Abstract
Background
High cigarette smoking prevalence and low quit rates in people with serious mental illness (SMI) contribute to disparate rates of chronic disease and premature death. This prospective trial tested the impact of switching to a potentially lower-harm nicotine-containing product on smoking in this population.
Aims and Methods
A total of 240 cigarette smokers with SMI who tried but were currently unwilling to quit were randomly assigned to receive disposable e-cigarettes for 8 weeks or not, with assessments at baseline, 2, 4, 6, 8, 13, and 26 weeks. Generalized linear mixed models examined the effects of e-cigarette provision on e-cigarette appeal, cigarettes per day (CPD), breath carbon monoxide (CO), nicotine dependence, and side effects. Clinical Trial registration: NCT03050853.
Results
Self-reported smoking was similar between groups at baseline (mean = 18.7 CPD). By week 2, 79% of the e-cigarette group were using e-cigarettes daily. During weeks 2–8, CPD and CO decreased in the e-cigarette versus assessment-only group (eg, 7.5 CPD [95% CI = 5.9, 9.2] vs. 18.1 CPD [CI = 16.4, 19.8] and 16.4 ppm [CI = 13.4, 19.5] vs. 25.4 ppm [CI = 22.4, 28.9], respectively, at week 2). Additionally, 19%–22% in the e-cigarette group reported smoking no cigarettes in weeks 2–8 compared to 0% in the assessment-only group. By 13 and 26 weeks, group differences in CPD, but not CO, remained significant. Nicotine dependence did not increase and side effects were minor.
Conclusions
Providing e-cigarettes for 8 weeks to smokers with SMI resulted in substantial reductions in CPD and CO. Enhancing and maintaining switching from cigarettes to e-cigarettes warrant further study.
Implications
This was the first prospective study to compare e-cigarette provision with assessments only to evaluate the appeal and impact of e-cigarettes on smoking behavior, carbon monoxide exposure, and nicotine dependence among smokers with SMI who had tried but were unable to quit and were not currently interested in cessation treatment. The finding that e-cigarette provision led to significant reductions in smoking and carbon monoxide without increasing nicotine dependence has implications for reducing harm not only among the millions of smokers with SMI who struggle to quit, but also for other vulnerable smokers who cannot achieve cessation.
Introduction
The rate of smoking among people with serious mental illness (SMI; disabling schizophrenia and bipolar disorder) is almost triple,1–3 and is declining at a slower rate,4 compared to the general population. Several factors contribute to high rates of smoking initiation and low rates of quitting (eg, dysfunctional nicotinic receptor sites,5,6 beliefs about the perceived benefits of smoking, and concerns about cessation held by smokers, families, and clinicians).7–10 Smokers with SMI thus have higher levels of nicotine dependence and tobacco-related carcinogens in their bodies compared to the general population,11 contributing to higher prevalence of chronic diseases and reduced life expectancy.12,13 Twenty years of research evaluating evidence-based cessation strategies demonstrates that, while treatment improves outcomes, people with SMI have great difficulty quitting and sustaining abstinence,14–17 warranting novel harm reduction strategies for this vulnerable group.
Although health effects of long-term use are unknown, e-cigarette aerosol contains dramatically lower levels of toxicants and carcinogens compared to combustible cigarettes.18–20 Several recent studies and reviews of the literature have concluded that e-cigarettes are substantially less harmful to smokers than combustible cigarettes.21–23 This justifies examination of e-cigarette switching among smokers who are unable to quit and otherwise continue exposing themselves to the known, dangerous effects of cigarettes.
Because e-cigarettes initially entering the U.S. market fell short with regard to nicotine delivery compared to combustibles they initially appeared to be poor substitutes for highly addicted smokers.24 However, designs subsequently evolved to deliver nicotine more quickly to the brain.25 Moreover, disposable “cigalikes,” like the one provided in this study, have the look and feel of a cigarette, replicate the hand-to-mouth smoking behavior that smokers are accustomed to, and are simple to use; thus e-cigarettes have become more appealing and popular.
Cross-sectional surveys have shown that smokers with mental illness are more likely than smokers without mental illness to try e-cigarettes, to use them regularly, and to indicate a willingness to use them in the future.26–28 Like other smokers, people with mental illness have chiefly used e-cigarettes to quit or cut down on cigarette smoking27,28 and perceive e-cigarettes as less harmful than cigarettes.29 Three pre–post, prospective, nonrandomized pilot studies among chronic smokers with SMI demonstrated that simply providing e-cigarettes was associated with significantly reduced use of cigarettes (up to 65%) and breath CO level,30–32 with 10%–14.3% of participants switching fully to e-cigarettes.30,31 However, we are unaware of any randomized controlled trials.
The aim of this randomized trial was to assess the effect of e-cigarette provision compared to assessment only on e-cigarette use, combustible cigarette consumption, breath carbon monoxide (CO), and nicotine dependence among chronic smokers with SMI. Based on our previous work,30 we hypothesized that e-cigarette provision would result in high subjective ratings of satisfaction and daily use of e-cigarettes among at least 50% of participants during the 8-week provision period coupled with reductions in daily cigarette use and exhaled CO (ClinicalTrials.gov number, NCT03050853). We also hypothesized that e-cigarettes would not increase nicotine dependence and would be associated with only minor side effects.
