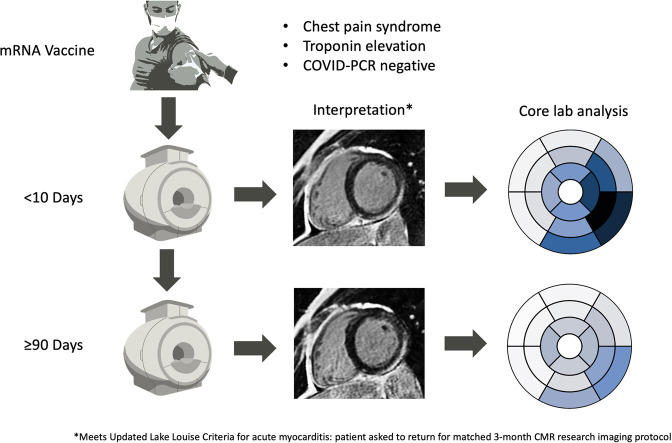
Abstract
Background
Acute myocarditis is a rare complication of mRNA-based COVID-19 vaccination. Little is known about the natural history of this complication.
Methods
Baseline and convalescent (≥ 90 days) cardiac magnetic resonance (CMR) imaging assessments were performed in 20 consecutive patients meeting Updated Lake Louise Criteria for acute myocarditis within 10 days of mRNA-based vaccination. CMR-based changes in left ventricular volumes, mass, ejection fraction (LVEF), markers of tissue inflammation (native T1 and T2 mapping), and fibrosis (late gadolinium enhancement [LGE] and extracellular volume [ECV]) were assessed between baseline and convalescence. Cardiac symptoms and clinical outcomes were captured.
Results
Median age was 23.1 years (range 18-39 years), and 17 (85%) were male. Convalescent evaluations were performed at a median (IQR) 3.7 (3.3-6.2) months. The LVEF showed a mean 3% absolute improvement, accompanied by a 7% reduction in LV end-diastolic volume and 5% reduction in LV mass (all P < 0.015). Global LGE burden was reduced by 66% (P < 0.001). Absolute reductions in global T2, native T1, and ECV of 2.1 ms, 58 ms, and 2.9%, repectively, were documented (all P ≤ 0.001). Of 5 patients demonstrating LVEF ≤ 50% at baseline, all recovered to above this threshold in convalescence. A total of 18 (90%) patients showed persistence of abnormal LGE although mean fibrosis burden was < 5% of LV mass in 85% of cases. No patient experienced major clinical outcomes.
Conclusions
COVID-19 mRNA vaccine–associated myocarditis showed rapid improvements in CMR-based markers of edema, contractile function, and global LGE burden beyond 3 months of recovery in this young patient cohort. However, regional fibrosis following edema resolution was commonly observed, justifying need for ongoing surveillance.
Graphical abstract
Résumé
Contexte
La myocardite aiguë est une complication rare de l’administration d’un vaccin à ARNm contre la COVID-19. Toutefois, on en sait peu sur l’histoire naturelle de cette complication.
Méthodologie
Des examens d’imagerie par résonance magnétique (IRM) cardiaque ont été réalisés au départ et lors de la convalescence (≥ 90 jours) chez 20 patients consécutifs répondant aux critères modifiés de Lake Louise relatifs à la myocardite aiguë dans les 10 jours suivant l’administration d’un vaccin à ARNm. Les changements à l’IRM cardiaque pour le ventricule gauche (VG) – volume, masse, fraction d’éjection (FEVG), marqueurs d’inflammation tissulaire (établissement du T1 natif et du T2) et fibrose (rehaussement tardif au gadolinium et volume extracellulaire) – ont été évalués au départ et lors de la convalescence. Les symptômes cardiaques et les résultats cliniques ont aussi été notés.
Résultats
L’âge médian était de 23,1 ans (min.-max. : 18-39 ans), et 17 participants (85 %) étaient des hommes. Les évaluations pendant la convalescence ont été effectuées à une médiane (écart interquartile) de 3,7 (3,3-6,2) mois. Une amélioration absolue moyenne de 3 % de la FEVG a été constatée, accompagnée d’une réduction de 7 % du volume diastolique du VG et d’une réduction de 5 % de la masse du VG (toutes les valeurs p < 0,015). Dans l’ensemble, le rehaussement tardif au gadolinium a été réduit de 66 % (p < 0,001). Des réductions absolues de 2,1 ms, 58 ms et 2,9 % ont respectivement été notées pour la T2 globale, la T1 native et le volume extracellulaire (toutes les valeurs p ≤ 0,001). Parmi les cinq patients présentant une FEVG ≤ 50 % au départ, tous ont connu une récupération surpassant ce seuil lors de la convalescence. Un rehaussement tardif anormal au gadolinium a persisté chez 18 patients (90 %), mais le fardeau moyen de la fibrose était inférieur à 5 % de la masse du VG dans 85 % des cas. Aucun patient n’a connu d’événement clinique majeur.
