Abstract
Introduction
This study examined whether smokers’ harm perceptions of nicotine replacement therapy (NRT) and nicotine vaping products (NVPs) relative to cigarettes predicted their subsequent use as smoking cessation aids during their last quit attempt (LQA).
Aims and Methods
We analyzed data from 1,315 current daily smokers (10+ cigarettes per day) who were recruited at Wave 1 (2016), and who reported making a quit attempt by Wave 2 (2018) of the International Tobacco Control Four Country Smoking and Vaping Surveys in Australia, Canada, England, and the United States. We used multinomial logistic regression models to examine prospective associations between harm perceptions of (a) NRT and (b) NVPs and their use at LQA, controlling for socio-demographic and other potential confounders.
Results
Smokers who perceive that (a) NRT and (b) NVPs are much less harmful than cigarettes were more likely to subsequently use the respective product as an aid than using no aid or other aids during LQA (adjusted relative risk ratio [aRRR] = 3.79, 95%CI = 2.16–6.66; and aRRR = 2.11, 95%CI = 1.29–3.45, respectively) compared to smokers who perceive these products as equally or more harmful. Additionally, those who perceive NVPs as much less harmful than cigarettes were less likely to use NRT as a quit aid (aRRR = 0.34, 95%CI = 0.20–0.60). No country variations for these associations were found.
Conclusions
This study found that smokers’ perceptions of the harmfulness of (a) NRT and (b) NVPs relative to cigarettes predicted the respective product use when trying to quit smoking. Corrective education targeting misperceptions of nicotine products’ relative harmfulness may facilitate their use for smoking cessation.
Implications
Nicotine replacement therapy and nicotine vaping products are two commonly used smoking cessation aids. This study demonstrates that misperceptions of the harms of nicotine products relative to cigarettes influence their use for smoking cessation. Believing that nicotine vaping products are much less harmful than cigarette smoking may lead some smokers to prefer these products over nicotine replacement therapy to aid smoking cessation. Education targeting misperceptions of nicotine products’ harmfulness relative to cigarettes may enable smokers to make informed choices about which are appropriate to aid smoking cessation.
Introduction
There is a continuum of risk across different nicotine products.1 Combustible tobacco products (eg, cigarettes) are the most harmful to health, while nicotine replacement therapy (NRT) products are the least harmful. While long-term epidemiological evidence is not available for nicotine vaping products (NVPs, known as e-cigarettes), toxicological evidence suggests their risk profile is likely to be somewhere in between cigarettes and NRT, but closer to the NRT level.1–4
Both NRT and NVPs are often used as smoking cessation aids.5 In many countries, NRT is a government-approved medical therapy for smoking cessation and is recommended in clinical practice guidelines as an effective cessation aid.6,7 Clinical trials have consistently demonstrated the safety and efficacy of NRT for smoking cessation.8 Smokers who quit with the help of NRT have 50%–60% greater likelihood of succeeding than those who try to quit without using an aid.8 However, the real-world effectiveness of NRT for smoking cessation appears lower than that found in clinical trials,9 which has been attributed to the way it is used in real life (eg, under-dosing and premature discontinuation of treatment).10 Past research has found widespread misperception of NRT harmfulness.11–13 Research studies have also found misperception of NRT harmfulness was associated with lower likelihood of using NRT in past quit attempts, lower consideration of using NRT for future quit attempts and lower compliance among those who used NRT during quit attempts.14,15 The findings suggest that many smokers are misinformed about the health harms of NRT and these misperceptions not only undermine NRT uptake but also its proper utilization for smoking cessation. Other research suggests that uncontrolled recall bias may also account for the lower effectiveness of NRT for smoking cessation often observed in real-world studies.16 Nevertheless, these past findings were based mainly on cross-sectional studies precluding the ability to determine the directionality of effect as use could also affect perception. Prospective cohort studies are needed to better understand how perceptions of NRT harmfulness would influence its use for smoking cessation in the real-world setting.
NVP use has increased rapidly in the last decade.3 Smoking cessation is one of the reasons smokers use NVPs.17 However, their effectiveness for smoking cessation is debated. The latest Cochrane systematic review provided moderate certainty evidence to indicate that NVPs are more effective than NRT for smoking cessation.18 Like NRT, harm perceptions of NVPs may impact the extent of NVP use. A growing body of literature suggests that misperceptions of the harmfulness of NVPs are substantial and increasing over time.19,20 Studies have also found that smokers who believe that NVPs are less harmful than smoking are more likely to use them than their counterparts who believe otherwise.20–22 However, it is unclear as to what extent the association between perceptions and use behavior applies to smoking cessation specifically. More studies are needed to understand how harm perceptions of NVPs influence its use for smoking cessation.
Recent research indicates that misperceptions of the harmfulness of nicotine are widespread23,24 and they contribute to misperceptions of NRT and NVPs.13 This may account for the high correlation found between harm perceptions of NRT and NVPs.25 Regardless of the mechanisms, harm perceptions are likely to generalize across all nicotine products, rather than be product specific. This cross-product relationship between harm perceptions of one nicotine product (eg, NRT) and use of other nicotine products (eg, NVPs), particularly for the purpose of smoking cessation, has not been studied before.
The regulatory context of a country has been shown to influence the extent of use of nicotine products.21 Differences in regulatory environments across countries also appear to affect harm perceptions of nicotine products.26,27 Access, availability and policy for NRT and NVPs vary across the countries studied here.28 For example, NRT is rarely accessed via prescription in Australia, Canada, and the United States, but is common in England.29,30 The regulatory environment for NVPs is more restrictive in Australia, where retail sales and marketing are banned and legal use requires a doctor’s prescription.31 In contrast, in England, the sale of NVPs is legal and their use for smoking cessation is supported, both by Public Health England and the National Institute for Health and Care Excellence (NICE) clinical practice guidelines.32 During data collection for our study (2016 and 2018), NVPs were not legal for sale in Canada, but were widely accessible due to poor enforcement of sales bans in retail stores.33 In the United States, NVPs were legal, but were not promoted for cessation by public health entities. While there is some evidence to suggest that regulatory environments influence harm perceptions of nicotine-containing products,26,27 further research is warranted to understand how regulations around NRT and/or NVPs would influence harm perceptions of these nicotine products and in turn, the extent of their use for smoking cessation.
