Abstract
Background
The ongoing COVID-19 pandemic is a major stressor that has been associated with increased risk for psychiatric illness in the general population. Recent work has highlighted that experiences of early-life stress (ELS) may impact individuals’ psychological functioning and vulnerability for developing internalizing psychopathology in response to pandemic-related stress. However, little is known about the neurobehavioral factors that may mediate the association between ELS exposure and COVID-related internalizing symptomatology. The current study sought to examine the mediating roles of pre-pandemic resting-state frontoamygdala connectivity and concurrent emotion regulation (ER) in the association between ELS and pandemic-related internalizing symptomatology.
Methods
Retrospective life-stress histories, concurrent self-reported ER strategies (i.e., reappraisal and suppression), concurrent self-reported internalizing symptomatology (i.e., depression- and anxiety-related symptomatology), and resting-state functional connectivity data from a sample of adults (N = 64, mean age = 22.12 years, female = 68.75%) were utilized.
Results
There were no significant direct associations between ELS and COVID-related internalizing symptomatology. Neither frontoamygdala functional connectivity nor ER strategy use mediated an association between ELS and COVID-related internalizing symptomatology (ps > .05). Exploratory analyses identified a significant moderating effect of reappraisal use on the association between ELS and internalizing symptomatology (β = −0.818, p = .047), such that increased reappraisal use buffered the impact of ELS on psychopathology.
Conclusions
While frontoamygdala connectivity and ER do not appear to mediate the association between ELS and COVID-related internalizing symptomatology, our findings suggest that the use of reappraisal may buffer against the effect of ELS on mental health during the pandemic.
Keywords: COVID-19 pandemic, Early-life stress, Emotion regulation, Frontoamygdala circuitry, Internalizing psychopathology, Resting-state functional connectivity
The ongoing COVID-19 pandemic is a major global stressor that poses an unprecedented threat to public mental health. Research suggests that greater COVID-19–related stress has been associated with increased risk for mental health disorders, such as depression and anxiety (1, 2, 3, 4). However, there is substantial variability in the degree to which individuals report psychological distress in response to COVID-related stressors (4). Though individual differences in reported outcomes following a stressor are common (5), the specific neurobiological and cognitive factors that account for this reported variance are not entirely understood. Given the enduring nature of the COVID-19 pandemic and its effects on mental health (6), further understanding of factors that increase risk for the development of psychopathology during the COVID-19 pandemic remains a critical public health need. Moreover, investigations of these factors during such a period of long-standing stress have the potential to yield novel insight into more basic neurobehavioral processes related to the impacts of stress on mental health.
To date, few studies have isolated factors that may attenuate the association between COVID-related stress and the development of psychopathology during the pandemic. However, work examining the association between exposure to early-life stress (ELS) and subsequent development of psychopathology sheds light on potential mediating factors. Decades of research have documented that experiences of ELS can exert detrimental and lasting effects on later neurobiological and behavioral development (7, 8, 9). Further, experiences of ELS have been shown to exacerbate the mental health effects of subsequent stress experienced in adulthood (10, 11, 12). This process, known as stress sensitization (13), is theorized to occur when chronic exposure to ELS dysregulates the functioning of neurobiological stress response systems, thus reducing an individual’s capacity for adaptive coping in response to subsequent stressful events (10). Of particular relevance to the present study, recent work from Gotlib et al. (14) found that greater exposure to pre-pandemic ELS experienced at or prior to age 13 years was associated with increased levels of depression-related symptomatology during the pandemic among adolescents, with individuals’ perceptions of stress during the COVID-19 pandemic mediating this association. Additionally, in the same cohort of adolescents, Chahal et al. (15) found that early pubertal maturation (notably, correlated with ELS among female participants) served as a risk factor for the onset of internalizing psychopathology during the pandemic and that coherence in the executive control network moderated this association. Collectively, this line of work suggests that previous exposure to ELS, particularly around or prior to pubertal development, may be a key determinant of mental health–related outcomes during a subsequent stressor such as the ongoing pandemic and that certain neurobehavioral factors may contribute to this association.
ELS-related alterations in frontoamygdala circuitry, neural pathways commonly implicated in emotion regulation (ER) processes, may influence later risk for developing psychopathology (16,17). Though variability in neurodevelopmental outcomes following ELS has been observed, cross-species models have consistently demonstrated the effects of ELS on both structural and functional frontoamygdala circuitry (18, 19, 20, 21, 22, 23). These effects can be far reaching and long lasting—exposure to adversity in childhood is associated with decreased structural integrity of white matter tracts linking corticolimbic regions (24) and negative static frontoamygdala resting-state functional connectivity (RSFC) (19,25) in adulthood. Furthermore, weaker functional connectivity between the amygdala and prefrontal regions following ELS exposure has been implicated in the development of psychopathology across the lifespan (24,26,27). As such, examinations of unique patterns of frontoamygdala RSFC in the general population may further our understanding of the relation between ELS and psychopathology. Moreover, though more recent work has used resting-state data to examine patterns of frontolimbic connectivity as a mediator of associations between ELS and psychopathology (25,28, 29, 30), much of the existing literature has relied on task-based paradigms (18,31). This underutilization of resting-state data has precluded our understanding of intrinsic functional organization in stress-exposed individuals in a more discernible (32,33) and more reliable (34) manner.
Additionally, difficulties with ER associated with alterations in frontoamygdala circuitry following ELS may have particularly important implications during times of heightened stress. The regulatory processes (e.g., reappraisal, suppression) by which individuals initiate, maintain, and modify their own reactions to the negative emotions that are often engendered during experiences of heightened stress may influence subsequent psychological states (35,36). Recent work has shown that disruptions in ER processes have been associated with increased risk for mental health issues during the COVID-19 pandemic (35,37,38). For example, Tyra et al. (35) demonstrated that greater use of reappraisal and lesser use of suppression was associated with reduced risk for developing stress-related symptomatology during the COVID-19 pandemic. Importantly, these findings lend support to the notion that reliance on distinct types of ER strategies may be associated with distinct mental health outcomes (39,40). Taken together, the extant literature suggests that both frontoamygdala RSFC and related ER processes may play mediating roles in the association between ELS exposure and the development of psychopathology during the COVID-19 pandemic.
Specific Aims and Hypotheses
The proposed registered report examined how exposure to ELS is associated with the development of internalizing symptomatology during the ongoing COVID-19 pandemic, as well as how neurobehavioral factors—assessed both prior to and during the ongoing COVID-19 pandemic—may mediate this association. Aim 1 examined associations between self-reported severity of ELS exposure (operationalized here as severity of stress experienced prior to age 12 years) and internalizing symptomatology during the COVID-19 pandemic (operationalized here as a sum of self-reported depression- and anxiety-related symptomatology). Aim 2 examined the distinct mediating roles of pre-pandemic frontoamygdala RSFC and concurrent ER tendencies on the association between ELS severity and pandemic-related internalizing symptomatology.