Conclusions
Une amélioration rapide des marqueurs à l’IRM cardiaque de l’œdème, de la fonction contractile et du fardeau global du rehaussement tardif au gadolinium après trois mois de récupération a été observée dans cette cohorte de jeunes patients atteints d’une myocardite associée aux vaccins à ARNm contre la COVID-19. Malgré tout, une fibrose régionale après la résolution de l’œdème a été observée fréquemment, ce qui justifie la nécessité d’une surveillance continue.
The rapid development, regulatory approval, and global distribution of mRNA-based vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or coronavirus disease 2019 (COVID-19), is considered one of the greatest contributions to public health in modern history. As of March 1, 2022, more than 10 billion vaccine doses have been administered across 184 countries.1 In this context, several case series2, 3, 4, 5, 6, 7, 8, 9, 10 have reported acute myocarditis occurring early after vaccination, particularly among younger male recipients.11, 12, 13 These observations have led to expanding interest and concern regarding the downstream sequelae of this potentially serious complication.
Cardiac magnetic resonance (CMR) imaging provides highly reproducible evaluations of chamber volumetry, mass, function, and tissue injury in the setting of acute myocarditis.14 Water-sensitive T2 (edema) imaging and fibrosis-sensitive late gadolinium enhancement (LGE) imaging can be complemented by parametric T1 and T2 mapping to permit the serial quantification of acute myocardial injury. Pre-pandemic CMR cohort studies of community-acquired acute myocarditis collectively suggest the acute inflammatory stage of myocarditis to resolve over a 3-month period, at which time parametric markers of tissue edema normalise and permit the reasonable evaluation of residual fibrosis.15, 16, 17, 18 However, COVID-19 vaccine–associated myocarditis is postulated to be related to an overaggressive immune response to host cell–manufactured mRNA nucleosides.19 Whether this unique and iatrogenic mechanism of immune-mediated cytotoxic injury carries similar natural history to active viral myocarditis is unknown.
In this study we recruited 20 consecutive patients presenting with acute myocarditis within 10 days of mRNA-based COVID-19 vaccination. All patients underwent baseline and ≥ 3-month convalescent assessments inclusive of clinical evaluation and comprehensive CMR imaging, the latter facilitating serial quantitative analysis of myocardial injury. Tissue injury findings were evaluated in the context of chamber remodelling, contractile recovery, symptom burden, and major clinical outcomes.
Methods
Twenty adult patients (≥ 18 years of age) diagnosed with acute myocarditis within 10 days of receiving an mRNA-based COVID-19 vaccine from June 2021 to December 2021 were enrolled. Patients were required to have a high clinical suspicion of acute myocarditis based on the European Society of Cardiology Diagnostic Criteria20 and meet CMR-based diagnostic criteria for acute myocarditis by the Updated Lake Louise Criteria.14 All subjects underwent CMR imaging, baseline blood collection, 12-lead electrocardiography, chest X-ray, and clinical evaluations. A detailed health questionnaire including demographics, current cardiac symptoms, previous history of inflammatory disease, and comorbid illnesses was completed. Patients were then asked to undergo repeated CMR imaging, questionnaires, and a review of medical records after a minimum 3 months of convalescence. Informed patient consent was obtained under the Cardiovascular Imaging Registry of Calgary (CIROC; NCT04367220).
CMR imaging was performed using 3 Tesla scanners (Prisma or Skyra, Siemens Healthineers). The imaging protocol included balanced steady-state free precession (bSSFP) cine imaging in sequential short- and long-axis planes followed by native T1 mapping using a modified lock-locker inversion recovery (MOLLI) technique, T2 mapping using a T2-prepared gradient echo technique, and black blood T2-weighted imaging using a spectral presaturation attenuated inversion recovery (SPAIR) technique before contrast infusion of 0.15 mmol/kg gadolinium (Gadovist, Bayer). Ten minutes after contrast administration, late gadolinium enhancement (LGE) imaging was performed in short- and long-axis views using a phase-sensitive inversion recovery (PSIR) pulse sequence, followed by repeated T1 mapping for the estimation of extracellular volume (ECV) fraction.
