POPULATION MIGRATION OF SERRATIA LIQUEFACIENS MG1 AS AN EXAMPLE OF MULTICELLULAR PROKARYOTIC BEHAVIOR
The view of bacteria as unicellular organisms has strong roots in the traditional way of culturing bacteria in liquid medium. Although studies of bacterial activities during conditions of balanced growth have lead to insight into basic life processes and have unraveled complex regulatory networks, it must be emphasized that in nature microbial activity is often associated with surfaces (16). In fact, it appears that the ability to form surface-associated structured and cooperative consortia (referred to as biofilms) is one of the most remarkable characteristics of bacteria. Moreover, the observation that bacteria undergo cell differentiation when they grow in colonies and the discovery of sophisticated intercellular communication systems have shown that bacteria are much more interactive than previously realized (81). Communication capabilities are considered to be essential prerequisites for coordinated bacterial activities. The communication language is in most cases chemical in nature. Signal molecules that are released by specialized cells are thought to modulate the activity of other cells in the vicinity, thus regulating collective activities (involving many different genes connected by joint control factors). This minireview focuses on one example of coordinated bacterial activity, namely, the migration of populations by means of swarming motility in the strain S. liquefaciens MG1.
SWARMING PHENOMENON
Bacterial surface translocation.
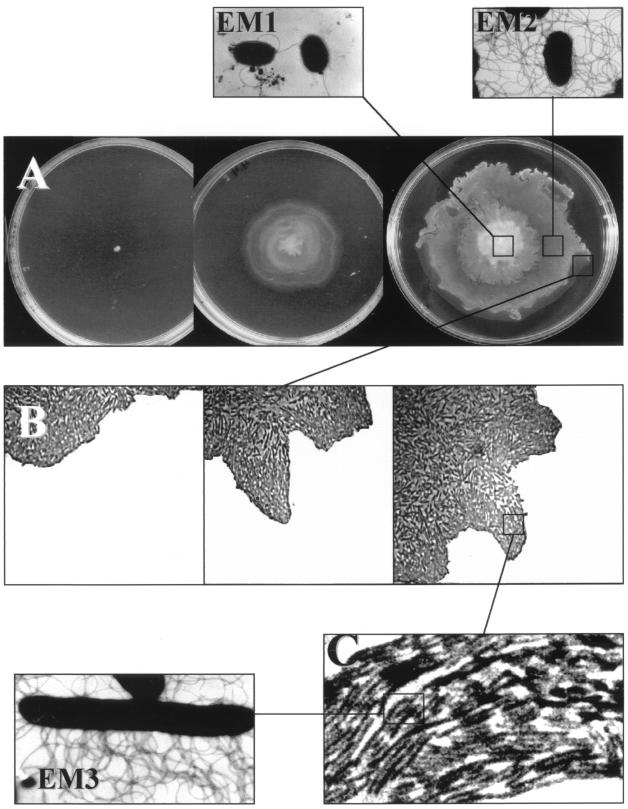
Swarming is one of six described forms of bacterial surface translocation (including swimming, gliding, twitching, darting, and sliding) (45). The ability of members of the genus Proteus to swarm on solid medium has interested many microbiologists since the phenomenon was described by Hauser more than a century ago (42). However, swarming is not limited to the genus Proteus but has been demonstrated for a wide range of diverse bacteria, and it is thought that this form of motility is ubiquitous among eubacteria (40). Swarming motility is driven by the operation of peritrichously arranged flagella, which are thought to function as helical propellers driven by a biological rotary motor (44). The development of a swarming colony on an agar plate follows three major steps (Fig. 1). First, a regular colony is formed at the inoculation point. Thereafter, the cells at the rim of the colony initiate a differentiation process resulting in long (up to 50-μm) multinucleated, aseptate, hyperflagellated cells, which have the unique ability to move on top of the agar surface (Fig. 1B). Microscopic inspection reveals that the differentiated cells organize in highly motile rafts that form an outer, motile layer that moves in a swirling fashion (Fig. 1C). By analogy to swarming bees, this type of multicellular bacterial behavior is referred to as swarming motility. The rapid outward movement of the swarm cells at the rim of the swarm colony is accompanied by bacterial growth inside the colony, resulting in extremely fast colonization of all available surface space. We have recorded velocities of colony expansion of up to 10 mm/h for S. liquefaciens MG1.
FIG. 1.
Growth of an expanding swarm culture on 0.6% agar. (A) Top view. (B) Microscopic inspection of the swirling cells at the outer region of the expanding colony. Cells have been sampled from the part of the colony in the squares and examined by electron microscopy (EM1 to -3). The central part of the colony contains cells that entered stationary phase, as judged from their appearance as round, less-flagellated cells (EM1). In the middle of the colony, the vegetative (biomass-producing) cells exhibit the swim cell morphology (EM2), and finally at the border, the highly motile, flagellated, and elongated swarm cells (EM3) (24) are organized in rafts (C).
Swarming motility is an intrinsically surface-linked and cell density-dependent phenomenon involving cell differentiation, extensive flagellation, contact between neighboring bacteria, and in particular, highly coordinated migration of swarm cells. Various extracellular compounds, such as biosurfactants and polysaccharides (2, 54, 58), facilitate surface translocation. The high degree of coordination between the cells within a swarm colony and the observation that separated swarm cells are unable to swarm suggest that this form of surface translocation has to be considered a social phenomenon. This is in sharp contrast to swimming, where cells move separately in periods of smooth runs interrupted by short tumbles in an apparently unorganized manner.
Medium effects.
S. liquefaciens MG1 is capable of swimming and swarming motility. The critical factor that determines whether cells swim, differentiate into swarm cells, or form a regular colony is the agar concentration and thus the viscosity of the medium. On media containing low agar concentrations (<0.4%), the strain exhibits swimming motility, while on media solidified with 0.4 to 1.2% agar (with an optimum colony expansion rate at 0.7%), the strain swarms atop the agar surface. On media with higher agar concentrations, migration of the strain is inhibited and consequently a normal-sized colony is formed. This is in sharp contrast to Proteus mirabilis and Vibrio parahaemolyticus, which are able to form swarming colonies on the surface of 2.0% agar. At the other extreme, Escherichia coli will form swarming colonies only on Eiken agar (41).
In P. mirabilis, swarming is cyclical in nature. The repetition of alternating phases of swarming and consolidation (dedifferentiation into vegetative cells) leads to the formation swarm colonies with regularly spaced concentric terraces (26). Swarming colonies of S. liquefaciens MG1 do not produce concentric zones of consolidation, but on some media like minimal medium containing gelatin, dentritic fractal consolidation patterns have been observed (23). As with Proteus, swarming of S. liquefaciens MG1 is strongly promoted by rich media (25, 48). Swarming on minimal medium is observed only when the medium is supplemented with a mixture of amino acids (such as Casamino Acids). V. parahaemolyticus will form swarm colonies on minimal medium (66). For P. mirabilis it has been shown that a single amino acid, glutamine, is sufficient to induce swarming motility (1). Swarming migration of S. liquefaciens MG1, however, cannot be promoted by the addition of any of the naturally occurring amino acids to minimal medium (24, 25). The doubling time of S. liquefaciens MG1 grown in liquid minimal medium was found to be significantly increased in the presence of even very low concentrations of Casamino Acids (0.01%), suggesting that the requirement of amino acids for swarming is likely to be attributed to the stimulation of growth (23). At present, we hypothesize that the indispensable requirement of amino acids may reflect a high demand for both building blocks and energy to synthesize and operate the hundreds of flagella produced during swarming differentiation.
