Abstract
Background
Levetiracetam (LEV) is one of the most frequently used antiepileptic drugs (AED) for brain tumor patients with seizures. We hypothesized that toxicity of LEV and temozolomide-based chemoradiotherapy may overlap.
Methods
Using a pooled cohort of patients with newly diagnosed glioblastoma included in clinical trials prior to chemoradiotherapy (CENTRIC, CORE, AVAglio) or prior to maintenance therapy (ACT-IV), we tested associations of hematologic toxicity, nausea or emesis, fatigue, and psychiatric adverse events during concomitant and maintenance treatment with the use of LEV alone or with other AED versus other AED alone or in combination versus no AED use at the start of chemoradiotherapy and of maintenance treatment.
Results
Of 1681 and 2020 patients who started concomitant chemoradiotherapy and maintenance temozolomide, respectively, 473 and 714 patients (28.1% and 35.3%) were treated with a LEV-containing regimen, 538 and 475 patients (32.0% and 23.5%) with other AED, and 670 and 831 patients (39.9% and 41.1%) had no AED. LEV was associated with higher risk of psychiatric adverse events during concomitant treatment in univariable and multivariable analyses (RR 1.86 and 1.88, P < .001) while there were no associations with hematologic toxicity, nausea or emesis, or fatigue. LEV was associated with reduced risk of nausea or emesis during maintenance treatment in multivariable analysis (HR = 0.80, P = .017) while there were no associations with hematologic toxicity, fatigue, or psychiatric adverse events.
Conclusions
LEV is not associated with reduced tolerability of chemoradiotherapy in patients with glioblastoma regarding hematologic toxicity and fatigue. Antiemetic properties of LEV may be beneficial during maintenance temozolomide.
Keywords: epilepsy, levetiracetam, seizure, temozolomide, toxicity
Key Points.
Antiemetic properties of levetiracetam may be beneficial in patients treated with temozolomide.
Psychiatric adverse events were associated with levetiracetam in concomitant phase.
No association of psychiatric adverse events with levetiracetam during maintenance.
Importance of the Study.
Levetiracetam is one of the most frequently used antiepileptic drugs in brain tumor patients suffering from epilepsy. Whether use of levetiracetam affects the tolerability of temozolomide-based chemoradiotherapy in patients with glioblastoma is unknown. This study shows that use of levetiracetam does not decrease the tolerability of chemoradiotherapy in patients with glioblastoma with regard to hematologic toxicity and fatigue. Psychiatric adverse events were associated with use of levetiracetam during the concomitant but not during the maintenance phase of chemoradiotherapy. Based on our finding of a reduced risk of nausea or emesis during maintenance treatment with temozolomide associated with use of levetiracetam, potential antiemetic properties of levetiracetam may be exploited for patients suffering from chemotherapy-induced nausea and emesis.
Symptomatic epileptic seizures represent a common comorbidity of patients with brain tumors.1 The need for effective and well tolerated antiepileptic treatment in these patients is evident. Levetiracetam (LEV) has emerged as the most frequently used antiepileptic drug (AED) for patients with brain tumors due to its efficacy as well as the rapid titration phase in line with current guidelines.1–4 The mechanism of action of LEV is not fully understood. Binding of LEV to the synaptic vesicular protein SV2A that controls neurotransmitter release is proposed as the main mechanism for its anticonvulsant effect.5–7 However, other targets have also been proposed including the presynaptic P/Q-type voltage-dependent calcium channel affecting glutamate release8 and the ryanodine and IP3 receptor in hippocampal neurons mediating calcium release from endoplasmic reticulum.9 The toxicity profile of LEV is considered as largely favorable and the property of the drug as a non-enzyme-inducing antiepileptic drug (AED) is appreciated for its lack of drug–drug interactions.3,10 However, the SANAD II trial, a multicenter randomized phase 4 study, comparing LEV and zonisamide with lamotrigine as first-line treatment for patients with newly diagnosed focal epilepsy failed to prove non-inferiority of LEV compared with lamotrigine with regard to 12-month remission from seizures.11 In this trial, the safety profile was also less favorable for LEV than for lamotrigine, including higher rates of psychiatric disorders in patients treated with LEV (30%) than in patients with lamotrigine (13%). In brain tumor patients, especially in the context of chemoradiotherapy, the tolerability of LEV may be further reduced. We hypothesized that there is some overlap of the toxicity profile of temozolomide-based chemoradiotherapy and LEV in patients with glioblastoma. Hematological toxicity is common with alkylating agents such as temozolomide12,13 and is occasionally described in patients receiving LEV.14–17 Temozolomide has a moderate emetogenic potential18 while antiemetic properties were suggested for LEV.19 Fatigue is a major burden for patients with brain tumors20 and also represents a common adverse event of LEV.21 Neuropsychiatric side effects represent one of the major limitations of the treatment of epilepsy with LEV and patients with brain tumors may be at increased risk of psychiatric comorbidity.22–24
Here we characterized the incidence of hematologic adverse events, nausea or emesis, fatigue, and psychiatric adverse events in patients with glioblastoma receiving combined chemoradiotherapy treated with LEV with or without other AED or treated with any other AED alone or in combination and compared to no AED use, analyzing a pooled patient cohort of the EORTC Brain tumor Group’s clinical trial database.