Image postprocessing was performed with the use of commercial software (cvi42TM version 5.13.5, Circle Cardiovascular Imaging). Baseline and follow-up studies were analysed by trained core laboratory personnel blinded to clinical data. Analysis was conducted in accordance with recommendations of the Society of Cardiovascular Magnetic Resonance.21 Semiautomated contours were applied to short-axis cine images to obtain biventricular left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volumes, ejection fraction (LVEF), and mass. Volumetric analyses were indexed to body surface area by means of the Mosteller formula. LGE images were analysed with the use of the signal threshold vs reference myocardium technique at 5 standard deviations above reference myocardium. Regional patterns of LGE were scored as subepicardial, mid-wall patchy, mid-wall striae, diffuse, RV insertion site, and subendocardial. The presence of regional edema (signal ≥ 2-fold that of skeletal muscle) was identified from T2-weighted spectral presaturation attenuated inversion-recovery (SPAIR) imaging. Finally, native T1 and T2 maps were analysed for the basal, mid, and apical views. Segmental values were generated for the 16-segment American Heart Association model with global values provided as the average of all segments. Identical methods were applied to reconstructed ECV maps.
Minor and major cardiovascular outcomes
At convalescent assessments, all patients were interviewed and medical records reviewed to identify clinical evidence of major and minor clinical outcomes. Major clinical outcomes were defined as cardiac hospitalisation, new-onset heart failure requiring diuretic use, atrial fibrillation, or ventricular arrhythmia. Minor clinical outcomes were defined as persistent chest pain or need for escalation in medical therapy.
Statistical analysis
Descriptive statistics were provided as percentages for discrete variables and mean ± SD or median (range or IQR) for continuous variables. Paired t test or Wilcoxon rank-sum tests were used to compare baseline and follow-up CMR parameters, depending on normality of variable distributions. A 2-sided P value of < 0.05 was set for statistical significance. All analyses were performed using IBM SPSS Statistics for Windows, version 28 (IBM Corp, Armonk, NY).
Results
Clinical characteristics
Patient characteristics are summarised in Table 1 . All patients were under 40 years of age, and a majority (85%) were male. Sixteen patients (80%) presented within 6 days (range 2-6 days) after a second mRNA vaccination, and 4 presented within 10 days (range 2-10 days) after a first mRNA vaccination. Of the former group, 4 received a second dose of BNT162b2 (Pfizer-BioNTech) and 12 (60%) a second dose of mRNA-1273 (Moderna). Of the latter group, 2 had received a first dose of BNT162b2 and 2 a first dose of mRNA-1273. Four patients described previous (> 6 months) history of a mild PCR-confirmed COVID-19 infection without chest pain or hospitalisation. Nineteen (95%) patients presented with chest pain, with 1 describing as upper epigastric pain, and none had any antecedent viral respiratory symptoms. No patient had a history of rheumatologic or connective tissue disease. One patient reported myocarditis 10 years earlier.
Table 1.
Baseline clinical characteristics of patients with COVID-19 vaccine–associated myocarditis (n = 20)
| Baseline clinical characteristics | |
|---|---|
| Age, y | 23.1 (20.3-29.4) |
| Male sex | 17 (85) |
| BMI, kg/m2 | 25 (23.2-27.6) |
| Diabetes | 0 (0) |
| Hypertension | 0 (0) |
| Dyslipidemia | 0 (0) |
| Current smoker | 0 (0) |
| Presenting symptomatology | |
| Chest pain | 19 (95) |
| Dyspnea | 2 (10) |
| Myalgias | 1 (5) |
| Sweating | 1 (5) |
| Epigastric discomfort | 1 (5) |
| Hospitalised | 18 (90) |
| CCU admission | 3 (15) |
| In-hospital clinical complications | |
| Hypotension | 0 (0) |
| Heart failure | 0 (0) |
| Respiratory failure/Intubation | 0 (0) |
| Atrial arrhythmia | 0 (0) |
| Ventricular arrhythmia | 0 (0) |
| Length of hospital stay, d | 3 (2-3) |
| Peak high-sensitivity troponin T, ng/L | 958 ± 627 |
| Peak NT-proBNP, ng/L (n = 4) | 576 (211-931) |
| Peak CRP, mg/L | 35.0 ± 24.1 |
| Leukocytosis (WBC > 11,000 per mm3) | 1 (5) |
| ECG at presentation | |
| Normal | 9 (45) |
| ST-segment elevation (diffuse or regional) | 11 (55) |
| PR depression | 4 (20) |
| Coronary artery angiography | |
| Abnormal | 0 (0) |
| Normal | 2 (10) |
| Not performed | 18 (90) |
| Medications at discharge | |
| Colchicine | 19 (95) |
| NSAIDs | 15 (75) |
| Steroids | 0 (0) |
| ACEi | 5 (25) |
| Beta-blocker | 4 (20) |
| Spironolactone | 1 (5) |
Values are presented as median (IQR), n (%), or mean ± SD.