GENETICS OF SWARMING
Two regulatory systems.
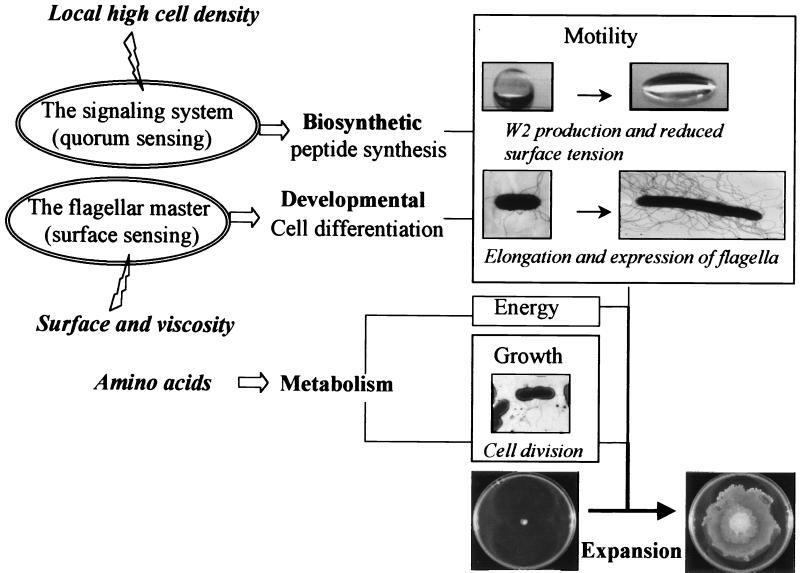
We have identified two key regulators in S. liquefaciens MG1 that are involved in the regulation of swarming behavior, namely, the flagellar master FlhD-FlhC and a N-acyl-l-homoserine lactone (AHL)-based quorum-sensing system (24, 25). In enteric bacteria, FlhD-FlhC controls expression of the entire flagellar hierarchy (56). Quorum-sensing systems based on AHL signal molecules have been reported for a variety of gram-negative bacteria and were demonstrated to control diverse physiological processes in concert with cell density (27, 28, 88). In most cases, these cell-cell communication circuits are involved in the production of extracellular products that are essential for the interaction of bacteria with each other and their surroundings. We recently demonstrated that the flagellar master and the quorum-sensing system control two separate regulons (33). In our model for control and development of a swarming colony, the flagellar master and the quorum-sensing system control two equally important pathways, a developmental pathway and a biosynthetic pathway, respectively (Fig. 2). In addition to this, energy-generating metabolism is required for swarming behavior of S. liquefaciens MG1.
FIG. 2.
Summary of the two major sensory, regulatory systems (ovals) involved in swarm cell differentiation and surface motility. Inducing stimuli (lightning) point to their respective sensory system. The fat horizontal arrows indicate the pathways targeted by the regulatory systems. The rectangles summarize the biological processes the combined action of which leads to expansion of the colony.
DEVELOPMENTAL PATHWAY
Regulation of the flagellar master.
In E. coli and Salmonella typhimurium, the flhDC operon encodes the transcriptional regulators FlhD and FlhC that controls the expression of approximately 50 genes related to flagellar structure, chemotaxis, and cell division (56). A recent analysis of transposon insertion mutants of S. liquefaciens MG1 that were isolated on the basis of their inability to swim indicates a substantial degree of homology with the E. coli flagellar hierarchy (13). In E. coli, expression of the flhDC operon is tightly regulated in response to environmental signals by the complex interplay of various regulatory systems and factors. Among those are catabolite repression (84), the phosphorylation status of the osmoregulator OmpR (77, 87), heat shock proteins (DnaK, DnaJ, and GrpE), acetyl phosphate (83), autogenous control, and cell cycle regulation (78, 79). In E. coli as in S. liquefaciens MG1, overexpression of flhDC causes inhibition of growth, and a connection between synthesis of flagella and cell division has been suggested (24, 78). In E. coli this coupling is mediated by FlhD which has been demonstrated to regulate the cell division rate via the acid response gene cadA (80).
Flagellar master and cell differentiation.
A flhDC null mutant of S. liquefaciens MG1 is devoid of flagella and is thus unable to swim or swarm (24). Controlled expression of the flhDC operon from an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Ptac promoter, however, leads to flagellar synthesis and restores both swimming and swarming. Moreover, overexpression of flhDC in liquid medium has been demonstrated to induce swarm cell differentiation. As a result, filamentous, multinucleated, and hyperflagellated cells are formed; these cells are indistinguishable from swarm cells isolated from the edge of a swarm colony (24). Thus, artificial stimulation of flhDC expression can overcome the otherwise obligatory requirement of surface contact. This indicates that at least the sensing of surface contact, which is the major stimulus for swarm cell differentiation, is channeled through the flhDC operon. Recent studies with P. mirabilis (29) and Yersinia enterolitica (96) were consistent with our results in S. liquefaciens MG1 and strengthened the view that the flhDC master operon is a major checkpoint for swarming behavior in different bacteria. The formation of a swarming colony would be most readily explained by assuming that the level of flhDC mRNA is specifically increased. In fact, Northern analysis of mRNA levels in P. mirabilis has demonstrated that the amount of flhDC mRNA is more than 30-fold higher in swarm cells than the amount found in vegetative cells (29). With S. liquefaciens MG1, we have not been able to detect any significant increase in flhDC transcription in differentiated swarm cells (89).
There are obvious differences among the enterics with respect to flhDC regulation. For example, in sharp contrast to E. coli, activation of the S. liquefaciens MG1 flhDC operon is independent of a functional Crp protein as crp mutants remain fully motile (13). Differentiated swarm cells of Proteus express a 50-fold increase in surface flagella, while MG1 displays only a modest increase (31, 53, 89). In contrast to S. liquefaciens MG1, several loci affecting flhDC expression at the transcriptional level such as ppaA and lrp have been identified in P. mirabilis (53). In P. mirabilis, expression of flhDC is positively regulated by the leucine-responsive regulatory protein Lrp (43). More recently four genes of unknown function have been identified in P. mirabilis that up-regulate expression of the flhDC operon when provided on a multicopy plasmid in trans (22). Inspection of the DNA sequences upstream of the flhDC genes from the different bacteria revealed a great degree of variation, while the coding regions were found to be highly conserved (29, 30).
Surface sensing.
V. parahemolyticus has been demonstrated to sense its presence on a surface with its polar flagellum that functions as a tactile sensor measuring external forces influencing its motion (65). Under conditions that render the polar flagellum nonfunctional, e.g., in highly viscous medium or on surfaces, expression of swarm cell-specific genes, in particular the lateral swarm flagella, is induced. As in P. mirabilis, S. liquefaciens MG1 produces only one type of flagellum, and so far the sensor of surface contact in these bacteria has not been identified.