Methods
Study Sites
Participants were recruited from two urban mental health agencies (Kentucky and Massachusetts) serving primarily Medicaid beneficiaries with SMI. We conducted the trial in an Eastern and a Southern state with significant geographically based difference in prevalence of smoking (30.2% vs. 16.3%, respectively)33 to increase study generalizability. We received IRB approval for the study from the Committee for the Protection of Human Subjects at Dartmouth College and the Central Office Research Review Committee of the Massachusetts Department of Mental Health. Trained research staff implemented the study protocol, including, at each site, a Research Interviewer blinded to group assignment and an unblinded Study Coordinator.
Participant Flow
Of the 959 individuals screened, 436 did not meet inclusion criteria, 133 could not be contacted, 106 declined to participate, and 44 consented but were not randomized (mostly screen failures), leaving 240 randomized participants. Among those participants, 210 (87.5%) were assessed at 8 weeks, and 214 (89.2%) were assessed at 26 weeks. All follow-up assessments concluded in January 2020.
Participants
Participants were adults aged 18 years or older with a DSM-V Axis I diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder, enrolled in services for at least 3 months, who met criteria for SMI (at least moderate impairment in multiple areas of psychosocial functioning), regular smoking for at least 5 years, currently smoking on average 10 cigarettes per day (CPD), breath CO ≥ 10 ppm, at least one quit attempt in the past 5 years using evidence-based treatment, and not currently engaged in a quit attempt by self-report. We excluded individuals with psychiatric instability (hospitalization in the past month), active substance use disorder, current e-cigarette use (more than four times in the past month), current pregnancy, or plans to become pregnant.
Procedures
Recruitment/Randomization
Participant recruitment began on March 9, 2017 via clinician referrals, posters/brochures, and mailings. After eligibility confirmation, potential participants were invited for an informational meeting, and interested individuals returned to review the consent form and provide written informed consent. After consent and baseline assessment, the unblinded Study Coordinator randomly assigned participants within site using an automated program that stratified by diagnosis (schizophrenia vs. bipolar disorder) and amount of daily smoking (>20 vs. ≤20 cigarettes), in blocks of four to assure balance between arms (1:1 ratio).
E-Cigarette Provision
The Study Coordinator provided participants with a 2-week supply of e-cigarettes and instructions on their safe use. Per product packaging, each disposable e-cigarette provided up to 300 puffs, roughly the equivalent of 20 cigarettes. Participants were given the opportunity to practice using the e-cigarette before leaving the appointment to ensure proper use. The Study Coordinator also provided brief information on safety (eg, keeping e-cigarettes out of the reach of children) and asked participants to try to replace all cigarettes with e-cigarettes but provided no further instructions or coaching. They gave participants additional 2-week supplies at 2, 4, and 6 weeks.
The disposable NJOY Daily e-cigarette was chosen for use in the study for several reasons. We used disposable e-cigarettes because we had previously found that, due to cognitive impairment, many people with SMI had difficulty with products that required charging and replacing cartridges.30 Also, this e-cigarette was the only one with publicly available safety data at the time. Additionally, the manufacturer partnered with the National Institute on Drug Abuse to develop an e-cigarette for use in research and had no ties to cigarette production. Finally, the product could be purchased at retail stores by participants after the study.
Assessment-Only Condition
Following randomization, Study Coordinators provided participants with appointments for follow-up study visits, asked them to refrain from using e-cigarettes, and reminded participants that they would receive a 4-week supply of e-cigarettes at the final follow-up visit.
Measures
Research staff obtained physician assessed DSM-5 mental illness diagnoses from the study site records and assessed demographics (listed in Table 1) and behavioral outcomes within a structured interview.
Table 1.
Baseline Demographics and Characteristics of 240 Study Participants
| Characteristics | No. (%) of participants | χ2 | p-value | ||
|---|---|---|---|---|---|
| Total (N = 240) | E-cigarette (n = 120) | Assessment (n = 120) | |||
| High school graduate or GED | 174 (72.5) | 91 (52.3) | 83 (47.7) | 1.34 | .25 |
| Male gender | 125 (52.1) | 60 (50.0) | 65 (54.2) | 0.42 | .52 |
| Racea | 3.63 | .60 | |||
| White | 130 (54.2) | 65 (54.2) | 65 (54.2) | ||
| Black | 79 (32.9) | 40 (33.3) | 39 (32.5) | ||
| Mixed and other | 30 (12.5) | 15 (11.7) | 16 (13.3) | ||
| Hispanic ethnicity | 24 (10.0) | 12 (10.0) | 12 (10.0) | 0.00 | 1.00 |
| Ever married | 95 (39.6) | 51 (42.5) | 44 (36.7) | 0.85 | .36 |
| Number of cigarettes | 0.02 | .69 | |||
| Less than 19 per day | 139 (57.9) | 71 (59.2) | 68 (56.7) | ||
| 20 or more per day | 101 (42.1) | 52 (43.3) | 49 (40.8) | ||
| Diagnosis | 0.02 | .90 | |||
| Schizophrenia spectrum | 113 (47.1) | 57 (47.5) | 56 (46.7) | ||
| Bipolar disorder | 127 (52.9) | 63 (52.5) | 64 (53.3) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | T test | p-value | |
| Age | 45.9 ± 11.9 | 46.3 ± 11.4 | 45.5 ± 12.5 | 0.60 | .60 |
| Carbon monoxide (ppm) | 26.9 ± 19.9 | 25.8 ± 17.8 | 27.9 ± 21.9 | 0.83 | .41 |
| FTCD score | 6.8 ± 1.5 | 6.7 ± 1.6 | 7.0 ± 1.5 | 1.30 | .20 |
| Brief Psychiatric Rating Scale score | 47.8 ± 14.2 | 47.1 ± 14.5 | 48.6 ± 13.8 | 0.80 | .60 |
| Age at first hospitalization | 21.6 ± 19.7 | 20.9 ± 10.0 | 22.3 ± 9.3 | 1.14 | .26 |
FTCD, Fagerström Test for Cigarette Dependence; GED, General Education Development.