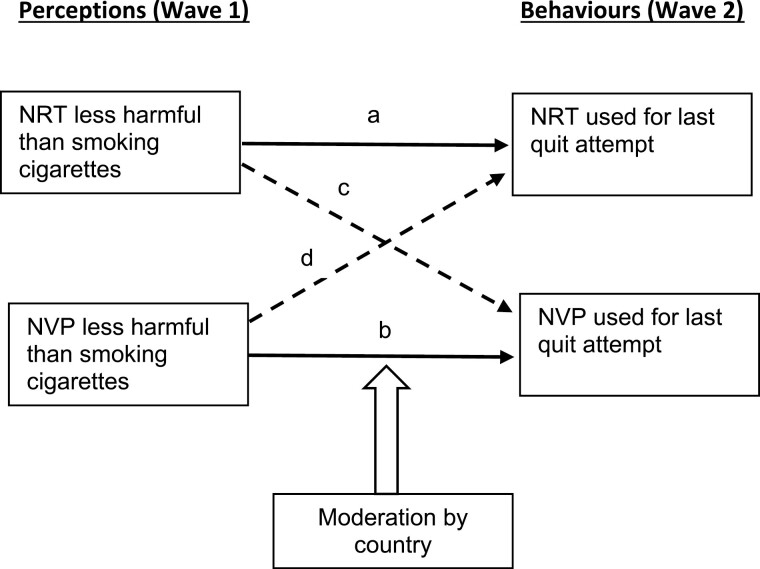
This prospective cohort study aimed to investigate: (1) how harm perceptions of NRT and NVPs among heavy daily smokers predicted their subsequent use during their last quit attempt (LQA); (2) whether harm perceptions of one nicotine product would predict use of the alternative product during LQA; and (3) whether country would moderate the above perception-behavior associations. As depicted in Figure 1, we hypothesized that accurate (ie, less harmful) perceptions of NRT or NVPs would predict a higher rate of use of the respective product as well as the alternate product during LQA; and that country would moderate the predictive associations with no clear expectation of how they might differ between countries.
Figure 1.
Hypothesized relationships of baseline harm perceptions with subsequent use behavior within and between products.
Methods
Sample and Design
We analyzed data from Waves 1 (2016) and 2 (2018) of the ITC Four Country and Vaping (ITC 4CV) Surveys, a cohort study consisting of four parallel online surveys conducted in Canada, the United States, England, and Australia. In addition to respondents retained from the ITC Four Country Survey (the predecessor of ITC 4CV), adults (≥ 18 years) were recruited by commercial panel firms in each country at Wave 1 (July–November 2016) as cigarette smokers (smoked at least 100 cigarettes in their lifetime), recent ex-smokers (quit within ≤2 years), or at-least-weekly NVP users (vapers). All Wave 1 respondents were invited to complete the Wave 2 survey (February–July 2018). The sample in each country was designed to be as representative as possible of smokers and vapers and used either probability-based sampling frames or nonprobability quota samples. Details of the conceptual framework and the survey methodology can be found in Fong et al.34 and Thompson et al.,35 and in the technical reports.36,37
We limited the analytical sample for this analysis to current cigarette smokers (regardless of whether they used any NVPs or not), who reported smoking at least 10 cigarettes per day in 2016, made a quit attempt by the 2018 follow-up, and completed both the 2016 and 2018 surveys (see Supplementary Figure S1). Overall, 1,315 individuals met these criteria across the four countries in Canada (n = 461), United States (n = 219), England (n = 353), and Australia (n = 282).
Measures
The survey questionnaires, with original response options, can be found at the ITC Project website: https://itcproject.org/surveys/. The following variables were used in the current study:
Outcomes:
Quit aids used during last quit attempt (LQA) were assessed by asking participants who had made a quit attempt at the follow-up survey (Wave 2) with the following questions: (1) if they had used any type of nicotine replacement product, such as patches, gum, or mouth spray, at the time of their last (smokers) or current (ex-smokers) quit attempt (referred to hereafter as last quit attempt [LQA]) (Yes/No); (2) if they had used a vaping product on their last/current quit attempt (Yes/No); and (3) if they had used any other aids/assistance which included stop-smoking medications (e.g., varenicline, bupropion), smokeless tobacco products (heated tobacco product, snus), stop-smoking service (eg, quitline, behavior therapy), online cessation help (eg, apps, website, support groups), self-help materials (pamphlets, brochures). Based on their responses to these survey questions, an outcome variable with four mutually exclusive categories was derived: (i) “No/other quit aids”; (ii) “Any use of NRT alone or with other aids excluding NVP”; (iii) “Any use of NVP alone or with other aids excluding NRT”; and (iv) “Any use of both NRT and NVP excluding prior categories”.
Baseline Measures
Predictor variables:
Perceptions of harm of NRT and NVP relative to cigarettes were assessed using the following respective questions: “Compared to smoking cigarettes, how harmful do you think nicotine replacement products are? Nicotine replacement products include patches, gum, inhalers, mouth spray, and various other nicotine products that have been approved as medicines” and “Compared to smoking cigarettes, how harmful do you think vaping (using e-cigarettes) is?” The original response options for both questions were: “much less harmful”, “somewhat less harmful”, “equally harmful”, “somewhat more harmful”, “much more harmful” or “don’t know”. For analysis purposes, responses to the first two response options were treated as accurate perceptions and the rest were treated as inaccurate perceptions. The “equally harmful”, “somewhat more harmful” and “much more harmful” categories were combined to ensure sufficient cases for analysis as the reference category. Both measures had been shown to work well in cognitive testing38 and past research.25
Covariates:
Age in years (18–25, 25–39, 40–54, or 55+), gender (male or female), education (low, moderate or high), income (low, moderate, high or not reported), ethnicity (ethnic majority or minority), country (Canada, United States, England, or Australia), beliefs about nicotine harmfulness (not at all through to extremely harmful), and knowledge of smoking health effects (derived using four items assessing whether respondents believed smoking causes strokes, blindness, breast cancer, or mouth cancer, and the affirmative responses were summed to give a total score ranging from 0 to 4).