BIOSYNTHETIC PATHWAY
Quorum sensing.
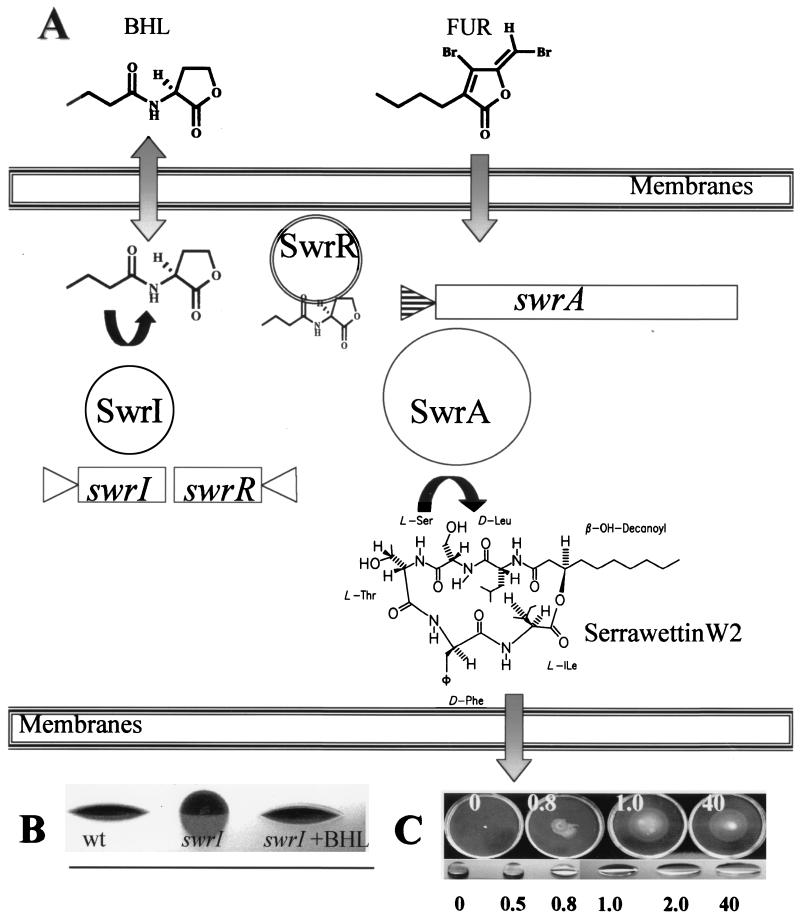
S. liquefaciens MG1 produces two extracellular signal molecules, N-butyrylhomoserine lactone (BHL) and N-hexanoylhomoserine lactone (HHL), that are used for sensing the density of the population (25). In recent years this type of regulatory system has been identified in various gram-negative bacteria and is referred to as the quorum-sensing system (27, 28, 88). It relies upon the presence of two proteins, a signal generator (AHL synthase) and a receptor for the specific AHL which functions as a transcriptional activator. In S. liquefaciens MG1, the swrI gene encodes an AHL synthase that directs the synthesis of BHL and HHL in a molar ratio of 10 to 1 (Fig. 3) (25). Downstream of swrI and transcribed convergently, an open reading frame codes for a polypeptide (SwrR) with substantial similarity to members of the LuxR family of AHL-dependent regulators (32). A knockout mutation of the swrI gene was found to strongly reduce the swarming capability of the strain. However, swarming motility could be restored to the wild-type level by supplementing the medium with 150 nM BHL or 900 nM HHL. Other signal molecules such as N-3-oxohexanoylhomoserine lactone (OHHL) or N-3-oxo-octanoylhomoserine lactone complement the swrI mutation at 9 μM (25). Thus, BHL is by far the most efficient in promoting swarming motility. The swrI mutation did not affect growth rate in liquid medium or swimming motility or the ability to differentiate into swarm cells when exposed to a surface (25, 31).
FIG. 3.
Quorum sensing and control of biosurfactant synthesis in S. liquefaciens MG1. (A) The divergently arranged genes swrI and swrR encode an AHL synthase (LuxI homologue) and a putative regulatory LuxR homologue, SwrR, respectively. The arrowheads indicate the direction of transcription. BHL is freely diffusible over the bacterial membranes as indicated by the shaded arrow pointing up and down. FUR, one of the furanone compounds produced by D. pulchra. The signal molecule, BHL, is thought to bind to SwrR which in turn up-regulates transcription of the swrA gene (the position of the swrA promoter is indicated by the arrowhead with horizontal lines). The signal inhibitor, FUR, is thought to pass through the bacterial membranes and compete with BHL for the binding site present on SwrR. SwrA encodes a peptide synthetase, SwrA, which catalyzes production of the surfactant serrawettin W2. It is not known whether passage of W2 through the bacterial membranes is passive or mediated by a transport system. (B) Side views of cultures by the drop-collapsing test (54). Small volumes of bacterial cultures were placed on the lid of a petri dish. wt, wild type; swrI, the swrI mutant; +BHL, the strain was grown in the presence of 200 nM BHL. (C) Swarming motility of the S. liquefaciens MG1 swrI swrA double mutant on medium supplemented with serrawettin W2 and drop-collapsing test (54) of water supplemented with serrawettin W2 at the concentrations (in micrograms per milliliter) indicated at the bottom of the panel.
Target genes of the quorum-sensing system.
By transposon mutagenesis, a nonswarming mutant was isolated. Insertion has occurred in a quorum sensing-controlled gene denoted swrA (54). DNA sequence analysis of the swrA gene revealed the presence of a translation product that exhibits homology to a large family of giant, multidomain enzyme complexes responsible for nonribosomal peptide synthesis (86, 92). The Srf complex of Bacillus subtilis, which catalyzes the synthesis of the biosurfactant surfactin (a small cyclic peptide consisting of seven amino acids and a 3-hydroxy-13-methyl-tetradecanoic fatty acid side chain), is one of the best-characterized examples of this type of enzyme complex (8, 14). The Srf complex contains seven highly homologous amino acid binding domains, which are encoded by four large open reading frames, that determine the seven specific amino acids and their order in the final surfactin molecule (11). B. subtilis mutants that are unable to produce surfactin have been demonstrated to be defective in swarming motility (68). From an evolutionary point of view, the ubiquitous peptide synthetases are highly interesting, being present in both gram-negative and -positive bacteria as well as in certain filamentous fungi (11, 86). The different products of the peptide synthetases display a range of powerful biological properties such as antibiotic, antifungal, hemolytic, antitumor, and surface-conditioning activities (50, 69, 70, 74).