ACEi, angiotensin-converting enzyme inhibitor; CCU, coronary care unit; CMR, cardiac magnetic resonance; CRP, C-reactive protein; ECG, electrocardiography; NT-proBNP, N-terminal pro–B-type natriuretic peptide; WBC, white blood cell count.
The results of non-CMR diagnostic testing are summarised in Table 1. All had negative PCR testing for COVID-19. Elevations in high-sensitive troponin-T were confirmed in all patients (peak levels 42-2320 ng/L, normal 0-13 ng/L), and 18 (90%) had elevation in C-reactive protein (10.5-96.2 mg/L, normal 0-8 mg/L). ST-segment elevation was observed on the initial electrocardiograms in 11 patients (55%). All chest X-ray results were normal.
Eighteen patients (90%) were hospitalised during their acute illness and were discharged without in-patient cardiac complications (median length of hospital stay 3 days [IQR 2-3 days]). All but 1 patient was treated with colchicine, combined with nonsteroidal antiinflammatory drugs in 75%. Five patients, all having an LVEF < 55%, were prescribed angiotensin-converting enzyme inhibitors (ACEis), 4 a beta-blocker in addition, and 1 spironolactone in addition. No patient was prescribed steroids. All patients reported > 50% symptom improvement within 48 hours of the first colchicine dose.
Clinical outcomes
Convalescent evaluations were conducted at a median of 111 days (range 92-224 days, IQR 99-186 days) from day of diagnosis. At this time, 4 patients (20%) reported a minor outcome due to ongoing chest pain, for which all were receiving extended colchicine and NSAID therapy without steroids. No major clinical outcome was documented.
CMR findings
All patients completed baseline and follow-up CMR imaging. All studies were of diagnostic quality.
Baseline CMR imaging findings are summarised in Table 2 . All patients met Updated Lake Louise Criteria for acute myocarditis with typical findings, as shown in Figure 1, Figure 2 . LVEF was below reference normal values22 (ie, ≤ 56%) in 10 patients (50%), with 5 (25%) having an LVEF < 50%. Regional elevation in T2 signal on SPAIR imaging was identified in 19 patients (95%). All patients showed subepicardial-pattern LGE involving the inferolateral and/or lateral wall segments, 2 patients (10%) being incrementally coded with mid-wall patchy LGE. Abnormal T1 and T2 signal elevations were commonly identified in segments without visible LGE, suggesting global myocardial edema. Postcontrast analyses identified a mean global LGE burden of 8.6 ± 5.3% of the LV mass. Regionally matched elevations in ECV were observed (Table 2; Figure 2, Figure 3 ).
Table 2.
Comparison of baseline and follow-up CMR quantitative markers in COVID-19 vaccine–associated myocarditis
| CMR variable | Baseline | Follow-up | P value |
|---|---|---|---|
| LVEDVi, mL/m2 | 81.7 (73.9-89.6) | 75.8 (71.15-84.9) | 0.015 |
| LVESVi, mL/m2 | 34.9 (31.4-42.0) | 32.6 (29.3-34.8) | 0.006 |
| LVEF, % | 54.7 ± 5.94 | 57.7 ± 3.48 | 0.014 |
| LVMI, g/m2 | 51.1 (45.8-57,4) | 48.4 (43.3-51.7) | 0.002 |
| RVEDVi, mL/m2 | 79.4 (70.0-79.4) | 82.1 (72.9-90.0) | 0.093 |
| RVESVi, mL/m2 | 36.4 (28.6-43.7) | 39 (34.0-42.7) | 0.048 |
| RVEF, % | 53.8 ± 5.91 | 54 ± 4.67 | 0.004 |
| LAVI-biplane, mL/m2 | 31.7 (26.4-37.9) | 31.9 (26.5-34.6) | 0.411 |
| LGE mass, g | 7.4 (3.24-12.1) | 1.7 (0.62-3.16) | < 0.001 |
| Global LGE, % of LV mass ≥ 5 SD | 8.6 ± 5.30 | 2.9 ± 2.01 | < 0.001 |
| Global T2, ms | 39.7 ± 2.39 | 37.6 ± 1.89 | 0.001 |
| Global native T1, ms | 1261.9 ± 45.5 | 1203.9 ± 28.2 | < 0.001 |
| Global ECV, % | 32.9 (30.9-37.0) | 30.0 (28.6-32.0) | 0.001 |
Values are presented as median (IQR) or mean ± SD.