Data Analysis
All statistical analyses were performed in Stata version 16 (StataCorp, TX, USA). Sample characteristics were examined using frequencies and (unweighted) percentages for categorical variables, and mean and standard deviations for continuous variables, separately by country. Multinomial logistic regression was employed to examine the association of daily smokers’ harm perceptions of nicotine products (ie, NRT and NVP) relative to cigarettes (much less harmful, somewhat less harmful or do not know vs. equally/more harmful) at Wave 1 (baseline) with choice of products used as a quit aid at their LQA initiated by Wave 2 (follow-up; ie, no/other aids as a reference compared with use of NRT, NVP or both products, while adjusting for the covariates listed above). Predictor by country interaction terms were added into the model to test for whether effects varied across countries. To explore the specificity of the predictive effect of these two product harm perceptions on choice of quit aid used for LQA, additional analyses were conducted using the outcome variable: no quit aids at LQA as the reference to compare with use of any nicotine products (which included a small number of heated tobacco products and smokeless tobacco products reported, n = 22) and use of only non-nicotine products as quit aids for LQA. Relative risk ratios (RRR) and 95% confidence intervals (CI) were estimated. Statistical significance was set to an alpha of 0.05.
Results
Sample Characteristics and Harm Perceptions
The unweighted baseline sample characteristics of smokers and their perceptions of the harmfulness of nicotine, NRT and NVPs are presented in Table 1. Overall, only 13% perceive that nicotine is either not at all or slightly harmful for health, 61% perceive NVPs as less harmful than cigarettes, and 76% perceive NRT as less harmful than cigarettes. Notably, the percentage who reported that NRT is less harmful than cigarettes was highest in England and lowest in the United States (79% and 71%, respectively), with similar country differences for NVPs being less harmful (67% and 54%, respectively). As seen in Table 1, one in five smokers in our sample (20%) reported using NRT (either exclusively or in combination with other aids) during their LQA, around one in four (26%) reported using NVP (either exclusively or in combination with other aids), and around 12% reported using both products (exclusively or in combination) during LQA, with the majority reporting using no aid (29%) and the rest using some other aids during their LQA (13%).
Table 1.
Sample Characteristics of Baseline (Wave 1) Current Daily (10+ cigs per day) Smokers* and Quit Aids used at Last Quit Attempts (Wave 2) by Country
| Variables | Canada N = 461 |
United States n=219 |
England n=353 |
Australia n=282 |
Total n=1,315 |
|---|---|---|---|---|---|
| W1 Age Group, n (%) | |||||
| 18–24 | 37 (8) | 13 (6) | 31 (9) | 5 (2) | 86 (7) |
| 25–39 | 109 (24) | 31 (14) | 57 (16) | 44 (16) | 241 (18) |
| 40–54 | 150 (33) | 63 (29) | 119 (34) | 106 (38) | 438 (33) |
| 55+ | 165 (36) | 112 (51) | 146 (41) | 127 (45) | 550 (42) |
| W1 Gender, n (%) | |||||
| Male | 219 (48) | 100 (46) | 163 (46) | 132 (47) | 614 (47) |
| Female | 242 (52) | 119 (54) | 190 (54) | 150 (53) | 701 (53) |
| W1 Education, n (%) | |||||
| Low | 160 (35) | 76 (35) | 131 (38) | 110 (39) | 477 (37) |
| Moderate | 213 (47) | 96 (44) | 123 (35) | 110 (39) | 542 (42) |
| High | 84 (18) | 47 (21) | 95 (27) | 80 (22) | 286 (22) |
| No answer | 4 (0) | 0 (0) | 4 (0) | 2 (0) | 10 (0) |
| W1 Income, n (%) | |||||
| Low | 173 (38) | 77 (35) | 81 (23) | 96 (34) | 427 (32) |
| Moderate | 130 (28) | 67 (31) | 162 (46) | 71 (25) | 430 (33) |
| High | 123 (27) | 73 (33) | 86 (24) | 101 (36) | 383 (29) |
| No information | 35 (8) | 2 (1) | 24 (7) | 14 (5) | 75 (6) |
| W1 Ethnicity, n (%) | |||||
| Ethnic majority | 410 (90) | 185 (84) | 332 (95) | 267 (95) | 1194 (92) |
| Ethnic minority | 41 (9) | 34 (16) | 14 (3) | 15 (5) | 104 (7) |
| Do not know | 10 (1) | 0 (0) | 7 (1) | 0 (0) | 17 (1) |
| W1 Knowledge of smoking harms (0–4) Mean (SD) | 2.8 (1.1) | 2.3 (1.2) | 2.5 (1.1) | 2.7 (1.2) | 2.6 (1.2) |
| W1 Belief of nicotine being harmful to health, n (%) | |||||
| Not at all | 9 (2) | 3 (1) | 21 (6) | 8 (3) | 41 (3) |
| Slightly | 35 (8) | 25 (11) | 51 (14) | 19 (7) | 130 (10) |
| Moderately | 119 (26) | 59 (27) | 79 (22) | 83 (29) | 340 (26) |
| Very | 185 (40) | 70 (32) | 100 (28) | 92 (33) | 447 (34) |
| Extremely | 105 (23) | 58 (26) | 92 (26) | 73 (26) | 328 (25) |
| Do not know | 8 (2) | 4 (2) | 10 (3) | 7 (2) | 29 (2) |
| W1 Belief of NRT harmfulness relative to cigarettes, n (%) | |||||
| Much Less | 163 (35) | 71 (32) | 137 (39) | 120 (43) | 491 (37) |
| Somewhat Less | 183 (40) | 86 (39) | 142 (40) | 99 (35) | 510 (39) |
| Equally | 57 (12) | 29 (13) | 25 (7) | 28 (10) | 139 (11) |
| More | 13 (3) | 6 (3) | 10 (3) | 7 (2) | 36 (3) |
| Do not know | 45 (10) | 27 (12) | 39 (11) | 28 (10) | 139 (11) |
| W1 Belief of NVP harmfulness relative to cigarettes, n (%) | |||||
| Much Less | 91 (20) | 38 (17) | 91 (26) | 52 (18) | 272 (21) |
| Somewhat Less | 188 (41) | 81 (37) | 145 (41) | 114 (40) | 528 (40) |
| Equally | 91 (20) | 50 (23) | 59 (17) | 45 (16) | 245 (19) |
| More | 20 (4) | 10 (5) | 18 (5) | 7 (2) | 55 (4) |
| Do not know | 71 (15) | 40 (18) | 40 (11) | 64 (23) | 215 (16) |
| W2 Nicotine product used as quit aids at LQA, n (%) | |||||
| No aid or other aids | 177 (38) | 115 (53) | 121 (34) | 140 (50) | 553 (42) |
| NRT only a | 103 (22) | 36 (16) | 46 (13) | 74 (26) | 259 (20) |
| NVP only b | 102 (22) | 45 (21) | 147 (41) | 42 (15) | 336 (26) |
| Both NRT and NVPc | 79 (17) | 22 (10) | 40 (11) | 26 (9) | 167 (12) |
| W2 Quit aids used at LQA, n (%) | |||||
| No aids | 118 (26) | 82 (37) | 91 (26) | 86 (31) | 377 (29) |
| Any nicotine aids^ | 286 (62) | 105 (48) | 233 (66) | 42 (50) | 766 (58) |
| Only non-nicotine aids | 57 (12) | 32 (15) | 29 (8) | 54 (19) | 172 (13) |
NB. Percentages and means are unweighted;
Among those who have made a quit attempt by Wave 2 (2018);
NRT = nicotine replacement therapy; NVP = nicotine vaping product; W1 = Wave 1; W2 = Wave 2; LQA = last quit attempt;
NRT only defined as any use of NRT either exclusively or in combination with other aids but exclude NVP for LQA.
