ABSTRACT
Background
Trimethylamine N-oxide (TMAO) and its precursors choline, betaine, and carnitine have been associated with cardiometabolic disease in nonpregnant adults. However, studies examining TMAO and its precursors in relation to cardiometabolic conditions during pregnancy are lacking.
Objectives
The primary objective was to estimate the association of TMAO and its precursors in maternal and cord plasma with gestational diabetes mellitus (GDM) and pre-eclampsia (PE) among women in the Boston Birth Cohort. A secondary objective was to determine whether associations vary by race/ethnicity.
Methods
ORs for each outcome according to tertiles and to an SD increment of TMAO, choline, betaine, and carnitine were estimated using logistic regression. Final models were adjusted for covariates.
Results
Among 1496 women, 115 women had GDM and 159 had PE during the index pregnancy. Intermetabolite correlations of TMAO and its precursors were stronger within cord plasma (r = 0.38–0.87) than within maternal plasma (r = 0.08–0.62). Maternal TMAO was associated with higher odds of GDM (third compared with first tertile OR: 1.75; 95% CI: 1.04, 2.94), whereas maternal choline, betaine, and carnitine were not associated with GDM. Maternal TMAO and choline were not associated with PE, whereas carnitine was associated with higher (OR: 1.86; 95% CI: 1.18, 2.94) and betaine with lower odds of PE (OR: 0.37; 95% CI: 0.23, 0.59). In cord plasma, TMAO was not associated with GDM or PE, but choline, betaine, and carnitine were associated with higher odds of PE (OR: 3.11; 95% CI: 1.62, 5.96; OR: 2.65; 95% CI: 1.42, 4.93; OR: 2.56; 95% CI: 1.39, 4.69, respectively). Cord choline was associated with lower odds of GDM (OR: 0.52; 95% CI: 0.27, 0.99), whereas other cord metabolites were not significantly associated with GDM. Associations did not vary by race/ethnicity.
Conclusions
TMAO and its precursors were associated with GDM and PE, but the associations differed based on the metabolite medium (maternal compared with cord plasma).
This trial was registered at clinicaltrials.gov as NCT03228875.
Keywords: trimethylamine N-oxide, choline, betaine, carnitine, pregnancy, metabolomics, gestational diabetes, pre-eclampsia
A circulating metabolite, trimethylamine N-oxide (TMAO), produced by intestinal microbes, was associated with gestational diabetes in a large urban cohort of racially and ethnically diverse women.
Introduction
Gestational diabetes mellitus (GDM) and pre-eclampsia (PE) are leading contributors to maternal and infant morbidity and mortality, which continue to rise in the United States and globally (1–5). In addition to contributing to perinatal morbidity and mortality, these conditions are risk factors for cardiometabolic disease later in life (1–9). Given their important contribution to health of the mother and the fetus, there is a critical need to identify factors that can be targeted for the early detection and, ultimately, prevention of these disorders.
Trimethylamine N-oxide (TMAO) and its precursors choline, betaine, and carnitine have been associated with type 2 diabetes, hypertension, stroke, major adverse cardiac events, and all-cause mortality in adults (10–14), and more recent research has linked them to GDM and PE during pregnancy. Although a thorough understanding of the determinants of circulating TMAO is the subject of ongoing research, current evidence points to both direct absorption of TMAO from dietary fish intake and endogenous production through an interaction between precursors (i.e., choline, betaine, and carnitine) and gut microbiota, whereby precursors are converted into trimethylamine (TMA) and then oxidized in the liver to TMAO. Choline, betaine, and carnitine are also both synthesized endogenously and supplemented by diet; choline and betaine are associated with egg consumption and carnitine is associated with red meat (15–18).
Existing studies on TMAO and its precursors and their associations with GDM and PE are limited. Several were case-control studies conducted in China and measured TMAO in maternal blood (plasma or serum). Many were relatively small, especially those examining PE (n = 60–264; 19–21). Results from the studies of GDM and TMAO were mixed, finding either a positive or an inverse association (22, 23). One of those studies also examined choline chloride, betaine, and l-carnitine with GDM and found a U-shaped association for choline and inverse associations for betaine and carnitine (23). Studies of PE and TMAO found a positive association between maternal TMAO and PE (19–21). As for the precursors, one study examining maternal concentrations of carnitine found that carnitine was elevated among women with PE compared with normotensive pregnant controls (24). Two studies found that carnitine in routine neonatal dried blood spots (25) and carnitine and choline in cord blood (26) were elevated among infants born to mothers with PE. In animal studies, TMAO contributed to some of the key pathologies of GDM and PE in mouse and rat models, respectively (27, 28).
To our knowledge, there has been just one study (25) of these metabolites in relation to pregnancy outcomes in a US population of pregnant women, and this study was not racially and ethnically diverse. Furthermore, no study has examined the group of precursors in addition to TMAO in both cord blood and maternal blood, which may be important because of cross-placental transfer of these metabolites (29–34). It is well-established that certain essential nutrients (e.g., folate) are preferentially allocated to the fetus, even in scenarios of maternal deficiency (35–37). Choline, which plays a role in fetal growth and neural development, and carnitine, which is involved in fatty acid metabolism and the removal of toxic metabolites, are known to be actively transported to the fetus (31, 32). Further, betaine, like choline, is an important methyl donor, playing a role in fetal gene expression and epigenetic regulation, and may well follow this pattern (30, 31). Given the aforementioned gaps in the literature, the purpose of the current study was to evaluate the associations of TMAO and its precursors with GDM and PE in a US prebirth cohort of urban women of predominantly black race or Hispanic ethnicity. A secondary objective was to examine whether these associations vary by race and ethnicity, because at least one previous study in nonpregnant adults found evidence for effect measure modification by race for TMAO and cardiovascular events (38).
Methods
Study participants
The Boston Birth Cohort (BBC; NCT03228875) began in 1998 with the original aim of studying the environmental and genetic determinants of preterm birth and low birth weight among women delivering at the Boston Medical Center (39). The Boston Medical Center sees a large proportion of low-income, city-dwelling patients, as well as a large proportion of patients from racial and ethnic backgrounds that have been historically underrepresented in research. The study population has been described previously (39–41). Briefly, women delivering singleton, live births were invited to participate. Pregnancies resulting in multiple gestations or major birth defects, as well as those conceived via in vitro fertilization, were excluded, as were women delivering preterm owing to nonobstetric factors (e.g., trauma).