Surface-grown cells of S. liquefaciens MG1 create a conditioning film that changes the wettability and surface tension of the medium (Fig. 3) (54). The formation of this film is dependent on functional swrI and swrA genes. In a swrI mutant, surface conditioning is restored when BHL is added exogenously to the medium (Fig. 3B) (54). Since swarming motility is a cell density-dependent phenomenon, the finding that S. liquefaciens MG1 employs a quorum-sensing mechanism in the process of surface conditioning was not unexpected (Fig. 3). Secretion of the extracellular lipopeptide serrawettin W2 causes reduction in the surface tension (54). Serrawettin W2 was originally isolated from spent culture supernatants of Serratia marcescens NS25, and its structure was proposed by Matsuyama et al. (59, 61). Detailed spectroscopic analyses identified the biosurfactant produced by S. liquefaciens MG1 as serrawettin W2 and at the same time confirmed the proposed chemical structure of this compound (54). Serrawettin W2 is a cyclic lipodepsipentapeptide carrying a 3-hydroxy C10 fatty acid side chain (Fig. 3). Interestingly, this biosurfactant is almost identical to kailuin A, a compound that was isolated from a gram-negative marine bacterium capable of swarming motility, except for the exchange of d-Leu in kailuin A with d-Phe in serrawettin W2 (39).
Although 18 transposon inserts were isolated in BHL-controlled genes (23) and expression of a minimum of 28 genes is regulated by the BHL signal molecule (33), the only identified gene involved in swarming motility is swrA. This suggests that, at least in our experimental setup, only a minor fraction of quorum sensing-responsive genes is involved in swarming motility. In P. aeruginosa, synthesis of the biosurfactant rhamnolipid is controlled by BHL-dependent quorum sensing (71).
Properties of serrawettins.
The function of serrawettin W2 can be visualized by its surface tension-reducing properties that cause water droplets to collapse (Fig. 3B). Media supplemented with pure W2 allows surfactant-defective S. liquefaciens MG1 (swrI mutant and the swrI swrA double mutant) cultures to travel atop the agar surface (Fig. 3C). The full effect of W2 is achieved within a narrow range around 1 μg/ml, probably reflecting the critical concentration of micelle formation. The biological importance of the biosurfactant is illustrated by fact that a nonflagellated mutant of S. liquefaciens MG1 is capable of colonizing the surface of plates with a low percentage of agar by means of spreading motility (which is solely driven by the biosurfactant), as has been observed previously with S. marcescens (58–63, 72).
Media supplemented with pure serrawettin W2, W1 (another biosurfactant produced by S. marcescens), or surfactin from B. subtilis restores the swarming phenotype of the S. liquefaciens MG1 swrI swrA double mutant. In fact, trace amounts of sodium dodecyl sulfate or Nonidet P-40 were found to be sufficient to promote swarming migration of the double mutant (34). This result demonstrates that the production of molecules lowering the surface tension of the medium is crucial for swarming motility of S. liquefaciens MG1. It also explains our previous observation that the swrI gene is dispensable for swarming motility on LB or brain heart infusion medium, since they were found to contain small amounts of surfactant (34). In P. mirabilis, a capsular polypeptide that facilitates swarming motility by reducing surface friction has been identified (38). Synthesis of this polypeptide is mediated by a 40.6-kDa enzyme that has strong homology to putative sugar transferases required for lipopolysaccharide core modification in Shigella and Salmonella.
EXOENZYMES
In S. liquefaciens MG1, expression of an extracellular phospholipase, which represents a potential virulence determinant, is coupled to the synthesis of flagella via the flhDC master regulator (30). This is reminiscent to the situation found with P. mirabilis for which it has been demonstrated that expression of virulence factors, such as intracellular urease, extracellular hemolysin, and metalloprotease, are differentially up-regulated in swarm cells (3, 4). However, in S. liquefaciens MG1, none of the other extracellular enzymes, which include two proteases, several chitinases, a lipase, and a nuclease, are coregulated with flagella. The quorum-sensing system in S. liquefaciens MG1 was found to be involved only in modulating expression of proteolytic and chitinolytic activity (23, 25). The regulation of the synthesis of the various exoenzymes in S. liquefaciens MG1 has been the subject of a recent review (32).
ORGANIZATION AND BEHAVIOR OF SPECIALIZED SUBCULTURES IN A SWARM COLONY
A swarming colony consists of specialized cells organized in subpopulations (Fig. 1). The immobilized, stationary-phase cells in the center do not contribute to the overall dynamics of the colony. However, the ability of the cells comprising the swimming and swarming subpopulations to go through cycles of differentiation and dedifferentiation is a major factor determining expansion of the moving culture. A mathematical approach to describe macroscopic pattern formation in P. mirabilis was recently presented by Esipov and Shapiro and demonstrated that the expansion rate and periodicity can be explained based on internal population dynamics of age structure (26). During the differentiation process, swarming cells arise from swimming cells that have ceased septation but continue to grow and form long, multinucleated, hyperflagellated cells. The model of Esipov and Shapiro assumes that once formed, the swarm cells do not give rise to new swarm cells. Instead, they age and reach a septation stage at which they divide into swimmers which then in turn gives rise to the formation of new swarm cells. In P. mirabilis, these interconversion cycles occur in a synchronized fashion, and as a result, the colony either grows or expands. Serratia differs from Proteus with respect to the secretion of serrawettins that enables continuous spreading of the growing culture (54, 60, 62, 63, 82).
Population analysis.
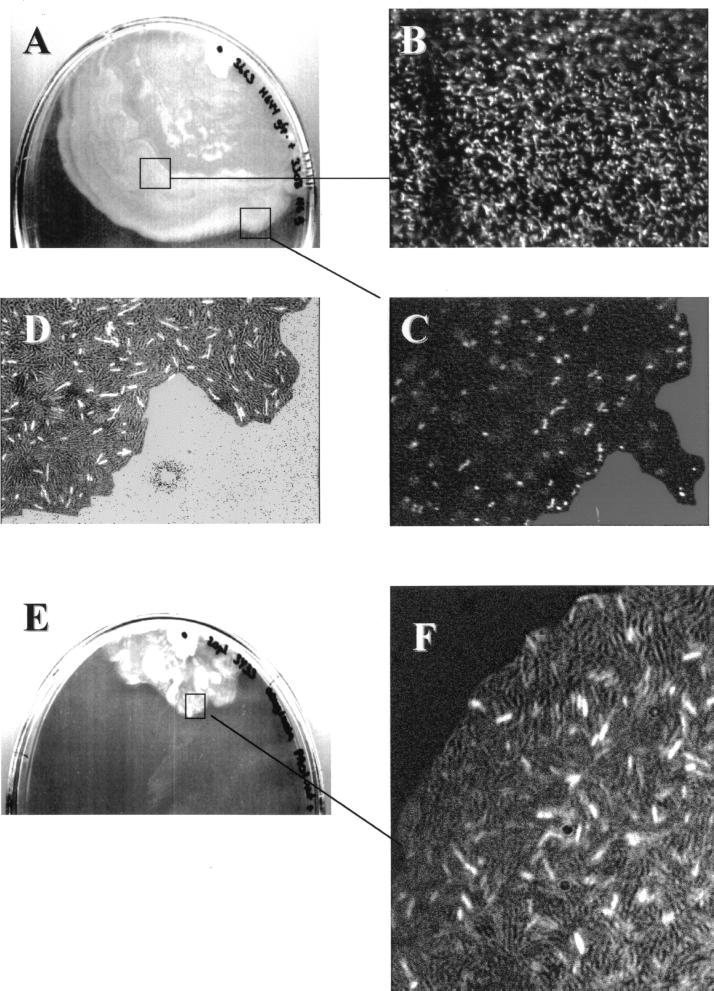
The following experiments provide novel information regarding community organization and population behavior in a swarming S. liquefaciens MG1 colony. Since the two major regulatory systems (Fig. 2) are organized separately, they can be disconnected in individual cells by means of mutations in key regulatory genes (flhD and swrI). A swarming culture composed of a mixture of green fluorescent protein (GFP)-tagged “green” flhD and “dark” swrI mutants (and vice versa) can be monitored in situ by combined phase-contrast and epifluorescence microscopy. This technique has shed some light on the function and significance of the swirling rafts in swarm colonies. The central part of the colony is densely populated with vegetative, nondifferentiated flhD cells (Fig. 4A and B). In addition, they are clearly present in the outer swirling layer that is dominated by differentiated swrI cells (Fig. 4A to C). Furthermore, the nonmotile flhD cells travel rapidly in the outer layer. We assume that the production of serrawettin creates a liquid interface layer in which the flow caused by the vigorous movement of the raft swrI cells distributes the flhD cells to the periphery of the expanding colony. Dividing cells are easily spotted among the transported flhD cells, demonstrating that growth and division are not restricted to the more-central parts of the expanding colony. We suggest that the behavior of the differentiated swarm cells serves two major purposes: it leads to the formation and spreading of a surface-conditioning film, and it circulates cells between the two specialized subcultures of swarmers and vegetative cells present at the border and the more-central parts of the colony, respectively. This in turn continuously creates new zones of growth and abolishes the formation of distinct consolidation and motility phases as seen with P. mirabilis.
