Abstract
Red blood cell distribution width (RDW) is a measure of the change in size of red blood cells and it is used in combination with other hematological parameters for the differential diagnosis of anemias. Recent evidence suggested that the change in RDW level may be a predictive biomarker of morbidity and mortality in cardiovascular diseases (CVDs). Cardiovascular diseases are the most common cause of death globally as compared to cancer and communicable diseases. Early diagnosis and prompt intervention of these diseases are very important to minimize their complications. Nowadays, the diagnosis of most cardiovascular diseases majorly depends on clinical judgment, electrocardiography and biochemical parameters. Red blood cell distribution width as a new predictive biomarker may play a pivotal role in assessing the severity and progression of CVDs. However, the underlying mechanisms for the association between RDW and CVDs are not clear. A deeper understanding of their association could help the physicians in more careful identification, early prevention, intervention, and treatment to prevent adverse cardiovascular events. This review aims to elaborate on the recent knowledge on the association between RDW and cardiovascular diseases and some possible pathophysiological mechanisms.
Keywords: biomarkers, cardiovascular diseases, inflammation, red blood cell distribution width, prognosis
Introduction
Red blood cells (RBCs) are non-nucleated blood cells characterized by having a typical oval biconcave shape, with a diameter of 6 to 8 μm and a thickness of 2 μm. The normal volume of RBCs ranges from 80 to 100 femtoliter (fL), but different physiological and pathological conditions may increase the degree of anisocytosis. The red blood cell distribution width (RDW) is a quantitative measure of variation in the size of circulating RBCs, which is receiving increasing interest as a diagnostic and prognostic marker in a vast array of human disorders.1 The RDW has been introduced as a novel inflammatory predictor in various disorders including functional bowel conditions,2 autoimmune diseases,3,4 malignancy,5 COVID-19 and multiple hospital admissions in subjects with chronic conditions.6
Red blood cell distribution width is calculated automatically by hematological analyzers by simply dividing the standard deviation (SD) of the mean corpuscular volume (MCV) by the MCV and multiplying by 100 to yield a percentage value. Since the methods of measuring the RBC size, the instrument, and statistical methods are varying in many laboratories, there is no established common reference range till now.7 The normal reference range of RDW most laboratories used is roughly ranging from 11% to 15%.
An RDW value below the reference range has been considered without clinical relevance, whereas an increased RDW value reflects a greater difference in the size of RBCs, which can be due to the presence of smaller or larger RBCs, or both. An elevated RDW usually results from increased or ineffective production of RBCs and excessive fragmentation or destruction of RBCs.8 Thus, RDW is increased in patients with iron deficiency anemia, megaloblastic anemia, myelodysplastic syndrome, hemolytic anemia, liver failure, sickle cell disease, and blood transfusions.9 Red blood cell distribution width together with MCV has been almost exclusively used for the differential diagnosis of anemias.1 However, it is almost an unknown parameter to which very few clinicians pay attention when examining blood cell count.
In addition to RBC disorders, various cardiovascular diseases (CVDs) such as heart failure (HF), ischemic cerebrovascular disease (including stroke), acute coronary syndrome (ACS), peripheral artery disease (PAD), hypertension, and atrial fibrillation (AF) are often associated with a high degree of anisocytosis.10 Since both RDW and cardiovascular conditions are associated with inflammation, RDW could also be associated with CV diseases. Cardiovascular diseases are a diverse group of disorders that affect the heart and blood vessels, and they are mostly interrelated with the underlying pathology of atherosclerosis.11 Compared to cancer and communicable diseases, CVD continues to be the most common cause of death globally (17.3 million death). Proper risk assessment and stratification, as well as prognosis evaluation for patients with CVDs, are critical to cardiologists. The diagnosis of CVDs mainly depends on clinical judgment, imaging, and biochemical parameters (Troponin, C-reactive protein, B type natriuretic peptide, myoglobin), but biomarkers that could be commonly used in different clinical practices are finite.12 In addition, noninvasive anatomical and electrophysiological (electrocardiography) cardiac biomarkers are commonly used as surrogate markers of CVDs, especially in its acute phase.13,14
The biomarker is defined as a characteristic objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention. Ideal diagnostic biomarkers should be highly specific, sensitive, rapidly available, inexpensive, and noninvasive.15 Clinicians have been trying for years to search for such a biomarker to aid the identification, early prevention, intervention, and therapy to prevent adverse cardiovascular events. One candidate is RDW, which is widely investigated and routine laboratory value reported as a component of standard complete blood count (CBC) without the need for extra procedures. Nowadays, scientific evidence has shown that RDW may provide valuable information for the diagnosis of different diseases and establishing the short- and long-term prognosis in patients with CVDs.16 Hence, recent evidence suggested that the change in RDW level may be a predictive marker of morbidity and mortality in CVDs.17 However, the underlying mechanisms of whether the increased heterogeneity is the cause or consequence of other pathophysiological conditions exceedingly remain unclear. This review aims to elaborate on the recent knowledge on the association between RDW and cardiovascular diseases and some possible pathophysiological mechanisms.
This review was conducted based on the relevant articles published in the English language on the current topic retrieved from electronic databases like PubMed, PubMed Central, ScienceDirect, Google Scholar, and google. Relevant articles were searched by the following words: red blood cell distribution width, cardiovascular diseases, coronary artery disease, myocardial infarction, acute coronary syndrome, heart failure, hypertension, and stroke, separately and in combination. Additionally, reference lists of publications in this field were searched to identify any studies for inclusion and the current review includes original peer-reviewed articles. Also, articles such as reviews, systematic reviews, and meta-analyses were considered.
Red Blood Cell Distribution Width and Cardiovascular Disease
Currently, a number of articles have been published regarding the role of RDW in clinical practice and the risk stratification of patients with CVDs. In this review, the most important and appropriate studies investigating the role of RDW in patients with CVDs are discussed here under and summarized in Table 1.
Table 1.