CMR, cardiac magnetic resonance; LAVI, left atrial volume indexed to body surface area. LGE, late gadolinium enhancement; LV, left ventricle; LVEDVi, left ventricular end-diastolic volume indexed to body surface area; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume indexed to body surface area; LVMI, indexed left ventricular mass; RV, right ventricle; RVEDVi, right ventricular end-diastolic volume indexed to body surface area; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end-systolic volume indexed to body surface area.
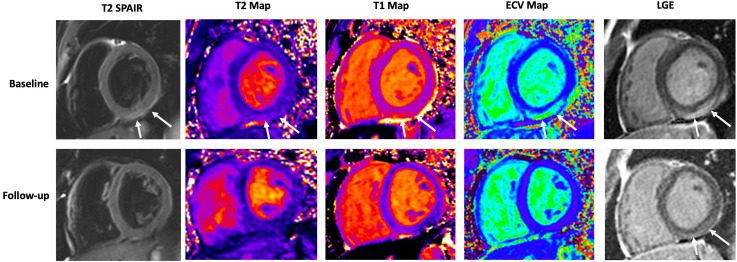
Figure 1.
Baseline and convalescent cardiac magnetic resonance findings in an 18-year-old man with acute myocarditis 3 days after a second dose of mRNA-based vaccine. (Top) Arrows indicate regional elevations in all tissue markers consistent with acute myocarditis. (Bottom) Substantial improvement observed at 105-day follow-up with mild persistent fibrosis seen on late gadolinium enhancement (LGE) imaging in the basal inferior wall (arrow). ECV, extracellular volume; SPAIR, spectral presaturation attenuated inversion-recovery.
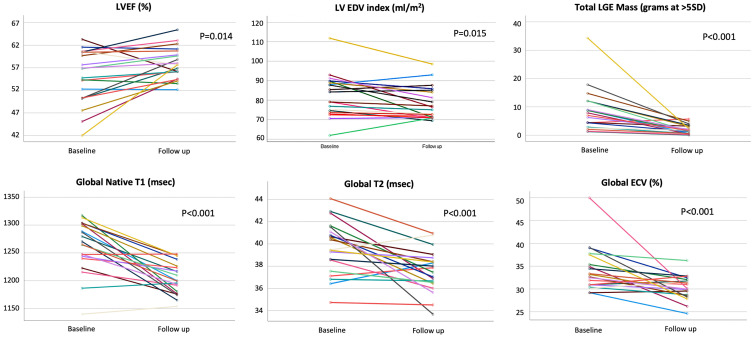
Figure 2.
Cardiac magnetic resonance quantitative imaging parameters measured at baseline and ≥ 3-month follow-up. ECV, extracellular volume; LGE, late gadolinium enhancement.
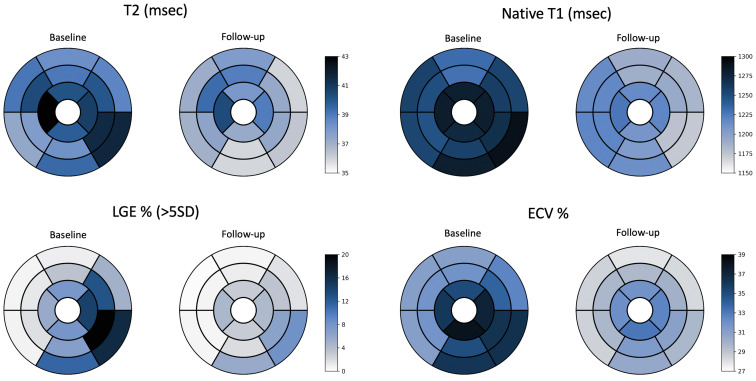
Figure 3.