The BBC continues to enroll women and their infants at birth and has followed participants for almost 20 y. It has also expanded its objectives to examine a variety of additional exposures (e.g., metabolites) and outcomes. We used data from women in the BBC who enrolled between 1998 and 2013, who had sufficient cord and/or maternal blood samples for metabolite analysis, and who had complete information on exposure variables, outcome variables, and key covariates (i.e., those that were included in the fully adjusted models). Institutional Review Boards at the Boston Medical Center and Johns Hopkins Bloomberg School of Public Health approved this study.
Human subjects
Participants underwent the informed consent process in their choice of English, Spanish, or Haitian Creole language. Participants gave written consent for their data to be published in aggregate and without identifiers. A signed copy of the consent form was given to participants to keep for their records. Confidentiality was maintained via the use of a study identifier; data were collected using study IDs, never using names or any other identifiable information. Study IDs are linked with parti-cipant identifiers in a master file at the Boston Medical Center, which is accessible only to authorized Boston Medical Center staff and the study principal investigator and requires a 2-step login process. No other data are stored in the master file. Data have not been anonymized because data collection is ongoing. There was no direct benefit to participants for their participation in this research. Participants were compensated with a small monetary incentive for both enrollment (at delivery) and longitudinal data collection.
Exposure variables
The exposures of interest were TMAO and its precursors choline, betaine, and carnitine. Metabolites were measured in venous umbilical cord blood (collected at birth) and maternal blood (collected within 1–3 d after delivery) plasma. Metabolites were analyzed by the Broad Institute of the Massachusetts Institute of Technology (MIT) and Harvard using LC-MS; metabolite concentrations were inversely normalized. Pooled samples were implemented as a quality control; the CVs of these metabolites were between 1% and 3%. Women with missing values on both maternal and cord metabolites were excluded from the analytic sample.
Outcome variables
The outcomes of interest included physician-diagnosed GDM and PE. GDM and PE were diagnosed during routine prenatal care visits and were recorded in electronic health records. Outcome data were extracted from medical records after study enrollment. Diagnoses were reviewed for consistency with contemporary American College of Obstetrics and Gynecology (ACOG) definitions. GDM was diagnosed between weeks 24 and 28 of gestation on the basis of a single oral-glucose-tolerance test (OGTT). PE was defined by new-onset hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg) plus proteinuria (excretion ≥300 mg in a 24-h urine collection) measured after 20 weeks of gestation; in the absence of proteinuria, PE may have been diagnosed if headache, blurred vision, abdominal pain, or abnormal laboratory results occurred (42, 43). One woman who had a diagnosis of eclampsia, but not PE, was classified as having PE for these analyses.
Covariates
Covariates were selected a priori based on a causal diagram of the hypothesized relations between these variables (Supplemental Figure 1) (44). Maternal age, parity, and prepregnancy hypertension and diabetes were extracted from medical records. Other demographic characteristics including self-reported race and ethnicity, educational attainment, marital status, and prepregnancy weight and height were ascertained via a questionnaire administered within 1–3 d after delivery, as was self-reported diet during pregnancy. Prepregnancy BMI (in kg/m2) was calculated as weight divided by height squared. Diet was not included as a covariate in the main analyses because the objective of the current study was to understand the associations of TMAO and its precursors with GDM and PE, regardless of whether these associations were driven by diet.
Statistical analyses
Exploratory data analysis was conducted to describe participant characteristics. Percentages were used to describe categorical variables and median [IQR] to describe continuous variables. Inversely normalized metabolite concentrations were modeled both in tertiles and as continuous variables. Spearman rank correlation coefficients were computed to examine the correlations between metabolites in cord and maternal blood. Available case analysis was implemented for each exposure–outcome pairing such that the crude and adjusted models for each pairing utilized the same number of observations. For example, if a mother had provided maternal blood, but not cord blood, she would be included in analyses of maternal metabolites. If a mother had missing information on GDM diagnosis, she could still be included in the analyses for PE. Mothers with missing covariate data were excluded from all analyses.
Maternal age, race and ethnicity, education, marital status, prepregnancy BMI, parity, and chronic prepregnancy hypertension were included as covariates in the models for GDM. The same variables, plus prepregnancy diabetes, were used in the analyses of PE. Women with prepregnancy diabetes were excluded from the GDM analyses because they were not at risk of developing new-onset gestational diabetes. Logistic regression was used to estimate the odds ratios (ORs) and 95% CIs of GDM and PE according to tertiles and to an SD increment of TMAO, choline, betaine, and carnitine. Finally, all analyses were run stratified by race and ethnicity (Black compared with all other race/ethnicities) to examine the consistency of results across groups. Potential race-by-exposure interactions were modeled to examine the possibility of effect measure modification by race/ethnicity.
Sensitivity analyses
To reduce the possibility that any observed associations were driven by prepregnancy conditions, we performed sensitivity analyses restricting to those without prepregnancy hypertension (for both GDM and PE), without prepregnancy diabetes (for PE only), and without either (for PE only). To try to disentangle the independent associations of each metabolite, we also performed sensitivity analyses including all 4 metabolites (as continuous variables) in a single regression model. To explore whether any observed associations persisted after accounting for dietary sources of TMAO and its precursors, all main analyses were run after additional adjustment for self-reported intake of meat, egg, and fish. To address the potential for selection bias from the larger cohort, participant characteristics of our analytic sample were first compared with the larger BBC, and then inverse probability weights of selection into the metabolite substudies (i.e., cord and maternal) were calculated, and these weights were applied to our logistic regression models. To estimate the weights, probability of selection into the study was modeled based on all variables in the final model plus gestational age at delivery (wk), delivery type (cesarean compared with vaginal), first sign of labor (contractions, membrane rupture, both, medically induced, or unknown), low birth weight, infant's sex, year of delivery, and maternal smoking (ever compared with never). Finally, to ensure that the eclampsia case did not drive our model results, we ran all models excluding this case from the analytic sample. Analyses were performed in Stata version 15 (StataCorp LLC). Significance levels were set at 0.05 for all main effects and interactions.