We hypothesized that adults who experienced more severe ELS would report higher levels of internalizing symptomatology during the pandemic. Further, we hypothesized that patterns of frontoamygdala RSFC and self-reported ER would mediate the association between ELS exposure and pandemic-related internalizing symptomatology. Specifically, we posited that weaker frontoamygdala connectivity patterns would be correlated with lower reliance on a prototypically adaptive ER strategy (i.e., reappraisal), and higher reliance on a prototypically maladaptive ER strategy (i.e., suppression). We additionally posited that weaker connectivity, lower use of reappraisal, and higher use of suppression would be associated with higher levels of pandemic-related internalizing symptomatology.
Methods and Materials
Participants
The present study includes 64 adults between ages 18 and 30 years who responded to community postings in New Haven, Connecticut, and study fliers posted online as part of recruitment efforts for a broader, ongoing study (described below) that began recruitment in 2016, and who also responded to a subsequent study invitation in spring 2020. Participant attributes are shown in Table 1. Inclusion criteria are detailed in Supplement 1: Participant inclusion criteria.
Table 1.
Participant Attributes (N = 64)
| Attribute | Mean ± SD (Range) or n (%) |
|---|---|
| Sex Assigned at Birth, Female/Male | 44 (68.75%)/19 (29.69%)a |
| Age at Time of Scan, Years | 22.12 ± 3.47 (18.0–30.8) |
| Race/Ethnicityb | |
| Asian | 14 (21.21%) |
| Black or African American | 7 (10.61%) |
| Hispanic or Latinx | 7 (10.61%) |
| Native American | 1 (1.51%) |
| Native Hawaiian or Pacific Islander | 0 |
| Non-Hispanic White | 37 (56.06%) |
| Other/unspecified | 0 |
| Highest Educational Degree Received | |
| Less than high school | 1 (1.56%) |
| High school diploma or GED | 34 (53.13%) |
| Bachelor’s degree | 23 (35.94%) |
| Master’s degree | 4 (6.25%) |
| Doctorate | 1 (1.56%) |
| Professional degree (MD, JD, DDS, etc.) | 1 (1.56%) |
| Total Combined Family Income Over the Past 12 Months, US$ | $69,991 ± 44,197 ($2500–$125,000)c |
| Time Elapsed Between Phase 1 and Phase 2, Months | 16 ± 10 (3–40) |
There was 1 participant with unknown sex assigned at birth.
Percentages for race/ethnicity do not sum to 100% due to multiracial reporting (i.e., some participants endorsed more than 1 race/ethnicity category).
Mean income calculated from averaging midpoint estimates of participants’ reported income brackets.
Procedure
The study protocol was approved by the Yale University Institutional Review Board, and all participants identified as being potentially eligible for the broader study provided written, informed consent according to the procedures set forth by the Human Investigation Committee at Yale University. The data used for this study were collected as a part of a broader, ongoing study of the neural mechanisms of fear reduction in children, adolescents, and adults. Phase 1 of the study consisted of 2 study visits. The first visit consisted of a clinical interview assessing lifetime history of stress exposure, a battery of questionnaires related to symptomatology, and a mock magnetic resonance imaging (MRI) scan [described in greater detail in Supplement 4: MRI acquisition protocol (additional information)]. The second visit consisted of an MRI scanning session during which RSFC data were collected on a research-dedicated 3T Siemens Prisma MRI scanner with a 32-channel head coil.
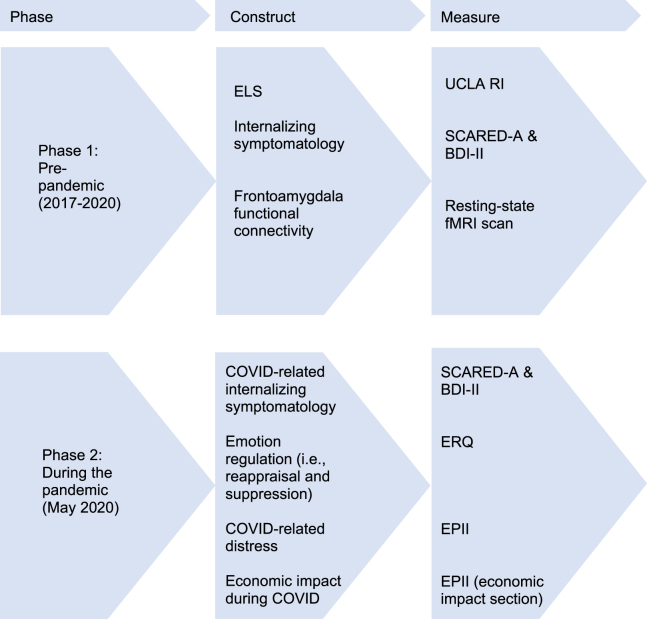
Participants who successfully completed phase 1 of the study were recontacted via email and telephone in spring 2020 following the onset of the COVID-19 pandemic and were offered the opportunity to participate in phase 2, a follow-up study that involved the completion of an additional set of questionnaires intended to assess coping and mental health during the pandemic. Specific measures completed at each phase are presented in Figure 1. Information about study timing is presented in Supplement 2: Study timing.
Figure 1.
Overview of study design and timing. Constructs that were assessed prior to the pandemic (phase 1) and during the pandemic (phase 2). BDI-II, Beck Depression Inventory-II; ELS, early-life stress; EPII, Epidemic-Pandemic Impacts Inventory; ERQ, Emotion Regulation Questionnaire; fMRI, functional magnetic resonance imaging; SCARED-A, Screen for Child Anxiety Related Emotional Disorders-Adult; UCLA RI, University of California at Los Angeles Posttraumatic Stress Disorder Reaction Index.
Self-report Measures
Scoring information and detailed information regarding psychometric properties of measures utilized in the proposed study are provided in Supplement 3: Measures (additional information).
Demographic Information
At phase 1, participants were asked to report their age, sex assigned at birth, race and ethnicity, highest education level, and annual household income.
Early-Life Stress
At phase 1, all participants completed an extended version of the University of California at Los Angeles Posttraumatic Stress Disorder Reaction Index (41), a clinician-administered interview regarding their lifetime history of exposure to stress. ELS severity for each participant was calculated by averaging the severity scores reported across all events endorsed prior to age 12 years.
Emotion Regulation
At phase 2, ER was assessed using the Emotion Regulation Questionnaire (42). The Emotion Regulation Questionnaire is a widely-used 10-item measure of ER that assesses individuals’ tendency to use 2 distinct ER strategies: reappraisal (6 items) and suppression (4 items). The scale scores for both reappraisal and suppression strategies were used in the current study to assess reliance on both prototypically adaptive and maladaptive ER strategies, respectively.
Internalizing Symptomatology
Pre-pandemic (phase 1) and concurrent (phase 2) levels of self-reported depression- and anxiety-related symptomatology were assessed using the Beck Depression Inventory-II (43) and the Screen for Child Anxiety Related Emotional Disorders-Adult (44), respectively. Total standardized scores (z scores) from these measures were summed to create a singular metric of COVID-related internalizing symptomatology.
COVID-Related Distress and Economic Impact
At phase 2, the Epidemic-Pandemic Impacts Inventory (45) was administered to assess the impact of the COVID-19 pandemic across 10 domains of personal and family life (e.g., work and employment, economic, education and training, home life, etc.). We added a single question at the end of each domain that assessed the general degree of distress that participants felt with regard to each specific domain, which we modeled after a line of questions included in the COPE (COVID-19 and Perinatal Experiences) study (46). A cumulative total of these distress-related questions and cumulative total of the number of economic impacts participants reported were used as covariates in the present study.