Comparison of baseline and follow-up mean segmental values of T2 mapping, native T1 mapping, extracellular volume (ECV) fraction, and late gadolinium enhancement (LGE) fibrosis burden using a 5-SD threshold. Data are presented according to the American Heart Association 16-segment model. Normal local reference values for T2, native T1, and ECV are 36-48 ms, 1103-1263 ms, and 23%-31%, respectively. LVEDV, left ventricular end-diastolic volumea; LVEF, left ventricular ejection fraction.
The findings of convalescent CMR imaging are summarised in Table 2 and graphically displayed in Figure 2, Figure 3. Significant reductions in LVEDV (7.2% relative; P = 0.015) and LVESV (6.6% relative; P = 0.006) were observed, these associated with a significant 3% absolute increase in mean LVEF (P = 0.014). A 5.2% relative reduction in LV mass was seen (P = 0.002). Five out of 10 patients (50%) showing abnormal LVEF at baseline experienced normalisation to normal values in convalescence. The remaining 5 patients had LVEF values of 52% to 55%. One patient experienced an absolute drop in LVEF of 7%, but remained within the normal range (Fig. 3).
Global measures of native T1 and T2 decreased significantly on convalescent imaging with respective mean global reductions of 58 ms (P ≤ 0.001) and 2.1 ms (P = 0.001). Based on local laboratory-specific reference values for each pulse sequence, no patient demonstrated a persistent elevation in global native T1 or T2 (ie, > 2 SD of reference mean). T2 SPAIR imaging was also reported as normal in all subjects. Mean global ECV decreased by an absolute value of 2.9% (P = 0.001), and 5 patients (25%) demonstrated a persistent elevation above the > 2-SD upper limit of normal (31%) for our laboratory (range 32.0% to 36.2%). LGE analysis showed a 66% relative reduction in the global enhanced myocardial mass (P < 0.001). Any residual LGE was visually coded in 18 patients (90%), its distribution consistently representing a reduced volume of injury observed at baseline.
The results of segmental LGE and tissue mapping–based analyses are shown in Figure 3, demonstrating robust resolution of tissue edema and mild persistent fibrosis of the inferolateral segments. A trivial pericardial effusion was observed in 2 patients. No patient showed pericardial thickening.
Patients with a baseline LVEF < 50% did not demonstrate a significantly higher burden of LGE at follow-up vs those with LVEF ≥ 50% (P = 0.75). Of the 4 patients with persistent chest pain at the time of convalescent evaluation, no statistically significant change in any CMR-based marker was observed.
Discussion
This study assessed the natural history of myocardial tissue injury associated with mRNA-based COVID-19 vaccination among a cohort of symptomatic hospitalised patients with CMR-confirmed disease. In this clinical population we documented no short-term major clinical adverse outcomes. At a median follow-up of 111 days, marked improvements were observed in all quantitative CMR-based measures of tissue injury and contractile function, with normalisation of tissue markers related to myocardial edema. At this convalescent stage we observed a 66% reduction in the volume of injured myocardium as assessed by LGE quantification; however, 90% of patients demonstrated evidence of residual myocardial fibrosis. These results support the natural history of COVID-19 vaccine–associated myocarditis to be transient with prompt resolution of myocardial inflammation when treated with standard medical therapy. However, the observed persistence of regional myocardial fibrosis in the majority of patients provides justification for long-term surveillance in this young patient population.