Results
Study sample
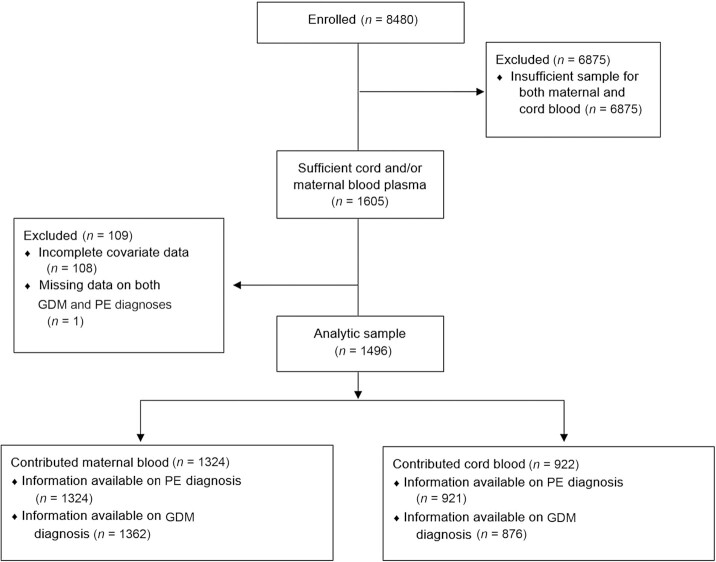
Among all 8480 women enrolled in the BBC up to 2013, 1605 women contributed maternal and/or cord blood (Figure 1). The 108 women who were missing information on covariates were excluded. An additional 1 woman was missing information on both GDM and PE diagnosis and was therefore excluded, leaving an analytic sample of 1496 women. Of these, 922 contributed cord blood and 1324 contributed maternal blood (750 contributed both). In total, 876 women contributed to the analyses of these metabolites in cord blood with GDM and 921 with PE; 1262 contributed to the analyses of the maternal blood metabolites with GDM and 1324 with PE.
FIGURE 1.
Participant flow diagram showing the flow of participants into the analytic sample from the parent study. Among 8480 women enrolled in the parent study, there were 1605 women with sufficient cord and/or maternal blood plasma; among those, 108 were excluded for incomplete covariate data and 1 was excluded for missing data on both GDM and PE diagnoses. Our analytic sample included the remaining 1496 women, 922 of whom contributed cord blood and 1324 of whom contributed maternal blood (750 contributed both). Among those included in the analytic sample who contributed cord blood, 46 were missing information on GDM diagnosis (n = 876 for GDM) and 1 was missing information on PE diagnosis (n = 921 for PE). Among those included in the analytic sample contributing maternal blood, 62 were missing information on GDM diagnosis (n = 1262 for GDM) and none were missing information on PE diagnosis (n = 1324 for PE). GDM, gestational diabetes mellitus; PE, pre-eclampsia.
Table 1 shows participant characteristics of the women contributing cord blood overall and stratified by cord TMAO tertile. The median age was 27.9 y. The majority of women self-identified as black (57.9%) or Hispanic (23.8%). Most had a high school education or equivalent (38.5%) or greater (33.5%) and approximately one-third were married (34.5%). The median prepregnancy BMI was 25.3. Most women (58.7%) had ≥1 previous live birth. Approximately 6% reported pre-existing chronic hypertension and 4.9% reported prepregnancy diabetes (type 1 or type 2). Women with higher cord blood TMAO were more likely to be older, have a history of diabetes, and have lower educational attainment. Women with higher cord TMAO were also more likely to be multiparous than nulliparous. Race/ethnicity, marital status, prepregnancy BMI, and the prevalence of prepregnancy chronic hypertension were similar across cord TMAO tertiles. Dietary sources of TMAO and its precursors, i.e., meat, eggs, and fish, were not associated with cord TMAO (data not shown). Supplemental Tables 1–7 show details of the study sample stratified by other metabolites (both in cord blood and in maternal blood).
TABLE 1.
Characteristics of study participants overall and by cord blood trimethylamine N-oxide tertile1
| Overall (n = 1000) | Tertile 1 (n = 334) | Tertile 2 (n = 333) | Tertile 3 (n = 333) | |
|---|---|---|---|---|
| Maternal age, y | 27.9 [22.9–33.4] | 26.6 [21.9–31.3] | 28.1 [23.1–33.5] | 29.7 [24.4–34.9] |
| Missing | 2 (0.2) | 1 (0.3) | 1 (0.3) | 0 (0.0) |
| Race/ethnicity | ||||
| Black | 579 (57.9) | 189 (56.6) | 185 (55.6) | 205 (61.6) |
| White | 54 (5.4) | 17 (5.1) | 19 (5.7) | 18 (5.4) |
| Hispanic | 238 (23.8) | 75 (22.5) | 84 (25.2) | 79 (23.7) |
| Other | 127 (12.7) | 52 (15.6) | 44 (13.2) | 31 (9.3) |
| Missing | 2 (0.2) | 1 (0.3) | 1 (0.3) | 0 (0.0) |
| Education | ||||
| Less than high school | 272 (27.2) | 106 (31.7) | 92 (27.6) | 74 (22.2) |
| High school or equivalent | 385 (38.5) | 114 (34.1) | 125 (37.5) | 146 (43.8) |
| More than high school | 335 (33.5) | 111 (33.2) | 112 (33.6) | 112 (33.6) |
| Missing | 8 (0.8) | 3 (0.9) | 4 (1.2) | 1 (0.3) |
| Marital status | ||||
| Unmarried | 642 (64.2) | 213 (63.8) | 207 (62.2) | 222 (66.7) |
| Married | 345 (34.5) | 118 (35.3) | 121 (36.3) | 106 (31.8) |
| Missing | 13 (1.3) | 3 (0.9) | 5 (1.5) | 5 (1.5) |
| Prepregnancy BMI, kg/m2 | 25.3 [22.3–29.8] | 24.9 [22.0–29.3] | 25.7 [22.2–30.2] | 25.2 [22.5–29.3] |
| Missing | 57 (5.7) | 14 (4.2) | 23 (6.9) | 20 (6.0) |
| Parity | ||||
| Nulliparous | 411 (41.1) | 151 (45.2) | 131 (39.3) | 129 (38.7) |
| Multiparous | 587 (58.7) | 182 (54.5) | 201 (60.4) | 204 (61.3) |
| Missing | 2 (0.2) | 1 (0.3) | 1 (0.3) | 0 (0.0) |
| Prepregnancy hypertension | 60 (6.0) | 22 (6.6) | 16 (4.8) | 22 (6.7) |
| Missing | 7 (0.7) | 2 (0.6) | 2 (0.6) | 3 (0.9) |
| Prepregnancy diabetes | 49 (4.9) | 13 (3.9) | 16 (4.8) | 20 (6.0) |
| Missing | 2 (0.2) | 1 (0.3) | 1 (0.3) | 0 (0.0) |
Values are median [IQR] for continuous variables and n (%) for categoric variables.