NVP only defined as any use of NVP either exclusively or in combination with other aids but exclude NRT for LQA.
Both NRT and NVP defined as use of both products for LQA either exclusively or in combination with other aids.
Include any use of heated tobacco products [HTP] and smokeless tobacco (asked only in Canada and the US).
Association of NRT and NVP Harm Perceptions Relative to Cigarettes with Own-product Use as a Quit Aid during LQA
Results from the multinomial logistic regression are presented in Table 2 (see Supplementary Table S1 for bivariate associations and Supplementary Table S2 for full details). After adjusting for covariates, smokers were more likely to use NRT as a quit aid for their LQA relative to use of other aids or no aids if they perceive NRT is much less harmful (adjusted relative risk ratio [aRRR] = 3.79, 95% confidence interval [CI] = 2.16–6.66), or somewhat less harmful (aRRR = 1.98, 95% CI = 1.15–3.42) compared to equally/more harmful than cigarettes (ie, approximately four times and two times as likely to do so, respectively).
Table 2.
Prospective Association Between Wave 1 Nicotine Product Harm Perceptions and Wave 2 Choice of Nicotine Product used as an Aid for Last Quit Attempts Among Baseline Daily Smokers Smoking 10+ Cigarettes per day who had made a Quit Attempt by Wave 2 (N = 1289^).
| Wave 1 Predictors | Wave 2 NRT use vs other/no aids a aRRR (95% CI) |
Wave 2 NVP use vs other/no aids b aRRR (95% CI) |
Wave 2 Both NRT & NVP use vs other/no aids c aRRR (95% CI) |
|---|---|---|---|
| NRT Relative Harm Perception | |||
| Much Less harmful | 3.79 (2.16, 6.66) *** | 1.51 (0.88, 2.61) | 1.96 (1.03, 3.73) * |
| Somewhat Less harmful | 1.98 (1.15, 3.42) * | 1.47 (0.89, 2.05) | 1.16 (0.62, 2.15) |
| Equal/More harmful | Reference | Reference | Reference |
| Do not know | 0.98 (0.45, 2.11) | 1.69 (0.85, 3.36) | 0.82 (0.29, 2.25) |
| NVP Relative Harm Perception | |||
| Much Less harmful | 0.34 (0.20, 0.60) *** | 2.11 (1.29, 3.45) ** | 1.29 (0.71, 2.35) |
| Somewhat Less harmful | 0.69 (0.46, 1.05) | 1.34 (0.88, 2.05) | 1.20 (0.72, 2.01) |
| Equal/More harmful | Reference | Reference | Reference |
| Do not know | 0.64 (0.38, 1.07) | 0.53 (0.29, 0.96) * | 0.32 (0.14, 0.75) ** |
Note: NRT = Nicotine Replacement Therapy, NVP = Nicotine Vaping Products;
aRRR = adjusted Relative Risk Ratio which estimates the likelihood of the outcome (e.g., NRT use vs other/no aids) for a variable, holding all other variables in the model constant; CI = Confidence Intervals;
Significant at p < .05
p < .01
p < .001.
Total N reduced due to the exclusion of the small number of Don’t Know responses on ethnicity and education from analysis.
Multinomial logistic regression model comparing between any use of NRT either exclusively or in combination with other aids but exclude NVP for LQA and no aid/other aids as the reference.
Multinomial logistic regression model comparing between any use of NVP either exclusively or in combination with other aids but exclude NRT for LQA and no aid/other aids as the reference.
Multinomial logistic regression model comparing between use of both NRT and NVP for LQA either exclusively or in combination with other aids and no aid/other aids as the reference.
All models adjusted for the other variable in the table, along with age, gender, income, education, ethnicity, country, knowledge of smoking harms and nicotine harm belief.
Smokers were 2.11 times as likely to use NVPs (vs. other/no aids) for quitting smoking during their LQA if they perceive that NVPs are much less harmful than cigarettes (aRRR = 2.11, 95% CI = 1.29–3.45) but were 0.53 times as likely to use NVPs (vs. other/no aids) if they did not know how harmful NVPs are relative to cigarettes (aRRR = 0.53, 95% CI = 0.29–0.96).
Association of NRT and NVP Harm Perceptions Relative to Cigarettes with the Other Product Use (ie, Cross-product Use) as a Quit Aid during LQA
Smokers were 0.34 times as likely to use NRT for LQA (vs. other/no aids) if they perceive that NVP is much less harmful than cigarettes (aRRR = 0.34, 95% CI = 0.20–0.60) compared to those who perceive NVP is equally/more harmful than cigarettes. However, NVP use was not predicted by perceptions about NRT harmfulness.
Country Differences in Predictive Associations
There was little statistical evidence for an interaction between country and relative harm perceptions of NRT or NVPs on use of NRT or NVPs during LQA (p = .24 and .21, respectively), with a similar relationship observed across all countries.