Summary of Studies Exploring Association Between RDW and CVDs
| Author, Year | Study Design | Study Population | Major Findings | References |
|---|---|---|---|---|
| Felker et al, 2007 | Retrospective cohort | 2679 chronic HF patients | Higher RDW in patient with CV event than without CV events (15.2 vs 14.4). HR for morbidity and mortality (1 SD increment of RDW): 1.17 (95% CI, 1.10–1.25, p<0.001) | [21] |
| Dai et al, 2014 | Cross-sectional study | 521 patients with acute HF | Higher RDW (16.2% vs 14.4%) in acute HF patients at admission were associated with worse short- and long-term outcomes and RDW values were more prognostically relevant than Hgb levels | [22] |
| Jenei et al, 2014 | Prospective cohort study | 195 patients with stable chronic HF | RDW >14.5% was independent predictor of 5-year mortality (HR 1 SD increment 1.46, 95% CI 1.221–1.733, p<0.001) | [23] |
| He et al, 2014 | Prospective cohort | 128 patients with acute HF | Both RDW and NT-proBNP are strong independent predictors of cardiovascular events | [24] |
| Liu et al, 2016 | Retrospective study | 179 chronic HF patients | RDW was markedly elevated in the mortality group compared with the survival group (15.8±1.8 vs 13.7±1.7, p<0.01). RDW was an independent risk factor for mortality (OR=2.531, 95% CI: 1.371–4.671) during hospitalization with AUC = 0.837 | [25] |
| Huang et al, 2014 | Meta-analysis of 17 studies | 18,288 patients with HF | RDW on admission and discharge, as well as its variation during treatment are prognostic markers in HF patients. In particular, each 1% increase in baseline RDW was associated with a 10% increased risk of all-cause mortality (OR, 1.10; 95% CI, 1.07–1.13). | [26] |
| Cemin et al, 2011 | Prospective study | 1971 patients admitted due to chest pain of suspected cardiac origin | Higher RDW value was obtained in patient with AMI compared to without AMI (14.4 vs 13.7) and RDW cut-off value of 13.7% showed a sensitivity and specificity of 75% and 52%, respectively | [18] |
| Hu et al, 2017 | Case–control study | 100 healthy and 300 patients with coronary heart disease | Stenocardia 121 cases, HF 65 cases and acute MI 114 cases were found. The result revealed that the RDW and HCY were both significantly higher in acute MI groups than in the 3 other groups | [19] |
| Lippi et al, 2009 | Prospective study | 2304 adult patients admitted for chest pain suggestive of ACS | The combined measurement of cardiac troponin T and RDW increases diagnostic sensitivity to 99% in diagnosing ACS (diagnostic sensitivity of cardiac troponin T alone was 94%). | [20] |
| Söderholm et al, 2015 | Prospective study | 26,879 participants without a history of coronary events or stroke | High RDW was associated with increased incidence of total stroke (HR for stroke 1.31 (1.11–1.54 the highest quartile compared to the lowest) | [29] |
| Ramírez et al, 2013 | Case-control study | 224 patients with ischemic stroke and 224 control subjects | Subjects who have RDW >14·61% were more likely to have a stroke compared with patients with RDW <13·27%, [OR 4·50; 95% CI: 2·50–8·01, P< 0·0001] | [30] |
| Wan et al, 2015 | Prospective study | 300 patients with AF | RDW was independently associated with all-cause mortality (HR: 1.024; 95% CI: 1.012–1.036, P <0.001) and major adverse events (HR: 1.012; 95% CI: 1.002–1.023, P=0.023). | [31] |
| Ertas et al, 2012 | Retrospective | 132 patients with no histories of AF undergoing coronary artery bypass grafting | Preoperative RDW levels were significantly higher in patients who developed AF than in those who did not (13.9 ± 1.4 vs 13.3 ± 1.2, p=0.03). Using a cut point of 13.45, the preoperative level correlated with the incidence of AF with a sensitivity of 61% and specificity of 60%. | [32] |
| Eryd et al, 2014 | Prospective study | 27,124 subjects from the general population without history of CVDs | HR for incidence of AF was 1.33 [95% CI 1.16–1.53] for the fourth versus first quartile of RDW P <0.001). | [33] |
| Osadnik et al, 2013 | Retrospective | 2550 consecutive patients with stable coronary artery disease | 4-fold increase (4.3% vs 17.1%, p < 0.0001) in mortality between the group of patients with RDW values <13.1% vs >14.1%). RDW is an independent predictor of mortality in patients with stable coronary artery disease | [34] |
| Ye et al, 2011 | Prospective study | 13,039 consecutive outpatients with PAD | Subjects in the highest quartile of RDW (>14.5%) had a 66% greater risk of mortality compared to those in the lowest quartile (RDW <12.8%; P<0.0001). A 1% increment RDW was associated with a 10% greater risk of all-cause mortality (HR: 1.10; 95% CI, 1.08–1.12) | [36] |
RDW in Myocardial Infarction
In addition to its routine index in clinical practice, the increased value of RDW has been proposed to be associated with the adverse outcomes of myocardial infarction. A study was conducted by Cemin et al,18 including 1971 patients admitted due to chest pain with suspected cardiac origin at the emergency department. The authors found that RDW was a significant predictor of acute myocardial infarction (AMI), showing the area under the curve (AUC) of 0.61 (95% CI, 0.54–0.68). The specificity and sensitivity of RDW at the 13.7% cut-off value were 52% and 75%, respectively.18 In addition, the diagnostic value of joint detection of RDW and homocysteine (HCY) variable coefficient on AMI were investigated by recruiting 400 study subjects (100 healthy and 300 patients with coronary heart disease). Among patients, 121 were stenocardia cases, 65 were HF cases and 114 were AMI cases.19 In this study, the authors found that RDW and HCY were increased in AMI groups than in the three other groups (P<0.05).
