Abstract
Background & aims
Sarcopenia, frailty, and COVID-19 appear to be intertwined. Preventive and intervention measures are required to break this link and mitigate the consequences of rising morbidity and mortality among older adults. This study aimed to identify and synthesize important factors related to the interaction of the devastating trio and their impact on the health and mortality of older adults.
Methods
Data were gathered via searches of PubMed, Cochrane Library, Google Scholar, and Elsevier Mendeley Website. Study selection and data extraction were conducted by the two authors independently. The primary outcome was mortality, secondary outcomes included hospitalization and risk of development of severe disease among older Covid-19 patients. The study results are presented as adjusted odds and hazard ratios with 95% CI.
Results
A total of 1725 studies were identified through our electronic databases searches. After screening and assessing for eligibility, 39 studies were included in this review, a total of 51,796 patients were included in the systematic review. Our results indicated that frail patients recorded a higher mean age compared to non-frail patients (p < 0.00001), and COVID-19 frail patients had significantly increased mortality rate compared to non-frail patients, the log adjusted OR was 2.10 (1.63, 2.71); I2 = 78%; p < 0.00001.
Conclusion
Age and frailty are important risk factors for mortality among older adults COVID-19 patients. COVID-19 patients with sarcopenia had a higher risk of developing severe conditions, including hospitalization and ICU admission. Findings that support the use of frailty and sarcopenia indicators to help in the decision-making process for medical care in older adults COVID-19 patients.
Keywords: Sarcopenia, Nutrition, Frailty, Physical activity, Systematic review
1. Introduction
Worldwide, people are living longer, the percentage of people ≥60 is growing faster than any other age group [1]. Older age is associated with multifaceted health conditions called geriatric syndromes, these conditions are often the consequence of multiple underlying factors including sarcopenia, frailty, falls and others [1,2].
With advanced age, older adults encounter major challenges in staying healthy and active; one of these major challenges is related to decrease lean body mass due to age-related changes. This condition is known as sarcopenia. Sarcopenia is defined as an age-related progressive loss of skeletal muscle mass and strength [3,4]. Evidence suggests that this decline in muscle mass may begin in the 3rd or 4th decades of life, it increases with growing older, and it becomes more prominent after the age of 60 years [[5], [6], [7]]. Sarcopenia has been associated with several chronic diseases [8], and it is a significant predictor of hospitalization among older adults [9]. Besides, sarcopenia may lead to overwhelming personal, social, and economic burdens influencing individuals, communities, and health care systems [4,7]. Globally, The prevalence of sarcopenia is rising due to the increased numbers of older adults [10], and it is accompanied by adverse health consequences including falls, functional decline, frailty, and mortality [3,4].
Sarcopenia and physical frailty are linked and often overlapped [11], these are two geriatric syndromes where sarcopenia usually precedes frailty, the devastating curve in this line starts with sarcopenia to frailty and consequently leads to disability and mortality [12,13]. Frailty was defined as a clinical syndrome that includes weight loss, exhaustion, weakness, and slowness [14]. A recent systematic review included studies from 62 countries [15], indicating that the estimated prevalence of frailty among older adults was 12%, and for pre-frailty was 46%, the prevalence was higher among females 15% compared to 11% in males. Older adults with frailty have a higher risk of physical and mental health decline, and they are encountering more challenges in terms of incidence of diseases and vulnerability to infection [16]. Frail older adults are more vulnerable to infectious diseases due to the increased inflammatory condition and physiological capacity decline which lead to increased susceptibility to stressors [17,18].
Recently, with the outbreak of the COVID-19 pandemic, compared to other age groups, older people are facing the most serious threats and challenges due to age-related physiological changes and prevailing health conditions that make them at a higher risk for contracting the COVID-19 virus and developing severe illness [19]. COVID-19 pandemic has been remarkably associated with sarcopenia [20], and muscle loss was found as a predictor of ICU hospitalization in COVID-19 patients [21]. Furthermore, frailty contributes independently to adverse outcomes in COVID-19 patients, as well as care needs were increased in survivors of COVID-19 with frailty or older age [22]. On the other hand, evidence suggests that the implications of COVID-19 including prolonged bed rest with hospitalization, quarantine, inadequate nutrient intake and physical inactivity would enhance the acute sarcopenia process due to the increased muscle wasting [[23], [24], [25]] which may lead to frailty if untreated.
The three sides of the devastating trio (sarcopenia, frailty, and COVID-19) seem to be convoluted and intertwined. Preventive and intervention measures are necessary to disrupt this connection and minimize the consequences in terms of the rising morbidity, disability, and mortality among older adults. Therefore, this study aimed to review all available studies to get a better insight into the association of the devastating trio of sarcopenia, frailty, and COVID-19.
2. Methods & materials
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) updated guidelines [26].The study protocol was registered at PROSPERO- International prospective register of systematic reviews (registration number is: CRD42022300812).
2.1. Eligibility criteria
The inclusion criteria were cohort studies, participants were women and men ≥50 years old, diagnosis of COVID-19, sarcopenia and/or frailty, and published full text studies in the English language. Exclusion criteria were other types of study design such as review and cross-sectional studies and preprints.
2.2. Information sources
Data were gathered via searches of PubMed, Cochrane, Google Scholars, and Elsevier Mendeley Library. The search targeted cohort studies addressing the association of COVID-19, sarcopenia and/or frailty. The date of publication was not limited.
2.3. Search strategy
The searching process was proceeded until the 9th of January 2022 by using the following portals’ databases, full article accessed 1. PubMed ((sarcopenia [Title/Abstract]) OR (Frailty [Title/Abstract])) AND (Covid-19 [Title/Abstract])) 2. Google Scholar (all entitled: sarcopenia OR frailty “Covid −19”) 3. Elsevier Mendeley website (frailty + covid-19) or (sarcopenia + covid-19) 4. Cochrane Library website using advanced search box using Keywords: Sarcopenia, Covid-19, Frailty.