Additional Analyses
Table 3 (see bivariate associations in Supplementary Table S3 and full details in Supplemental Table S4) shows the results of additional analyses exploring whether and how nicotine product harm perceptions might predict use of any nicotine product(s) and sole use of nonnicotine products for LQA, with no aids as the comparator. Relative to use of no aids for LQA, smokers were 2.19 times as likely to use any nicotine product(s) during LQA if they perceived that NRT is much less harmful relative to cigarettes compared to perceiving that NRT is equally/more harmful relative to cigarettes (aRRR = 2.19, 95% CI = 1.39–3.47). Use of any nicotine product(s) during LQA compared to use of no aids was not associated with NVP relative harm perceptions except for the subgroup who did not know the relative harmfulness of NVPs being 0.54 times as likely to do so (aRRR = 0.54, 95% CI = 0.34–0.86). Neither NRT nor NVP relative harm perceptions were associated with the likelihood of using only nonnicotine aids (vs. no aids) during LQA.
Table 3.
Prospective Association of Wave 1 Nicotine Product Harm Perceptions with Wave 2 Use of Nicotine and Nonnicotine Aids for Last Quit Attempts Among Baseline Daily Smokers Smoking 10+ Cigarettes per day who had Made a Quit Attempt by Wave 2 (N = 1289^).
| Wave 1 Predictors | Wave 2 any nicotine aids# vs. no aids a aRRR (95% CI) |
Wave 2 nonnicotine aids only vs. no aidsb aRRR (95% CI) |
|---|---|---|
| NRT Relative Harm Perception | ||
| Much Less harmful | 2.22 (1.40, 3.52) *** | 0.93 (0.49, 1.73) |
| Somewhat Less harmful | 1.47 (0.95, 2.25) | 0.86 (0.48, 1.55) |
| Equal/More harmful | Reference | Reference |
| Do not know | 0.97 (0.55, 1.72) | 0.57 (0.25, 1.26) |
| NVP Relative Harm Perception | ||
| Much Less harmful | 1.12 (0.72, 1.76) | 1.32 (0.69, 2.54) |
| Somewhat Less harmful | 1.01 (0.70, 1.45) | 1.14 (0.69, 1.95) |
| Equal/More harmful | Reference | Reference |
| Do not know | 0.53 (0.33, 0.84) ** | 0.92 (0.48, 1.74) |
Note: NRT = Nicotine Replacement Therapy, NVP = Nicotine Vaping Products;
aRRR = adjusted Relative Risk Ratio, CI = Confidence Intervals;
Significant at p < .01
p < .001.
Total N reduced due to the exclusion of the small number of Don’t Know responses on ethnicity and education from analysis.
Include use of heated tobacco products [HTP] and smokeless tobacco (asked only in Canada and the US).
Model comparing no aid (reference) with any nicotine aids (ie, any use of NRT, NVP, HTP or smokeless tobacco, either alone or in combination with other aids for LQA).
Model comparing no aid (reference) with exclusively nonnicotine aids for LQA.
All models adjusted for the other variable in the table, along with age, gender, income, education, ethnicity, country, knowledge of smoking harms and nicotine harm belief;
Discussion
As hypothesized, the results indicated that daily smokers’ perceptions of harm of NRT and NVPs relative to cigarettes were predictive of subsequent use of that same product. Smokers who perceived NRT as either much less or somewhat less harmful than cigarettes were more likely to use NRT for their LQA but were no more or less likely to use NVPs. Those who perceived NVPs as much less harmful were more likely to use it for their LQA. Those who did not know how harmful NVPs are, were less likely to do so. Interestingly, perceptions of NVPs being much less harmful than cigarettes also predicted a lower likelihood of NRT use at their LQA. We also found that use of both products together during their LQA was more likely if smokers perceived NRT is much less harmful than cigarettes, but they were less likely to do so if they did not know the relative harmfulness of NVPs. No country differences were observed in these relationships.
Consistent with past studies,11,15,20 our findings confirm that harm perceptions influence nicotine product use, with accurate perceptions associated with greater likelihood of use as a smoking cessation aid, whereas inaccurate perceptions appear to deter their use for this purpose. The finding of a weaker association between harm perception and use for NVP than for NRT is noteworthy and may suggest that harm perceptions contribute less as a determinant of product use for NVPs than for NRT. This may be due to NVP novelty and less well-established efficacy and safety as a cessation product as compared to NRT. In this regard, our finding that believing NVPs are much less harmful than cigarettes is associated with less NRT use. This suggests NVPs may be the preferred option when perceived as much less harmful than cigarettes and hence, they are considered acceptable substitutes for NRT as a smoking cessation aid.39
Our finding of a predictive relationship between NVP/NRT harm perceptions and their use has important implications as it suggests that use of these products for smoking cessation could be undermined by misperceptions of their harmfulness. Past research suggests that potential sources of harm misperceptions of nicotine-containing products include inaccurate beliefs about the links between nicotine and cancer,13 exposure to misinformation from social media, government websites and tobacco industry,25,40 and interpreting uncertainty about the long-term health effects of product use as indicator of significant unknown risks.41 Given that NRT and NVPs are the two most popular smoking cessation aids,5 and that there was still a substantial number of smokers who remain either misinformed or unaware of the relative harmfulness of NRT and NVPs compared to cigarettes, education and accurate messaging around the harms of these nicotine products is needed. An accurate understanding of NRT/NVP harms would ensure that smokers are able to make informed choices about whether these products are appropriate for them to use as quit aids.
The finding of cross-product influences of NVP harm perceptions on the use of NRT as an aid for smoking cessation is novel and suggests that harm perceptions of one nicotine product may influence use of another. Our study suggests that an accurate understanding of NRT harmfulness relative to cigarettes may promote not only NRT use but also other nicotine products. An accurate understanding of NVPs, on the other hand, appears to promote mainly its own use for smoking cessation. This suggestion is consistent with an accurate NRT harm perception being associated with use of any forms of nicotine aids (vs. no aids) but not that of NVP, thus providing further support for the generality of NRT harm perception effect as opposed to the more product-specific effect of NVP harm perception. Taken together, these findings suggest that the influence of NRT harm perceptions on choice of nicotine products for smoking cessation may be much less discriminating compared to that of NVPs. This could be because NVPs are perceived to possess other qualities that make them a more attractive cessation aid than NRT, such as providing better nicotine delivery and behavioral substitutability than NRT.42 Nevertheless, our findings require replication to confirm using other samples and across a broader range of nicotine products (eg, heated tobacco products).