Intermetabolite correlations
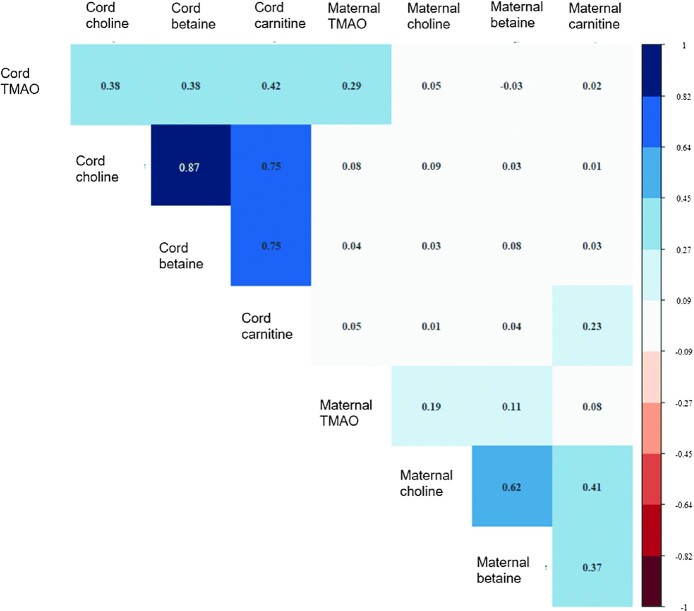
Spearman rank correlation coefficients among metabolites were stronger within cord blood (r = 0.38–0.87) than within maternal blood (r = 0.08–0.62) (see Figure 2). Correlations between the same metabolite measured in cord and maternal blood were low for choline (r = 0.09) and betaine (r = 0.08) and moderate for TMAO (r = 0.29) and carnitine (r = 0.23). There was low correlation between other metabolites across cord and maternal blood (r = −0.03 to 0.09).
FIGURE 2.
Heat map of Spearman rank correlation matrix for the included metabolites. Correlations between metabolites measured in cord blood ranged from 0.38 to 0.87 and correlations between metabolites measured in maternal blood ranged from 0.08 to 0.62. Correlations between the same metabolite measured in cord compared with maternal blood were 0.29 for TMAO, 0.09 for choline, 0.08 for betaine, and 0.23 for carnitine. Correlations between other metabolites across cord and maternal blood ranged from −0.03 to 0.09. TMAO, trimethylamine N-oxide.
GDM and PE
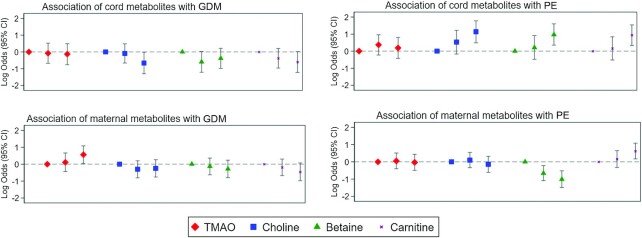
In our analytic sample, there were 115 GDM cases and 159 PE cases (Supplemental Table 8). Among the maternal metabolites analyzed, TMAO was associated with higher odds of GDM (third compared with first tertile multivariable-adjusted OR: 1.75; 95% CI: 1.04, 2.94), whereas TMAO precursors (choline, betaine, and carnitine) were not associated with GDM (OR: 0.78; 95% CI: 0.47, 1.30; OR: 0.76; 95% CI: 0.45, 1.28; OR: 0.63; 95% CI: 0.37, 1.07, respectively) (see Figure 3, Table 2). Concentrations of TMAO and choline in maternal blood were not associated with PE (OR: 0.97; 95% CI: 0.61, 1.55; OR: 0.87; 95% CI: 0.54, 1.38, respectively), whereas maternal blood carnitine was associated with higher odds of PE (OR: 1.86; 95% CI: 1.18, 2.94) and betaine was associated with lower odds of PE (OR: 0.37; 95% CI: 0.23, 0.59).
FIGURE 3.
Log ORs and 95% CIs for the association of cord and maternal blood metabolites with GDM and PE, comparing the middle and highest tertiles with the lowest tertile. These are log transformations of the results shown in Table 2. GDM, gestational diabetes mellitus; PE, pre-eclampsia; TMAO, trimethylamine N-oxide.
TABLE 2.