Furthermore, a study done by Lippi et al20 showed that RDW had a diagnostic value in patients admitted to the ICU for chest pain suggestive of ACS. On admission, the sensitivity and specificity of troponin T were 94% (25 false-negative results) and 100%, respectively. With a 14% cut-off value, the sensitivity and specificity of RDW were 79% and 50%, respectively. The measurements of troponin T and RDW help to diagnose MI with greater sensitivity (99%) than the analysis of troponin T alone. This implicated that RDW and other parameters like HCY and troponin T joint detection of AMI has a higher sensitivity and specificity, which may highly improve the accurate diagnosis of MI. However, homocysteine is expensive and not widely available. If the use of RDW in resource poor settings are considered, homocysteine–RDW combination may not be useful.
RDW in Heart Failure
As mentioned earlier, the RDW and anemia relationship has been studied since the sixties. The RDW has also been suggested as a useful tool for predicting morbidity and mortality in patients with acute and chronic heart failure.21–25 In 2007 Felker et al21 reported for the first time in two cohorts (n = 2679) of patients with chronic HF. They demonstrated that the RDW was the most powerful predictor of adverse follow-up outcomes, both in cardiovascular mortality and in hospitalizations due to HF. Hazard ratio (HR) for morbidity and mortality (RDW increased in 1 SD): was 1.17 (95% confidence interval [CI], 1.10–1.25, P<0.001).21 A recent meta-analysis reported by Huang et al26 showed that RDW is a prognostic marker in HF patients on admission, discharge, and during treatment. A 1% higher RDW in baseline was associated with a 10% increased risk of future mortality events (HR, 1.10; 95% CI, 1.07–1.13). This indicates that 1% increment in RDW during admission can predict the patient’s future outcome. Several studies have shown a positive relationship between RDW and different markers that have been applied over the last decade in predictive models of survival for patients with HF including C-reactive protein, in-hospital mortality,22 hospital readmissions,23 and type B natriuretic peptide (BNP).24
Regarding the latter blood marker highly studied in HF, He et al24 conducted a prospective study to evaluate the predictive value of RDW for a cardiovascular event during hospitalization of acute HF patients and compared it with that of NT-proBNP. The main result of this study was a significant weak positive correlation existed between the RDW and NT-proBNP levels (r = 0.217; P<0.05), the cutoff point for NT-proBNP was 1471.5 pg/mL with a 93.5% sensitivity and 32.9% specificity. For RDW, the cutoff point was 14.1%, with 87.0% sensitivity and 54.9% specificity and survival analysis revealed that patients with an RDW level >14.5% and NT-proBNP >1471.5 pg/mL were at the highest risk for cardiovascular event (P<0.001). This investigation suggests that the combination of RDW and other validated biomarkers such as NT-proBNP can help cardiologists improve the accurate rate of diagnosis and reduce misdiagnosis.24
RDW in Ischemic Cerebrovascular Disease
A piece of evidence showed that the RDW value is increased in patients with stroke compared to non-stroke patients and is also a significant and independent predictor of both cardiovascular and all-cause mortality in stroke patients.27 In addition, RDW is associated with the risk of stroke in patients with HF28 and the general population in the community.29 Kaya et al compared HF patients with and without stroke, they found that significantly increased basal RDW (16.9 ± 1.14 vs 14.6 ± 1.4, P<0.001). An RDW ≥ 15.2% measured on admission had 87% sensitivity and 74% specificity in predicting stroke in patients with HF (AUC: 0.923, 95% CI: 0.852–0.994, P<0.001).28
A population-based study conducted involving 26,879 participants without a history of coronary events or stroke29 showed that increased RDW was associated with a higher incidence of total stroke. Study participants grouped in the highest quartile of RDW showed a significantly higher incidence of stroke (HR, 1.31; 95% CI, 1.11–1.54). Ramírez et al30 conducted a case–control study, found that RDW was higher in the stroke group as compared to controls 14.48 ± 1.76 vs 13.9 ± 1.43, P=0.001). Patients in the fourth quartile (RDW>14.61%) were significantly more likely to have a stroke compared with the lower quartile [RDW< 13.27%, odds ratio (OR) 4.50; 95% CI: 2.50–8.01, P< 0.0001]. In summary, these data suggest RDW may be a predictive marker in stroke.
RDW in Atrial Fibrillation
Likewise in patients with other CVDs, an increased RDW is an independent predictor of unfavorable clinical outcomes, such as all-cause mortality and the incidence of major adverse events in patients with AF.31–33 In brief, Wan et al31 followed 300 patients with AF and they found that those study subjects in the higher RDW quartile (RDW≥14.4%) had a 13.77% increased risk of mortality as compared to the lowest quartile (RDW<12.8%, P<0.001). In addition, RDW was independently associated with all-cause mortality and major adverse events (HR: 1.024; 95% CI: 1.012–1.036, P<0.001) and (HR: 1.012; 95% CI: 1.002–1.023, P=0.023), respectively. The analysis of receiver operating characteristics (ROC) showed that RDW predicted both mortality and major adverse events with AUC of 0.682 and 0.617; the best cutoff points were 13.85% with a sensitivity of 63.3% and a specificity of 70.4%. And 13.55%, with a sensitivity of 62.0% and a specificity of 60.6%, respectively.31
RDW in Coronary Artery Disease
Several studies have shown that the value of RDW is higher in patients with coronary artery disease.34,35 The study by Osadnik et al34 analyzed 2550 consecutive patients with stable coronary artery disease (CAD). The patients were grouped by their RDW values at the lower quartile (<13.1%) and higher quartile (>14.1%) and followed for a mean of 2.5 years. During the follow-up, there was an almost fourfold increase (4.3% vs 17.1%, P<0.0001) in mortality between the group. Interestingly, RDW remained significantly associated with mortality in the entire cohort (HR-1.23 [95% CI (1.13–1.35), P<0.0001]) after adjusting for other variables. Additionally, Lapp et al35 studied 1489 patients with CAD and 449 healthy controls. They reported that RDW strongly predicts all-cause mortality in patients with CAD and the control population (HR=1.37 per quintile; 95% CI=1.29, 1.46; P<0.001) and (HR=1.33 per quintile, CI=1.15, 1.55; P<0.001), respectively. However, highly sensitive C-reactive protein (hs-CRP) did not predict mortality in the control group. Thus, the association of RDW with mortality in patients with CAD may provide potentially useful prognostication.