FIG. 4.
Swarm colonies consisting of two strains. (A) Top view of a mixed culture of the S. liquefaciens MG1 flhD strain and the swrI mutant. The strains were applied at the dark spot in a 50:50 ratio. (B and C) Detection of GFP-tagged flhD cells by means of epifluorescence microscopy in the more-central part (B) and in the outer swirling layer of the colony (C). (D) Detection of swrI cells harboring a LuxR-based AHL monitor system (PluxI-gfp fusion) expressing GFP in response to the presence of extracellular AHL signal molecules by epifluorescence-light microscopy. (E) Top view of a mixed culture of P. aeruginosa PAO1 and S. liquefaciens MG1 swrI harboring the AHL monitor. (F) Microscopic inspection of the square in the outer part of the colony by epifluorescence-light microscopy.
Cell-cell signaling in the swarm.
Similar experiments highlight the assignment of the AHL molecules as messengers between specialized subcultures. “Dark” flhD cells are mixed with swrI cells harboring a plasmid-borne AHL monitor system in which expression of the GFP is controlled by LuxR (7). Many bright green, differentiated swrI cells are seen in the swarm, indicating that AHL signals originating from the transported flhD cells are received by the swrI cells and transformed into gene expression (Fig. 4D). E. coli or Pseudomonas putida strains (non-AHL producers), harboring a swrI+-containing plasmid can form swarming colonies in conjunction with the S. liquefaciens MG1 swrI mutant. Similarly, swarming colonies can form among Pseudomonas aeruginosa PAO1 cells (AHL producers) and the swrI mutant (Fig. 4E). The appearance of bright green swrI cells harboring the AHL monitor system is indicative of interspecies communication (Fig. 4F). Neither the Pseudomonas nor the E. coli strains produce serrawettin, and they are unable to differentiate into swarm cells in this particular setup. This indicates that AHL signals originating from the AHL producers trigger surfactant synthesis in the population of swrI cells. Thus, the organisms interact by means of chemical signals originating from the Pseudomonas cells. An additional level of community complexity arises from the interaction of surface, cells, and cellular exoproducts, which in turn drives the community members to self-organize into a functional community which expresses its complex phenotypic traits.
Although the above-mentioned organisms might not meet under natural conditions, such random communities may display activities and functionalities indicative of coordinated performance. On the other hand, Erwinia carotovora regulates carbanapem synthesis by means of an OHHL-based quorum-sensing system (67) and are able to eliminate bacterial competitors such as Serratia. It does not form a swarming colony with the swrI mutant, indicating that in nature quorum-sensing systems can be employed to favor either collaboration or competition.
ECOLOGICAL RELEVANCE
Swarm cells and virulence.
Many pathogenic members of the genera Serratia, Proteus, Vibrio, Bacillus, Clostridium, Escherichia, and Salmonella are able to swarm (2, 41). Serratia is a common cause of infections in insects and cold-blooded vertebrates (37). S. marcescens and S. liquefaciens are opportunistic human pathogens that cause respiratory and urinary tract infections (17). Does the ability of swarm cell differentiation in mucus or in urinary or respiratory tracts contribute to the pathogenicity of Serratia strains? For the uropathogen Proteus, swarming behavior is closely associated with modulation of virulence characteristics and the ability to invade human urothelial cells (2–5). For V. parahaemolyticus, differentiation into swarm cells plays an important role in adsorption and colonization of chitinaceous shells of crustaceans (9, 10). Taken together with our recent observation that differentiated S. liquefaciens MG1 cells are resistant to predation (6), it suggests that the ability of bacteria to differentiate into the swarm cell state is a general and ecologically important phenomenon not necessarily related to motility. Based on the experiments described above, it might be considered an important social phenomenon, since cultures of different species in certain conditions might be able to collaborate in the process of surface colonization.
Biofilm formation.
Pratt and Kolter (76) suggested recently a dual role for flagellum-mediated motility in E. coli and P. aeruginosa biofilm formation in which flagella promote initial cell-to-surface contact and also contribute to the spreading of a growing biofilm along an abiotic surface. In the formation of P. aeruginosa biofilms, the buildup of microcolonies on the tightly packed monolayer is highly dependent on type IV pili-mediated twitching motility (73). For P. aeruginosa, it has been recently demonstrated that both the formation of biofilms and twitching motility are dependent on the quorum-sensing system operating in this organism (18, 36). These results argue in favor of functional overlaps between factors necessary for biofilm formation, bacterial pathogenesis, and attachment in vivo as suggested by Kolter and coworkers.
Eukaryotic defense systems.
The ability of pathogenic bacteria to form biofilms within the human body is a major medical problem, since this growth mode substantially increases the resistance level of bacteria to antibiotics (15, 47, 51). Biofilm formation is also a major challenge for marine eukaryotes (52, 55). Bacteria can be highly detrimental to marine algae and other eukaryotes (55). Moreover, because bacteria are generally the first colonizers of submerged surfaces, the abundance and composition of the bacterial community on the surface will significantly affect the subsequent development of a macrofouling community (9, 46). To cope with this, eukaryotes have developed chemical defense mechanisms (19, 20, 93, 94), which in several cases are based on nontoxic secondary metabolites that specifically inhibit bacterial colonization-relevant phenotypes (50, 64, 85, 95). Such secondary metabolites (furanones) are produced by the marine alga Delisea pulchra (20, 31, 49). The effects of furanones on bacterial colonization phenotypes are due to interference with specific cell processes rather than to toxicity or general surface modification (49, 64). Several of the furanone compounds that exhibit structural similarity to the short-chain AHL molecules (Fig. 3) inhibit swarming motility of S. liquefaciens MG1 (31, 57). The D. pulchra compounds reduce the motility of the swarm cells by means other than influencing flagellar synthesis or growth rate (31). Our data strongly suggest that furanone compounds inhibit the communication system and reduce serrawettin W2 production (31, 35). The direct interaction with the AHL signaling systems has gained further strength by the displacement of labeled OHHL from LuxR by specific furanones (57). The furanones are likely competitive inhibitors of the AHL signal molecules competing for the same site of the receptor protein. In other words, furanone compounds enable growth and cell differentiation but disable the expansion. Defense systems based on nontoxic metabolites may have the advantage of allowing formation of discrete surface-bound bacterial colonies whose presence is beneficial to the eukaryotic host.