Associations of metabolites with GDM and PE in reference to the lowest tertile1
| Unadjusted | Multivariable adjusted | |||||
|---|---|---|---|---|---|---|
| Lowest | Middle | Highest | Lowest | Middle | Highest | |
| GDM | ||||||
| Cord TMAO | Ref. | 1.11 (0.63, 1.94) | 0.94 (0.52, 1.69) | Ref. | 0.92 (0.51, 1.68) | 0.88 (0.47, 1.63) |
| Cord choline | Ref. | 0.99 (0.58, 1.71) | 0.61 (0.33, 1.11) | Ref. | 0.92 (0.52, 1.62) | 0.52 (0.27, 0.99) |
| Cord betaine | Ref. | 0.69 (0.38, 1.23) | 0.82 (0.47, 1.42) | Ref. | 0.55 (0.30, 1.03) | 0.68 (0.37, 1.24) |
| Cord carnitine | Ref. | 0.78 (0.45, 1.37) | 0.68 (0.38, 1.21) | Ref. | 0.68 (0.38, 1.23) | 0.55 (0.30, 1.02) |
| Maternal TMAO | Ref. | 1.19 (0.69, 2.03) | 1.92 (1.16, 3.17) | Ref. | 1.12 (0.64, 1.94) | 1.75 (1.04, 2.94) |
| Maternal choline | Ref. | 0.87 (0.53, 1.42) | 0.89 (0.54, 1.45) | Ref. | 0.74 (0.45, 1.22) | 0.78 (0.47, 1.30) |
| Maternal betaine | Ref. | 0.98 (0.61, 1.59) | 0.81 (0.49, 1.34) | Ref. | 0.88 (0.53, 1.43) | 0.76 (0.45, 1.28) |
| Maternal carnitine | Ref. | 0.95 (0.59, 1.53) | 0.72 (0.43, 1.20) | Ref. | 0.83 (0.51, 1.35) | 0.63 (0.37, 1.07) |
| PE | ||||||
| Cord TMAO | Ref. | 1.45 (0.83, 2.55) | 1.44 (0.82, 2.53) | Ref. | 1.45 (0.80, 2.62) | 1.21 (0.66, 2.23) |
| Cord choline | Ref. | 2.01 (1.03, 3.92) | 3.66 (1.96, 6.81) | Ref. | 1.70 (0.85, 3.40) | 3.11 (1.62, 5.96) |
| Cord betaine | Ref. | 1.38 (0.71, 2.71) | 3.32 (1.84, 5.98) | Ref. | 1.24 (0.62, 2.48) | 2.65 (1.42, 4.93) |
| Cord carnitine | Ref. | 1.29 (0.67, 2.49) | 3.16 (1.77, 5.64) | Ref. | 1.17 (0.59, 2.31) | 2.56 (1.39, 4.69) |
| Maternal TMAO | Ref. | 1.09 (0.71, 1.69) | 1.15 (0.75, 1.76) | Ref. | 1.06 (0.67, 1.67) | 0.97 (0.61, 1.55) |
| Maternal choline | Ref. | 1.08 (0.71, 1.64) | 0.86 (0.55, 1.32) | Ref. | 1.11 (0.71, 1.73) | 0.87 (0.54, 1.38) |
| Maternal betaine | Ref. | 0.57 (0.38, 0.85) | 0.37 (0.24, 0.58) | Ref. | 0.52 (0.34, 0.80) | 0.37 (0.23, 0.59) |
| Maternal carnitine | Ref. | 1.09 (0.68, 1.73) | 1.44 (0.82, 2.53) | Ref. | 1.18 (0.72, 1.92) | 1.86 (1.18, 2.94) |
Values are ORs (95% CIs). Multivariable-adjusted models adjusted for maternal age (y; continuous), race/ethnicity (black, white, Hispanic, other), educational attainment (less than high school, high school or equivalent, greater than high school), marital status (unmarried, married), prepregnancy BMI (in kg/m2; continuous), parity (0, ≥1), chronic prepregnancy hypertension (no/yes), and prepregnancy diabetes (no/yes; for PE models only). GDM, gestational diabetes mellitus; PE, pre-eclampsia; TMAO, trimethylamine N-oxide.
In cord blood, TMAO was not associated with GDM or PE (OR: 0.88; 95% CI: 0.47, 1.63 for GDM; OR: 1.21; 95% CI: 0.66, 2.23 for PE), but the TMAO precursors choline, betaine, and carnitine were associated with higher odds of PE (OR: 3.11; 95% CI: 1.62, 5.96; OR: 2.65; 95% CI: 1.42, 4.93; OR: 2.56; 95% CI: 1.39, 4.69, respectively). Cord blood choline was associated with lower odds of GDM (OR: 0.52; 95% CI: 0.27, 0.99), whereas cord blood betaine and carnitine showed U-shaped and inverse trends, respectively (second tertile compared with first tertile OR: 0.55; 95% CI: 0.30, 1.03; third compared with first tertile OR: 0.68; 95% CI: 0.37, 1.24 for betaine; third compared with first tertile OR: 0.55; 95% CI: 0.30, 1.02 for carnitine) (see Table 2). Cord blood TMAO was not associated with GDM (OR: 0.88; 95% CI: 0.47, 1.63). Results modeling these metabolites as continuous variables were consistent with the results according to tertiles (Supplemental Table 9). Findings were also consistent across strata of self-reported race/ethnicity (black compared with all other race/ethnicities) and there was no statistical evidence of exposure-by-race interactions (all P ≥ 0.05) (see Supplemental Table 10).
Sensitivity analyses
Among women entering pregnancy without hypertension, diabetes, or either, odds of PE were still increased among those in the highest compared with the lowest tertile of cord choline, betaine, and carnitine (Supplemental Table 11). Results for maternal blood betaine and carnitine with PE, and maternal blood TMAO with GDM, were also consistent with the main analyses. After adjusting for precursor metabolites, the association of maternal TMAO with GDM persisted (multivariable-adjusted OR per SD increment: 1.23; 95% CI: 1.00, 1.52) (Supplemental Table 12). The inverse association of cord choline with GDM was also robust to adjustment for other cord metabolites (OR: 0.62; 95% CI: 0.35, 1.07). The associations of maternal betaine and carnitine with PE were both preserved when adjusting for all other exposures (OR: 0.46; 95% CI: 0.36, 0.59 and OR: 1.84; 95% CI: 1.48, 2.29, respectively). Finally, the associations of cord blood betaine and carnitine with PE disappeared when adjusting for other metabolites, but the association of choline with PE remained (OR: 1.69; 95% CI: 1.02, 2.79). Adjustment for diet yielded similar results to the main analyses across all exposure–outcome pairings (Supplemental Table 13).
In terms of potential selection bias, we found some differences in participant characteristics between women in the entire cohort and women selected for the metabolite substudy (Supplemental Tables 14 and 15), including differences in race, education, prepregnancy BMI, prepregnancy chronic hypertension, and prepregnancy diabetes. Those who had metabolites analyzed were also more likely to be black and less likely to be white or Hispanic than those who did not have metabolites analyzed. They were also more likely to have a high school education or equivalent. They had higher BMI and were more likely to enter into pregnancy with type 1 or type 2 diabetes. Those who contributed cord blood were more likely to enter into pregnancy with chronic hypertension than those who did not contribute cord blood. To address this potential selection bias we conducted inverse probability weighting for selection into the sample, and we found that the estimates were similar to those of our main model (Supplemental Table 16). Finally, when omitting the eclampsia case from the analytic sample, results of the main analyses were unchanged (data not shown).