RDW in Peripheral Artery Disease
Recent studies have shown that the value of RDW is increased in patients with peripheral arterial disease. Ye et al36 employed an epidemiological study to investigate the prognostic role of RDW in patients with peripheral artery disease (PAD). In this study, the authors reported that those patients in the upper quartile of RDW (>14.5%) had a 66% higher risk of mortality compared to those in the lower quartile (RDW <12.8%; P<0.0001). One percent increment in RDW was associated with a 10% higher risk of all-cause mortality (HR: 1.10; 95% CI, 1.08–1.12).
RDW in Hypertension
It has been reported that the value of RDW is increased in hypertensive patients. Tanindi et al37 investigated the role of RDW in apparently healthy individuals (n = 36), prehypertensive patients (n = 74), and hypertensive patients (n = 128). Following adjusting for confounding variables, they found the mean value of RDW was 15.26 ± 0.82 in prehypertensive, 16.54 ± 0.91 in hypertensive, and 13.87 ± 0.94 in control groups (P<0.05). Additionally, RDW showed a strongly positive and statistically significant correlation with systolic and diastolic blood pressures (r=0.848 and r= 0.748, respectively, P<0.01), independent of confounders such as age, inflammatory status, and anemia. Another comparative cross-sectional study conducted by Enawugaw et al38 showed that RDW increased significantly in hypertensive groups compared to normotensive individuals (P< 0.001).
Pathophysiological Mechanisms
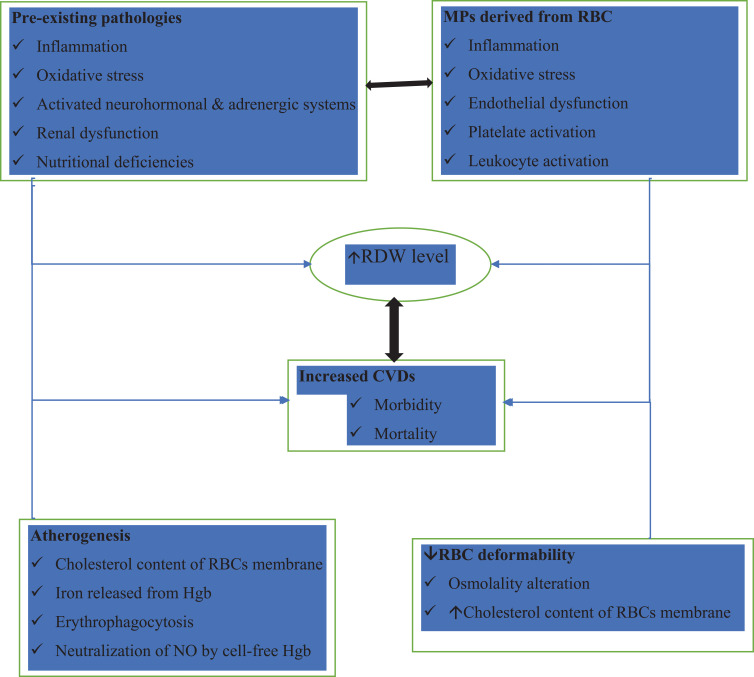
Despite the numerous clinical evidence available, that shows the increase in RDW as a potential biomarker in CVD to be taken into account in the coming years, the underlying pathophysiological mechanisms have not been studied and can generate strong attention in the medical and biomedical community. The main question we can ask ourselves is how a greater heterogeneity of RBC in the blood can directly cause CVD or if this greater heterogeneity is simply an epiphenomenon, secondary to other deleterious processes, previously activated in these pathologies. The existing hypothesis mainly includes the rheological properties of RBCs, microparticles derived from RBCs, anemia, oxidative stress, and inflammatory cytokines (Figure 1).
Figure 1.
Hypothetical model that would explain the mechanisms for increase in RDW in patients with cardiovascular diseases. All these mechanisms as a whole and separately, would perpetuate deleterious processes that would spread and generate cardiovascular diseases by having a high RDW.
Abbreviations: CVDs, cardiovascular diseases; RBCs, red blood cells; RDW, red blood cell distribution width; Hgb, hemoglobin; NO, nitric oxide; ↓, decrease; ↑, increase.
Rheological Properties of Red Blood Cells
Red blood cells play a major role in humans and are responsible for the transportation of oxygen and carbon dioxide between blood and tissues. The red blood cells perform this activity because of their ability to deform and flow in the vascular system. High deformability that allows red blood cells to flow through microcapillaries that are smaller in size than the cells themselves is attributed to several factors, including cell shape, the viscosity of the intracellular fluid, and rheological properties of the cell membrane.39 There are many morphological abnormalities that affect the deformability and elasticity of RBCs including drepanocyte (sickle shape RBC), spherocyte and acanthocyte. Sickle cell anemia is a type of inherited hemolytic anemia due to hemoglobin S (Hgb S) variant. In the presence of Hgb S, the morphology of RBCs can be affected which results in less deformable sickle-shaped RBCs.40 Cardiovascular diseases are common in sickle cell anemia, including cardiac enlargement, myocardial infarction, stroke, and pulmonary hypertension.41
Moreover, change in osmolality under some pathological and physiological conditions can affect the deformability of RBCs and microvascular blood flow and result in CVDs eventually.42 One study shows that RDW values above 14.0% were significantly associated with decreased RBC deformability, which can compromise blood flow through microcirculation.43 From the perspective of hematology, the predictive role of osmolality in cardiovascular prognostication may be evaluated by the deformability of RBCs, which is assessed by the value of RDW and the resulting hypoxia may be used to explain the higher risk for CVD events related to higher RDW.