One participant in the e-cigarette group refused to report racial category.
Cigarette and E-Cigarette Use
The unblinded Study Coordinators used the Timeline Follow-Back (TLFB) method,34 a structured interview that employs a calendar and participant-specific memory anchors to obtain self-reported substance use, to assess daily cigarettes smoked and vape sessions since the prior assessment. We also asked participants about use of other combusted tobacco and non-tobacco products, including cannabis. This method is reliable and valid to document substance use in the general population35 and among people with SMI.35,36 We also asked participants at all post-baseline visits (yes/no) whether they used any non-study e-cigarettes. Daily use of e-cigarettes was defined as using the product at least 6 days per week. At the time of the study, there were no validated methods for quantifying e-cigarette use. Thus, we relied on both vape sessions from the TLFB and Study Coordinators also instructed participants to bring all used and unused e-cigarettes to each study visit to assess use. Sample size was determined based on the hypothesis that mean daily use in the first 8 weeks of the study would be greater than 50% against a null percentage of 43.6% or less.
Breath CO
Breath CO was measured by the blinded Research Interviewers at each visit using the Smokerlyzer Breath Carbon Monoxide Monitor (Bedfont Scientific)37 as a biologic measure of toxin exposure.
Psychological Appeal of E-Cigarettes
Because no validated scales existed that would permit evaluation of the aspects of satisfaction with e-cigarettes we included in our funded study plan (ie, enjoyment from vaping, enjoyment compared to cigarettes, ease of use, and willingness to purchase e-cigarettes), at each post-baseline visit, the unblinded Study Coordinators used the four-item index of psychological appeal we developed in our pilot study.30 Items were rated on a 5-point Likert scale from 1 (not at all satisfied) to 5 (very satisfied). At the 8-week assessment, the Coordinators also used open-ended questions to obtain qualitative feedback (positive and negative) on e-cigarette use. We used the “funnel structure” method of interviewing,38 with broad questions at the beginning, followed by more specific probes related to the objectives of the inquiry.
Self-Reported Nicotine Dependence
Self-reported nicotine dependence was assessed by the blinded Research Interviewers at each visit with the Fagerström Test for Cigarette Dependence (FTCD),39 which has been shown to have reasonable internal consistency and test–retest reliability among smokers with SMI.40 Participants were instructed to consider all tobacco products, including combustible tobacco and e-cigarettes, when responding to this measure.
Side Effects/Adverse Events
The blinded Research Interviewers used a checklist to assess nicotine-related side effects (nausea, dizziness, vomiting, palpitations) and smoking- or vaping-related side effects (cough, throat irritation, bad taste in the mouth) at each visit. They also collected self-reported serious adverse events (hospitalizations and emergency room visits).
Statistical Analyses
Descriptive Analyses
Baseline characteristics in each group were compared using t tests for continuous variables and chi-squared tests for categorical variables. Qualitative responses to open-ended questions regarding appeal of e-cigarettes were coded, categorized for their content, and collated. We used descriptive statistics to assess use of e-cigarettes, adverse effects, and impressions based on qualitative responses.
Longitudinal Analysis of E-cigarette Use and Impact on Smoking
We assessed primary outcomes (CPD and breath CO) and side effects, using Mallinckrodt and Lipkovich’s41 approach, which focuses on contrasting significant group differences at each assessment point within the GLMM framework. The standard GLMM typically includes group, time, and Group × Time interaction effects in the model, with a significant Group × Time interaction term indicating the effect of the study manipulation. This method examines the general pattern of group differences over time, but does not test group differences at specific assessment points, so effects can be missed when the pattern of mean group changes are non-linear. We used the Mallinckrodt and Lipkovich approach because we expected the group differences to change over time, particularly from the e-cigarette provision period (baseline to 8 weeks) to the follow-up period (8–26 weeks). Because almost no participants in the assessment-only group used e-cigarettes during the study, statistical modeling to compare e-cigarette use between the groups over time was not appropriate. All analyses were performed on originally assigned groups.