2.4. Study selection
After excluding duplicates, the retrieved studies were reviewed by the two authors independently, titles and abstracts were evaluated at the initial screening stage, then selected the studies that met the inclusion criteria. In the second stage, full texts were extracted for all potentially eligible studies in this review.
2.5. Data extraction
Data were extracted from the studies included authors, journal name, year of publication, country, study tools and outcomes, socio-demographic and clinical characteristics of the participants (e.g., sample size, sex ratio, and mean age). All data were cross-checked by the two authors, any dissimilarities were discussed until consensus was reached.
2.6. Assessment of risk bias
Assessment of risk of bias was performed according to the Newcastle Ottawa Scale (NOS) [27] which evaluates the quality of cohort studies based on three categories: selection, comparability, and outcomes. The score ranges from 0 to 9, a higher score indicates better quality.
2.7. Statistical analysis
At the initial stage, a narrative synthesis was carried out, descriptive findings related to the participants' demographic clinical characteristics and outcomes were included. Consequently, all included studies in the systematic review were screened for data eligible for meta-analysis such as frailty status based on clinical frailty scale and age data according to frailty status. The quantitative synthesis was carried out for outcomes reported by more than four studies with the same outcome measure. The study's findings were presented as odds ratios (OR) and hazard ratios (HR) with a 95% confidence interval (CI). For odds ratios and mean ages with frailty status meta-analysis, all appropriate data were collected and entered into IBM SPSS Statistics v28. The extracted values of odds ratios with their 95% confidence interval were converted to log odds ratios with Standard Error for processing in different meta-analysis programs [28].
We used Cohen's d statistics to determine the effect size of the included studies [29]. Subgroup analyses were performed, patients were classified according to their (Clinical frailty scale) CFS score [30], where patients with a score of six and above were considered frail, a score of 4 and 5 (pre-frail), and scores 1–3 (non-frail). Heterogeneity was assessed using the I-squared test. Publication bias was assessed using Egger's test and presented by the funnel plots. All forest and funnel plots were designed using Cochrane review manager, version 5.4.1.
3. Results
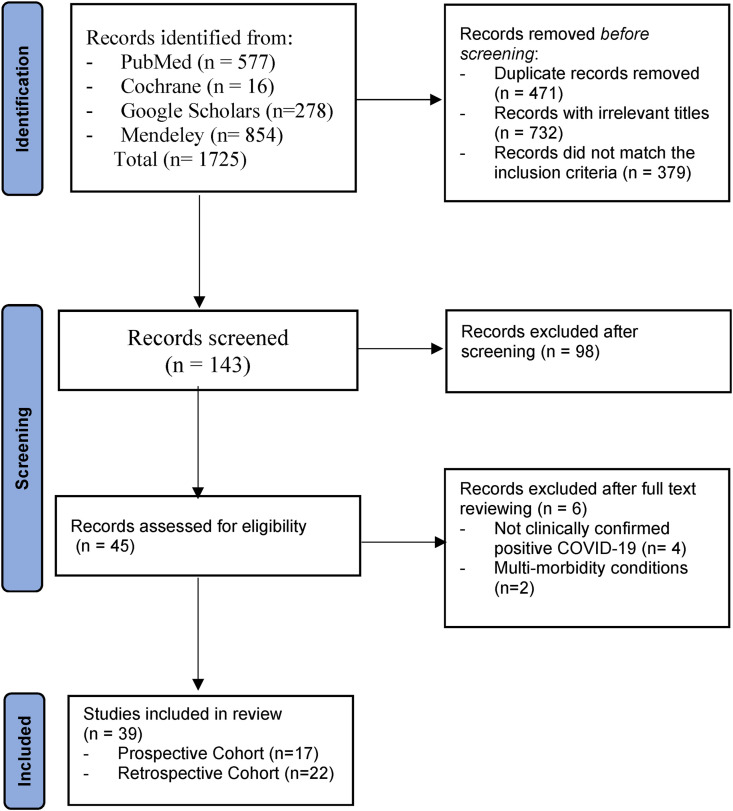
A total of 1725 studies were identified through our electronic databases searches. After removing not relevant titles and duplicates, a total of 143 articles were screened for the title and abstract. Accordingly, a total of 98 articles were excluded because they were not cohort studies (e.g., cross-sectional study, review articles), or included different age groups. Of the remaining 45 cohort studies which were assessed for eligibility, we have included 39 articles [[31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]]. The remaining six articles were excluded because, in four of them, participants were not clinically confirmed positive COVID-19 [[70], [71], [72], [73]], and two articles included participants mainly with multi-morbidity conditions such as cancer and renal diseases [74,75] which were not within the scope of this study. Figure 1 illustrates the flow diagram of the literature search and selection process.
Fig. 1.
PRISMA flow chart of the search strategy.
Table 1 (Supplementary) shows the characteristics of the 39 included cohort studies [[31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]] in our systematic review. The majority of the studies were conducted in Europe (n = 31, 79%) [[31], [32], [33], [34], [35], [36],[39], [40], [41], [42], [43], [44], [45],[48], [49], [50], [51], [52], [53],[55], [56], [57], [58], [59], [60],[63], [64], [65], [66], [67], [68]], while 3 were performed in Asia [46,47,69], 2 studies in South America [61,62] and 3 were conducted in USA [37,38,54]. Four of the included studies were multicenter international [36,43,45,64] which included 11,055 patients from 266 hospitals and intensive care units in Europe (included countries ranging from 11 to 28 countries), and four studies were a national multicenter cohort in the Netherlands [32], England [57], Denmark [65], and Ireland [34].