In addition, an increased cholesterol erythrocyte membrane level is another cause of decreased membrane fluidity and deterioration of erythrocyte deformability. This can affect the microcirculation, resulting in short survival of circulating erythrocyte, and higher RDW values. In recent studies, a strong and direct relationship was observed between the RDW value and the cholesterol content of erythrocyte membranes (CEM), and the CEM is also positively and independently associated with clinical instability in patients with CVDs.44,45
The excess free cholesterol within the necrotic core of atherosclerotic plaque can be derived from different cellular sources, including RBCs.46 The RBCs entrapped within the atherosclerotic plaque core and directly participate in the accelerated atherogenesis and CVDs through a variety of mechanisms.47 First, the assembly of free and crystallized cholesterol originating from the RBC membrane promotes the expansion of the lipid core. Second, injury of RBCs within the atherosclerotic plaque results in release of iron found in the hemoglobin content that triggers the generation of reactive oxygen species, tissue injury, and activation of many pro-inflammatory cytokine pathways. Third, erythrophagocytosis mediated by the interaction of erythrocytes with scavenger receptors on macrophages and other phagocytes may also amplify the formation of foam cells and facilitate the excrescence of the atherosclerotic plaque. Fourth, the neutralization of nitric oxide by cell-free hemoglobin released from injured erythrocytes inside the necrotic core of the atherosclerotic plaque may also contribute to inhibiting endothelium-dependent nitric oxide-mediated vasodilation.1,47
Microparticles Derived from Red Blood Cells
Microparticles (MPs) are active subcellular structures (0.1 to 1 μm), derived from the plasma membrane of different types of cells, mainly platelets, RBCs, monocytes, lymphocytes, neutrophils, and endothelial cells during the processes of cellular activation or apoptosis. Microparticles have various receptors and molecules inside, allowing them to activate mainly negative processes in other cell groups such as endothelial cells, platelets, and white blood cells.48 In comparison to healthy individuals, blood levels of MPs derived from RBCs are elevated in patients with CVDs; secondary to nutritional deficiency, pro-inflammatory, and oxidative states, where its excessive increase has been associated with different cardiovascular pathologies.49 Several research groups have shown that the deleterious participation of MPs in CVDs would be mainly related to their high proinflammatory, prooxidatidant and prothrombotic potential.48–50 It is possible to propose that there are increased MPs derived from RBCs due to nutritional deficiency, pro-inflammatory and oxidative states, and these MPs once released into the blood can interact directly with processes and cells generating a greater proinflammatory, prothrombotic and oxidative state, which could increase the heterogeneity of RBCs, resulting in a higher RDW, which could be the potential factor in the pathogenesis and poor prognosis in patients with CVDs.
Anemia and Iron Metabolism
Anemia has been recognized as a well-documented risk factor for an increased mortality rate in patients with CVDs including chronic HF and ACS. The potential mechanisms for the development of anemia are multifactorial including inflammation, decreased production of erythropoietin, ineffective erythropoiesis, nutritional deficiencies, and comorbidities (chronic kidney disease, diabetes mellitus). Because many of these mechanisms can cause variation in the size of the erythrocyte, the association between these mechanisms and outcomes can be demonstrated by RDW in an integrated fashion.51 Most chronic anemias are associated with left ventricular hypertrophy and heart failure. Patients with chronic anemia and hemoglobin <10 g/dL show some hemodynamic compensatory responses like decreased systemic vascular resistance, increased cardiac output, water, and sodium retention, and low renal blood flow and GFR. These responses may cause an increased cardiac workload to maintain sufficient oxygen supply because of an increase in blood volume and consequent left ventricular remodeling.52
Red blood cell distribution width is affected by iron metabolism. It is noted that impaired iron metabolism is characterized by the presence of higher RDW, low hemoglobin, low MCV, high erythropoietin, normal iron-binding capacity, and ferritin. It is understood that the ability to mobilize and use stored iron may be impaired even in the presence of sufficient total body iron. This condition is known as “reticuloendothelial block” mediated by overproduction of hepcidin (the master regulator of iron metabolism) in response to inflammatory cytokine (IL-6), which is strongly associated with higher RDW. Also, IL-6 increases the expression of ferritin and iron retention within macrophages, leading to a decreased iron level in the circulation and limited availability of iron for erythroid cells.53 Studies indicated that increased baseline RDW and MCV has been associated with the formation of anemia during hospitalization in patients with AMI and with normal hemoglobin levels at admission.54 Furthermore, increased RDW levels increase mortality in patients with cardiovascular disease regardless of the baseline hemoglobin value and RDW has a good prognostic role in non-anemic patients.34
Oxidative Stress
Oxidative stress is a condition developed from an imbalance between the formation of free radicals and body antioxidant defenses, resulting in damage to macromolecules (nucleic acids, proteins, and lipids) and tissue injury.55 It is commonplace in most chronic diseases such as cancer, diabetes mellitus, CVDs, inflammation, liver failure, and chronic kidney disease.56 Oxidative stress exerts a profound effect on RBC’s homeostasis and survival. Some studies show a close association between the increase in RDW and biomarkers of oxidative stress.57,58 In brief, Az et al showed that the addition of vitamin C (500 mg/day) to 22 soccer players under 25 years of age generated a decrease in RDW levels compared to another group that did not receive vitamin C.57
Additionally, Semba et al have reported that a decrease in plasma levels of selenium and carotenoids (potent antioxidants in the blood) was associated with the increase in RDW in 786 women.58 These studies allow the preliminary hypothesis that oxidative stress can generate changes in the size of the RBCs, increasing its heterogeneity and therefore the RDW. Moreover, smoking can result in increased oxidative stress and higher RDW is correlated with the number of cigarettes smoked per day and the duration of smoking.59 In addition, it has been recognized that smoking is a well-known risk factor in the development of CVDs including myocardial infarction. Some reports showed a stronger relation between RDW and myocardial infarction in smokers, which supports the hypothesis that RDW value can reflect inflammation conditions.60
Inflammatory Cytokine
Some studies have reported a univariate and multivariate statistical relationship between the RDW with various established systemic inflammatory parameters.51,61 Lippi et al published for the first time in a cohort of 3845 subjects who were monitored with routine blood count for 3 years, that hsCRP predicted the increase in RDW levels regardless of age, gender, MCV, hemoglobin, and ferritin.61 Inflammation can increase RDW levels by altering iron metabolism, decreasing RBCs’ survival, impairing RBC maturation, and causing immature RBC to enter the peripheral circulation. The production of erythrocytes in bone marrow can be inhibited by proinflammatory cytokines as a result of the blockage of erythroid progenitor cell proliferation and proerythroblast maturation.51,62
The chronic inflammatory state is one of the roots of atherosclerosis and resultant complications including CVDs.63 Cardiovascular disease is accompanied by increased circulating pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which can affect erythropoiesis, reflected by an increase of RDW.64 The process of such conditions mainly resulted from desensitizing erythroid progenitor cells to erythropoietin (EPO), blocking antiapoptotic and maturation effects of EPO, and decreased renal EPO synthesis by inflammatory cytokines. Additionally, the synthesis of EPO is lower in patients with heart failure and impaired kidney function.64 An increased RDW value highlights the presence of immature RBC in the peripheral circulation, which could be due to reduced functional iron availability or inhibition in the synthesis or activity of EPO. So RDW may be a representative of an integrative indicator of different pathological processes in CVDs.