The incidence of acute myocarditis associated with the administration of mRNA-based vaccines to SARS-CoV-2 is estimated to be from 1.4 to 2.7 cases per 100,000 exposures,23, 24, 25 although increased rates have been observed in younger male recipients.11 , 12 , 23 Several studies to date have described short-term estimates of major cardiovascular complications, these consistently identifying a low incidence rate.4 , 5 , 7 , 26 , 27 Despite this, concern has been raised because of the potential for residual myocardial injury to become a nidus for downstream complications, including heart failure or ventricular arrhythmias.28, 29, 30 The mechanism of myocardial injury following mRNA-based vaccination remains uncertain, but is postulated to reflect overaggressive T-cell activation following host-cell manufacturing of modified mRNA nucleosides of the SARS-CoV-2 virus,31 subsequently leading to myocardial injury through molecular mimicry. Whether this iatrogenic catalyst of myocardial injury results in a similar duration and cumulative burden of inflammatory injury compared with community-acquired viral myocarditis was previously unknown. Recently, a single case series of 5 subjects was published casting preliminary insights from convalescent CMR-based findings following mRNA COVID-19 vaccination–induced injury.32 In that study by Cavalcante et al., all 5 patients showed LVEF normalisation and resolution of myocardial edema (by T2 mapping) after a median follow-up of 106 days. Similar to our reported larger cohort study, persistent fibrosis was seen on LGE imaging in 4 (80%) of these patients following the complete resolution of edema. The extent of injury encountered during vaccine-associated myocarditis was recently studied by Hanneman et al. in 21 patients and compared with the burden of injury observed in a historic myocarditis cohort.27 That study suggested that a lower mean burden of LGE may be encountered in this setting compared with historic community-acquired myocarditis. However, in our study we identified a mean acute LGE burden of 8.6 ± 5.3% using a > 5 SD threshold, this being identical to that which we observed in a historic acute myocarditis cohort series of 100 patients studied at our institution using identical core laboratory–based analysis techniques (8.5 ± 9.2% at > 5 SD threshold).33 In addition, vs the 65% reduction in global LGE burden that we observed at 12 months in this historic cohort, we identified an identical 66% reduction at ≥ 3 months of follow-up, further supporting this hypothesis is that all objective markers of tissue edema were normalised at our preselected ≥ 90 day period of convalescence. Although this suggests that extended follow-up beyond this period is unlikely to yield further reductions in LGE burden, this requires confirmation and we accordingly plan to reassess this patient population at 12 months.
The presence of persistent fibrosis in the absence of edema has been described to be a predictor of long-term outcomes in patients with non-COVID acute myocarditis.30 A subcohort analysis of the Italian Study in Myocarditis (ITAMY) registry studying those patients who underwent repeated imaging at 6 months, demonstrated that persistence of LGE after resolution of edema was associated with a 4.5-fold increased risk (P = 0.008) of death, ventricular arrhythmia, or heart failure hospitalisation in long-term follow-up.30 Accordingly, despite the present and previous studies33 , 34 demonstrating significant involution of tissue injury volume after edema resolution, any degree of residual fibrosis may indicate need for long-term surveillance.
Limitations
This study is recognised to have limitations due to its modest sample size and available duration of clinical surveillance. We chose a minimum duration of 3 months to define convalescence based on previous studies describing satisfactory resolution of tissue mapping markers of edema by that time in community-acquired myocarditis.15, 16, 17, 18 Supporting this time period, we observed a normalisation of T2 mapping values and T2-weighted black blood imaging findings in all subjects. While supporting a state of postinjury recovery, we recognise the potential for incremental injury involution and remodelling to occur beyond this period, warranting studies of longer duration for the evaluation of tissue remodelling in this population. Histologic confirmation of acute myocarditis was not performed owing to a low suspicion for alternative diagnoses and lack of clinical indication, based on contemporary recommendations.31 , 35 , 36 Finally, convalescent sampling of serum biomarkers was not undertaken in this study, limiting serial comparisons of imaging and serum-based markers of injury.
Conclusion
Acute myocarditis after COVID-19 mRNA vaccination is associated with a prompt resolution in myocardial edema, reduction in tissue injury volume, and improvement in systolic function when treated with standard medical therapy. However, a dominant proportion of patients show residual fibrosis following the resolution of edema, this being previously recognised as a risk marker of future cardiovascular outcomes in non-COVID community-acquired myocarditis. Future studies evaluating long-term clinical outcomes in this patient population are required.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgements
We thank all of the personnel of the Stephenson Cardiac Imaging Centre, Alberta Health Sciences, Calgary, Canada, for their contributions to this study.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1682 for disclosure information.