All of the articles included subjects who were clinically confirmed positive COVID-19. A total of 51, 796 patients were included in the systematic review ranged from 30 participants in a study conducted in France [44] to 27,961 participants in a study conducted in USA [54]. The median age of the participants ranged from 50 to 89 years. All studies had both sexes with the percentage of women varying between 19.2% [44] and 65.7% [37].
The outcomes of the studies included mortality which was recorded in 32 studies [[31], [32], [33], [34], [35], [36], [37],[39], [40], [41], [42], [43],45,[47], [48], [49],[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62],[64], [65], [66], [67]] and frailty status was recorded in 35 studies [[31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43],45,[47], [48], [49],[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62],[64], [65], [66], [67], [68], [69]]. However, Sarcopenia was recorded in 5 studies [44,46,50,63,68].
3.1. Sarcopenia and frailty measurements
Sarcopenia was measured in 3 studies [44,46,50] using strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F) questionnaire. In 2 studies [63,68], sarcopenia was measured via muscle strength with a hand-held handgrip dynamometer and body composition. The percentage of older adults with sarcopenia ranged from 33% [46] to 75% [44].
For frailty measurement, the majority of the studies (28/35, 77%) used the clinical frailty scale (CFS) [[31], [32], [33], [34], [35], [36],39,[41], [42], [43],45,48,49,[51], [52], [53],[55], [56], [57], [58], [59], [60], [61], [62],64,66,67,69], frailty index was used in 4 studies [37,38,54,69], the Frail Nondisabled Survey (n = 1) [40], Fried's frailty phenotype (n = 1) [68], Geriatric Frailty symptoms including confusion, difficulty walking and falls (n = 1) [65], and Frail scale (n = 1) [47].
The percentage of older adults with frailty ranged from 21% [64] to 76.6% [33] of the total participants in each study.
3.2. Risk of development of severe disease among older Covid-19 patients
The majority of the studies included hospitalized COVID-19 patients (n = 33/39, 82%) [[31], [32], [33], [34],36,[39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53],[55], [56], [57],[59], [60], [61], [62], [63], [64], [65], [66], [67],69], community dwelling elderly (n = 2) [38,68], USA Medicare fee-for service–enrolled beneficiaries (n = 1) [54], and long stay and care home residents (n = 3) [35,37,58].
Older age and frailty were described in all studies that addressed mortality and frailty as foremost risk factors associated with mortality in COVID-19 patients. Two studies indicated that the increased age is an independent risk factor for the development of critical illness and increased mortality among COVID-19 patients [33,43]. However, other studies (n = 7) [39,45,53,55,60,67,69] stated that COVID-19 disease outcomes in terms of the development of severe conditions, critical illness, and mortality were better predicted by frailty than either age or comorbidity.
3.3. Mortality and frailty
A higher mortality rate has been recorded amongst Covid-19 frail patients. The majority of the conducted studies in Europe 27/31 addressed the association between frailty and mortality rate among Covid-19 patients, where 25 studies [[31], [32], [33], [34], [35], [36],39,[41], [42], [43],45,48,[51], [52], [53],[55], [56], [57], [58], [59], [60],[64], [65], [66], [67]] indicated that there was an association between frailty and mortality, and (n = 2) [40,49] indicated that the frailty was not correlated with mortality. Two of three studies conducted in the USA indicated that among Covid-19 patients, the mortality increased with frailty [37,54] with a significant difference according to the level of frailty (16.7% in pre-frail, 22.2% in moderately frail, and 50.0% in frail; P < 0.001) [37]. One study in Asia (China) [47] indicated that frailty would increase the risk of development of severe disease among older Covid-19 patients as well as increasing mortality rate. In addition, one study in Brazil [62] stated that frailty measures have provided valuable prognostic values among COVID-19 patients.
3.4. Mortality and sarcopenia
From the five studies that recorded sarcopenia [44,46,50,63,68], one study has reported a mortality rate among COVID-19 patients with sarcopenia [68], and the same study has recorded frailty as well, results indicated that frailty but not sarcopenia increases the risk of developing COVID-19 in older community-dwelling adults. In four studies, results stated that a high percentage of hospital-admitted COVID-19 patients had a high risk of sarcopenia ranging from 33% [46] to 75% [44] of the participants in each study.