The level of misperceptions of nicotine product harmfulness varied across our studied countries. We showed that smokers in England were the least, while those in the United States were the most, misinformed about the harmfulness of NRT and NVPs relative to cigarettes. This is consistent with prior research.27 There was little evidence of any by-country interactions for our main findings, suggesting the effect is likely related to beliefs, not to aspects of the country context although low statistical power to detect an interaction effect means this finding should be interpreted with caution. Given past efforts to educate smokers about NRT safety, the intransigence of a proportion of the smoking population to continue believing NRT is more harmful than the evidence calls for more efforts to address this issue. It is similarly important to correct any misperceptions of the harmfulness of NVPs relative to cigarettes so that smokers are not dissuaded from switching to a lower risk nicotine product, either as a short-term cessation aid17 or a longer-term substitute where warranted, such as for highly dependent smokers.
Past research has indicated that corrective health education can help improve smokers’ knowledge about nicotine health effects but there are challenges as well.43 Recent research indicates the need for absolute and comparative risk communication around nicotine and nicotine-containing products to be well-designed, evidence-based and preferably tailored to a specific target audience (eg, smokers) to avoid unintended consequences such as uptake among non-smokers.44–46 Pack inserts may be an efficient and effective way to educate smokers about the health harms of nicotine-containing products such as NRT.47 Offering a trial of NRT to all smokers including those who are reluctant to use NRT may help dispel any safety concerns they may have about NRT. This is best done by health professionals as they are in an ideal position to provide education, given that smokers are much more likely to visit a health professional than the general nonsmoking population.48 An advantage of providing education through pack inserts and health professionals is that these channels avoid exposure among nonsmokers, thus minimizing any unintended consequences. In many countries, NRT is the medically approved therapy for smoking cessation and is included in clinical practice guidelines.6,7 In countries like Australia, NVPs are cautiously recommended in clinical practice guidelines as a second line treatment for smokers to help them quit smoking.6 From October 1, 2021, Australian smokers can only access nicotine liquid/pods with a doctor’s prescription which can be dispensed at a local or online pharmacy.49 However, this option was not available at the time of the survey. While this new access arrangement could increase the opportunities for doctors and pharmacists to educate smokers about the use of NVPs for quitting smoking, including their relative harmfulness, it is currently unclear what proportion of Australian prescribers and dispensers will support the use of NVPs for smoking cessation.
Study Strengths and Limitations
Study strengths include prospective cohort design over 2 years and cross-country comparisons. Nevertheless, several study limitations warrant some discussion. First, our survey did not assess perception of harms of NRT against NVPs. It remains unclear how any perceived differences in comparative harms of the two products might affect their use for the purpose of smoking cessation. Second, findings may be biased by the reliance on self-report data which may underestimate brief unassisted quit attempts as they are less memorable and tend to be forgotten. Third, it is unclear how participants interpreted the survey question because some may have considered addictiveness to be a harm, or they may have considered short-term side-effects of product use. Fourth, our findings predate e-cigarette or vaping use associated lung injury (EVALI), which has been shown to influence NVP harm perceptions and NVP use,50 and thus may not generalize beyond the study period. Relatedly, our findings may also not generalize to populations outside of the four countries studied, or to smokers who had not recently attempted to quit. Finally, although the associations were longitudinal, this of itself does not demonstrate causality given there might be other potential confounders not controlled for in our analysis, thus our causal interpretations should be treated as possibilities, not demonstrated realities.
Conclusions
This study found that smokers’ perceptions of the harmfulness of NRT and NVPs relative to smoking predicted their respective use as an aid for smoking cessation. Accurate perceptions of NRT may further promote use of any nicotine product for smoking cessation, but accurate perceptions of NVPs may deter use of NRT. Given misperceptions of these two most popular quit aids remain unacceptably high, provision of corrective education to smokers is clearly required.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Hua-Hie Yong, Department of Psychology, Deakin University, Geelong, VIC, Australia.
Shannon Gravely, Department of Psychology, University of Waterloo, Waterloo, ON, Canada.
Ron Borland, Melbourne Centre for Behaviour Change, School of Psychological Sciences, The University of Melbourne, Melbourne, VIC, Australia.
Coral Gartner, School of Public Health, The University of Queensland, Brisbane, QLD, Australia.
K Michael Cummings, Department of Psychiatry & Behavioral Sciences, Medical University of South Carolina, Charleston, SC, USA.
Katherine East, Department of Psychology, University of Waterloo, Waterloo, ON, Canada; National Addiction Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Scott Tagliaferri, Department of Psychology, Deakin University, Geelong, VIC, Australia.
Tara Elton-Marshall, School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON, Canada.
Andrew Hyland, Department of Health Behavior, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Maansi Bansal-Travers, Department of Health Behavior, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Geoffrey T Fong, Department of Psychology, University of Waterloo, Waterloo, ON, Canada; Ontario Institute for Cancer Research, MaRS Centre, Toronto, ON, Canada.
Funding
This study was supported by grants from the National Cancer Institute of the US (P01CA200512), the Canadian Institutes of Health Research (FDN-148477), and by the National Health and Medical Research Council of Australia (GNT1106451). Additional support to GTF was provided by a Senior Investigator Award from the Ontario Institute for Cancer Research and the Canadian Cancer Society O. Harold Warwick Prize and AH was supported by a Tobacco Centers of Regulatory Science US National Cancer Institute grant (U54CA238110). CG receives support from the National Health and Medical Research Council of Australia (GNT1198301). KE is the recipient of Fellowship funding from the UK Society for the Study of Addiction (SSA). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit for publication.
Declaration of Interests
KMC has served on advisory committees for Pfizer, Inc. to assist them in ways to promote access to smoking cessation treatments. He has also served as a paid expert witness in litigation filed against the tobacco industry. GTF has served as an expert witness or consultant for governments defending their country’s policies or regulations in litigation. GTF and SG served as paid expert consultants to the Ministry of Health of Singapore in reviewing the evidence on plain/standardized packaging. None of the other authors has any conflict of interest to declare.
Ethics Approval
The survey protocols and all materials, including the survey questionnaires, were approved by the Research Ethics committee at the University of Waterloo, Canada (ORE#20803/30570, ORE#21609/30878), King’s College London, UK (RESCM-17/18-2240), Cancer Council Victoria, Australia (HREC1603), University of Queensland, Australia (20160000330/HREC1603), Deakin University, Australia (DUHREC2018-346) and Medical University of South Carolina, US (waived due to minimal risk).