Discussion
In our urban, racially and ethnically diverse population, we found that TMAO concentration in maternal blood was associated with higher odds of GDM, whereas maternal blood TMAO precursors were not associated with GDM. Cord blood choline was associated with lower odds of GDM, whereas cord blood betaine and carnitine showed similar (or U-shaped, for betaine) trends. Unlike maternal blood TMAO, cord blood TMAO was not associated with GDM. Further, TMAO in either cord or maternal blood was not associated with odds of PE; however, higher concentrations of cord blood choline, betaine, and carnitine, as well as maternal carnitine, were associated with greater odds of PE, whereas higher maternal blood betaine was associated with lower odds of PE.
The finding of a positive dose-response-like association of maternal TMAO with GDM adds to a mixed literature base on this association. Two studies, both case-control studies conducted in China using maternal blood, have reported on TMAO and GDM. One conducted both a nested (433 cases and 433 controls) and a nonnested (276 cases and 552 controls) matched case-control study (22). Both the nested and nonnested studies found higher odds of GDM among those in the highest quartile of maternal TMAO, which was measured concurrently with GDM (24–32 weeks of gestation) in the nonnested study and at the first prenatal care visit (≤16 weeks of gestation) in the nested study (22). Contrary to the findings of the current study, and to those of the aforementioned study, a second study of GDM and TMAO found an inverse association between maternal TMAO and GDM, which tended toward being U-shaped (23). In addition, that study found inverse associations of maternal betaine and l-carnitine with GDM, and a U-shaped association for choline chloride (23). The current study found similar patterns for betaine and carnitine. It may be possible that the TMAO precursors are not related to GDM, but it is their conversion to TMAO, based on the composition of the gut microbiome, that is associated with GDM. Future studies might aim to disentangle whether these associations are modified by diet or by the gut microbiome.
Contrary to existing studies, we did not find an association of maternal TMAO with PE. Other studies, again case-control studies conducted in China using maternal blood, have found positive associations between TMAO and PE. One found that third-trimester TMAO was higher among women with PE, and tended to be higher in a dose-dependent manner with severity of PE (19). A second found that TMAO was higher among women with PE than among their gestational age–matched controls (20). The third found that TMAO measured during the second trimester was not associated with PE but TMAO measured at delivery was strongly associated with PE, early-onset PE, and severe PE (21). Discrepant findings for both GDM and PE may be partially explained by differences in the timing of blood collection, assay methods, or underlying confounding structure between the study populations, or by unmeasured effect modifiers that may differ between populations. In addition to TMAO, our finding that cord carnitine was associated with higher odds of PE in both maternal and cord blood is in line with the results of existing studies (19–21). Our finding that, after adjustment for other cord metabolites, only choline remained significantly associated with PE suggests that, of choline, betaine, and carnitine, choline may be the most related to PE pathology—a finding that deserves further investigation.
The finding that several metabolites trended in opposite directions, depending on whether they were measured in cord blood or maternal blood, was an unexpected observation that merits further attention. This was most evident for betaine, which was positively associated with the odds of PE when measured in cord blood, but inversely associated with PE when measured in maternal blood. It is known that certain nutrients are preferentially allocated to the fetus, even when maternal supply is low. For example, folate, an essential nutrient for both mother and fetus, has been demonstrated to follow this pattern, perhaps because of its involvement in neural tube development and other essential functions (35–37). As such, it is possible that other essential nutrients including choline, betaine, and carnitine are allocated similarly. Alternatively, it is possible that this finding occurred by chance and therefore it needs to be replicated in other studies.
As a point of caution, it is important to note that circulating TMAO, choline, betaine, and carnitine are not equivalent to TMAO, choline, betaine, and carnitine consumed through the diet. Given the limitations of our study, we cannot say, for example, whether the association of cord blood choline with PE is due to differences in choline intake, differences in the metabolic breakdown of choline, or even due to PE pathology itself (i.e., reverse causation). To consider limiting dietary choline, or making any dietary changes based on the results of the current study, is premature and potentially harmful. The body's demand for choline increases during pregnancy as the nutrient supports the fetus's central nervous system development. Further, most pregnant women do not currently meet the recommended amount of choline in their diets (45). Further research is needed both to establish the temporality of the observed associations and to determine, if causal, whether GDM and PE risk can be reduced by a modified diet early in pregnancy.
This study is not without limitations. First, blood was taken at or shortly after delivery rather than before or simultaneously with diagnosis of GDM or PE. As such, it is not possible to know whether these metabolite profiles preceded disease pathology and diagnosis; it is possible that the metabolite concentrations are the result of the cardiometabolic conditions, rather than vice versa, or that changes to the diet during pregnancy or hospitalization are reflected in the measured values. Mothers with GDM or PE may have been hospitalized longer before delivery than mothers with uncomplicated pregnancies, potentially inducing an association between the metabolites and these conditions.
Second, although the overarching goal of the current study was to examine associations of TMAO and its precursors with GDM and PE, the ability to disentangle whether these associations were influenced or modified by potentially modifiable factors such as diet and/or the gut microbiome was limited by the dietary measures available in this cohort as well as the lack of microbiome data. Although the temporality of the measurements of exposures and outcomes in this study precluded the ability to draw any inferences about causality, it is still of interest to examine whether these associations persist independently of diet. Like exposure data, dietary data would ideally be collected throughout pregnancy, including before outcome ascertainment, allowing for any changes during pregnancy to be reflected in the data. Further, differentiation between any meat and red meat would more specifically reflect carnitine intake. Sources of TMAO may matter for disease risk. For example, TMAO is contained in fish and other seafoods, although fish has been paradoxically associated with lower cardiometabolic disease risk (46–49). In the current study, dietary sources of TMAO and its precursors were not strongly associated with any of the metabolites measured in either cord or maternal blood. This could be due to limitations of the dietary assessment, or it could be an indication that something unmeasured, e.g., the composition of the gut microbiome, is more important for determining circulating metabolite concentrations. Recent evidence suggests the latter is likely (15, 18).
Third, because TMAO, choline, betaine, and carnitine are in the same metabolic pathway, regression adjustment looking at each metabolite in isolation, or holding all others constant, may not be the most biologically relevant model for these associations. Methods for assessing exposure mixtures, such as quantile g-computation or Bayesian kernel machine regression, should be considered to assess the effects of summed exposures and their components (50–54). These more advanced methods alone would not negate the limitations of the current study, but, combined with solutions to the aforementioned limitations, may be useful in future studies.