3.5. Meta-analysis results
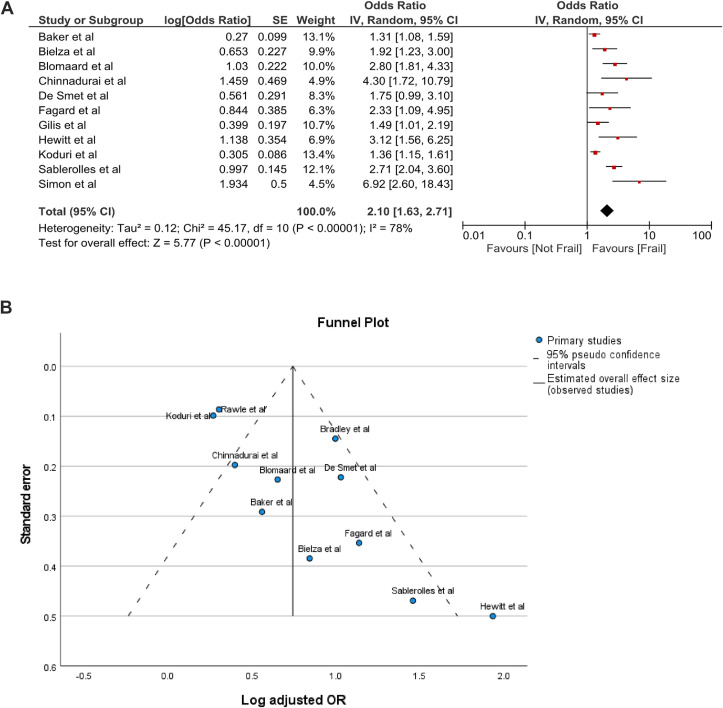
Of the 28 studies that used the clinical frailty scale (CFS), a total of 20 studies [31,32,36,39,[41], [42], [43],45,48,49,52,53,55,56,[58], [59], [60],62,64,67] were included in the meta-analysis based on the odds ratio and/or hazard ratio related to mortality according to the frailty status. In 8 of the 28 studies, mortality and/or frailty were not documented in CFS increments or crude patient numbers were unavailable, therefore they were excluded from the meta-analysis. Our results indicated that COVID-19 frail patients had a significantly increased mortality rate compared to non-frail patients, the log adjusted OR was 2.10 (1.63, 2.71); I2 = 78%; p < 0.00001 (Fig. 2 A). Egger's test indicated publication bias for adjusted OR among the included studies (P = 0.0073), as illustrated in the funnel plot (Fig. 2 B).
Fig. 2.
A. Forest plot of adjusted OR of mortality related to frailty status. B. Funnel plot of adjusted OR of mortality related to frailty status.
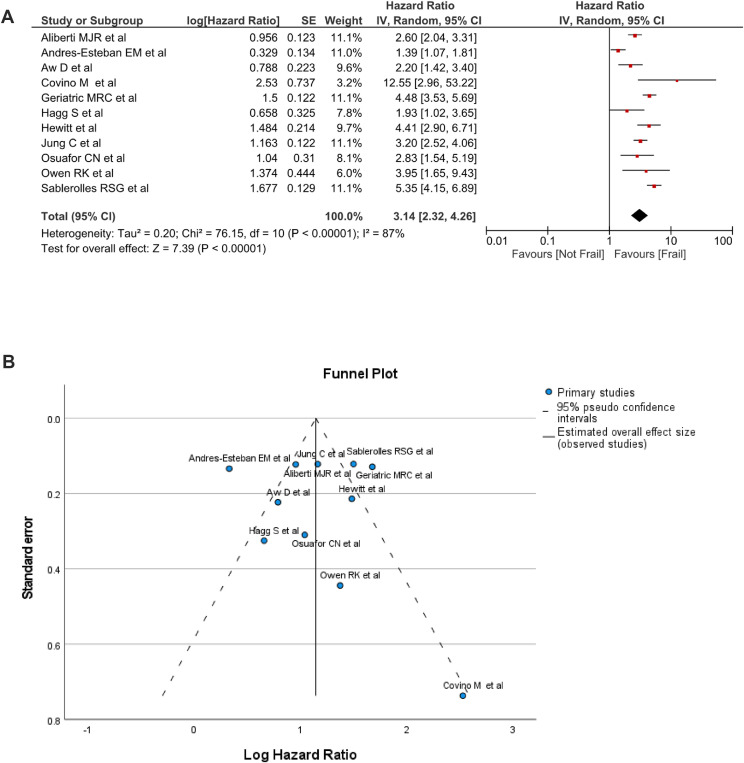
Also, the log adjusted hazard ratio (HR) data recorded significant differences related to mortality among the frail and non-frail groups, the total (95% CI) HR was 3.14 (3.32, 4.26); I2 = 87%; p < 0.00001 (Fig. 3 A). Egger's test indicated no publication bias for adjusted HR among the included studies (P = 0.9069) as illustrated in the funnel plot (Fig. 3 B).
Fig. 3.
A. Forest plot of adjusted HR of mortality related to frailty status. B. Funnel plot of adjusted HR of mortality related to frailty status.
In terms of age, frail patients recorded a higher mean age compared to non-frail patients (p < 0.00001) as shown in Fig. 4 A.
Fig. 4.
A. Mean age analysis according to the frailty status.
Egger's test indicated no publication bias related to the mean age among the included studies (P = 0.6614), as illustrated in the funnel plot Fig. 4. B (Supplementary).
3.6. Risk of bias
Results of risk of bias are presented in Table 2 (Supplementary) according to the Newcastle Ottawa Scale (NOS). The results were based on three categories: selection, comparability, and outcomes. 24 studies scored 8–9 indicating good quality with low risk of bias, and 15 studies scored 7 indicating fair quality with moderate risk of bias.
4. Discussion
Due to age-related physiological changes and persisting health problems, older persons confront the most acute hazards and challenges compared to other age groups, putting them at a larger risk of contracting the COVID-19 virus and experiencing severe disease [19]. This was consistent with our findings, which revealed that the majority of the studies (82%) comprised hospitalized COVID-19 patients. In which of them were four multicenter international studies [36,43,45,64] that included 11,055 patients from 266 hospitals and intensive care units in Europe. Indicating that the risk of development of severe disease, hospitalization, and ICU admission is prominent among older Covid-19 patients. Globally, the rising number of older adults is accompanied by a multifaceted of health conditions, many of which are the result of various underlying factors such as sarcopenia and frailty [1].