Newly discovered signals.
Recently, a group of cyclic dipeptides were found to cross talk with quorum-sensing systems (12). Cyclo(ΔAla–l-Val) and cyclo(l-Pro–l-Tyr) were found to be produced by unrelated gram-negative bacteria including P. aeruginosa, Enterobacter agglomerans, P. mirabilis, and Citrobacter freundii. A third peptide, cyclo(l-Phe–l-Pro) was identified in P. fluorescens and P. alcaligenes. Cyclo(l-Pro–l-Met), cyclo(l-Pro–l-Val), and cyclo(l-Pro–l-Leu) were identified in E. coli (21). Some of the peptides were found to react with different AHL monitor strains such as E. coli harboring the bioluminescent LuxR-based system and Chromobacterium violaceum. Cyclo(l-Pro–l-Tyr) showed competitive (to BHL) inhibition of swarming motility in the swrI mutant, whereas cyclo(l-Pro–l-Met) stimulated swarming motility of the swrI mutant as efficiently as OHHL. Importantly, the observed responses were found to be specific to individual molecules, which indicates that cyclic peptides do cross talk with AHL-based quorum-sensing systems. Cyclo(l-Phe–l-Pro) and cyclo(l-Pro–l-Tyr) have been shown to act on the central nervous system (75). Based on the structural similarity between some cyclic dipeptides and for example the thyrotropin-releasing hormone, Chhabra et al. suggest the possibility that these cyclic dipeptides may influence interactions between bacterial pathogens and their hosts (12).
FUTURE PERSPECTIVES
Many interesting questions remain to be answered. For example, what is the underlying molecular mechanism by which the Serratia quorum-sensing system operates? A direct involvement of SwrR in quorum sensing, such as binding of BHL and other signal molecules, and its function as a transcriptional regulator remain to be elucidated. Serrawettin W2 is produced in vast amounts when S. liquefaciens MG1 is grown on plates but is hardly detectable in liquid-grown cultures (54). Is the attachment to a surface the primary signal for the expression of the biosurfactant? Is this signal then just boosted by the quorum-sensing system that would function as a signal amplifier? Alternatively, population densities that can be attained in liquid medium may be insufficient to trigger the quorum-sensing system, in contrast to the high cell density within a plate-grown colony. Is surfactant production differentially up-regulated in swarm cells? Investigation to address these questions will to a large degree depend on the possibility to analyze gene expression in single cells. A recently developed technique that allows detection of mRNA levels in single cells (90, 91) and GFP-based reporter gene technology (Fig. 4) appear to be suitable tools for this purpose.
So far, only a few of the S. liquefaciens MG1 quorum-sensing target genes have been identified (23, 54), and work in progress aims at the identification of these genes. Some of these genes may encode potential virulence determinants whose expression is sensitive to furanone compounds and cyclic dipeptides. This may form a valuable model system in the process of gaining knowledge of the structure and function of bacterial signaling systems. The study of the interaction with cognate signals as well as other modulatory signals may help develop new strategies in the battle against infectious diseases.
ACKNOWLEDGMENTS
We acknowledge the contributions of many people to the work on surface motility of S. liquefaciens MG1. Special thanks to Staffan Kjelleberg, Gordon Stuart, Paul Williams, and James Shapiro for suggestions and for sharing unpublished results with us and to Gunna Christiansen for making electron micrograph pictures available to us.
Work on quorum sensing in biofilms is supported by grants from the Deutsche Forschungsgemeinschaft (EB 2051/1-1) (to L.E.), The Danish Biotechnology Program, and The Danish Medical Research Council (to M.G. and S.M.). Work on signal inhibitors has been supported by travel grants from The Danish Plasmid Foundation, The Carlsberg Foundation, and Løvens Kemiske Foundation to M.G.
REFERENCES
- 1.Allison C, Lai H C, Gygi D, Hughes C. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol Microbiol. 1993;8:53–60. doi: 10.1111/j.1365-2958.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 2.Allison C, Hughes C. Bacterial swarming: an example of procaryotic differentiation and multicellular behavior. Sci Prog. 1991;75:403–422. [PubMed] [Google Scholar]
- 3.Allison C, Jones P, Coleman N, Hughes C. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect Immun. 1992;60:4740–4746. doi: 10.1128/iai.60.11.4740-4746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison C, Lai H C, Hughes C. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol Microbiol. 1992;6:1583–1591. doi: 10.1111/j.1365-2958.1992.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 5.Allison C, Emody L, Coleman N, Hughes C. The role of swarm-cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J Infect Dis. 1994;69:1155–1158. doi: 10.1093/infdis/169.5.1155. [DOI] [PubMed] [Google Scholar]
- 6.Ammendola A, Geisenberger O, Andersen J B, Givskov M, Schleifer K H, Eberl L. Serratia liquefaciens swarm cells exhibit enhanced resistance to predation by Tetrahymena sp. FEMS Microbiol Lett. 1998;164:69–75. doi: 10.1111/j.1574-6968.1998.tb13069.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen, J. B., L. Eberl, S. Molin, and M. Givskov. Unpublished data.
- 8.Arima K, Kakinuma A, Tamura G. Surfactin, a crystalline peptide lipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun. 1968;31:488–494. doi: 10.1016/0006-291x(68)90503-2. [DOI] [PubMed] [Google Scholar]
- 9.Belas M R. The swarming phenomenon of Proteus mirabilis. ASM News. 1992;58:15–22. [Google Scholar]
- 10.Belas M R, Colwell R R. Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol. 1982;151:1568–1580. doi: 10.1128/jb.151.3.1568-1580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cane D E, Walsh C T, Khosla C. Harnessing the biosynthetic code: combinations, permutations and mutations. Science. 1998;282:63–68. doi: 10.1126/science.282.5386.63. [DOI] [PubMed] [Google Scholar]
- 12.Chhabra, S. R., M. T. G. Holden, R. de Nys, P. Stead, N. J. Bainton, P. J. Hill, M. Givskov, S. Kjelleberg, G. P. C. Salmon, G. S. A. B. Stewart, B. Bycroft, and P. Williams. Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria: evidence for a new family of diffusible prokaryotic signal molecules. Submitted for publication.
- 13.Christensen, A. C., T. B. Rasmussen, and M. Givskov. Unpublished data.