Conclusions and Future Directions
Currently,a piece of evidence that supports a higher degree of heterogeneity is common in patients with different CVDs such as stroke, PAD, hypertension, ACS, HF, and AF. A higher RDW value significantly and independently predicts a worse prognosis in patients with CVDs and the general population. As compared to other biochemical parameters in CVDs, RDW is an easily available biomarker as is a rapid and inexpensive test without requiring specialized skills. However, the specificity of RDW is not very high, its high sensitivity and correlation with other biomarkers like NT-proBNP, troponin, HCY, and CRP can support cardiologists in the diagnosis and management of CVDs. Generally, the scientific evidence available so far indicated that the role of RDW may be expanded beyond the routine boundaries of RBC dysfunctions, especially it helps in the diagnosis and prognosis of patients with CVDs. However, an interesting and yet unanswered question exists is the relationship between increased red cell distribution width and CVDs is causal or occurs as a result of the underlying pathophysiological state. As, the data reviewed in this manuscript are preliminary and observational in nature, they cannot investigate the underlying mechanisms. If RDW is considered as a new biomarker and risk factor, it must meet a series of requirements to be recognized such as being modified by the effect of therapies. Future studies should focus on investigating the most precise biological mechanisms that explain this increase seen in clinical practice, how to standardize its determination in a more unified way, and also the evaluation of the RDW and its potential capacity to be modified by the use of therapies.
Funding Statement
No funding was received for this study or publication of this article.
Abbreviations
ACS, acute coronary syndrome; AF, atrial fibrillation; AMI, acute myocardial infarction; AUC, area under the curve; CAD, coronary artery disease; CBC, complete blood cell count; CEM, cholesterol content of erythrocyte membranes; CI, confidence interval; CVDs, cardiovascular diseases; EPO, erythropoietin; HCY, homocysteine; HF, heart failure; HR, hazard ratio; hsCRP, highly sensitive C-reactive protein; IL-1β, interleukin 1 beta; IL-6, interleukin 6; MCV, mean corpuscular volume; MPs, microparticles; NO, nitric oxide; NT-proBNP; N-terminal pro-B-type natriuretic peptide; OR, odds ratio; PAD, peripheral artery disease; RBC, red blood cell; RDW, red blood cell distribution width; ROC, receiver operating characteristic; SD, standard deviation; TNF-α, tumor necrosis factor-alpha.
Compliance with Ethics Guideline
This review is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- 1.Salvagno GL, Sanchis-gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2014;52:1–20. [DOI] [PubMed] [Google Scholar]
- 2.Aktas G, Alcelik A, Tekce BK, Tekelioglu V, Sit M, Savli H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz Gastroenterol. 2014;9(3):160–163. doi: 10.5114/pg.2014.43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aktas G, Sit M, Dikbas O, et al. Could red cell distribution width be a marker in Hashimoto’s thyroiditis? Exp Clin Endocrinol Diabetes. 2014;122(10):572–574. doi: 10.1055/s-0034-1383564 [DOI] [PubMed] [Google Scholar]
- 4.Akır L, Aktas G, Mercimek OB, Enginyurt O, Kaya Y, Mercimek K. Are red cell distribution width and mean platelet volume associated with rheumatoid arthritis? Biomed Res. 2016;27(2):292–294. [Google Scholar]
- 5.Aktas G, Sit M, Karagoz I, et al. Could red cell distribution width be a marker of thyroid cancer? J Coll Physicians Surg Pak. 2017;27:556–558. [PubMed] [Google Scholar]
- 6.Tel BMA, Kahvecib G, Bilgina S, et al. Haemoglobin and red cell distribution width levels in internal medicine patients indicate recurrent hospital admission during COVID-19. Fam Med Prim Care Rev. 2022;24(1):32–36. doi: 10.5114/fmpcr.2022.113011 [DOI] [Google Scholar]
- 7.Lippi G, Pipitone S, Favaloro EJ. Harmonization of red blood cell distribution width (RDW): an attainable target? Ann Blood. 2017;2(15):1–5. doi: 10.21037/aob.2017.09.01 [DOI] [Google Scholar]
- 8.Lee H, Kim J, Oh S, Kim S, Kim H. Red blood cell distribution width is associated with severity of leukoaraiosis. PLoS One. 2016;11(2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Plebani M. Red blood cell distribution width (RDW) and human pathology. One size fits all. Clin Chem Lab Med. 2014;52(9):1247–1249. doi: 10.1515/cclm-2014-0585 [DOI] [PubMed] [Google Scholar]
- 10.Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis. 2015;7(10):402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luepker RV, Lakshminarayan K, Detels R, Beaglehole R, Lansang MA. Cardiovascular and Cerebrovascular Diseases. 5th ed. CRC Press. 2009:971–996 p. [Google Scholar]
- 12.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;34:1–14. [DOI] [PubMed] [Google Scholar]
- 13.Parsanathan R, Jain SK. Novel invasive and noninvasive cardiac-specific biomarkers in obesity and cardiovascular diseases. Metab Syndr Relat Disord. 2020;18(1):10–30. doi: 10.1089/met.2019.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krittayaphong R, Muenkaew M, Chiewvit P, et al. Electrocardiographic predictors of cardiovascular events in patients at high cardiovascular risk: a multicenter study. J Geriatr Cardiol. 2019;16(8):630–638. doi: 10.11909/j.issn.1671-5411.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhelst XPD, Troisi RI, Colle I, Geerts A, Van VH. Biomarkers for the diagnosis of acute cellular rejection in liver transplant recipients: a review. Hepatol Res. 2013;43(2):165–178. doi: 10.1111/hepr.12012 [DOI] [PubMed] [Google Scholar]