References
- 1.Bloomberg. Covid-19 tracker. https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/ Available at:
- 2.Abu Mouch S., Roguin A., Hellou E., et al. Myocarditis following Covid-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim R.J., Kim H.W., Jenista E.R., et al. Patients with acute myocarditis following mrna covid-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan M., Montgomery J., Engler R., et al. Myocarditis following immunization with mRNA Covid-19 vaccines in members of the us military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel Y.R., Louis D.W., Atalay M., Agarwal S., Shah N.R. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA Covid-19 vaccination: a case series. J Cardiovasc Magn Reson. 2021;23:1–8. doi: 10.1186/s12968-021-00795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw K.E., Cavalcante J.L., Han B.K., Gössl M. Possible association between Covid-19 vaccine and myocarditis: clinical and CMR findings. JACC Cardiovasc Imaging. 2021;14:1856–1861. doi: 10.1016/j.jcmg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosner C.M., Genovese L., Tehrani B.N., et al. Myocarditis temporally associated with Covid-19 vaccination. Circulation. 2021;10:1–9. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David Hana K.P., Sherif Roman B.G., Sofka S. Clinical cardiovascular adverse events reported post–Covid-19 vaccination: are they a real risk? Curr Probl Cardiol. 2022;47 doi: 10.1016/j.cpcardiol.2021.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson R.A.I.V. Myocarditis and pericarditis after vaccination for Covid-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simone A., Herald J., Chen A., et al. Acute myocarditis following Covid-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based Covid-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazlollahi A., Zahmatyar M., Noori M., et al. Cardiac complications following mRNA Covid-19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2022;32:e2318. doi: 10.1002/rmv.2318. [DOI] [PubMed] [Google Scholar]
- 13.Tijmes F.S., Thavendiranathan P., Udell J.A., Seidman M., Hanneman K. Cardiac mri assessment of nonischemic myocardial inflammation: State of the art review and update on myocarditis associated with covid-19 vaccination. Radiol Cardiothorac Imaging. 2021;3:e210252. doi: 10.1148/ryct.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 15.Luetkens J.A., Homsi R., Dabir D., et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 17.Bohnen S., Radunski U.K., Lund G.K., et al. Tissue characterisation by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitormyocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging. 2017;18:744–751. doi: 10.1093/ehjci/jex007. [DOI] [PubMed] [Google Scholar]
- 18.Von Knobelsdorff-Brenkenhoff F., Schüler J., Dogangüzel S., et al. Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2017;10:1–10. doi: 10.1161/CIRCIMAGING.116.005242. [DOI] [PubMed] [Google Scholar]
- 19.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with Covid-19 mRNA vaccines. Circulation 2021. 2019:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caforio A.L.P., Pankuweit S., Arbustini E., et al. Current state of knowledge on etiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 21.Schulz-Menger J., Bluemke D.A., Bremerich J., et al. Standardised image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardised Post-Processing. J Cardiovasc Magn Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawel-Boehm N., Maceira A., Valsangiacomo-Buechel E.R., et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witberg G., Barda N., Hoss S., et al. Myocarditis after Covid-19 vaccination in a large health care organisation. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gellad W.F. Myocarditis after vaccination against Covid-19. BMJ. 2021;375:n3090. doi: 10.1136/bmj.n3090. [DOI] [PubMed] [Google Scholar]
- 25.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dionne A., Sperotto F., Chamberlain S., et al. Association of myocarditis with BNT162b2 messenger RNA Covid-19 vaccine in a case series of children. JAMA Cardiol. 2021;6:1446–1450. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fronza M., Thavendiranathan P., Chan V., et al. Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology. 2022;304:553–562. doi: 10.1148/radiol.212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanguineti F., Garot P., Mana M., et al. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson. 2015;17:78. doi: 10.1186/s12968-015-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gräni C., Eichhorn C., Bière L., et al. Prognostic Value of cardiac magnetic resonance tissue characterisation in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aquaro G.D., Perfetti M., Camastra G., et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017;70:1977–1987. doi: 10.1016/j.jacc.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 31.Bozkurt B., Colvin M., Cook J., et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 32.Cavalcante J., Shaw K., Gössl M., et al. Cardiac magnetic resonance imaging midterm follow up of Covid-19 vaccine–associated myocarditis. JACC Cardiovasc Imaging. 2022;15:1821–1824. doi: 10.1016/j.jcmg.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White J.A., Hansen R., Abdelhaleem A., et al. Natural history of myocardial injury and chamber remodelling in acute myocarditis: a 12-month prospective cohort study using cardiovascular magnetic resonance imaging. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.118.008614. [DOI] [PubMed] [Google Scholar]
- 34.Grun S., Schumm J., Greulich S., et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Ammirati E., Frigerio M., Adler E.D., et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luk A., Clarke B., Dahdah N., et al. Myocarditis and pericarditis after Covid-19 mRNA vaccination: practical considerations for care providers. Can J Cardiol. 2021;37:1629–1634. doi: 10.1016/j.cjca.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.