Data Availability
The data are jointly owned by a third party in each country that collaborates with the International Tobacco Control Policy Evaluation (ITC) Project. Data from the ITC Project are available to approved researchers 2 years after the date of issuance of cleaned data sets by the ITC Data Management Centre. Researchers interested in using ITC data are required to apply for approval by submitting an International Tobacco Control Data Repository (ITCDR) request application and subsequently to sign an ITCDR Data Usage Agreement. To avoid any real, potential, or perceived conflict of interest between researchers using ITC data and tobacco-related entities, no ITCDR data will be provided directly or indirectly to any researcher, institution, or consultant that is in current receipt of any grant monies or in-kind contribution from any tobacco manufacturer, distributor, or other tobacco-related entity. The criteria for data usage approval and the contents of the Data Usage Agreement are described online (http://www.itcproject.org). The authors of this paper obtained the data following this procedure. This is to confirm that others would be able to access these data in the same manner as the authors. The authors did not have any special access privileges that others would not have.
References
- 1. Abrams DB, Glasser AM, Pearson JL, et al. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu Rev Public Health. 2018;39:193–213. doi: 10.1146/annurev-publhealth-040617-013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Czoli CD, Fong GT, Mays D, Hammond D.. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49–e58. [DOI] [PubMed] [Google Scholar]
- 3. National Academies of Sciences Engineering and Medicine. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press; 2018. http://nationalacademies.org/hmd/Reports/2018/public-health-consequences-of-e-cigarettes.aspx [PubMed] [Google Scholar]
- 4. Royal College of Physicians. Nicotine without smoke: Tobacco harm reduction. London: RCP; 2016. [Google Scholar]
- 5. Gravely S, Cummings KM, Hammond D, et al. Self-reported quit aids and assistance used by smokers at their most recent quit attempt: Findings from the 2020 International Tobacco Control Four Country Smoking and Vaping Survey. Nicotine Tob Res. 2021;23(10):1699–1707. doi: 10.1093/ntr/ntab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Royal Australian College of General Practitioners. Supporting smoking cessation: a guide for health professionals. 2019. https://www.racgp.org.au/getattachment/00185c4e-441b-45a6-88d1-8f05c71843cd/Supporting-smoking-cessation-A-guide-for-health-professionals.aspx. Accessed February 18, 2022.
- 7. US DHHS. Smoking cessation a report of the Surgeon General. 2020. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf. Accessed February 18, 2022.
- 8. Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T.. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5:CD000146. doi: 10.1002/14651858.CD000146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stanley TD. Yes, nicotine replacement therapy’s effectiveness is much lower than often reported. J Clin Epidemiol. 2017;81:144–145. doi: 10.1016/j.jclinepi.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 10. Beard E, Bruguera C, McNeill A, Brown J, West R.. Association of amount and duration of NRT use in smokers with cigarette consumption and motivation to stop smoking: A national survey of smokers in England. Addict Behav 2015;40:33–38. doi: 10.1016/j.addbeh.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 11. Bansal MA, Cummings KM, Hyland A, Giovino GA.. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6(Suppl 3):S303–S310. [DOI] [PubMed] [Google Scholar]
- 12. Villanti AC, Naud S, West JC, et al. Prevalence and correlates of nicotine and nicotine product perceptions in U.S. young adults, 2016. Addict Behav 2019;98:106020. doi: 10.1016/j.addbeh.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson S, Partos T, McNeill A, Brose LS.. Harm perceptions of e-cigarettes and other nicotine products in a UK sample. Addiction 2019;114(5):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carpenter MJ, Ford ME, Cartmell K, Alberg AJ.. Misperceptions of nicotine replacement therapy within racially and ethnically diverse smokers. J Natl Med Assoc. 2011;103(9-10):885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiffman S, Ferguson SG, Rohay J, Gitchell JG.. Perceived safety and efficacy of nicotine replacement therapies among US smokers and ex-smokers: Relationship with use and compliance. Research Support, Non-U.S. Gov’t. Addiction 2008;103(8):1371–1378. [DOI] [PubMed] [Google Scholar]
- 16. Kasza KA, Hyland AJ, Borland R, et al. Effectiveness of stop-smoking medications: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction 2013;108(1):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yong HH, Borland R, Cummings KM, et al. Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: Findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction 2019;114(Suppl 1):35–48. doi: 10.1111/add.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartmann-Boyce J, McRobbie H, Butler AR, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2021;9:CD010216. doi: 10.1002/14651858.CD010216.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, Feng B, Weaver SR, et al. Changing perceptions of harm of e-cigarette vs cigarette use among adults in 2 US National Surveys from 2012 to 2017. JAMA Netw Open 2019;2(3):e191047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elton-Marshall T, Driezen P, Fong GT, et al. Adult perceptions of the relative harm of tobacco products and subsequent tobacco product use: longitudinal findings from waves 1 and 2 of the population assessment of tobacco and health (PATH) study. Addict Behav 2020;106:106337. doi: 10.1016/j.addbeh.2020.106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yong HH, Borland R, Balmford J, et al. Trends in e-cigarette awareness, trial, and use under the different regulatory environments of Australia and the United Kingdom. Nicotine Tob Res. 2015;17(10):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgan JC, Cappella JN.. Harm perceptions and beliefs about potential modified risk tobacco products. Int J Environ Res Public Health. 2021;18(2):576. doi: 10.3390/ijerph18020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denlinger-Apte RL, Pacek LR, Ross JC, et al. Risk perceptions of low nicotine cigarettes and alternative nicotine products across priority smoking populations. Int J Environ Res Public Health. 2021;18(10):5311. doi: 10.3390/ijerph18105311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Villanti AC, Naud S, West JC, et al. Latent classes of nicotine beliefs correlate with perceived susceptibility and severity of nicotine and tobacco products in US young adults. Nicotine Tob Res. 2019;21(Suppl 1):S91–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yong HH, Karmakar C, Motin MA, et al. Identifying factors that conjointly influence nicotine vaping product relative harm perception among smokers and recent ex-smokers: findings from the 2016 ITC Four Country Smoking and Vaping Survey. Drug Alcohol Depend. 2021;218:108370. doi: 10.1016/j.drugalcdep.2020.108370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yong HH, Borland R, Balmford J, et al. Prevalence and correlates of the belief that electronic cigarettes are a lot less harmful than conventional cigarettes under the different regulatory environments of Australia and the United Kingdom. Nicotine Tob Res. 2017;19(2):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borland R, Cooper J, McNeill A, O’Connor R, Cummings KM.. Trends in beliefs about the harmfulness and use of stop-smoking medications and smokeless tobacco products among cigarettes smokers: findings from the ITC four-country survey. Harm Reduct J 2011;8:21. doi: 10.1186/1477-7517-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennedy RD, Awopegba A, De Leon E, Cohen JE.. Global approaches to regulating electronic cigarettes. Tob Control. 2017;26(4):440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. UK National Health Service. Stop smoking treatments, https://www.nhs.uk/conditions/stop-smoking-treatments/. Accessed 18 February, 2022.