In this systematic review, we found that sarcopenia was recorded in 5 studies [44,46,50,63,68], all studies stated that COVID-19 patients with higher sarcopenia risk were more likely to develop severe conditions. In four studies [44,46,50,63], results stated that a high percentage of hospital-admitted COVID-19 patients had a high risk of sarcopenia ranging from 33% [46] to 75% [44] of the participants in each study. On the other hand, one study stated that COVID-19 infection combined with ICU admission and related complications can lead to acute sarcopenia [63], this opposite direction in the devastating trio might be related to prolonged bed rest. inadequate nutrient intake and physical inactivity throughout the quarantine period as well as during hospitalization [23,76]. Indicating that COVID-19 infection also may exacerbate sarcopenia due to increased muscle wasting caused by systematic inflammation among COVID-19 patients [24].
In terms of mortality, no mortality rate was clearly recorded in the five studies addressing sarcopenia. A finding that was also evident in a study conducted among COVID-19 older community-dwelling adults [68], stated that frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19. This might be attributed to that sarcopenia is being considered as a less severe condition compared to frailty where sarcopenia usually precedes frailty [11]. The devastating curve in this line starts with sarcopenia to frailty and consequently leads to disability and mortality [12,13].
All of the included studies in this review that recorded mortality and frailty in COVID-19 patients identified older age and frailty as the leading causes of mortality. Increasing age was found as an independent risk factor for critical illness and higher mortality in COVID-19 patients [33,43]. Our results indicated that a significant difference was recorded (p < 0.001) according to age differences between frail and non-frail COVID 19 patients. However, frailty, rather than age or comorbidity, was found to be a stronger predictor of COVID-19 disease outcomes in terms of the development of severe diseases, critical illness, and mortality [39,45,53,55,60,67,69]. Therefore, frailty was described as an important predictor of COVID-19 prognosis and should not be overlooked.
Evidence suggests that frailty contributes independently to adverse outcomes in COVID-19 patients, as well as care needs were increased in survivors of COVID-19 with frailty or older age [22,45]. This might be related to increased inflammatory conditions and physiological capability decline, frail older persons are more susceptible to infectious diseases, resulting in greater vulnerability to stressors [17,18]. Corresponding with our findings, the majority of the included studies in this systematic review indicated that a higher mortality rate has been recorded amongst Covid-19 frail patients, and the mortality increased with frailty [37,54], with a significant difference according to the level of frailty. Our results indicated that COVID-19 frail patients had a significantly increased mortality rate compared to non-frail patients (p < 0.00001). Findings that highlight the importance and support the use of frailty indicators to help in the decision-making process for medical care in older adults COVID-19 patients.
Several instruments were used to assess frailty among COVID-19 patients, however, the clinical frailty scale (CFS) seems to be the most used instrument in the literature [77], and this finding was prominent in our study, as we found that the majority of the articles addressing frailty 28/36 have used the CSF. This could be related to the datum that CFS scoring may allow for differential frailty stratification in the context of the COVID-19 pandemic; CFS was identified as a prognostic indicator of mortality in COVID-19 as shown in a recent systematic review [77], with CFS 1–3 patients being considered at low risk, CFS 4–5 at moderate risk, and CFS 6–9 patients being considered at high risk of mortality regardless of age. The differential diagnosis characteristics of the CFS in addition to the abundance of the studies that used CFS as a scale for identifying the level of frailly have contributed to proceeding with the meta-analysis in this study.
This study aimed to get a better insight into the association of the devastating trio of sarcopenia, frailty, and COVID-19. To our knowledge, this is the first study that addresses the association of the three components among older adults patients. However, recent systematic reviews have addressed the association of frailty with adverse outcomes including mortality in patients with COVID-19 [[78], [79], [80]]. There is still a need to address this topic, particularly in older persons, because the COVID-19 adverse outcomes and mortality rate are higher in this age group [19], and more research is needed to identify sarcopenia and frailty markers at the prevention level [81].
We thought that addressing sarcopenia is a vital added value to our study, it's evidenced that sarcopenia is a primary component of physical frailty; where sarcopenia usually precedes frailty [12,13]. Therefore, preventing sarcopenia was considered as a major modifiable cause of frailty in the elderly, where it can be reversed by interventions such as physical activity, exercise [82], and nutrition [50]. Acute sarcopenia, on the other hand, occurs in COVID-19 survivors; and older persons are the most vulnerable group [25]. This line in the devastating trio might be delinked if preventive measures of tailored physical activity and adequate nutrition are taken to prevent muscle wasting in older adults that may occur due to systematic inflammation [23,24,76]. The interventions that target skeletal muscles in terms of physical activity, exercises and protein intake, in particular, are expected to disrupt the cycle in this devastating trio and assist the treatment of both disorders, where sarcopenia can be reversed and the prevention and treatment of COVID-19 can be enhanced [24].
A possible limitation of this study was that several instruments were used to assess frailty or sarcopenia, since the clinical frailty scale (CFS) was the most used instrument, our quantitative analysis was confined to the studies that used it. More review studies are needed to address the association of frailty with adverse outcomes among Covid-19 patients, where frailty is being assessed with different instruments. Also, regarding sarcopenia, still limited evidence is available, further studies are needed for studying the association of sarcopenia and adverse outcomes among older adults Covid-19 patients in the two directions.
5. Conclusions and implications
We found that age and frailty are important risk factors for mortality among older adults COVID-19 patients. A significant difference was recorded according to age between frail and non-frail COVID 19 patients. Frail patients had a significantly increased mortality rate compared to non-frail. Also, COVID-19 patients with sarcopenia were more likely to develop severe conditions in terms of hospitalization and ICU admission. Findings that highlight the importance and support the use of frailty and sarcopenia indicators to help in the decision-making process for medical care in older adults COVID-19 patients. Interventions including nutritional therapy in terms of adequate nutrition and protein intake, as well as tailored physical activity are vital to disrupt the cycle in this devastating trio, which can enhance the prevention and treatment of sarcopenia, frailty and COVID-19 in older adults.