RS Functional MRI Acquisition
At the end of the initial visit to the lab (phase 1), in order to desensitize participants to the scanner environment, all participants completed a 20-minute mock scan session in a dedicated simulator at the scanning facility. During their second visit to the lab (phase 1), participants completed a 3-hour MRI scanning session that included 2 resting-state functional MRI (fMRI) scans, which lasted 5 minutes each. Information regarding mock scan procedures, RS scan procedures, MRI acquisition parameters, and preprocessing of imaging data is presented in Supplement 4: MRI acquisition protocol (additional information).
Proposed Analyses
Given strong a priori hypotheses about the effects of stress on frontoamygdala circuitry, we conducted seed-based analyses of resting-state fMRI data to examine RSFC between the basolateral amygdala and the ventromedial prefrontal cortex (regions of interest presented in Figure 2). The mask for the basolateral amygdala was derived from the Jülich histological atlas (47), and the mask for the anterior ventromedial prefrontal cortex was derived from the Mackey and Petrides atlas (48). The CONN Toolbox (49) was used to examine seed-based connectivity between the basolateral amygdala and anterior ventromedial prefrontal cortex. The blood oxygen level–dependent time course of each region of interest was calculated as the average of the time courses of its constituent voxels. RSFC between the 2 regions of interest was calculated as the Fisher z-transformed correlation coefficient of their time courses. Additional information on our neuroimaging analytic plan is presented in Supplement 5: Analytic plan (additional information).
Figure 2.
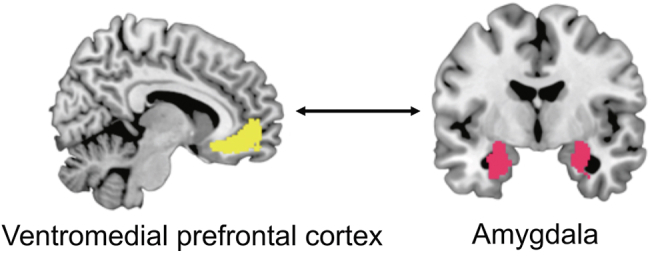
Regions of interest. Regions of interest that were used to examine resting-state functional connectivity between the ventromedial prefrontal cortex (left) and basolateral amygdala (right).
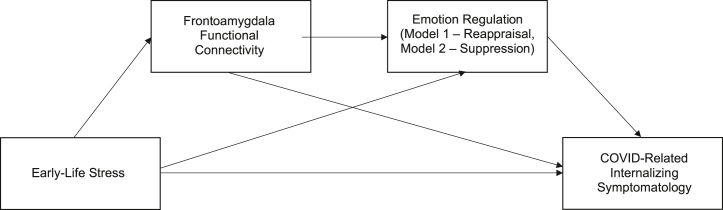
Power considerations are reported in Supplement 6: Power considerations. Serial mediation models were conducted using the PROCESS macro (50,51) in R version 4.1.2 (R Foundation for Statistical Computing). ELS was specified as the independent variable, with RSFC as the first mediator and ER strategy (i.e., either reappraisal or suppression) as the second mediator (illustrated in Figure 3). Reappraisal and suppression scores were entered as mediators in two separate models. In both models, internalizing symptomatology (composite of anxiety and depression symptoms) was specified as the dependent variable. The following covariates were included in all models: pre-pandemic internalizing symptomatology, age at time of scan, COVID-related distress, economic-related impact experienced during COVID-19, and elapsed time between fMRI scan and completion of pandemic-related questionnaires. All variables were fixed, and nonnormally distributed variables were log-transformed. Within this model, 3 indirect effects were tested sequentially with bootstrapped CIs with 10,000 iterations: 1) the effect of ELS on internalizing symptomatology via RSFC, 2) the effect of ELS on internalizing symptomatology via ER (i.e., reappraisal in model 1 and suppression in model 2), and 3) the effect of ELS on internalizing symptomatology via RSFC and ER (i.e., reappraisal in model 1 and suppression in model 2). The indirect effects were considered significant if the 95% CI did not include zero. All analyses were preregistered on the Open Science Framework (https://osf.io/pvam9/?view_only=9cdd3a08acef41bbbaa195c3a60e7973).
Figure 3.
Analytical models. Hypothesized serial mediation model testing the indirect effect of early-life stress on internalizing symptomatology via resting-state functional connectivity and emotion regulation.
In addition, we ran several supplementary analyses to examine the robustness of our findings. The first set of supplementary analyses employed a redefined age cutoff for ELS exposure occurring before age 18 years (as compared with ELS exposure occurring before age 12, as operationalized in the primary models). The second set of supplementary analyses examined the effect of the cumulative number of ELS events that an individual experienced as an index of exposure to ELS, rather than as an average of self-reported severity of ELS events, across all models.
Exploratory Analyses
In addition to the registered mediation models, we examined a set of moderation models to further elucidate the way in which neurobehavioral factors may influence the relationship between ELS and COVID-related symptomatology. We conducted 3 separate single-variable moderation models, with prepubertal ELS severity scores as the independent variable, COVID-related internalizing symptomatology as the dependent variable, and frontoamygdala RSFC, reappraisal, and suppression each serving as moderating variables in separate models.
Results
Descriptive statistics and correlations between the key variables in our primary models are shown in Table 2. Additional descriptive statistics and correlations are presented in Supplement 7: Descriptive and correlation analyses (additional information).
Table 2.
Descriptive Statistics and Correlations for Primary Variables (N = 64)
| Mean (SD) | ELS Severity (Prepubertal) | Frontoamygdala Resting-State Functional Connectivity | Reappraisal | Suppression | COVID-Related Internalizing Symptomatology | |
|---|---|---|---|---|---|---|
| ELS Severity (Prepubertal) | 3.24 (2.40) | – | ||||
| Frontoamygdala Resting-State Functional Connectivity | 0.16 (0.13) | −0.087 | – | |||
| Reappraisal | 28.33 (6.87) | 0.142 | −0.163 | – | ||
| Suppression | 4.92 (5.47 | 0.196 | −0.102 | 0.098 | – | |
| COVID-Related Internalizing Symptomatology | 1.77 (1.65) | 0.045 | 0.104 | −0.210 | 0.104 | – |
ELS, early-life stress.
Mediation Models
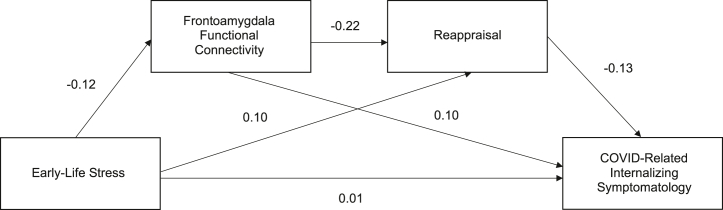
The first primary serial mediation model examined the mediating effect of frontoamygdala RSFC and the use of reappraisal on the relationship between prepubertal ELS severity and COVID-related internalizing symptomatology. Table 3 and Figure 4 display the standardized coefficients for total and direct effects on frontoamygdala connectivity, reappraisal, and COVID-related internalizing symptomatology in the serial mediation model. The direct and positive association between ELS severity and COVID-related internalizing symptomatology was nonsignificant (p > .05). All additional total and direct effects on frontoamygdala connectivity, reappraisal, and COVID-related internalizing symptomatology in this primary model were also nonsignificant. Table 4 shows total, individual, and serial indirect effects for ELS severity on COVID-related internalizing symptomatology via frontoamygdala connectivity and reappraisal with bias-corrected 95% CIs. There were no significant indirect effects of ELS severity on COVID-related internalizing symptomatology via frontoamygdala connectivity or via reappraisal.