Despite these limitations, this study has a number of strengths. To our knowledge, this is the first to have considered the group of precursors in addition to TMAO as potential markers of GDM and PE and, further, the first to have conducted a targeted analysis of these metabolites in both cord blood and maternal blood. Second, the study used objective measures of these metabolites which, although potentially influenced by diet, unlike diet are not subject to recall or social desirability biases. Third, the sample size was larger than those of many existing similar studies, which allowed for adjustment for a number of covariates and allowed us to conduct race-specific analyses and to test for effect measure modification by race/ethnicity. Fourth, and relatedly, our study population contained a large proportion of Black and Hispanic mothers, low-income mothers, and mothers with urban residence. These populations have been historically underrepresented in research in general, and in studies of these associations in particular. Further, it is important to examine these associations in a cohort of predominantly black mothers because Black mothers experience pregnancy complications at disproportionate rates in the United States (55–59).
In conclusion, these findings add to the evidence that TMAO and its precursors may play a role in cardiometabolic disease pathology during pregnancy. Future studies that measure these metabolites throughout pregnancy, especially before GDM and PE development and diagnosis, are warranted to establish the temporality of these associations. Future research would benefit from detailed, trimester-specific measures of relevant dietary data in order to assess the extent of potential confounding by dietary intake and to determine whether diet during pregnancy is a modifiable risk factor for these conditions. In conjunction with improved dietary data collection and early metabolite measurement, microbiome data would allow for the investigation of potential effect measure modification in the relation between diet and TMAO. Future studies may also wish to examine the associations of these metabolites with the components of GDM and PE (e.g., blood glucose, blood pressure, proteinuria) in order to disentangle potential etiologic mechanisms for these associations. As our understanding of dietary and microbiome contributors to TMAO and these related metabolites advances, we hope this line of research can help to prevent cardiometabolic disease in pregnancy and beyond through targeted interventions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—KLM and NTM: conceived of the project idea and wrote the paper; KLM: performed the statistical analysis; MZ, XH, GW, and XW: provided their expert knowledge of the cohort and study conduct; JPB: consulted on the sensitivity analyses and discussion; and all authors: read and approved the final manuscript.
Notes
Supported by National Heart, Lung, and Blood Institute grant K01HL141589 (to NTM) and American Heart Association award 827990 (to MZ). The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605); National Institutes of Health grants (R21ES011666, 2R01HD041702, R21HD066471, R01HD086013, R01HD098232, R01 ES031272, and R01ES031521); and the Health Resources and Services Administration of the US Department of Health and Human Services (UJ2MC31074). . This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, any funding agencies.
Author disclosures: NTM is on the scientific advisory board of Tiny Health Inc. Otherwise, the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplemental Figure 1 and Supplemental Tables 1–16 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: BBC, Boston Birth Cohort; GDM, gestational diabetes mellitus; PE, pre-eclampsia; TMAO, trimethylamine N-oxide.
Contributor Information
Kristen L McArthur, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Mingyu Zhang, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, MD, USA.
Xiumei Hong, Department of Population, Family and Reproductive Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Guoying Wang, Department of Population, Family and Reproductive Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Jessie P Buckley, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Xiaobin Wang, Department of Population, Family and Reproductive Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Pediatrics, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Noel T Mueller, Email: noeltmueller@jhu.edu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, MD, USA.
Data Availability
Data described in the article, codebook, and analytic code will be made available upon request pending Institutional Review Board review and approval.
References
- 1. Centers for Disease Control and Prevention . Pregnancy Mortality Surveillance System [Internet]. Atlanta, GA: CDC; 2020; [cited April 2021]. Available from: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. [Google Scholar]
- 2. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64. [DOI] [PubMed] [Google Scholar]
- 3. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237–60. [DOI] [PubMed] [Google Scholar]
- 4. Catalano PM. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J Dev Orig Health Dis. 2010;1(4):208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallis AB, Saftlast AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–6. [DOI] [PubMed] [Google Scholar]
- 6. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JAet al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications: systematic review and meta-analysis. Circulation. 2019;139(8):1069–79. [DOI] [PubMed] [Google Scholar]
- 7. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. [DOI] [PubMed] [Google Scholar]
- 8. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton Cet al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497. [DOI] [PubMed] [Google Scholar]
- 10. Zhuang R, Ge X, Han L, Yu P, Gong X, Meng Qet al. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes Rev. 2019;20(6):883–94. [DOI] [PubMed] [Google Scholar]
- 11. Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu Get al. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose–response meta-analysis. Adv Nutr. 2020;11(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farhangi MA, Vajdi M, Asghari-Jafarabadi M. Gut microbiota-associated metabolite trimethylamine N-oxide and the risk of stroke: a systematic review and dose–response meta-analysis. Nutr J. 2020;19(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FBet al. Long-term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J Am Coll Cardiol. 2020;75(7):763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farhangi MA. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: findings from an updated systematic review and meta-analysis. Nutrition. 2020;78:110856. [DOI] [PubMed] [Google Scholar]
- 15. Tilves C, Mueller NT. Trimethylamine N-oxide variation in humans: the product of a diet–microbiota interaction?. Am J Clin Nutr. 2021;113(6):1400–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamaya R, Ivey KL, Lee DH, Wang M, Li J, Franke Aet al. Association of diet with circulating trimethylamine-N-oxide concentration. Am J Clin Nutr. 2020;112(6):1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu BC, Hullar MAJ, Randolph TW, Franke AA, Monroe KR, Cheng Iet al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020;111(6):1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mei Z, Chen G-C, Wang Z, Usyk M, Yu B, Baeza YVet al. Dietary factors, gut microbiota, and serum trimethylamine-N-oxide associated with cardiovascular disease in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2021;113(6):1503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen Y, Peng L, Xu R, Zang N, Huang Q, Zhong M. Maternal serum trimethylamine-N-oxide is significantly increased in cases with established preeclampsia. Pregnancy Hypertens. 2019;15:114–17. [DOI] [PubMed] [Google Scholar]