- 16.Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Mark. 2017;2017:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montagnana M, Cervellin G, Meschi T, et al. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2012;50(4):635–641. doi: 10.1515/cclm.2011.831 [DOI] [PubMed] [Google Scholar]
- 18.Cemin R, Donazzan L, Lippi G, Clari F, Daves M. Blood cells characteristics as determinants of acute myocardial infarction. Clin Chem Lab Med. 2011;49(7):1231–1236. doi: 10.1515/CCLM.2011.183 [DOI] [PubMed] [Google Scholar]
- 19.Hu GX, Zhang J, Tian YG, et al. Diagnostic value of joint detection of homocysteine and RDW CV on acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2017;20(19). [PubMed] [Google Scholar]
- 20.Lippi G, Filippozzi L, Salvagno GL, Guidi GC, Clinica C. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009;47(3):353–357. doi: 10.1515/CCLM.2009.066 [DOI] [PubMed] [Google Scholar]
- 21.Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure. J Am Coll Cardiol. 2007;50(1):40–47. doi: 10.1016/j.jacc.2007.02.067 [DOI] [PubMed] [Google Scholar]
- 22.Dai Y, Konishi H, Takagi A, Miyauchi K, Daida H. Red cell distribution width predicts short- and long-term outcomes of acute congestive heart failure more effectively than hemoglobin. Exp Ther Med. 2014;8(2):600–606. doi: 10.3892/etm.2014.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenei ZM, Förhécz Z, Gombos T, Pozsonyi Z, Jánoskuti L, Prohászka Z. Red cell distribution width as predictive marker in CHF: testing of model performance by reclassification methods. Int J Cardiol. 2014;2:15–16. [DOI] [PubMed] [Google Scholar]
- 24.He W, Jia J, Chen J, et al. Comparison of prognostic value of red cell distribution width and NT-proBNP for short-term clinical outcomes in acute heart failure patients. Int Hear J. 2014;55(1):58–64. doi: 10.1536/ihj.13-172 [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Wang P, Shen P, Zhou J-H. Predictive values of red blood cell distribution width in assessing severity of chronic heart failure. Med Sci Monit. 2016;22:2119–2125. doi: 10.12659/MSM.898103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Hu Z, Liu S, et al. Prognostic value of red blood cell distribution width for patients with heart failure: a systematic review and meta-analysis of cohort studies. PLoS One. 2014;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herna V, Herna L, Espan F. Red blood cell distribution width in patients with cryptogenic stroke. Clin Appl Thromb. 2014;2014:1–5. [DOI] [PubMed] [Google Scholar]
- 28.Kaya A, Isik T, Kaya Y, et al. Relationship between red cell distribution width and stroke in patients with stable chronic heart failure: a propensity score matching analysis. Clin Appl Thromb. 2015;21(2):160–165. doi: 10.1177/1076029613493658 [DOI] [PubMed] [Google Scholar]
- 29.Söderholm M, Borné Y, Hedblad B, Persson M, Red Cell EG. Distribution width in relation to incidence of stroke and carotid atherosclerosis: a population-based cohort study. PLoS One. 2015;44:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramírez-Moreno JM, Gonzalez-Gomez M, Ollero-Ortiz A, Roa-Montero AM, Gómez-Baquero MJ, Constantino-Silva AB. Relation between red blood cell distribution width and ischemic stroke: a case-control study. Int J Stroke. 2013;8(36):2013. doi: 10.1111/ijs.12091 [DOI] [PubMed] [Google Scholar]
- 31.Wan H, Yang Y, Zhu J, et al. The relationship between elevated red cell distribution width and long-term outcomes among patients with atrial fibrillation. Clin Biochem. 2015;48(12):762–767. doi: 10.1016/j.clinbiochem.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Ertas GÖK, Aydin C, SÖNmez O, ErdoGAN E, Zeybek R, GÖKtekin Ö. Red cell distribution width predicts new-onset atrial fibrillation. Scand Cardiovasc J. 2012;90:1–4. [DOI] [PubMed] [Google Scholar]
- 33.Eryd SA, Born Y, Melander O, et al. Red blood cell distribution width is associated with incidence of atrial fibrillation. J Intern Med. 2014;275(1):84–92. doi: 10.1111/joim.12143 [DOI] [PubMed] [Google Scholar]
- 34.Osadnik T, Strzelczyk J, Hawranek M, et al. Red cell distribution width is associated with long-term prognosis in patients with stable coronary artery disease. BMC Cardiovasc Disord. 2013;13(113):1–8. doi: 10.1186/1471-2261-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lappé JM, Horne BD, Shah SH, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 2011;412(23–24):2094–2099. doi: 10.1016/j.cca.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 36.Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011;107(8):1241–1245. doi: 10.1016/j.amjcard.2010.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanindi A, Topal FE, Topal F, Celik B. Red cell distribution width in patients with prehypertension and hypertension. Blood Press. 2012;21(3):177–181. doi: 10.3109/08037051.2012.645335 [DOI] [PubMed] [Google Scholar]