Table 3.
Standardized Coefficients for Total and Direct Effects of Prepubertal ELS Severity on Frontoamygdala Resting-State Connectivity, Reappraisal, and COVID-Related Internalizing Symptomatology
| Frontoamygdala Connectivity Total/Direct Effecta | Reappraisal |
Internalizing Symptomatology |
|||
|---|---|---|---|---|---|
| Total Effect | Direct Effect | Total Effect | Direct Effect | ||
| ELS Severity (Prepubertal) | −0.1158 | 0.1260 | 0.1012 | −0.0135 | 0.0141 |
| Frontoamygdala Connectivity | – | – | −0.2150 | 0.1245 | 0.0964 |
| Reappraisal | – | – | – | – | −0.1306 |
| R2 | 0.0483 | 0.1894 | 0.4824 | ||
ELS, early-life stress.
Total and direct effect are considered to be equivalent when examining effects between the independent variable and the first mediator in a serial mediation analysis.
Figure 4.
Serial mediation model results: mediating roles of frontoamygdala connectivity and reappraisal. Association between early-life stress and COVID-related internalizing symptomatology, with frontoamygdala connectivity and reappraisal serving as serial mediators. All effects displayed are standardized, direct effects. No effects were significant (ps > .05).
Table 4.
Total, Individual, and Serial Indirect Effects for Prepubertal ELS Severity on Frontoamygdala Connectivity, Reappraisal, and COVID-Related Internalizing Symptomatology
| Pathway | Indirect Effect | SE | Bias-Corrected 95% CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Total Indirect Effect | −0.0277 | 0.0370 | −0.1051 | 0.0464 |
| Individual Indirect Effects | ||||
| ELS → frontoamygdala connectivity → COVID-related internalizing symptomatology | −0.0112 | 0.0215 | −0.0631 | 0.0252 |
| ELS → reappraisal → COVID-related internalizing symptomatology | −0.0132 | 0.0243 | −0.0580 | 0.0448 |
| Serial Indirect Effects | ||||
| ELS → frontoamygdala connectivity → reappraisal → COVID-related internalizing symptomatology | −0.0033 | 0.0096 | −0.0293 | 0.0105 |
ELS, early-life stress.
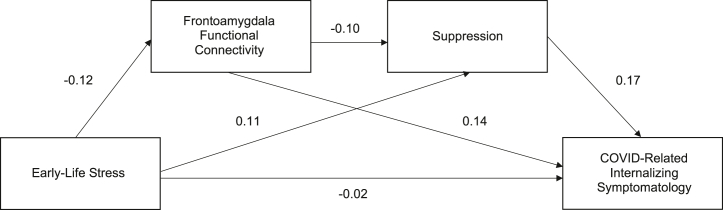
The second primary serial mediation model examined the mediating effect of frontoamygdala RSFC and the use of suppression on the relationship between prepubertal ELS severity and COVID-related internalizing symptomatology. The standardized coefficients for total and direct effects on frontoamygdala connectivity, suppression, and COVID-related internalizing symptomatology are shown in Table 5 and in Figure 5. All total and direct effects on frontoamygdala connectivity, reappraisal, and COVID-related internalizing symptomatology in this primary model were nonsignificant (ps > .05). Table 6 shows total, individual, and serial indirect effects for ELS stress severity on COVID-related internalizing symptomatology via frontoamygdala connectivity and suppression with bias-corrected 95% CIs. No significant indirect effects were found in this model.
Table 5.
Standardized Coefficients for Total and Direct Effects of Prepubertal ELS Severity on Frontoamygdala Resting-State Connectivity, Suppression, and COVID-Related Internalizing Symptomatology
| Frontoamygdala Connectivity Total/Direct Effecta | Suppression |
Internalizing Symptomatology |
|||
|---|---|---|---|---|---|
| Total Effect | Direct Effect | Total Effect | Direct Effect | ||
| ELS Severity (Prepubertal) | −0.1158 | 0.1193 | 0.1074 | −0.0135 | −0.0170 |
| Frontoamygdala Connectivity | – | – | −0.1027 | 0.1246 | 0.1417 |
| Suppression | – | – | – | – | 0.1664 |
| R2 | 0.0483 | 0.2098 | 0.4905 | ||
ELS, early-life stress.
Total and direct effect are considered to be equivalent when examining effects between the independent variable and the first mediator in a serial mediation analysis.
Figure 5.
Serial mediation model results: mediating roles of frontoamygdala connectivity and suppression. Association between ELS and COVID-related internalizing symptomatology, with frontoamygdala connectivity and suppression serving as serial mediators. All effects displayed are standardized, direct effects. No effects were significant (ps > .05).
Table 6.
Total, Individual, and Serial Indirect Effects for Prepubertal ELS Severity on Frontoamygdala Connectivity, Suppression, and COVID-Related Internalizing Symptomatology
| Pathway | Indirect Effect | SE | Bias-Corrected 95% CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Total Indirect Effect | 0.0034 | 0.0327 | −0.0642 | 0.0731 |
| Individual Indirect Effects | ||||
| ELS → frontoamygdala connectivity → COVID-related internalizing symptomatology | −0.0165 | 0.0288 | −0.0883 | 0.0272 |
| ELS → suppression → COVID-related internalizing symptomatology | 0.0179 | 0.0262 | −0.0233 | 0.0821 |
| Serial Indirect Effects | ||||
| ELS → frontoamygdala connectivity → suppression → COVID-related internalizing symptomatology | 0.0020 | 0.0062 | −0.0045 | 0.0192 |
ELS, early-life stress.
Results from supplementary models (i.e., with different operationalizations of ELS exposure) are presented in Supplement 8: Supplemental analyses. Results were highly consistent with primary models in that there were no significant indirect effects across different operationalizations of ELS exposure. However, we did find that cumulative ELS exposure prior to age 12 years had a significant direct and positive association with use of reappraisal (β = 0.277, p = .037).