Results
Study Participants
All participants were smokers with SMI (47.1% schizophrenia; 52.9% bipolar disorder) who reported an average of 11.1 lifetime psychiatric hospitalizations. Mean age of participants was 45.9 years. Approximately half (52.1%) were male, 45.4% were Black or mixed race, and 10% were Hispanic. Mean monthly income was $838 per month, and 82% were not employed. Participants reported smoking an average of 18.7 (±9.5) CPD, with mean CO of 26.9 ppm (±19.9). Nicotine dependence for the entire group was high (mean FTCD score 6.9 ± 1.5). As shown in Table 1, study groups did not differ on baseline demographics or smoking characteristics.
E-Cigarette Appeal
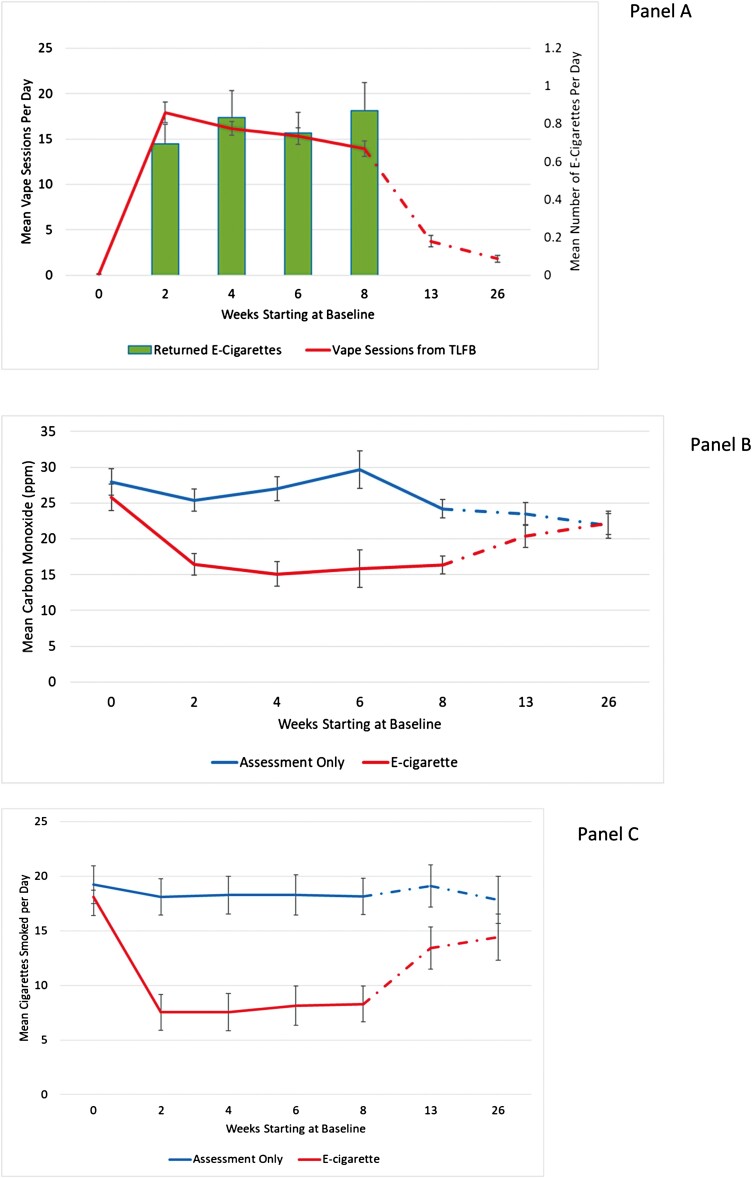
Within the first 2 weeks of the study, 79% of the e-cigarette group reported using e-cigarettes daily. Daily use remained high while e-cigarettes were provided by the study (70%, 66%, and 68% of participants at weeks 4, 6, and 8). Only 12 participants in the e-cigarette group and two assessment-only participants reported using a non-study e-cigarette during the trial. The correlation between self-reported vape sessions and returned used product ranged from r = .25 to r = .65 during the e-cigarette provision phase (Figure 1). We suspect concordance was low at times because: (1) participants lost or forgot to return product, (2) participants struggled to recall all vape sessions, and (3) the amount of an e-cigarette used in a “vape session” varies within and among individuals. E-cigarette participants found the product very appealing. Specifically, from weeks 2 to 8, mean ratings ranged from 4.0 to 4.2 for enjoyment from vaping; from 3.8 to 3.9 for enjoyment compared to cigarettes ranged from; from 4.3 to 4.5 for ease of use; and from 3.6 to 3.8 for willingness to replace cigarettes with e-cigarettes.
Figure 1.
Effectsa of e-cigarette provision compared to assessment only: (A) mean e-cigarette consumption (treatment group only), (B) mean cigarettes per day, and (C) mean CO. aError bars represent 95% confidence limits for means from generalized linear mixed models.