- 14.Cosmina P, Rodriguez F, de Ferra F, Grandi G, Perego M, Venema G, van Sinderen D. Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol Microbiol. 1993;8:821–831. doi: 10.1111/j.1365-2958.1993.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 15.Costerton J W, Cheng K-J, Geesey G G, Ladd T L, Nickel J C, Dasgupta M, Marrie T J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 16.Costerton J W, Lewandowski Z, Caldweil D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 17.Daschner F D. The epidemiology of Serratia marcescens. In: von Graevenitz A, Rubin S J, editors. The Genus Serratia. Boca Raton, Fla: CRC Press, Inc.; 1980. pp. 187–196. [Google Scholar]
- 18.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 19.Davis A R, Targett N M, McConnell O J, Young C M. Epibiosis of marine algae and benthic invertebrates: natural products chemistry and other mechanisms inhibiting settlement and overgrowth. Bioorg Mar Chem. 1989;3:86–114. [Google Scholar]
- 20.de Nys R, Steinberg P D, Willemsen P, Dworjanyn S A, Gabelish C L, King R J. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling. 1995;8:259–271. [Google Scholar]
- 21.de Nys, R., K. Yamamoto, M. Givskov, N. Kumar, P. Williams, G. S. A. B. Stewart, R. Read, R. Utsumi, and S. Kjelleberg. A new class of cyclic dipeptide signalling molecules in Escherichia coli which influence the expression of stationary phase genes. Submitted for publication.
- 22.Dufour A, Furness R B, Hughes C. Novel genes that upregulate the Proteus mirabilis flhDC master operon controlling flagellar biogenesis and swarming. Mol Microbiol. 1998;29:741–751. doi: 10.1046/j.1365-2958.1998.00967.x. [DOI] [PubMed] [Google Scholar]
- 23.Eberl, L., and M. Givskov. Unpublished data.
- 24.Eberl L, Christiansen G, Molin S, Givskov M. Differentiation of Serratia liquefaciens into swarm cells is controlled by the expression of the flhD master operon. J Bacteriol. 1996;178:554–559. doi: 10.1128/jb.178.2.554-559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberl L, Winson M K, Sternberg C, Stewart G S A B, Christiansen G, Chhabra S R, Daykin M, Williams P, Molin S, Givskov M. Involvement of N-acyl-L-homoserine lactone autoinducers in control of multicellular behavior of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 26.Esipov S E, Shapiro J A. Kinetic model of Proteus mirabilis swarm colony development. J Math Biol. 1998;36:249–268. [Google Scholar]
- 27.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuqua W C, Winans S, Greenberg E. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 29.Furness R B, Fraser G M, Hay N A, Hughes C. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J Bacteriol. 1997;179:5585–5588. doi: 10.1128/jb.179.17.5585-5588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Givskov M, Eberl L, Christiansen G, Benedik M J, Molin S. Induction of phospholipase- and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 31.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated procaryotic signaling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Givskov M, Eberl L, Molin S. Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol Lett. 1997;148:115–122. [Google Scholar]
- 33.Givskov M, Östling J, Lindum P W, Eberl L, Christiansen G, Molin S, Kjelleberg S. The participation of two separate regulatory systems in controlling swarming motility of Serratia liquefaciens. J Bacteriol. 1998;180:742–745. doi: 10.1128/jb.180.3.742-745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Givskov, M. Unpublished data.
- 35.Givskov, M., and S. Kjelleberg. Unpublished data.
- 36.Glessner A, Iglewski B H, Robinson J B. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. The role of Pseudomonas aeruginosa las and rhl quorum sensing systems in control of twitching motility, abstr. D-88; p. 227. [Google Scholar]
- 37.Grimont P A D, Grimont F. The genus Serratia. Annu Rev Microbiol. 1978;32:221–248. doi: 10.1146/annurev.mi.32.100178.001253. [DOI] [PubMed] [Google Scholar]
- 38.Gygi D, Bailey M J, Allison C, Hughes C. Requirement for FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol Microbiol. 1995;15:761–770. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 39.Harrigan G G, Harrigan B L, Davidson B S. Kailuins A-D, new cyclic acyldepsipeptides from cultures of a marine-derived bacterium. Tetrahedron. 1997;53:1577–1582. [Google Scholar]
- 40.Harshey R M. Bees aren’t the only ones: swarming in Gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 41.Harshey R M, Matsuyama T. Dimorphic transition in E. coli and S. typhimurium: surface induced differentiation into hyperflagellated swarmer cells. Proc Natl Acad Sci USA. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauser G. Über Fäulnisbakterien und deren Beziehung zur Septicämie. Leipzig, Germany: F. G. W. Vogel; 1885. [Google Scholar]
- 43.Hay N A, Tipper D J, Gygi D, Hughes C. A nonswarming mutant of Proteus mirabilis lacks the Lrp global transcriptional regulator. J Bacteriol. 1997;176:4741–4746. doi: 10.1128/jb.179.15.4741-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazelbauer G L, Berg H C, Matsumura P. Bacterial motility and signal transduction. Cell. 1993;73:5–22. doi: 10.1016/0092-8674(93)90156-k. [DOI] [PubMed] [Google Scholar]
- 45.Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henschel J R, Cook P A. The development of a marine fouling community in relation to the primary film of microorganisms. Biofouling. 1990;2:1–11. [Google Scholar]
- 47.Hoyle B D, Costerton W J. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 48.Jones H E, Park R W A. The influence of medium composition on the growth and swarming of Proteus. J Gen Microbiol. 1967;47:369–378. doi: 10.1099/00221287-47-3-369. [DOI] [PubMed] [Google Scholar]
- 49.Kjelleberg S, Steinberg P D, Givskov M, Manefield M, de Nys R. Do marine products interfere with procaryotic AHL regulatory systems? Aquat Microbiol Ecol. 1997;13:85–93. [Google Scholar]
- 50.Kleinkauf H, von Döhren H. Biosynthesis of peptide antibiotics. Annu Rev Microbiol. 1987;41:259–289. doi: 10.1146/annurev.mi.41.100187.001355. [DOI] [PubMed] [Google Scholar]
- 51.Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 52.Kushmaro A, Loya Y, Fine E, Rosenberg E. Bacterial infection and coral bleaching. Nature. 1996;380:396. [Google Scholar]
- 53.Lai H-C, Gygi D, Fraser G M, Hughes C. A swarming-defective mutant of Proteus mirabilis lacking a putative cation-transporting membrane P-type ATPase. Microbiology. 1998;144:1957–1961. doi: 10.1099/00221287-144-7-1957. [DOI] [PubMed] [Google Scholar]
- 54.Lindum P W, Anthoni U, Christoffersen C, Eberl L, Molin S, Givskov M. N-acyl-l-homoserine lactone autoinducers control production of an extracellular surface-active lipopeptide required for swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:6384–6388. doi: 10.1128/jb.180.23.6384-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Littler M M, Littler D S. Impact of CLOD pathogen on Pacific coral reefs. Science. 1995;267:1356–1360. doi: 10.1126/science.267.5202.1356. [DOI] [PubMed] [Google Scholar]