- 20. Wang J, Gu X, Yang J, Wei Y, Zhao Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front Cell Infect Microbiol. 2019;9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang X, Li Z, Gao Z, Wang D, Li X, Li Yet al. Association between risk of preeclampsia and maternal plasma trimethylamine-N-oxide in second trimester and at the time of delivery. BMC Pregnancy Childbirth. 2020;20(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li P, Zhong C, Li S, Sun T, Huang H, Chen Xet al. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr. 2018;108(3):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huo X, Li J, Cao Y-F, Li S-N, Shao P, Leng Jet al. Trimethylamine N-oxide metabolites in early pregnancy and risk of gestational diabetes: a nested case-control study. J Clin Endocrinol Metab. 2019;104(11):5529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thiele IG, Niezen-Koning KE, van Gennip AH, Aarnoudse JG. Increased plasma carnitine concentrations in preeclampsia. Obstet Gynecol. 2004;103(5 Pt 1):876–80. [DOI] [PubMed] [Google Scholar]
- 25. Ryckman KK, Shchelochkov OA, Cook DE, Berberich SL, Copeland S, Dagle JMet al. The influence of maternal disease on metabolites measured as part of newborn screening. J Matern Fetal Neonatal Med. 2013;26(14):1380–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jääskeläinen T, Kärkkäinen O, Jokkala J, Litonius K, Heinonen S, Auriola Set al. A non-targeted LC-MS profiling reveals elevated levels of carnitine precursors and trimethylated compounds in the cord plasma of pre-eclamptic infants. Sci Rep. 2018;8(1):14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin X, Zhang Y, He X, Chen Y, Chen N, Liu Jet al. The choline metabolite TMAO inhibits NETosis and promotes placental development in GDM of humans and mice. Diabetes. 2021;70(10):2250–63. [DOI] [PubMed] [Google Scholar]
- 28. Chen H, Li J, Li N, Liu H, Tang J. Increased circulating trimethylamine N-oxide plays a contributory role in the development of endothelial dysfunction and hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy. 2019;38(2):96–104. [DOI] [PubMed] [Google Scholar]
- 29. Bernhard W, Raith M, Kunze R, Koch V, Heni M, Maas Cet al. Choline concentrations are lower in postnatal plasma of preterm infants than in cord plasma. Eur J Nutr. 2015;54(5):733–41. [DOI] [PubMed] [Google Scholar]
- 30. Bernhard W, Poets CF, Franz AR. Choline and choline-related nutrients in regular and preterm infant growth. Eur J Nutr. 2019;58(3):931–45. [DOI] [PubMed] [Google Scholar]
- 31. Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grube M, Meyer zu Schwabedissen H, Draber K, Präger D, Möritz KU, Linnemann Ket al. Expression, localization, and function of the carnitine transporter OCTN2 (SLC22A5) in human placenta. Drug Metab Dispos. 2005;33(1):31–7. [DOI] [PubMed] [Google Scholar]
- 33. Korsmo HW, Jiang X, Caudill MA. Choline: exploring the growing science on its benefits for moms and babies. Nutrients. 2019;11(8):1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Brenna JTet al. Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am J Clin Nutr. 2013;98(6):1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacquemyn Y, Ajaji M, Karepouan N, Jacquemyn N, Van Sande H. Vitamin B12 and folic acid status of term pregnant women and newborns in the Antwerp region, Belgium. Clin Exp Obstet Gynecol. 2014;41(2):141–3. [PubMed] [Google Scholar]
- 36. Wallace JM, Bonham MP, Strain J, Duffy EM, Robson PJ, Ward Met al. Homocysteine concentration, related B vitamins, and betaine in pregnant women recruited to the Seychelles Child Development Study. Am J Clin Nutr. 2008;87(2):391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Antony AC. In utero physiology: role of folic acid in nutrient delivery and fetal development. Am J Clin Nutr. 2007;85(2):598S–603S. [DOI] [PubMed] [Google Scholar]
- 38. Shafi T, Powe NR, Meyer TW, Hwang S, Hai X, Melamed MLet al. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol. 2017;28(1):321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai H-J, Liu X, Mestan K, Yu Y, Zhang S, Fang Yet al. Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on G×E interactions and pathogenic pathways. Hum Genet. 2008;123(4):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong X, Zhang B, Liang L, Zhang Y, Ji Y, Wang Get al. Postpartum plasma metabolomic profile among women with preeclampsia and preterm delivery: implications for long-term health. BMC Med. 2020;18(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang Get al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. [DOI] [PubMed] [Google Scholar]
- 42. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 43. Bustamante Helfrich B, Chilukuri N, He H, Cerda SR, Hong X, Wang Get al. Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort. Placenta. 2017;52:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 45. USDA, US Department of Health and Human Services (DHHS) . Dietary Guidelines for Americans, 2020–2025. 9th ed. Washington (DC): USDA and US DHHS; 2020. [Google Scholar]
- 46. Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer ARet al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–11. [DOI] [PubMed] [Google Scholar]
- 48. Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123(24):2870–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Landfald B, Valeur J, Berstad A, Raa J. Microbial trimethylamine-N-oxide as a disease marker: something fishy?. Microb Ecol Health Dis. 2017;28(1):1327309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamra GB, Buckley JP. Environmental exposure mixtures: questions and methods to address them. Curr Epidemiol Rep. 2018;5(2):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valeri L, Mazumdar MM, Bobb JF, Claus HB, Rodrigues E, Sharif OIAet al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20–40 months of age: evidence from rural Bangladesh. Environ Health Perspect. 2017;125(6):067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bobb JF, Valeri L, Claus HB, Christiani DC, Wright RO, Mazumdar Met al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Keil AP, Buckley JP, O'Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4):047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmidt S. Quantile g-computation: a new method for analyzing mixtures of environmental exposures. Environ Health Perspect. 2020;128(10):104004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The Black–White disparity in pregnancy-related mortality from 5 conditions: differences in prevalence and case-fatality rates. Am J Public Health. 2007;97(2):247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosenthal L, Lobel M. Explaining racial disparities in adverse birth outcomes: unique sources of stress for Black American women. Soc Sci Med. 2011;72(6):977–83. [DOI] [PubMed] [Google Scholar]
- 58. Liese KL, Mogos M, Abboud S, Decocker K, Koch AR, Geller SE. Racial and ethnic disparities in severe maternal morbidity in the United States. J Racial Ethn Health Disparities. 2019;6(4):790–8. [DOI] [PubMed] [Google Scholar]
- 59. Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, codebook, and analytic code will be made available upon request pending Institutional Review Board review and approval.