Effect of E-Cigarette Provision on Cigarette Smoking
Generalized linear mixed models (GLMMs) were fit to assess the effect of e-cigarette provision on cigarette smoking. Group (e-cigarette vs. assessment only), time (0, 2, 4, 6, 8, 13, and 26 weeks), and the Group × Time interaction were included as fixed effects. Based on model fit, instead of using random effects to take the correlated nature of the data into account, we allowed the variance and covariance to be fully estimated using an unstructured variance–covariance matrix parameterized through its Cholesky root. The Group × Time interaction term tests the overall manipulation effect. However, for the reason we stated above, we focused on group comparisons at each assessment point within the GLMM framework using specific estimates/contrasts. In the model testing the effect of e-cigarette provision on CPD, the Group × Time interaction effect was significant (F-value = 18.14, p < .0001), indicating that there was a significant manipulation effect over the entire study period. As shown in Figure 1, at baseline, mean self-reported CPD did not differ between the e-cigarette group (18.1 [95% CI = 16.4–19.8]) and the assessment-only group (19.2 [95% CI = 17.5–20.9]) (p = .3636). By week 2, mean CPD was substantially reduced in the e-cigarette group (7.5 [95% CI = 5.9–9.2]) and unchanged in the assessment-only group (18.1 [95% CI = 16.4, 19.8]), a reduction maintained through week 8 for the e-cigarette (8.2 [95% CI = 6.6, 9.9]) versus the assessment-only group (18.2 [95% CI = 16.5, 19.8]). Group differences were highly significant for weeks 2–8 (p < .001). By week 26, mean CPD trended upwards for the e-cigarette group (14.4 [95% CI = 12.3, 16.5]) but remained significantly lower than the assessment-only group (18.7 [95% CI = 16.7, 20.7]) (p = .0275).
We also examined self-reported combustible cigarette use categorically in order to examine how many participants in each group eliminated (0 cigarettes) or substantially reduced (one to five cigarettes) daily cigarettes. The remainder of participants were categorized as smoking six or more CPD. Because of zero or very small values, we could not test differences between groups for 0 CPD or one to five CPD. The group difference for six or more CPD was tested within the same GLMM framework as described above. As shown in Table 2, 18.6%–22.1% of participants in the e-cigarette group reported smoking no cigarettes between weeks 2 and 8 versus 0% in the assessment-only group. This difference remained substantial at week 13 (14.7% vs. 0.9%), but narrowed at week 26 (10.7% vs. 5.7%). Similarly, a larger proportion of participants in the e-cigarette group reported smoking only one to five CPD at weeks 2–8 (27.4%–38.1%) compared to the assessment-only group (2.6%–6.4%). The difference was still substantial at week 13 (11.0% vs. 3.7%), but slightly favored the assessment-only group by week 26 (2.7% vs. 5.7%). Significantly, fewer participants in the e-cigarette group reported smoking six or more CPD from weeks 2 to 13 (p < .001), but the groups were no longer significantly different at week 26.
Table 2.
Self-Reported Combustible Cigarette Use Over 26 Weeks (N = 240)
| Timepoint | 0 Cigs/day and CO < 6 | 0 Cigs/day and CO ≥ 6 | 1–5 Cigs/day | 6+ Cigs/day | Test statistica | ||||
|---|---|---|---|---|---|---|---|---|---|
| E-cig n (%) |
Assessment only n (%) |
E-cig n (%) |
Assessment only n (%) |
E-cig n (%) |
Assessment only n (%) |
E-cig n (%) |
Assessment only n (%) |
||
| Baseline (n = 239) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.5) | 3 (2.5) | 117 (97.5) | 116 (97.5) | ns |
| 2 wk (n = 230) | 13 (11.2) | 0 (0.0) | 10 (8.6) | 0 (0.0) | 38 (32.8) | 3 (2.6) | 55 (47.4) | 111 (97.4) | ** |
| 4 wk (n = 225) | 14 (12.4) | 0 (0.0) | 7 (6.2) | 0 (0.0) | 43 (38.1) | 3 (2.7) | 49 (43.4) | 109 (97.3) | ** |
| 6 wk (n = 224) | 18 (15.9) | 0 (0.0) | 7 (6.2) | 0 (0.0) | 32 (28.3) | 7 (6.3) | 56 (49.6) | 104 (93.7) | ** |
| 8 wk (n = 223) | 12 (10.6) | 0 (0.0) | 9 (8.0) | 0 (0.0) | 31 (27.4) | 7 (6.4) | 61 (54.0) | 103 (93.6) | ** |
| 13 wk (n = 217) | 9 (8.3) | 1 (0.9) | 7 (6.4) | 0 (0.0) | 12 (11.0) | 4 (3.7) | 81 (74.3) | 103 (95.4) | ** |
| 26 wk (n = 213) | 6 (5.7) | 2 (1.9) | 3 (2.8) | 2 (1.9) | 10 (9.3) | 8 (7.6) | 89 (82.4) | 93 (88.6) | ns |
Cigs/day, cigarettes per day; ns, not significant.
Group difference at each assessment point was tested within GLMM: **p < .01.
In the model testing the effect of e-cigarette provision on CO, the Group × Time interaction effect was significant (F-value = 6.30 and p < .0001), indicting an overall manipulation effect over the study period. As shown in Figure 1, mean CO at baseline did not differ between the e-cigarette group (25.8 [95% CI = 10.7–21.1]) and the assessment-only group (27.9 [95% CI = 21.6, 26.7]) (p = .4057). By week 2, mean breath CO was significantly lower in the e-cigarette (16.4 [95% CI = 13.4, 19.5]) compared to the assessment-only group (25.4 [95% CI = 22.4, 28.4]), which was maintained through week 8 (16.3 [95% CI = 13.8, 18.9] vs. 24.2 [95% CI = 21.6, 26.3]). Group differences were all highly significant from weeks 2 to 8 (p < .001). Group differences in mean breath CO were not significantly different at weeks 13 (p = .1500) or 26 (p = .8605). These group contrasts were conducted within the GLMM framework and thus adjusted for within-subject correlation and missing data (12.5% at week 8; 10.8% at week 12).