- 30. Shahab L, Cummings KM, Hammond D, et al. The impact of changing nicotine replacement therapy licensing laws in the United Kingdom: findings from the International Tobacco Control Four Country Survey. Addiction 2009;104(8):1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Douglas H, Hall W, Gartner C.. E-cigarettes and the law in Australia. Aust Fam Physician. 2015;44(6):415–418. [PubMed] [Google Scholar]
- 32. McNeill A, Brose L, Calder R, Bauld L, Robson D.. Vaping in England: an evidence update February 2019. A report commissioned by Public Health England. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/781748/Vaping_in_England_an_evidence_update_February_2019.pdf/. Accessed February 18, 2022.
- 33. Braak DC, Cummings KM, Nahhas GJ, et al. Where do vapers buy their vaping supplies? Findings from the International Tobacco Control (ITC) 4 Country Smoking and Vaping Survey. Int J Environ Res Public Health. 2019;16(3):338. doi: 10.3390/ijerph16030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fong GT, Cummings KM, Borland R, et al. The conceptual framework of the International Tobacco Control (ITC) Policy Evaluation Project. Tob Control. 2006;15(Suppl. 3):iii3–11. doi: 10.1136/tc.2005.015438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thompson ME, Fong GT, Boudreau C, et al. Methods of the ITC Four Country Smoking and Vaping Survey, Wave 1 (2016). Addiction 2019;114(Suppl 1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ITC Project. ITC Four Country Smoking and Vaping Survey Wave 1 (2016) Technical Report. 2018. https://itcproject.s3.amazonaws.com/uploads/documents/4CV1_Technical_Report_Nov23201.pdf. Accessed February 18, 2022.
- 37. ITC Project. ITC Four Country Smoking and Vaping Survey, Wave 2 (2018) Technical Report. 2018. https://itcproject.org/methods/technical-reports/itc-four-country-smoking-and-vaping-survey-wave-2-4cv2-technical-report/. Accessed February 18, 2022.
- 38. Thrasher JF, Quah ACK, Dominick G, et al. Using cognitive interviewing and bBehavioral coding to determine measurement equivalence across linguistic and cultural groups: an example from the International Tobacco Control Policy Evaluation Project. Field Method. 2011;23(4):439–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beard E, West R, Michie S, Brown J.. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. BMJ 2016;354:i4645. doi: 10.1136/bmj.i4645. [DOI] [PubMed] [Google Scholar]
- 40. Tan ASL, Lee CJ, Nagler RH, Bigman CA.. To vape or not to vape? Effects of exposure to conflicting news headlines on beliefs about harms and benefits of electronic cigarette use: results from a randomized controlled experiment. Prev Med. 2017;105:97–103. doi: 10.1016/j.ypmed.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Owusu D, Lawley R, Yang B, et al. “The lesser devil you don’t know”: a qualitative study of smokers’ responses to messages communicating comparative risk of electronic and combusted cigarettes. Tob Control. 2020;29(2):217–223. doi: 10.1136/tobaccocontrol-2018-054883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Brien B, Knight-West O, Walker N, Parag V, Bullen C.. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis 2015;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Erku DA, Bauld L, Dawkins L, et al. Does the content and source credibility of health and risk messages related to nicotine vaping products have an impact on harm perception and behavioural intentions? A systematic review. Addiction 2021;116(12):3290–3303. doi: 10.1111/add.15473. [DOI] [PubMed] [Google Scholar]
- 44. Duong HT, Loud EE, Thrasher JF, et al. “It brings light to what you really put into your body”: A focus group study of reactions to messages about nicotine reduction in cigarettes. Tob Control. 2021. doi: 10.1136/tobaccocontrol-2020-056312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Byron MJ, Hall MG, King JL, Ribisl KM, Brewer NT.. Reducing nicotine without misleading the public: descriptions of cigarette nicotine level and accuracy of perceptions about nicotine content, addictiveness, and risk. Nicotine Tob Res. 2019;21(Suppl 1):S101–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang B, Popova L.. Communicating risk differences between electronic and combusted cigarettes: the role of the FDA-mandated addiction warning and a nicotine fact sheet. Tob Control. 2020;29(6):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bansal-Travers M, Cummings KM, Hyland A, Brown A, Celestino P.. Educating smokers about their cigarettes and nicotine medications. Health Educ Res. 2010;25(4):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Australian Institute of Health and Welfare. Australia’s health 2016. Canberra, Australia: AIHW. http://www.aihw.gov.au/publication-detail/?id=60129555544 [Google Scholar]
- 49. Australian Government Department of Health. Nicotine vaping products. https://www.tga.gov.au/nicotine-vaping-products. Accessed 1 October 2021.
- 50. Tattan-Birch H, Brown J, Shahab L, Jackson SE.. Association of the US outbreak of vaping-associated lung injury with perceived harm of e-cigarettes compared with cigarettes. JAMA Netw Open. 2020;3(6):e206981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are jointly owned by a third party in each country that collaborates with the International Tobacco Control Policy Evaluation (ITC) Project. Data from the ITC Project are available to approved researchers 2 years after the date of issuance of cleaned data sets by the ITC Data Management Centre. Researchers interested in using ITC data are required to apply for approval by submitting an International Tobacco Control Data Repository (ITCDR) request application and subsequently to sign an ITCDR Data Usage Agreement. To avoid any real, potential, or perceived conflict of interest between researchers using ITC data and tobacco-related entities, no ITCDR data will be provided directly or indirectly to any researcher, institution, or consultant that is in current receipt of any grant monies or in-kind contribution from any tobacco manufacturer, distributor, or other tobacco-related entity. The criteria for data usage approval and the contents of the Data Usage Agreement are described online (http://www.itcproject.org). The authors of this paper obtained the data following this procedure. This is to confirm that others would be able to access these data in the same manner as the authors. The authors did not have any special access privileges that others would not have.