Exploratory Analyses
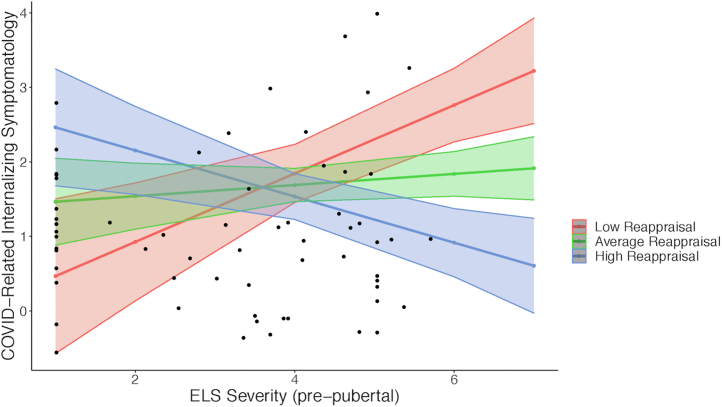
The first model in our exploratory analyses examined the moderating effect of reappraisal use on the association between average prepubertal ELS severity and COVID-related internalizing symptomatology. We found that a significant interaction between ELS severity and reappraisal (B = −0.818, p = .047) predicted COVID-related symptomatology. There were no significant interaction effects between average ELS severity and use of suppression, or between average prepubertal ELS severity and frontoamygdala connectivity, on COVID-related internalizing symptomatology (ps > .05). The unstandardized coefficients for the effects of ELS severity × neurobehavioral measures on COVID-related symptomatology are displayed in Table 7 and are plotted in Figure 6.
Table 7.
Potential Neurobehavioral Moderators of the Association Between Prepubertal ELS Severity and Internalizing Symptomatology Reported During the COVID-19 Pandemic
| Dependent Variable | Independent and Moderating Variables | B (95% CI)a | SE | t55 | p Value |
|---|---|---|---|---|---|
| COVID-Related Internalizing Symptomatology | ELS severity (prepubertal) | 0.901 (−0.031 to 1.833) | 0.460 | 1.958 | .058 |
| Reappraisal | 2.941 (−0.889 to 6.772) | 1.890 | 1.556 | .128 | |
| ELS severity × reappraisal | −0.818 (−1.625 to −0.010) | 0.398 | −2.052 | .047b | |
| COVID-Related Internalizing Symptomatology | ELS severity (prepubertal) | 0.094 (−0.519 to 0.707) | 0.303 | 0.312 | .757 |
| Suppression | 0.854 (−1.320 to 3.027) | 1.073 | 0.796 | .413 | |
| ELS severity × suppression | −0.075 (−0.541 to 0.390) | 0.230 | −0.328 | .745 | |
| COVID-Related Internalizing Symptomatology | ELS severity (prepubertal) | 0.103 (−0.444 to 0.650) | 0.270 | 0.382 | .704 |
| Frontoamygdala connectivity | 3.034 (−8.999 to 15.064) | 5.937 | 0.511 | .612 | |
| ELS severity × frontoamygdala connectivity | −0.326 (−2.776 to 2.125) | 1.210 | −0.269 | .789 |
ELS, early-life stress.
Unstandardized beta.
Significant (p < .05).
Figure 6.
Exploratory model: moderating role of reappraisal. Plot for the significant interaction between early-life stress (ELS) severity and reappraisal use on COVID-related internalizing symptomatology. Association between ELS and COVID-related internalizing symptomatology is plotted at mean, low (−1 SD), and high (+1 SD) reappraisal use. SE bands represent ±1 SE from the fitted values.
Discussion
The current registered report did not find evidence that frontoamygdala connectivity or use of reappraisal or suppression plays a mediating role in the relationship between ELS and COVID-related internalizing symptomatology. However, exploratory analyses demonstrated a significant moderating effect of reappraisal use on the association between prepubertal ELS severity and COVID-related internalizing symptomatology, such that higher reappraisal use buffered the impact of ELS on symptomatology. These findings contribute to a growing literature on specific factors that may serve to buffer against psychopathology during the COVID-19 pandemic.
ELS and COVID-Related Internalizing Symptomatology
Past research demonstrates that ELS exposure predicts depression and anxiety in adulthood (52, 53, 54) and that ELS is associated with internalizing symptomatology during the pandemic (14,55,56). In the present study, we did not observe any significant direct associations between COVID-related symptomatology and ELS. The lack of significant relationships between ELS and COVID-related mental health was unexpected but not entirely surprising. Several previous studies have identified null, weak, or inconsistent associations between ELS exposure and the presence of psychopathology in later adulthood (57,58). One possibility is that the null associations in the current study may reflect multifinality––the phenomenon by which the same risk factors (e.g., exposure to adversity early in life) can lead to different developmental trajectories and outcomes (59,60). The present findings may also stem from empirical and theoretical work that suggests that heterogeneity in ELS, such as differences in chronicity (61) or type of stress (62, 63, 64), may moderate the association between stress exposure and subsequent vulnerability. Our lack of accounting for these differences in our models may be obfuscating present associations between ELS and COVID-related symptomatology.
Mediating Effects of Frontoamygdala RSFC and Emotion Regulation
In all tested serial mediation models, there were no significant indirect associations between ELS and COVID-related symptomatology through frontoamygdala RSFC, use of reappraisal, or use of suppression. Direct effects of ELS severity on frontoamygdala connectivity, reappraisal, and suppression were nonsignificant, as were direct effects of frontoamygdala connectivity on reappraisal and suppression. By contrast, supplemental analyses showed that cumulative ELS exposure prior to age 12 years had a significant direct and positive association with use of reappraisal. Though the directionality of this finding is inconsistent with both our hypotheses and past evidence of a negative relation between adversity exposure and reappraisal use (65), it may be explained in part by past literature suggesting that individuals with a history of adversity exposure may habitually engage in cognitive reappraisal as a coping strategy (66). Additionally, factors unaccounted for in the current analyses, such as past psychotherapy (67), may contribute to the positive association observed here. Future work should continue to examine how exposure to stress early in life may relate to the extent to which one engages in reappraisal-based strategies during the pandemic.
The lack of observed mediating effects of frontoamygdala connectivity is inconsistent with previous work that has identified a mediating role of corticolimbic circuitry on the association between ELS and psychopathology (28,29). Additionally, in contrast to the current findings, previous work has shown associations between frontoamygdala RSFC and ER abilities (68,69). Several factors may have precluded the identification of associations between frontoamygdala connectivity and other key variables such as ELS and ER use in the current study. First, though shown to be more reliable than task-activation paradigms (32,33), fMRI has demonstrated greater variance within and between scanning sessions in comparison with other metrics of connectivity, such as structural connectivity (70,71). Additionally, between-subject spatial differences in amygdala subdivisions (72) may have contributed to the null findings. While we examined predefined anatomical partitions of the amygdala defined across subjects, alternative approaches, such as subject-by-subject connectivity-based parcellation (73), may allow for a more precise examination of frontoamygdala interactions that better accounts for individual differences in cytoarchitecture. The current null findings likely also point to a need for future examination of a broader network of connections that extend beyond the basolateral amygdala and ventromedial prefrontal cortex, particularly ventrolateral and dorsolateral prefrontal regions that have been implicated in cognitive reappraisal (74, 75, 76). Finally, variations in neuroimaging preprocessing pipelines and methodological differences can contribute to distinct findings (77). Future work could examine a mediating effect of frontoamygdala circuitry in stress and psychopathology in a multiverse fashion (78,79) to assess the robustness of findings.