Nicotine Dependence
There were no differences in mean FTCD scores between the e-cigarette and assessment-only groups at baseline (6.7 vs. 7.0) or at 8 weeks (5.8 vs. 6.4).
Side Effects and Serious Adverse Events
The same modeling approach described for our primary outcomes was used to analyze side effects. The group difference at each assessment point was tested within the GLMM framework for several side effects. As shown in Table 3, significantly fewer participants in the e-cigarette group self-reported cough at baseline and through 8 weeks, itchy/sore throat at baseline and through 4 weeks, and total symptoms at 2 weeks through 8. Significantly fewer participants in the e-cigarette group also self-reported bad taste in the mouth, but only at 2, 8, and 13 weeks. Changes in other symptoms, including nausea, dizziness, vomiting, and palpitations, were more variable, with no significant declines or increases for either group during the study (not shown).
Table 3.
Self-Reported Side Effects
| Timepoint | Cougha | Itchy/sore throata | Bad tastea | Total symptomsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E-cigarette n (%) |
Assessment n (%) |
p-value | E-cigarette n (%) |
Assessment n (%) |
p-value | E-cigarette n (%) |
Assessment n (%) |
p-value | E-cigarette M ± SD |
Assessment M ± SD |
p-value | |
| Baseline (n = 239) | 80 (67.2) | 98 (81.7) | ** | 45 (37.8) | 63 (52.5) | * | 68 (57.1) | 67 (55.8) | ns | 2.9 ± 2.2 | 3.3 ± 2.0 | ns |
| 2 wk (n = 220) | 54 (48.7) | 76 (69.7) | ** | 26 (23.4) | 40 (36.7) | * | 37 (33.6) | 55 (50.5) | ** | 1.9 ± 1.9 | 2.7 ± 1.9 | ** |
| 4 wk (n = 209) | 50 (48.5) | 70 (66.0) | ** | 23 (22.3) | 39 (36.8) | * | 42 (40.8) | 50 (47.2) | ns | 1.9 ± 1.8 | 2.6 ± 2.0 | ** |
| 6 wk (n = 203) | 43 (43.0) | 67 (65.1) | ** | 28 (28.0) | 40 (38.8) | * | 32 (32.0) | 37 (35.9) | ns | 1.8 ± 1.9 | 2.3 ± 1.8 | * |
| 8 wk (n = 204) | 48 (45.3) | 72 (70.6) | ** | 28 (26.4) | 28 (27.5) | ns | 31 (29.3) | 43 (42.2) | * | 1.7 ± 1.9 | 2.4 ± 1.9 | ** |
| 13 wk (n = 204) | 49 (48.0) | 59 (57.8) | ns | 24 (23.5) | 27 (26.5) | ns | 25 (24.5) | 39 (38.2) | * | 1.9 ± 1.8 | 2.1 ± 1.9 | ns |
| 26 wk (n = 213) | 47 (46.5) | 49 (53.3) | ns | 25 (24.8) | 25 (27.2) | ns | 37 (36.6) | 33 (35.9) | ns | 1.9 ± 2.2 | 2.1 ± 2.0 | ns |
ns, not significant.
Group difference at each assessment point was tested: **p < .01, *p < .05.
Most of the qualitative comments by participants in the e-cigarette group were focused on respiratory symptoms, followed by physical and mental health. With respect to respiratory symptoms, 70% of participants made at least one or more positive comment (n = 209) such as, “not using my inhaler as much as before,” and “I can breathe better and I’m not doing all that coughing and wheezing,” while only 33% of participants made a negative comment about respiratory symptoms (n = 39), such as, “I didn’t like the cough, it was more severe than…with the cigarettes.”
With respect to physical and mental health, 33% of participants made a positive comment (n = 58), such as, “I feel better physically,” and “I noticed that my heart does not race when walking now.” In contrast, only 12% of participants made a negative comment (n = 18), such as, “I noticed headache, nausea, anxiety when I vape the e-cig for 40 minutes straight,” and “… it doesn’t fill the emptiness I feel inside as much.”
During the first 8 weeks of the study, seven out of 240 (2.9%) study participants had a total of nine serious adverse events (emergency room admission or hospitalization) involving respiratory symptoms: two occurred in the e-cigarette group and seven occurred in the assessment-only group.
Discussion
This randomized trial found that provision of disposable “cigalike” e-cigarettes to smokers with SMI was followed by rapid uptake and high rates of daily use. The e-cigarette group markedly reduced their cigarette use and CO compared to the assessment-only group across the 8-week e-cigarette provision period and into the 13-week follow-up. Absent a desire to quit smoking, and without coaching, 19%–22% of the e-cigarette group fully substituted e-cigarettes for cigarettes during the e-cigarette provision period. In spite of concerns that e-cigarette provision could increase intake of and dependence on nicotine, CPD were significantly lower in the e-cigarette group compared to the assessment-only group at every timepoint and scores on the FTCD did not increase in the e-cigarette group compared to the assessment-only group. Also, neither side effects nor adverse events were more common in the e-cigarette group.