Funding statement
“This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.”
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Author contributions
We acknowledge that all authors (HH and IG) contributed to the study conceptualization and design, data curation, and formal analysis. HH contributed to the writing of the original draft, and HH and IG read and gave final approval for the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2022.07.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization . 2021. Ageing and health.https://www.who.int/news-room/fact-sheets/detail/ageing-and-health accessed December 10, 2021. [Google Scholar]
- 2.Bauer J.M., Sieber C. Sarcopenia, frailty, and intrinsic capacity. Sarcopenia. 2021:115–125. doi: 10.1002/9781119597896.ch10. [DOI] [Google Scholar]
- 3.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaudart C., Zaaria M., Reginster J. 2017. Health outcomes of sarcopenia : a systematic review and meta-analysis; pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walston J.D. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mijnarends D.M., Luiking Y.C., Halfens R.J.G., Evers S.M.A.A., Lenaerts E.L.A., Verlaan S., et al. Muscle, health and costs: a glance at their relationship. J Nutr Health Aging. 2018;22:766–773. doi: 10.1007/s12603-018-1058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deschenes M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Zhang W., Wang C., Tao W., Dou Q., Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. 2018;18:188. doi: 10.1186/s12877-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper C., Dere W., Evans W., Kanis J.A., Rizzoli R., Sayer A.A., et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23:1839–1848. doi: 10.1007/s00198-012-1913-1. A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. [DOI] [PubMed] [Google Scholar]
- 12.Wilson D., Jackson T., Sapey E., Lord J.M. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Cederholm T. Overlaps between frailty and sarcopenia definitions. Nestle Nutrition Institute Workshop Series. 2015;83:65–69. doi: 10.1159/000382063. [DOI] [PubMed] [Google Scholar]
- 14.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., et al. Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.O'Caoimh R., Sezgin D., O'Donovan M.R., Molloy D.W., Clegg A., Rockwood K., et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96–104. doi: 10.1093/ageing/afaa219. [DOI] [PubMed] [Google Scholar]
- 16.Turner G., Clegg A. Best practice guidelines for the management of frailty: a British geriatrics society, age UK and royal college of general practitioners report. Age Ageing. 2014;43:744–747. doi: 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 17.Ekiz T., Kara M., Özçakar L. Fighting against frailty and sarcopenia - as well as COVID-19? Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dent E., Martin F.C., Bergman H., Woo J., Romero-Ortuno R., Walston J.D. Management of frailty: opportunities, challenges, and future directions. Lancet (London, England) 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . 2021. Health care considerations for older people during COVID-19 pandemic.https://www.euro.who.int/en/health-topics/Life-stages/healthy-ageing/data-and-statistics/health-care-considerations-for-older-people-during-covid-19-pandemic accessed December 10, 2021. [Google Scholar]
- 20.Ali A.M., Kunugi H. Screening for sarcopenia (physical frailty) in the COVID-19 era. International Journal of Endocrinology. 2021;2021 doi: 10.1155/2021/5563960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giraudo C., Librizzi G., Fichera G., Motta R., Balestro E., Calabrese F., et al. Reduced muscle mass as predictor of intensive care unit hospitalization in COVID-19 patients. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50:617–630. doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piotrowicz K., Gąsowski J., Michel J.-P., Veronese N. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33(10):2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P.-Y., Li Y., Wang Q. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. 2021;84 doi: 10.1016/j.nut.2020.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carly Welch, Tahir Masud, Daisy Wilson, Thomas A Jackson CG. COVID-19 and acute sarcopenia. Aging and Disease n.d.;11:1345–1351. [DOI] [PMC free article] [PubMed]
- 26.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed) 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells G.A., Shea B., O’Connel D., Peterson J., Welch V., Losos M., et al. Oxford; 2009. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses.http://ohrica/programs/clinical_epidemiology/oxford_htm_Feb_1_2009 [Google Scholar]
- 28.Cochrane Handbook for Systematic Reviews of Interventions n.d. https://handbook-5-1.cochrane.org/index.htm.