ER as a Moderating Factor
While we did not identify mediating effects of frontoamygdala connectivity or ER strategy use in our registered models of ELS and symptomatology, exploratory analyses showed that reappraisal use significantly moderated the relationship between prepubertal ELS severity and COVID-related internalizing symptomatology. Specifically, individuals who reported higher levels of reappraisal use displayed a negative association between ELS severity and symptomatology during the pandemic. By contrast, individuals who reported lower levels of reappraisal use showed a positive association between ELS severity and psychopathology during the pandemic. This finding is consistent with literature that has identified the use of cognitive reappraisal as a buffer against the effects of stress on mental health outcomes (40,80, 81, 82, 83), as well as with more recent work that has identified links between stress exposure, ER, and COVID-related psychopathology (83, 84, 85, 86, 87, 88). Additionally, the current findings lend support to existing frameworks that are more consistent with a moderating role (as opposed to a mediating role) of ER in the association between stress and psychopathology (89, 90, 91). Though stressful life events have been associated with difficulties with ER (16), conceptualizing ER as a moderating factor may be consistent with frameworks proposing that pre-existing strengths and vulnerabilities (e.g., cognitive reappraisal abilities) interact with stress exposure to predict later mental health. Future research will be important for better distinguishing specific conditions in which ER strategies may be acting as modulatory compared with explanatory factors in the association between stress exposure and mental health outcomes.
Limitations and Conclusions
In part owing to the unique circumstances of conducting this research in the context of a global pandemic, this study had several limitations. First, although our post hoc power calculation estimated that the sample size would be sufficient to achieve desired power, our sample size was limited by the nature of longitudinal data collection during the pandemic. It is important to consider the null findings in the context of a sample size far smaller than that recommended for examining brain-behavior associations (92). Second, our post hoc power analysis was conducted for the preregistered mediation analyses and not for the exploratory moderation models. Third, our observational study design limits the ability to draw causal inferences. Assessing ELS prior to adulthood and employing a longitudinal design with additional time points and stricter temporal precedence would allow for a clearer understanding of associations between early experiences, neurobehavioral development, and stress-related psychopathology. Fourth, the average age of participants differed significantly between those that completed all measures required from phase 1 compared with phase 2, indicating a potential source of attrition bias in our sample (detailed further in Supplement 1: Participant inclusion criteria. Last, the majority of our sample identified as non-Hispanic White, were of medium-high socioeconomic status, and had completed or were currently completing a bachelor’s degree. The extent to which these findings generalize to more racially and socioeconomically diverse samples, especially to individuals who may have experienced more financial strain and disproportionate health impacts during the pandemic (93, 94, 95), is unknown. Despite these limitations, the current work adds to an emerging literature documenting that engagement in specific ER strategies may buffer against mental health consequences during stress exposure. Moreover, the registered report format of the current work contributes to a growing effort to reduce publication and research bias in hypothesis-driven deductive scientific research. Finally, our null findings should not dissuade continued efforts to improve the environments in which children develop. The current work should instead motivate researchers to continue to examine how stress exposure impacts later mental health outcomes and should motivate clinicians and policymakers to work to intervene whenever possible.
Acknowledgments and Disclosures
This work was supported by a National Institutes of Health Director’s Early Independence Award (Grant No. DP5OD021370 [to DGG]), a Brain & Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression) Young Investigator Award (to DGG), a Jacobs Foundation Early Career Research Fellowship (to DGG), the Society for Clinical Child and Adolescent Psychology (Division 53 of the American Psychological Association) Richard “Dick” Abidin Early Career Award and Grant (to DGG), a National Science Foundation Graduate Research Fellowship Program Award (Grant No. DGE1752134 [to EMC]), a Society for Clinical Child and Adolescent Psychology (Division 53 of the American Psychological Association) Donald Routh Dissertation Grant (to EMC), an American Psychological Foundation Elizabeth Munsterberg Koppitz Child Psychology Graduate Fellowship (to EMC), a Dissertation Funding Award from the Society for Research in Child Development (to EMC), a Dissertation Research Award from the American Psychological Association (to EMC), a National Science Foundation Graduate Research Fellowship Program Award (to JCF), and a Susan Nolen-Hoeksema Postdoctoral Fellowship (to AEB).
We thank the Yale Center for Research Computing, particularly Dr. Kaylea Nelson, for assistance with analyses conducted on the Milgram cluster. We acknowledge the many contributions of the undergraduate research assistants who coded and entered the early-life stress data used in analyses, including Isabel Santiuste, Lindiwe Mayinja, Elizabeth Kitt, Brandon Lopez, Reta Behnam, Jenny Wang, and Marisa Rogers. Finally, we are grateful to the study participants for their time and participation.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
JCF and EMC contributed equally to this work as joint first authors.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.07.006.
Supplementary Material
References
- 1.Pfefferbaum B., North C.S. Mental health and the Covid-19 pandemic. N Engl J Med. 2020;383:510–512. doi: 10.1056/NEJMp2008017. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar R.P. COVID-19 and mental health: A review of the existing literature. Asian J Psychiatry. 2020;52 doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17:1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonanno G.A., Mancini A.D. Beyond resilience and PTSD: Mapping the heterogeneity of responses to potential trauma. Psychol Trauma Theory Res Pract Policy. 2012;4:74–83. [Google Scholar]
- 6.Gruber J., Prinstein M.J., Clark L.A., Rottenberg J., Abramowitz J.S., Albano A.M., et al. Mental health and clinical psychological science in the time of COVID-19: Challenges, opportunities, and a call to action. Am Psychol. 2021;76:409–426. doi: 10.1037/amp0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr C.P., Martins C.M.S., Stingel A.M., Lemgruber V.B., Juruena M.F. The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. J Nerv Ment Dis. 2013;201:1007–1020. doi: 10.1097/NMD.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 8.Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manyema M., Norris S.A., Richter L.M. Stress begets stress: The association of adverse childhood experiences with psychological distress in the presence of adult life stress. BMC Public Health. 2018;18:835. doi: 10.1186/s12889-018-5767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin K.A., Conron K.J., Koenen K.C., Gilman S.E. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearlin L.I., Schieman S., Fazio E.M., Meersman S.C. Stress, health, and the life course: Some conceptual perspectives. J Health Soc Behav. 2005;46:205–219. doi: 10.1177/002214650504600206. [DOI] [PubMed] [Google Scholar]
- 13.Hammen C., Henry R., Daley S.E. Depression and sensitization to stressors among young women as a function of childhood adversity. J Consult Clin Psychol. 2000;68:782–787. [PubMed] [Google Scholar]