Most participants discontinued e-cigarette use after the provision period. We suspect this occurred for several reasons: (1) the high cost of e-cigarettes compared to inexpensive tobacco (roll-your-own, generic, mini-cigars) commonly used by this impoverished group and (2) low motivation of people with SMI to invest effort to buy a nicotine product other than usual brand cigarettes. Nevertheless, our results suggest that e-cigarette use may represent a feasible harm reduction strategy for smokers with SMI who are unwilling or unable to quit. This study included free access to e-cigarettes. However, despite high appeal ratings, a minority of participants fully discontinued combustible cigarette use. Further research is needed to develop strategies to facilitate and support complete switching to e-cigarettes, including relative risk reduction education, training on how to maximize enjoyment of e-cigarettes, and coaching on specific ways to replace smoking with vaping.
There are no prospective randomized studies in the general population utilizing a simple design such as ours in which smokers without cessation intentions were randomly assigned to either receive e-cigarettes plus assessments or assessments only. This study has many strengths: random assignment to study groups, equivalence between groups on baseline characteristics, biological validation of self-reported cigarette smoking, and validation of self-reported e-cigarette use through physical counts of e-cigarettes returned. Random assignment to groups allowed greater certainty that the behavioral changes observed resulted from provision of e-cigarettes versus the Hawthorne effect, which cannot be ruled out in pre–post studies of e-cigarette use in people with SMI.30–32 Another strength is 89% retention in follow-up assessments over a 6-month period. Finally, the large, racially diverse group of individuals from two U.S. states with very different population prevalence of smoking increases generalizability of these findings.
Some limitations are worthy of mention. First, although breath CO provides biological verification of smoking, it only reflects cigarette use over 24–48 hours, and we did not require a drug test to screen for use of other combustibles. Second, the study did not include cotinine as an indicator of the potential increase in nicotine exposure from e-cigarette provision. Third, the measurement of cigarette use, and e-cigarette use and appeal, required unblinded assessment, allowing for the possibility of observer bias based on knowledge about group assignment. However, blinded assessment of breath CO and nicotine dependence mitigates this limitation. Finally, this study was not designed to assess the long-term safety of e-cigarettes.
This is the first fully powered, prospective randomized controlled study comparing e-cigarette provision with continued cigarette smoking as usual. E-cigarette provision to smokers with SMI compared to assessment only resulted in high levels of switching; e-cigarette uptake in concert with reduced cigarette smoking. The effects of e-cigarette provision were large, reducing cigarette consumption by more than 50%, along with significant reductions in CO. Because only 19%–22% of e-cigarette participants were able to completely switch to e-cigarettes, and switching attenuated after e-cigarette provision ended, future research should examine whether teaching smokers with SMI skills to fully substitute e-cigarettes for cigarettes could enhance and extend switching, leading to greater harm reduction over time.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Sarah I Pratt, Department of Psychiatry, Geisel School of Medicine at Dartmouth, Concord, NH, USA; C. Everett Koop Institute, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Joelle C Ferron, Department of Psychiatry, Geisel School of Medicine at Dartmouth, Concord, NH, USA.
Mary F Brunette, Department of Psychiatry, Geisel School of Medicine at Dartmouth, Concord, NH, USA; C. Everett Koop Institute, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Meghan Santos, Department of Psychiatry, Geisel School of Medicine at Dartmouth, Concord, NH, USA.
James Sargent, C. Everett Koop Institute, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Haiyi Xie, Department of Biomedical Data Sciences, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Funding
The study was funded by the National Institute on Drug Abuse (NIDA, 1R01DA041416) in the United States.