- 29.McGrath S., Zhao X., Steele R., Thombs B.D., Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020 doi: 10.1177/0962280219889080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ (Can Med Assoc J) : Canadian Medical Association Journal = Journal de l’Association Medicale Canadienne. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andres-Esteban E.M., Quintana-Diaz M., Ramirez-Cervantes K.L., Benayas-Pena I., Silva-Obregon A., Magallon-Botaya R., et al. Outcomes of hospitalized patients with COVID-19 according to level of frailty. PeerJ. 2021;9 doi: 10.7717/peerj.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blomaard L.C., van der Linden C.M.J., van der Bol J.M., Jansen S.W.M., Polinder-Bos H.A., Willems H.C., et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in The Netherlands: the COVID-OLD study. Age Ageing. 2021;50:631–640. doi: 10.1093/ageing/afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniero C., Patel D., Pavithran A., Naran P., Ng F.L., Prowle J., et al. A retrospective cohort study of risk factors and outcomes in older patients admitted to an inner-city geriatric unit in London during first peak of COVID-19 pandemic. Ir J Med Sci. 2021;191(3):1037–1045. doi: 10.1007/s11845-021-02679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moloney Eustace J., O’ Caoimh R., O’connor K., O’sullivan C., Jackson A., McGrath K., et al. vol. 113. 2020. (Frailty, covid-19 disease severity and outcome among hospitalised older adults). [Google Scholar]
- 35.Rawle M.J., Bertfield D.L., Brill S.E. Atypical presentations of COVID-19 in care home residents presenting to secondary care: a UK single centre study. Aging Medicine. 2020;3:237–244. doi: 10.1002/agm2.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sablerolles R.S.G., Lafeber M., van Kempen J.A.L., van de Loo B.P.A., Boersma E., Rietdijk W.J.R., et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. The Lancet Healthy Longevity. 2021;2:e163–e170. doi: 10.1016/s2666-7568(21)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi S.M., Bakaev I., Chen H., Travison T.G., Berry S.D. Risk factors, presentation, and course of coronavirus disease 2019 in a large, academic long-term care facility. J Am Med Dir Assoc. 2020;21:1378–1383. doi: 10.1016/j.jamda.2020.08.027. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi S., Lo O.Y., Newmeyer N., Bakaev I., Kim D.H. Recovery from coronavirus disease 2019 among older adults in post-acute skilled nursing facilities. J Am Med Dir Assoc. 2021;22:1138–1141. doi: 10.1016/j.jamda.2021.04.003. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon N.R., Jauslin A.S., Rueegg M., Twerenbold R., Lampart M., Osswald S., et al. Association of frailty with adverse outcomes in patients with suspected covid-19 infection. J Clin Med. 2021;10 doi: 10.3390/jcm10112472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinmeyer Z., Vienne-Noyes S., Piau A., Sourdet S., Bernard M., Steinmeyer A., et al. Acute care of older patients with COVID-19: clinical characteristics and outcomes. Geriatrics. 2020;5:1–11. doi: 10.3390/geriatrics5040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Covino M., Russo A., Salini S., De Matteis G., Simeoni B., Della Polla D., et al. Frailty assessment in the emergency department for risk stratification of COVID-19 patients aged ≥80 years. J Am Med Dir Assoc. 2021;22:1845–1852. doi: 10.1016/j.jamda.2021.07.005. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagard K., Gielen E., Deschodt M., Devriendt E., Flamaing J. Risk factors for severe COVID-19 disease and death in patients aged 70 and over: a retrospective observational cohort study. Acta Clin Belg: International Journal of Clinical and Laboratory Medicine. 2021:1–8. doi: 10.1080/17843286.2021.1890452. [DOI] [PubMed] [Google Scholar]
- 43.Geriatric Medicine Research C., Covid C., Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50:617–630. doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerard M., Nguyen-Thi P.-L., Malgras A., Bermand T., Jaussaud R., Quilliot D. Assessment of muscle function in severe and malnourished COVID-19 patients. Int J Nutr Food Sci. 2020;9:132. doi: 10.11648/j.ijnfs.20200905.12. [DOI] [Google Scholar]
- 45.Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Y., He M., Hou L.S., Xu S., Huang Z.X., Zhao N., et al. The role of SARC-F scale in predicting progression risk of COVID-19 in elderly patients: a prospective cohort study in Wuhan. BMC Geriatr. 2021;21:355. doi: 10.1186/s12877-021-02310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y., Hou L., Yang X., Huang Z., Yang X., Zhao N., et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: a prospective cohort study. BMC Med. 2020;18:274. doi: 10.1186/s12916-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osuafor C.N., Davidson C., Mackett A.J., Goujon M., Van Der Poel L., Taylor V., et al. Clinical features, inpatient trajectories and frailty in older inpatients with covid-19: a retrospective observational study. Geriatrics. 2021;6:1–13. doi: 10.3390/geriatrics6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owen R.K., Conroy S.P., Taub N., Jones W., Bryden D., Pareek M., et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2021;50:307–316. doi: 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramos A., Joaquin C., Ros M., Martin M., Cachero M., Sospedra M., et al. Impact of COVID-19 on nutritional status during the first wave of the pandemic. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izzi-Engbeaya C., Distaso W., Amin A., Yang W., Idowu O., Kenkre J.S., et al. Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diabetes Research and Care. 2021;9 doi: 10.1136/bmjdrc-2020-001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hägg S., Jylhävä J., Wang Y., Xu H., Metzner C., Annetorp M., et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559. doi: 10.1016/j.jamda.2020.08.014. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilis M., Chagrot N., Koeberle S., Tannou T., Brunel A.-S., Chirouze C., et al. Older adults with SARS-CoV-2 infection: utility of the clinical frailty scale to predict mortality. J Med Virol. 2021;93:2453–2460. doi: 10.1002/jmv.26766. [DOI] [PubMed] [Google Scholar]
