: 10.1136/bjsports-2016-096743. [DOI] [PubMed] [Google Scholar]
- 7.Faber BG, Ebsim R, Saunders FR, Frysz M, Gregory JS, Aspden RM, Harvey NC, Davey Smith G, Cootes T, Lindner C, Tobias JH. Cam morphology but neither acetabular dysplasia nor pincer morphology is associated with osteophytosis throughout the hip: findings from a cross-sectional study in UK Biobank. Osteoarthritis Cartilage. 2021;29(11):1521-9. 10.1016/j.joca.2021.08.002. [DOI] [PMC free article] [PubMed]
- 8.Murphy NJ, Eyles JP, Hunter DJ. Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv Ther. 2016;33(11):1921–1946. doi: 10.1007/s12325-016-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin DR, Dickenson EJ, Wall PDH, Achana F, Donovan JL, Griffin J, et al. Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): a multicentre randomised controlled trial. Lancet. 2018;391(10136):2225–2235. doi: 10.1016/S0140-6736(18)31202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Klij P, Heerey J, Waarsing JH, Agricola R. The Prevalence of Cam and Pincer Morphology and Its Association With Development of Hip Osteoarthritis. J Orthop Sports Phys Ther. 2018;48(4):230–238. doi: 10.2519/jospt.2018.7816. [DOI] [PubMed] [Google Scholar]
- 11.van Klij P, Heijboer MP, Ginai AZ, Verhaar JAN, Waarsing JH, Agricola R. Cam morphology in young male football players mostly develops before proximal femoral growth plate closure: a prospective study with 5-yearfollow-up. Br J Sports Med. 2019;53(9):532–538. doi: 10.1136/bjsports-2018-099328. [DOI] [PubMed] [Google Scholar]
- 12.Harris EC, Coggon D. HIP osteoarthritis and work. Best Pract Res Clin Rheumatol. 2015;29(3):462–482. doi: 10.1016/j.berh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Klij P, Reiman MP, Waarsing JH, Reijman M, Bramer WM, Verhaar JAN, et al. Classifying Cam Morphology by the Alpha Angle: A Systematic Review on Threshold Values. Orthop J Sports Med. 2020;8(8):2325967120938312. doi: 10.1177/2325967120938312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholls AS, Kiran A, Pollard TC, Hart DJ, Arden CP, Spector T, et al. The association between hip morphology parameters and nineteen-year risk of end-stage osteoarthritis of the hip: a nested case-control study. Arthritis Rheum. 2011;63(11):3392–3400. doi: 10.1002/art.30523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas GE, Palmer AJ, Batra RN, Kiran A, Hart D, Spector T, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504–10. doi: 10.1016/j.joca.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Edwards K, Leyland KM, Sanchez-Santos MT, Arden CP, Spector TD, Nelson AE, et al. Differences between race and sex in measures of hip morphology: a population-based comparative study. Osteoarthritis Cartilage. 2020;28(2):189–200. doi: 10.1016/j.joca.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Fioruzzi A, Acerbi A, Jannelli E, Ivone A, Fontana A. Interobserver and intraobserver reliability of a new radiological classification for femoroacetabular impingement syndrome. Musculoskelet Surg. 2020;104(3):279–284. doi: 10.1007/s12306-019-00618-x. [DOI] [PubMed] [Google Scholar]
- 18.Faber B, Ebsim R, Saunders F, Frysz M, Davey Smith G, Cootes T, et al. Deriving alpha angle from anterior-posterior dual-energy x-ray absorptiometry scans: an automated and validated approach [version 1; peer review: awaiting peer review]. Wellcome Open Res. 2021;6(60). [DOI] [PMC free article] [PubMed]
- 19.Gregson CL, Steel SA, O'Rourke KP, Allan K, Ayuk J, Bhalla A, et al. 'Sink or swim': an evaluation of the clinical characteristics of individuals with high bone mass. Osteoporos Int. 2012;23(2):643–654. doi: 10.1007/s00198-011-1603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardcastle SA, Dieppe P, Gregson CL, Hunter D, Thomas GE, Arden NK, et al. Prevalence of radiographic hip osteoarthritis is increased in high bone mass. Osteoarthritis Cartilage. 2014;22(8):1120–1128. doi: 10.1016/j.joca.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley A, Hardcastle SA, Paternoster L, McCloskey E, Poole KES, Javaid MK, et al. Individuals with high bone mass have increased progression of radiographic and clinical features of knee osteoarthritis. Osteoarthritis Cartilage. 2020;28(9):1180–1190. doi: 10.1016/j.joca.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Hartley A, Hardcastle SA, Frysz M, Parkinson J, Paternoster L, McCloskey E, et al. Increased development of radiographic hip osteoarthritis in individuals with high bone mass: a prospective cohort study. Arthritis Res Ther. 2021;23(1):4. doi: 10.1186/s13075-020-02371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregson CL, Hardcastle SA, Murphy A, Faber B, Fraser WD, Williams M, et al. High Bone Mass is associated with bone-forming features of osteoarthritis in non-weight bearing joints independent of body mass index. Bone. 2017;97:306–313. doi: 10.1016/j.bone.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartley A, Sanderson E, Granell R, Paternoster L, Zheng J, Smith GD, Southam L, Hatzikotoulas K, Boer CG, van Meurs J, Zeggini E; Genetics of Osteoarthritis Consortium, Gregson CL, Tobias JH. Using multivariable Mendelian randomization to estimate the causal effect of bone mineral density on osteoarthritis risk, independently of body mass index. Int J Epidemiol. 2021:dyab251. 10.1093/ije/dyab251. [DOI] [PMC free article] [PubMed]
- 25.Hardcastle SA, Dieppe P, Gregson CL, Arden NK, Spector TD, Hart DJ, et al. Osteophytes, enthesophytes, and high bone mass: a bone-forming triad with potential relevance in osteoarthritis. Arthritis Rheumatol. 2014;66(9):2429–2439. doi: 10.1002/art.38729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregson CL, Duncan EL. The Genetic Architecture of High Bone Mass. Front Endocrinol (Lausanne) 2020;11:595653. doi: 10.3389/fendo.2020.595653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anjali Patel DB, Sarah Hardcastle, Jenny Gregory, Richard Aspden, Ben Faber, George Smith, Jon Tobias, Celia Gregson. Do alterations in hip shape explain the increased risk of hip osteoarthritis in individuals with High Bone Mass? Bone Res Soc Annu Meeting. 2016:12.