Declaration of Interests
None declared.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Diaz FJ, James D, Botts S, Maw L, Susce MT, de Leon J.. Tobacco smoking behaviors in bipolar disorder: a comparison of the general population, schizophrenia, and major depression. Bipolar Disord. 2009;11(2):154–165. [DOI] [PubMed] [Google Scholar]
- 2. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH.. Smoking and mental illness: a population-based prevalence study. J Am Med Assoc. 2000;284(20):2606–2610. [DOI] [PubMed] [Google Scholar]
- 3. McClave AK, McKnight-Eily LR, Davis SP, Dube SR.. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100(12):2464–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steinberg ML, Williams JM, Li Y.. Poor mental health and reduced decline in smoking prevalence. Am J Prev Med. 2015;49(3):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole RD, Parikh V.. Nicotine dependence in schizophrenia: contributions of nicotinic acetylcholine receptors. In: Preedy VR, ed. Neuroscience of Nicotine. London: Academic Press; 2019:135–143. [Google Scholar]
- 6. Parikh V, Kutlu MG, Gould TJ.. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: current trends and perspectives. Schizophr Res. 2016;171(1–3):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aschbrenner KA, Dixon LB, Naslund JA, et al. . An online survey of family members’ beliefs and attitudes about smoking and mental illness. J Dual Diagn. 2017;13(3):179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aschbrenner KA, Naslund JA, Gill L, et al. . Preferences for smoking cessation support from family and friends among adults with serious mental illness. Psychiatr Q. 2017;88(4):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown CH, Medoff D, Dickerson FB, et al. Factors influencing implementation of smoking cessation treatment within community mental health centers. J Dual Diagn. 2015;11(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Himelhoch S, Daumit G.. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228–2230. [DOI] [PubMed] [Google Scholar]
- 11. DeAtley T, Denlinger-Apte RL, Cioe PAet al. . Biopsychosocial mechanisms associated with tobacco use in smokers with and without serious mental illness. Prev Med. 2020;140:106190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colton C, Manderscheid R.. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):1–14. [PMC free article] [PubMed] [Google Scholar]
- 13. Druss BG, Zhao L, Von Esenwein S, Morrato EH, Marcus SC.. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599–604. [DOI] [PubMed] [Google Scholar]
- 14. Banham L, Gilbody S.. Smoking cessation in severe mental illness: what works? J Addict. 2010;105(7):1176–1189. [DOI] [PubMed] [Google Scholar]
- 15. Ferron JC, Devitt T, McHugo GJ, Jonikas AJ, Cook JA, Brunette MF.. Abstinence and use of community-based cessation treatment after a motivational intervention among smokers with severe mental illness. Community Ment Health J. 2016;52(4):446–456. [DOI] [PubMed] [Google Scholar]
- 16. Evins AE, Cather C, Pratt SAet al. . Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. J Am Med Assoc. 2014;311(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsoi DT, Porwal M, Webster AC.. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stephens WE. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob Control. 2018;27(1):10–17. [DOI] [PubMed] [Google Scholar]
- 19. Margham J, McAdam K, Forster M, et al. . Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol. 2016;29(10):1662–1678. [DOI] [PubMed] [Google Scholar]
- 20. Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H.. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. J Addict. 2014;109(11):1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 22. McNeill A, Brose LS, Calder R, Bauld L, Robson D.. Evidence Review of E-cigarettes and Heated Tobacco Products 2018. London: Public Health England; 2018. [Google Scholar]
- 23. Hartmann-Boyce J, McRobbie H, Lindson N, et al. . Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2020;10(10):CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H.. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology. 2017;234(5):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wagener TL, Floyd EL, Stepanov I, et al. . Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2017;26(e1):e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bianco CL, Pratt SI, Ferron JC, Brunette MF.. Electronic cigarette use during a randomized trial of interventions for smoking cessation among Medicaid beneficiaries with mental illness. J Dual Diagn. 2019;15(3):184–191. [DOI] [PubMed] [Google Scholar]
- 27. Peckham E, Mishu M, Fairhurst Cet al. . E-cigarette use and associated factors among smokers with severe mental illness. Addict Behav. 2020;108:106456. [DOI] [PubMed] [Google Scholar]
- 28. Prochaska JJ, Grana RA.. E-cigarette use among smokers with serious mental illness. PLoS One. 2014;9(11):e113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller ME, Tidey JW, Rohsenow DJ, Higgins ST.. Electronic cigarette expectancies in smokers with psychological distress. Tob Regul Sci. 2017;3(1):108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pratt SI, Sargent J, Daniels L, Santos MM, Brunette M.. Appeal of electronic cigarettes in smokers with serious mental illness. Addict Behav. 2016;59:30–34. [DOI] [PubMed] [Google Scholar]
- 31. Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R.. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hickling LM, Perez-Iglesias R, McNeill Aet al. . A pre–post pilot study of electronic cigarettes to reduce smoking in people with severe mental illness. Psychol Med. 2019;49(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 33. McCarthy J. In U.S., Smoking Rate Lowest in Utah, Highest in Kentucky. Gallup [Internet]. 2014. https://news.gallup.com/poll/167771/smoking-rate-lowest-utah-highest-kentucky.aspx. Accessed November 30, 2020. [Google Scholar]
- 34. Sobell LC, Sobell MB.. Alcohol Timeline Followback (TLFB) Users’ Manual. Toronto, Ontario, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 35. Sobell LC, Agrawal S, Sobell MB, et al. . Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. J Stud Alcohol Drugs. 2003;64(6):858–861. [DOI] [PubMed] [Google Scholar]
- 36. Sobell LC, Sobell MB.. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, eds. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992:41–72. [Google Scholar]
- 37. Jarvis MJ, Russell MA, Saloojee Y.. Expired air carbon monoxide: a simple breath test of tobacco smoke intake. Br Med J. 1980;281(6238):484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krueger RA. Focus Groups. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994. [Google Scholar]
- 39. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 40. Weinberger AH, Reutenauer EL, Allen TM, et al. . Reliability of the Fagerstrom Test for Nicotine Dependence, Minnesota Nicotine Withdrawal Scale, and Tiffany Questionnaire for smoking urges in smokers with and without schizophrenia. Drug and Alcohol Depend. 2007;86:278–282. [DOI] [PubMed] [Google Scholar]
- 41. Mallinckrodt C, Lipkovich I.. Chapter 8: Modeling means over time. In: Hall C, ed. Analyzing Longitudinal Clinical Trial Data. Boca Raton, FL: Taylor & Francis; 2017:91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.