- 14.Gotlib I.H., Borchers L.R., Chahal R., Gifuni A.J., Teresi G.I., Ho T.C. Early life stress predicts depressive symptoms in adolescents during the COVID-19 pandemic: The mediating role of perceived stress. Front Psychol. 2021;11 doi: 10.3389/fpsyg.2020.603748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chahal R., Kirshenbaum J.S., Miller J.G., Ho T.C., Gotlib I.H. Higher executive control network coherence buffers against puberty-related increases in internalizing symptoms during the COVID-19 pandemic. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:79–88. doi: 10.1016/j.bpsc.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demir Z., Böge K., Fan Y., Hartling C., Harb M.R., Hahn E., et al. The role of emotion regulation as a mediator between early life stress and posttraumatic stress disorder, depression and anxiety in Syrian refugees. Transl Psychiatry. 2020;10:371. doi: 10.1038/s41398-020-01062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee D.G. Sensitive periods of emotion regulation: Influences of parental care on frontoamygdala circuitry and plasticity. New Dir Child Adolesc Dev. 2016;2016:87–110. doi: 10.1002/cad.20166. [DOI] [PubMed] [Google Scholar]
- 18.Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser R.H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., et al. Childhood stress, grown-up brain networks: Corticolimbic correlates of threat-related early life stress and adult stress response. Psychol Med. 2018;48:1157–1166. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatini M.J., Ebert P., Lewis D.A., Levitt P., Cameron J.L., Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teicher M.H., Samson J.A. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uliana D.L., Gomes F.V., Grace A.A. Stress impacts corticoamygdalar connectivity in an age-dependent manner. Neuropsychopharmacology. 2021;46:731–740. doi: 10.1038/s41386-020-00886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanson J.L., Knodt A.R., Brigidi B.D., Hariri A.R. Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev Psychopathol. 2015;27:1611–1619. doi: 10.1017/S0954579415000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Y., Herrera-Melendez A.L., Pestke K., Feeser M., Aust S., Otte C., et al. Early life stress modulates amygdala-prefrontal functional connectivity: Implications for oxytocin effects. Hum Brain Mapp. 2014;35:5328–5339. doi: 10.1002/hbm.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peverill M., Sheridan M.A., Busso D.S., McLaughlin K.A. Atypical prefrontal–amygdala circuitry following childhood exposure to abuse: Links with adolescent psychopathology. Child Maltreat. 2019;24:411–423. doi: 10.1177/1077559519852676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin L.M. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 28.Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C., et al. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J Abnorm Psychol. 2015;124:817–833. doi: 10.1037/abn0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brieant A.E., Sisk L.M., Gee D.G. Associations among negative life events, changes in cortico-limbic connectivity, and psychopathology in the ABCD Study. Dev Cogn Neurosci. 2021;52 doi: 10.1016/j.dcn.2021.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S., et al. Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J Neurosci. 2016;36:6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 33.Smitha K., Akhil Raja K., Arun K., Rajesh P., Thomas B., Kapilamoorthy T., Kesavadas C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30:305–317. doi: 10.1177/1971400917697342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shehzad Z., Kelly A.C., Reiss P.T., Gee D.G., Gotimer K., Uddin L.Q., et al. The resting brain: Unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyra A.T., Ginty A.T., John-Henderson N.A. Emotion regulation strategies predict PTSS during the COVID-19 pandemic in an American Indian population. Int J Behav Med. 2021;28:808–812. doi: 10.1007/s12529-021-09964-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M., Saudino K. Emotion regulation and stress. J Adult Dev. 2011;18:95–103. [Google Scholar]
- 37.Panayiotou G., Panteli M., Leonidou C. Coping with the invisible enemy: The role of emotion regulation and awareness in quality of life during the COVID-19 pandemic. J Context Behav Sci. 2021;19:17–27. [Google Scholar]
- 38.Restubog S.L.D., Ocampo A.C.G., Wang L. Taking control amidst the chaos: Emotion regulation during the COVID-19 pandemic. J Vocat Behav. 2020;119 doi: 10.1016/j.jvb.2020.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dryman M.T., Heimberg R.G. Emotion regulation in social anxiety and depression: A systematic review of expressive suppression and cognitive reappraisal. Clin Psychol Rev. 2018;65:17–42. doi: 10.1016/j.cpr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Moore S.A., Zoellner L.A., Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behav Res Ther. 2008;46:993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg A.M., Brymer M.J., Decker K.B., Pynoos R.S. The University of California at Los Angeles post-traumatic stress disorder reaction index. Curr Psychiatry Rep. 2004;6:96–100. doi: 10.1007/s11920-004-0048-2. [DOI] [PubMed] [Google Scholar]
- 42.Gross J.J., John O.P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 43.Beck A.T., Steer R.A., Brown G. Manual for the Beck Depression Inventory-II. Psychological Corporation: San Antonio, TX. 1996 [Google Scholar]
- 44.Bögels S.M., van Melick M. The relationship between child-report, parent self-report, and partner report of perceived parental rearing behaviors and anxiety in children and parents. Personal Individ Differ. 2004;37:1583–1596. [Google Scholar]
- 45.Grasso D.J., Briggs-Gowan M.J., Ford J.D., Carter A.S. University of Connecticut School of Medicine; 2020. The Epidemic–Pandemic Impacts Inventory (EPII)https://health.uconn.edu/psychiatry/research/family-adversity-and-resilience-research-program/epii/ Available at: [Google Scholar]
- 46.Thomason M.E., Graham A., VanTieghem M.R. COPE: Coronavirus Perinatal Experiences-Impact Survey (COPE-IS). Available at: 2020. https://www.nlm.nih.gov/dr2/COPE-Impact_Survey_Perinatal_Pandemic_Survey.pdf
- 47.Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 48.Mackey S., Petrides M. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 2014;40:2777–2796. doi: 10.1111/ejn.12654. [DOI] [PubMed] [Google Scholar]
- 49.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 50.Hayes A.F. Guilford Press; New York: 2017. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- 51.Hayes A.F. Partial, conditional, and moderated moderated mediation: Quantification, inference, and interpretation. Commun Monogr. 2018;85:4–40. [Google Scholar]
- 52.Clark C., Caldwell T., Power C., Stansfeld S.A. Does the influence of childhood adversity on psychopathology persist across the lifecourse? A 45-year prospective epidemiologic study. Ann Epidemiol. 2010;20:385–394. doi: 10.1016/j.annepidem.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Benjet C., Borges G., Medina-Mora M.E. Chronic childhood adversity and onset of psychopathology during three life stages: Childhood, adolescence and adulthood. J Psychiatr Res. 2010;44:732–740. doi: 10.1016/j.jpsychires.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin K.A., Kubzansky L.D., Dunn E.C., Waldinger R., Vaillant G., Koenen K.C. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depress Anxiety. 2010;27:1087–1094. doi: 10.1002/da.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X., Lv Q., Tang W., Deng W., Zhao L., Meng Y., et al. Psychological stresses among Chinese university students during the COVID-19 epidemic: The effect of early life adversity on emotional distress. J Affect Disord. 2021;282:33–38. doi: 10.1016/j.jad.2020.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doom J.R., Seok D., Narayan A.J., Fox K.R. Adverse and benevolent childhood experiences predict mental health during the COVID-19 pandemic. Advers Resil Sci. 2021;2:193–204. doi: 10.1007/s42844-021-00038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grover R.L., Ginsburg G.S., Ialongo N. Childhood predictors of anxiety symptoms: A longitudinal study. Child Psychiatry Hum Dev. 2005;36:133–153. doi: 10.1007/s10578-005-3491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isaksson J., Deyessa N., Berhane Y., Högberg U. Early adversity and psychiatric symptoms – a prospective study on Ethiopian mothers and their children. BMC Psychiatry. 2017;17:344. doi: 10.1186/s12888-017-1500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLaughlin K.A. Future directions in childhood adversity and youth psychopathology. J Clin Child Adolesc Psychol. 2016;45:361–382. doi: 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cicchetti D., Rogosch F.A. Equifinality and multifinality in developmental psychopathology. Dev Psychopathol. 1996;8:597–600. [Google Scholar]