- 56.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 57.Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjellebjerg S. Halogenated furanones from Delisea pulchra inhibit AHL mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 58.Matsuyama T, Fujita M, Yano I. Wetting agent produced by Serratia marcescens. FEMS Microbiol Lett. 1985;28:125–129. [Google Scholar]
- 59.Matsuyama T, Murakami T, Fujita M, Fujita S, Yano I. Extracellular vesicle formation and biosurfactant production by Serratia marcescens. J Gen Microbiol. 1986;132:865–875. [Google Scholar]
- 60.Matsuyama T, Kaneda K, Ishizuka I, Toida T, Yano I. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J Bacteriol. 1990;172:3015–3022. doi: 10.1128/jb.172.6.3015-3022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuyama T, Bhasin A, Harshey R M. Mutational analysis of flagellum-independent surface spreading of Serratia marcescens 274 on a low-agar medium. J Bacteriol. 1995;177:987–991. doi: 10.1128/jb.177.4.987-991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuyama T, Nakagawa Y. Surface-active exolipids: analysis of absolute chemical structures and biological functions. J Microbiol Methods. 1996;25:165–175. [Google Scholar]
- 64.Maximilien R, de Nys R, Holmström C, Gram L, Givskov M, Crass K, Kjelleberg S, Steinberg P D. Chemical mediation of bacterial surface colonisation by secondary metabolites from the red alga Delisea pulchra. Aquat Microbiol Ecol. 1998;15:233–246. [Google Scholar]
- 65.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 66.McCarter L. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J Bacteriol. 1998;180:3166–3173. doi: 10.1128/jb.180.12.3166-3173.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGowan S, Sebaihia M, Jones S, Yu B, Bainton N, Chan P F, Bycroft B, Stewart G S A B, Williams P, Salmond G P. Carbapenem antibiotic production in Erwinia carotovora is regulated by CarR, a homologue of the LuxR transcriptional activator. Microbiology. 1995;141:541–550. doi: 10.1099/13500872-141-3-541. [DOI] [PubMed] [Google Scholar]
- 68.Mendelson N H, Salhi B. Patterns of reporter gene expression in the phase diagram of Bacillus subtilis colony forms. J Bacteriol. 1996;178:1980–1989. doi: 10.1128/jb.178.7.1980-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neu T R, Hartner T, Poralla K. Surface active properties of viscosin—a peptidolipid antibiotic. Appl Microbiol Biotechnol. 1990;32:518–520. [Google Scholar]
- 70.Neu T R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ochsner U A, Koch A K, Fiechter A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Rear J, Alberti L, Harshey R M. Mutations that impair swarming motility in Serratia marcescens 274 include but are not limited to those affecting chemotaxis or flagellar function. J Bacteriol. 1992;174:6125–6137. doi: 10.1128/jb.174.19.6125-6137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 74.Pospiech A, Cluzel B, Bietenhader J, Schupp T. A new Myxococcus xanthus gene cluster for the biosynthesis of the antibiotic saframycin Mx1 encoding a peptide synthetase. Microbiology. 1995;141:1793–1803. doi: 10.1099/13500872-141-8-1793. [DOI] [PubMed] [Google Scholar]
- 75.Prasad C. Bioactive cyclic dipeptides. Peptides. 1995;16:151–165. doi: 10.1016/0196-9781(94)00017-z. [DOI] [PubMed] [Google Scholar]
- 76.Pratt A L, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 77.Prüß B M, Wolfe A J. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol Microbiol. 1994;12:973–984. doi: 10.1111/j.1365-2958.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 78.Prüß B M, Matsumura P. A regulator of the flagellar regulon of Escherichia coli, flhDC, also affects cell division. J Bacteriol. 1996;178:668–674. doi: 10.1128/jb.178.3.668-674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prüß B M, Matsumura P. Cell cycle regulation of flagellar genes. J Bacteriol. 1997;179:5602–5604. doi: 10.1128/jb.179.17.5602-5604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prüß B M, Markovic D, Matsumura P. The Escherichia coli flagellar transcriptional activator flhD regulates cell division through induction of the acid response gene cadA. J Bacteriol. 1997;179:3818–3821. doi: 10.1128/jb.179.11.3818-3821.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shapiro J A. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- 82.Shapiro, J. A., and M. Givskov. Unpublished data.
- 83.Shi W, Zhou Y, Wild J, Adler J, Gross C A. DnaK, DnaJ, and GrpE are required for flagellum synthesis in Escherichia coli. J Bacteriol. 1992;174:6256–6263. doi: 10.1128/jb.174.19.6256-6263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silverman M, Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974;120:1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slattery M, McClintoch J B, Heine J N. Chemical defences in Antarctic soft corals: evidence for antifouling compounds. J Exp Mar Biol Ecol. 1995;190:61–77. [Google Scholar]
- 86.Stachelhaus T, Marahiel M A. Modular structures of genes encoding multifunctional peptide synthetases required for non-ribosomal peptide synthesis. FEMS Microbiol Lett. 1995;125:3–14. doi: 10.1111/j.1574-6968.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 87.Stock J B, Ninfa A J, Stock M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swift S, Throup J, Williams P, Salmond G, Stewart G. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 89.Tolker-Nielsen, T., A. B. Christensen, K. Holmstrøm, L. Eberl, T. B. Rasmussen, C. Sternberg, S. Molin, and M. Givskov. Assessment of flhDC mRNA levels in individual Serratia liquefaciens swarm cells. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 90.Tolker-Nielsen T, Holmstrøm K, Molin S. Visualization of specific gene expression in Salmonella typhimurium by in situ PCR. Appl Environ Microbiol. 1997;63:4196–4203. doi: 10.1128/aem.63.11.4196-4203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolker-Nielsen T, Holmstrøm K, Boe L, Molin S. Non-genetic population heterogeneity studied by in situ polymerase chain reaction. Mol Microbiol. 1998;27:1099–1105. doi: 10.1046/j.1365-2958.1998.00760.x. [DOI] [PubMed] [Google Scholar]
- 92.Turgay K, Krause M, Marahiel M A. Four homologous domains in the primary structure of GrsB are related to domains in a superfamily of adenylate-forming enzymes. Mol Microbiol. 1992;6:529–546. doi: 10.1111/j.1365-2958.1992.tb01498.x. [DOI] [PubMed] [Google Scholar]
- 93.Wahl M. Marine epibiosis. Fouling and antifouling: some basic aspects. Mar Ecol Prog Ser. 1989;58:175–189. [Google Scholar]
- 94.Wahl M. Living attached: fouling, epibiosis. In: Nagabhushanam R, Thompson M F, editors. Fouling organisms of the Indian Ocean: biology and control technology. New Delhi, India: Oxford & IBH Publishers; 1996. pp. 31–83. [Google Scholar]
- 95.Wahl M, Jensen P R, Fenical W. Chemical control of bacterial epibiosis on ascidians. Mar Ecol Prog Ser. 1994;110:45–57. [Google Scholar]
- 96.Young G M, Miller V L. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. The flagellar master operon flhDC affects expression of invasin and integrates expression of swimming motility, swarming motility, and phospholipase in Yersinia enterolitica, abstr. B-149; p. 80. [Google Scholar]