- 54.Izurieta H.S., Graham D.J., Jiao Y., Hu M., Lu Y., Wu Y., et al. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. JID (J Infect Dis) 2021;223:945–956. doi: 10.1093/infdis/jiaa767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S., et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932. doi: 10.1016/j.jamda.2020.06.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chinnadurai R., Ogedengbe O., Agarwal P., Money-Coomes S., Abdurrahman A.Z., Mohammed S., et al. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatr. 2020;20:409. doi: 10.1186/s12877-020-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradley P., Frost F., Tharmaratnam K., Wootton D.G. Utility of established prognostic scores in COVID-19 hospital admissions: multicentre prospective evaluation of CURB-65, NEWS2 and qSOFA. BMJ Open Respiratory Research. 2020;7 doi: 10.1136/bmjresp-2020-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bielza R., Sanz J., Zambrana F., Arias E., Malmierca E., Portillo L., et al. Clinical characteristics, frailty, and mortality of residents with COVID-19 in nursing homes of a region of madrid. J Am Med Dir Assoc. 2021;22:245–252. doi: 10.1016/j.jamda.2020.12.003. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker K.F., Hanrath A.T., van der Loeff I.S., Tee S.A., Capstick R., Marchitelli G., et al. vol. 9. Medical Sciences; Basel, Switzerland: 2021. (COVID-19 management in a UK NHS foundation trust with a high consequence infectious diseases centre: a retrospective analysis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aw D., Woodrow L., Ogliari G., Harwood R. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age Ageing. 2020;49:915–922. doi: 10.1093/ageing/afaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poco P.C.E., Aliberti M.J.R., Dias M.B., Takahashi S. de F., Leonel F.C., Altona M., et al. Divergent: age, frailty, and atypical presentations of COVID-19 in hospitalized patients. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2021;76:e46–e51. doi: 10.1093/gerona/glaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aliberti M.J.R., Szlejf C., Avelino-Silva V.I., Suemoto C.K., Apolinario D., Dias M.B., et al. COVID-19 is not over and age is not enough: using frailty for prognostication in hospitalized patients. J Am Geriatr Soc. 2021;69:1116–1127. doi: 10.1111/jgs.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gobbi M., Bezzoli E., Ismelli F., Trotti G., Cortellezzi S., Meneguzzo F., et al. Skeletal muscle mass, sarcopenia and rehabilitation outcomes in post-acute COVID-19 patients. J Clin Med. 2021;10 doi: 10.3390/jcm10235623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung C., Flaatten H., Fjølner J., Bruno R.R., Wernly B., Artigas A., et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021;25:149. doi: 10.1186/s13054-021-03551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karlsson L.K., Jakobsen L.H., Hollensberg L., Ryg J., Midttun M., Frederiksen H., et al. Clinical presentation and mortality in hospitalized patients aged 80+ years with COVID-19–A retrospective cohort study. Arch Gerontol Geriatr. 2021;94 doi: 10.1016/j.archger.2020.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knights H., Mayor N., Millar K., Cox M., Bunova E., Hughes M., et al. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, UK. Clinical Medicine. J Roy Coll Phys Lond. 2020;20:E148–E153. doi: 10.7861/CLINMED.2020-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koduri G., Gokaraju S., Darda M., Warrier V., Duta I., Hayes F., et al. Clinical frailty score as an independent predictor of outcome in COVID-19 hospitalised patients. European Geriatric Medicine. 2021;12:1065–1073. doi: 10.1007/s41999-021-00508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lengele L., Locquet M., Moutschen M., Beaudart C., Kaux J.F., Gillain S., et al. Frailty but not sarcopenia nor malnutrition increases the risk of developing COVID-19 in older community-dwelling adults. Aging Clin Exp Res. 2022;34(1):223–234. doi: 10.1007/s40520-021-01991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim J.P., Low K.Y.H., Lin N.J.J., Lim C.Z.Q., Ong S.W.X., Tan W.Y.T., et al. Predictors for development of critical illness amongst older adults with COVID-19: beyond age to age-associated factors. Arch Gerontol Geriatr. 2021;94 doi: 10.1016/j.archger.2020.104331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasegawa Y., Takahashi F., Hashimoto Y., Munekawa C., Hosomi Y., Okamura T., et al. Effect of covid-19 pandemic on the change in skeletal muscle mass in older patients with type 2 diabetes: a retrospective cohort study. Int J Environ Res Publ Health. 2021;18 doi: 10.3390/ijerph18084188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saraiva M.D., Apolinario D., Avelino-Silva T.J., De Assis Moura Tavares C., Gattás-Vernaglia I.F., Marques Fernandes C., et al. The impact of frailty on the relationship between life-space mobility and quality of life in older adults during the COVID-19 pandemic. J Nutr Health Aging. 2021;25:440–447. doi: 10.1007/s12603-020-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shinohara T., Saida K., Tanaka S., Murayama A., Higuchi D. Did the number of older adults with frailty increase during the COVID-19 pandemic? A prospective cohort study in Japan. European Geriatric Medicine. 2021;12:1085–1089. doi: 10.1007/s41999-021-00523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y., Fu P., Li J., Jing Z., Wang Q., Zhao D., et al. Changes in psychological distress before and during the COVID-19 pandemic among older adults: the contribution of frailty transitions and multimorbidity. Age Ageing. 2021;50:1011–1018. doi: 10.1093/ageing/afab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neradova A., Vajgel G., Hendra H., Antonelou M., Kostakis I.D., Wright D., et al. Frailty score before admission as risk factor for mortality of renal patients during the first wave of the COVID pandemic in London. G Ital Nefrol : Organo Ufficiale Della Societa Italiana Di Nefrologia. 2021;38 [PubMed] [Google Scholar]
- 75.Mahmoud M., Carmisciano L., Tagliafico L., Muzyka M., Rosa G., Signori A., et al. Patterns of comorbidity and in-hospital mortality in older patients with COVID-19 infection. Front Med. 2021;8 doi: 10.3389/fmed.2021.726837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim J.-W., Yoon J.S., Kim E.J., Hong H.-L., Kwon H.H., Jung C.Y., et al. Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus disease 2019. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2021;76 doi: 10.1093/gerona/glab085. e110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kastora S., Kounidas G., Perrott S., Carter B., Hewitt J., Myint P.K. Clinical frailty scale as a point of care prognostic indicator of mortality in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dumitrascu F., Branje K.E., Hladkowicz E.S., Lalu M., McIsaac D.I. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc. 2021;69:2419–2429. doi: 10.1111/jgs.17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kow C.S., Hasan S.S., Thiruchelvam K., Aldeyab M. Association of frailty and mortality in patients with COVID-19: a meta-analysis. Br J Anaesth. 2021;126:e108–e110. doi: 10.1016/j.bja.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2021;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee L., Patel T., Hillier L.M., Maulkhan N., Slonim K., Costa A. Identifying frailty in primary care: a systematic review. Geriatr Gerontol Int. 2017;17:1358–1377. doi: 10.1111/ggi.12955. [DOI] [PubMed] [Google Scholar]
- 82.Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–142. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.