- 38.Enawgaw B, Adane N, Terefe B, Asrie F, Melku M. A comparative cross-sectional study of some hematological parameters of hypertensive and normotensive individuals at the university of Gondar hospital, Northwest Ethiopia. BMC Hematol. 2017;17(21):1–7. doi: 10.1186/s12878-017-0093-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guido S, Tomaiuolo G. Microconfined flow behavior of red blood cells in vitro. Med Eng Phys. 2009;10(8):751–763. [DOI] [PubMed] [Google Scholar]
- 40.Inusa BPD, Hsu LL, Kohli N, Patel A, Ominu-evbota K. Sickle cell disease_genetics, pathophysiology, clinical presentation and treatment. Int J Neonatal Screen. 2019;5(20):1–15. doi: 10.3390/ijns5020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mozos I. Mechanisms linking red blood cell disorders and cardiovascular diseases. Biomed Res Int. 2015;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinhart WH, Piety NZ, Goede JS, Shevkoplyas SS. Effect of osmolality on erythrocyte rheology and perfusion of an artificial microvascular network. Microvasc Res. 2015;98:102–107. doi: 10.1016/j.mvr.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the Red Cell Distribution Width with Red Blood Cell Deformability. New York, NY, USA: springer; 2013: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tziakas DN, Chalikias GK, Stakos D, et al. Independent and additive predictive value of total cholesterol content of erythrocyte membranes with regard to coronary artery disease clinical presentation. Int J Cardiol. 2011;150(1):22–27. doi: 10.1016/j.ijcard.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 45.Tziakas D, Chalikias G, Grapsa A, Gioka T. Red blood cell distribution width – a strong prognostic marker in cardiovascular disease – is associated with cholesterol content of erythrocyte membrane. Clin Hemorheol Microcirc. 2012;51(4):243–254. doi: 10.3233/CH-2012-1530 [DOI] [PubMed] [Google Scholar]
- 46.Michel J, Martin-Ventura JL. Red blood cells and hemoglobin in human atherosclerosis and related arterial diseases. Int J Mol Sci. 2020;21(18):1–20. doi: 10.3390/ijms21186756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tziakas DN, Chalikias GK, Stakos D, Boudoulas H. The role of red blood cells in the progression and instability of atherosclerotic plaque. Int J Cardiol. 2010;142(1):2–7. doi: 10.1016/j.ijcard.2009.10.031 [DOI] [PubMed] [Google Scholar]
- 48.Loyer X, Vion A, Tedgui A, Boulanger CM. Microvesicles as cell–cell messengers in cardiovascular diseases. Circ Res. 2014;114(2):345–353. doi: 10.1161/CIRCRESAHA.113.300858 [DOI] [PubMed] [Google Scholar]
- 49.Agouni A, Andriantsitohaina R, Martinez MC. Microparticles as biomarkers of vascular dysfunction in metabolic syndrome and its individual components. Curr Vasc Pharmacol. 2014;12(3):1–30. doi: 10.2174/1570161112666140423223148 [DOI] [PubMed] [Google Scholar]
- 50.Andriantsitohaina R, Gaceb A, Vergori L, Martínez MC. Microparticles as regulators of cardiovascular inflammation. Trends Cardiovasc Med. 2012;22(4):88–92. doi: 10.1016/j.tcm.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 51.Forhecz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohaszka Z, Janoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Hear J. 2009;158(4):659–666. doi: 10.1016/j.ahj.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 52.Lee W, Hsu P, Chu C, et al. Anemia as an independent predictor of adverse cardiac outcomes in patients with atrial fibrillation. Int J Med Sci. 2015;12(8):618–624. doi: 10.7150/ijms.11924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16(3):230–238. doi: 10.1016/j.cardfail.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salisbury AC, Amin AP, Reid KJ, et al. Red blood cell indices and development of hospital-acquired anemia during acute myocardial infarction. AJC. 2012;109(8):1104–1110. doi: 10.1016/j.amjcard.2011.11.045 [DOI] [PubMed] [Google Scholar]
- 55.Jat D, Nahar M. Oxidative stress and antioxidants: an overview. Int J Adv Res Rev. 2017;2:9. [Google Scholar]
- 56.Islam MMT, Shekhar HU Impact of Oxidative Stress on Human Health. Springer. 2015. 59–73. [Google Scholar]
- 57.Karakilcik AZ, Halat R, Zerin M, Celik H, Nazligul Y. Effects of vitamin C and exercise on lipid profile, platelet and erythrocyte indices in young soccer players. J Sports Med Phys Fitness. 2014;54(5):665–671. [PubMed] [Google Scholar]
- 58.Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s health and aging study I. Clin Nutr. 2010;29(5):600–604. doi: 10.1016/j.clnu.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korkmaz H, Erdem K. Elevated red blood cell distribution width in healthy smokers. Arch Turk Soc Cardiol. 2013;41(3):199–206. [DOI] [PubMed] [Google Scholar]
- 60.Buchanan DM, Arnold SV, Gosch KL, et al. Association of smoking status with angina and health- related quality of life after acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2015;8(5):493–500. doi: 10.1161/CIRCOUTCOMES.114.001545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133(4):628–633. doi: 10.5858/133.4.628 [DOI] [PubMed] [Google Scholar]
- 62.Osterholm EA, Georgieff MK. Chronic inflammation and iron metabolism. J Pediatr. 2015;166(6):1351–1357.e1. doi: 10.1016/j.jpeds.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zyga S. Cardiovascular disease and chronic inflammation in end stage kidney disease. Int J Caring Sci. 2013;6(1):29–36. [Google Scholar]
- 64.Inuzuka R, Abe J. Red blood cell distribution width as a link between ineffective erythropoiesis and chronic inflammation in heart failure. Circ J. 2015;79(5):974–975. doi: 10.1253/circj.CJ-15-0254 [DOI] [PubMed] [Google Scholar]