- 61.Phillips N.K., Hammen C.L., Brennan P.A., Najman J.M., Bor W. Early adversity and the prospective prediction of depressive and anxiety disorders in adolescents. J Abnorm Child Psychol. 2005;33:13–24. doi: 10.1007/s10802-005-0930-3. [DOI] [PubMed] [Google Scholar]
- 62.Cohodes E.M., Kitt E.R., Baskin-Sommers A., Gee D.G. Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Dev Psychobiol. 2021;63:153–172. doi: 10.1002/dev.21969. [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin K.A., Sheridan M.A. Beyond cumulative risk: A dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016;25:239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller A.B., Sheridan M.A., Hanson J.L., McLaughlin K.A., Bates J.E., Lansford J.E., et al. Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. J Abnorm Psychol. 2018;127:160–170. doi: 10.1037/abn0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gruhn M.A., Compas B.E. Effects of maltreatment on coping and emotion regulation in childhood and adolescence: A meta-analytic review. Child Abuse Negl. 2020;103 doi: 10.1016/j.chiabu.2020.104446. [DOI] [PubMed] [Google Scholar]
- 66.El Khawli E., Fan Y., Aust S., Wirth K., Bönke L., Stevense A., et al. Early-Life stress modulates neural networks associated with habitual use of reappraisal. Behav Brain Res. 2018;337:210–217. doi: 10.1016/j.bbr.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 67.Troy A.S., Shallcross A.J., Davis T.S., Mauss I.B. History of mindfulness-based cognitive therapy is associated with increased cognitive reappraisal ability. Mindfulness. 2013;4:213–222. doi: 10.1007/s12671-012-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 69.Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala–frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honey C.J., Sporns O., Cammoun L., Gigandet X., Thiran J.P., Meuli R., Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osmanlıoğlu Y., Alappatt J.A., Parker D., Verma R. Connectomic consistency: A systematic stability analysis of structural and functional connectivity. J Neural Eng. 2020;17 doi: 10.1088/1741-2552/ab947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sylvester C.M., Yu Q., Srivastava A.B., Marek S., Zheng A., Alexopoulos D., et al. Individual-specific functional connectivity of the amygdala: A substrate for precision psychiatry. Proc Natl Acad Sci U S A. 2020;117:3808–3818. doi: 10.1073/pnas.1910842117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eickhoff S.B., Thirion B., Varoquaux G., Bzdok D. Connectivity-based parcellation: Critique and implications. Hum Brain Mapp. 2015;36:4771–4792. doi: 10.1002/hbm.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R.E., et al. vlPFC–vmPFC–Amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb Cortex. 2017;27:3502–3514. doi: 10.1093/cercor/bhw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H., et al. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Botvinik-Nezer R., Holzmeister F., Camerer C.F., Dreber A., Huber J., Johannesson M., et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steegen S., Tuerlinckx F., Gelman A., Vanpaemel W. Increasing transparency through a multiverse analysis. Perspect Psychol Sci. 2016;11:702–712. doi: 10.1177/1745691616658637. [DOI] [PubMed] [Google Scholar]
- 79.Bloom P.A., VanTieghem M., Gabard-Durnam L., Gee D.G., Flannery J., Caldera C., et al. Age-related change in task-evoked amygdala-prefrontal circuitry: A multiverse approach with an accelerated longitudinal cohort aged 4-22 years. Hum Brain Mapp. 2022;43:3221–3244. doi: 10.1002/hbm.25847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson J., O’Connor D.B., Jones C., Jackson C., Hughes G.J., Ferguson E. Reappraisal buffers the association between stress and negative mood measured over 14 days: Implications for understanding psychological resilience. Eur J Pers. 2016;30:608–617. [Google Scholar]
- 81.Rodriguez M., Bellet B.W., McNally R.J. Reframing time spent alone: Reappraisal buffers the emotional effects of isolation. Cogn Ther Res. 2020;44:1052–1067. doi: 10.1007/s10608-020-10128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu C., Xu Y., Xu S., Zhang Q., Liu X., Shao Y., et al. Cognitive reappraisal and the association between perceived stress and anxiety symptoms in COVID-19 isolated people. Front Psychiatry. 2020;11:858. doi: 10.3389/fpsyt.2020.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Janiri D., Moccia L., Dattoli L., Pepe M., Molinaro M., De Martin V., et al. Emotional dysregulation mediates the impact of childhood trauma on psychological distress: First Italian data during the early phase of COVID-19 outbreak. Aust N Z J Psychiatry. 2021;55:1071–1078. doi: 10.1177/0004867421998802. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Y., MacGeorge E.L., Myrick J.G. Mental health and its predictors during the early months of the COVID-19 pandemic experience in the United States. Int J Environ Res Public Health. 2020;17:6315. doi: 10.3390/ijerph17176315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Low R.S., Overall N.C., Chang V.T., Henderson A.M., Sibley C.G. Emotion regulation and psychological and physical health during a nationwide COVID-19 lockdown. Emotion. 2021;21:1671–1690. doi: 10.1037/emo0001046. [DOI] [PubMed] [Google Scholar]
- 86.Kalia V., Knauft K., Hayatbini N. Cognitive flexibility and perceived threat from COVID-19 mediate the relationship between childhood maltreatment and state anxiety. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siegel A., Lahav Y. Emotion regulation and distress during the COVID-19 pandemic: The role of childhood abuse. J Interpers Violence. 2022;37(17--18):NP16302–NP16326. doi: 10.1177/08862605211021968. [DOI] [PubMed] [Google Scholar]
- 88.Weissman D.G., Rodman A.M., Rosen M.L., Kasparek S., Mayes M., Sheridan M.A., et al. Contributions of emotion regulation and brain structure and function to adolescent internalizing problems and stress vulnerability during the COVID-19 pandemic: A longitudinal study. Biol Psychiatry Glob Open Sci. 2021;1:272–282. doi: 10.1016/j.bpsgos.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Troy A.S., Wilhelm F.H., Shallcross A.J., Mauss I.B. Seeing the silver lining: Cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion. 2010;10:783–795. doi: 10.1037/a0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krkovic K., Krink S., Lincoln T.M. Emotion regulation as a moderator of the interplay between self-reported and physiological stress and paranoia. Eur Psychiatry. 2018;49:43–49. doi: 10.1016/j.eurpsy.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Voon D., Hasking P., Martin G. The roles of emotion regulation and ruminative thoughts in non-suicidal self-injury. Br J Clin Psychol. 2014;53:95–113. doi: 10.1111/bjc.12030. [DOI] [PubMed] [Google Scholar]
- 92.Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shippee T.P., Akosionu O., Ng W., Woodhouse M., Duan Y., Thao M.S., Bowblis J.R. COVID-19 pandemic: Exacerbating racial/ethnic disparities in long-term services and supports. J Aging Soc Policy. 2020;32:323–333. doi: 10.1080/08959420.2020.1772004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Condon E.M., Dettmer A.M., Gee D.G., Hagan C., Lee K.S., Mayes L.C., et al. Commentary: COVID-19 and mental health equity in the United States. Front Sociol. 2020;5 doi: 10.3389/fsoc.2020.584390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hooper M.W., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.