- 28.Roels P, Agricola R, Oei EH, Weinans H, Campoli G, Zadpoor AA. Mechanical factors explain development of cam-type deformity. Osteoarthritis Cartilage. 2014;22(12):2074–2082. doi: 10.1016/j.joca.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Speirs AD, Beaulé PE, Rakhra KS, Schweitzer ME, Frei H. Bone density is higher in cam-type femoroacetabular impingement deformities compared to normal subchondral bone. Osteoarthritis Cartilage. 2013;21(8):1068–1073. doi: 10.1016/j.joca.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Gregson C.L. SS, Yoshida K., Reid D.M., Tobias J.H. An investigation into the impact of osteoarthritic changes on bone mineral density measurements in patients with high bone mass. (SA257). ASBMR 30th Annual Meeting, Montreal. 2008.
- 31.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindner C, Thiagarajah S, Wilkinson JM, Wallis GA, Cootes TF. Development of a fully automatic shape model matching (FASMM) system to derive statistical shape models from radiographs: application to the accurate capture and global representation of proximal femur shape. Osteoarthritis Cartilage. 2013;21(10):1537–1544. doi: 10.1016/j.joca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Lindner C, Thiagarajah S, Wilkinson JM, Wallis GA, Cootes TF. Fully automatic segmentation of the proximal femur using random forest regression voting. IEEE Trans Med Imaging. 2013;32(8):1462–1472. doi: 10.1109/TMI.2013.2258030. [DOI] [PubMed] [Google Scholar]
- 34.Croft P, Cooper C, Wickham C, Coggon D. Defining osteoarthritis of the hip for epidemiologic studies. Am J Epidemiol. 1990;132(3):514–522. doi: 10.1093/oxfordjournals.aje.a115687. [DOI] [PubMed] [Google Scholar]
- 35.Burnett S HD, Cooper C, Spector T. A Radiographic Atlas of Osteoarthritis. 1994. [Google Scholar]
- 36.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 38.Faber BG, Frysz M, Tobias JH. Unpicking observational relationships between hip shape and osteoarthritis: hype or hope? Curr Opin Rheumatol. 2020;32(1):110–118. doi: 10.1097/BOR.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 39.Agricola R, Heijboer MP, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Waarsing JH. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK) Ann Rheum Dis. 2013;72(6):918–923. doi: 10.1136/annrheumdis-2012-201643. [DOI] [PubMed] [Google Scholar]
- 40.Bergink AP, Rivadeneira F, Bierma-Zeinstra SM, Zillikens MC, Ikram MA, Uitterlinden AG, et al. Are Bone Mineral Density and Fractures Related to the Incidence and Progression of Radiographic Osteoarthritis of the Knee, Hip, and Hand in Elderly Men and Women? The Rotterdam Study Arthritis Rheumatol. 2019;71(3):361–369. doi: 10.1002/art.40735. [DOI] [PubMed] [Google Scholar]
- 41.Gosvig KK, Jacobsen S, Sonne-Holm S, Gebuhr P. The prevalence of cam-type deformity of the hip joint: a survey of 4151 subjects of the Copenhagen Osteoarthritis Study. Acta Radiol. 2008;49(4):436–441. doi: 10.1080/02841850801935567. [DOI] [PubMed] [Google Scholar]
- 42.Dickenson E, Wall PD, Robinson B, Fernandez M, Parsons H, Buchbinder R, et al. Prevalence of cam hip shape morphology: a systematic review. Osteoarthritis Cartilage. 2016;24(6):949–961. doi: 10.1016/j.joca.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 43.Hanzlik S, Riff AJ, Wuerz TH, Abdulian M, Gurin D, Nho SJ, et al. The Prevalence of Cam Morphology: A Cross-Sectional Evaluation of 3,558 Cadaveric Femora. Front Surg. 2020;7:588535. doi: 10.3389/fsurg.2020.588535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank JM, Harris JD, Erickson BJ, Slikker W, 3rd, Bush-Joseph CA, Salata MJ, et al. Prevalence of Femoroacetabular Impingement Imaging Findings in Asymptomatic Volunteers: A Systematic Review. Arthroscopy. 2015;31(6):1199–1204. doi: 10.1016/j.arthro.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 45.Clohisy JC, Carlisle JC, Beaulé PE, Kim YJ, Trousdale RT, Sierra RJ, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4(Suppl 4):47–66. doi: 10.2106/JBJS.H.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albers CE, Wambeek N, Hanke MS, Schmaranzer F, Prosser GH, Yates PJ. Imaging of femoroacetabular impingement-current concepts. J Hip Preserv Surg. 2016;3(4):245–261. doi: 10.1093/jhps/hnw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary table 1. Demographics of the total study population by high bone mass status.
Data Availability Statement
The data used in this study is available for all researchers. Please contact the corresponding author to arrange access.