Patients and Methods
Patient Cohort
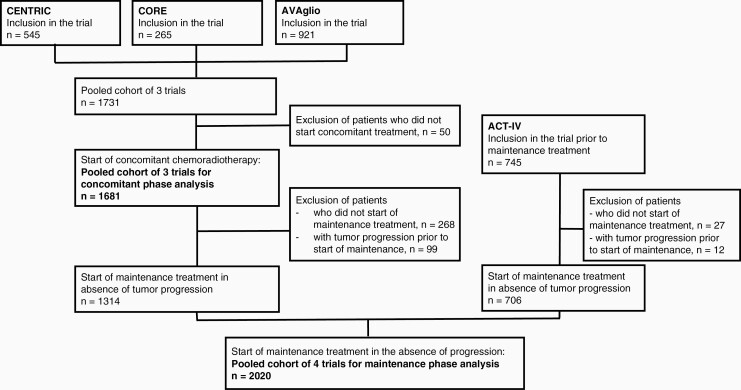
We performed a retrospective analysis of individual patient data (n = 2476) of four randomized clinical trials in patients with newly diagnosed glioblastoma: CENTRIC (Clinicaltrials.gov number NCT00689221),25 CORE (Clinicaltrials.gov number NCT00813943),26 AVAglio (Clinicaltrials.gov number NCT00943826)27 and ACT-IV (Clinicaltrials.gov number NCT01480479)28 [see Figure 1 for the Consolidated standards of reporting trials (CONSORT) diagram]. Approval for trial participation was obtained for all sites by their institutional review boards, and informed consent was available for all patients. In CENTRIC, CORE, and AVAglio, patients were enrolled prior to the start of standard radiotherapy with concomitant temozolomide, while ACT-IV enrolled patients after completing standard radiotherapy with concomitant temozolomide, i.e. prior to the maintenance phase of treatment. Therefore, for analysis of adverse events during concomitant treatment, only data of the trials CENTRIC, CORE, and AVAglio were included while for the period of maintenance treatment, data of all trials were included from patients who started maintenance in the absence of tumor progression. Data on lymphocyte counts were not available in AVAglio, and therefore for analysis of lymphopenia, the dataset S (small) including the trials CENTRIC, CORE, and ACT-IV was used, i.e. without the patients of AVAglio, while for all other analysis the dataset L (large) including all four trials was used.
Figure 1.
Consolidated standards of reporting trials (CONSORT) diagram.
Definition of Terms
Baseline patient characteristics including clinical data, WHO performance status and the use of corticosteroids and antidepressants at the start of concomitant and maintenance treatment were derived from the period of 14 days prior to the start of concomitant temozolomide and 28 days prior to the start of maintenance treatment, respectively. In AVAglio, WHO performance status was computed from Karnofsky performance status.29 The type of AED at the start of concomitant and maintenance temozolomide was defined as drug used within 14 days prior to the start of concomitant and maintenance treatment, respectively, and classified into use of LEV alone or in combination with other AED, use of any other AED alone or in combination, or no AED use. Adverse events were collected during concomitant treatment defined as the start of concomitant temozolomide chemoradiotherapy until one day prior to start of maintenance temozolomide and during maintenance temozolomide defined as the start of maintenance treatment with temozolomide until day 28 of the sixth cycle of temozolomide. Common Terminology Criteria for Adverse Events (CTCAE) were used with the version applied for the respective clinical trial, i.e. CTCAE version 3.0 for CENTRIC, CORE, and AVAglio and version 4.0 for ACT-IV, respectively. For hematologic adverse events, re-grading according to CTCAE version 4.0 was done in a retrospective manner. For the hematologic adverse events, anemia, thrombocytopenia, neutropenia, and lymphopenia were considered separately. Based on an expected high frequency but limited clinical significance of grade 1 and 2 thrombocytopenia, lymphopenia, and neutropenia during chemoradiotherapy, we focused the analysis of these items on severe, i. e. grade 3 or 4 toxicity compared with grade 0, 1, 2 toxicity. Since severe anemia is not expected in this patient population, we compared the incidence of any grade of anemia versus no toxicity. With the term psychiatric adverse events, we summarized different items among others affective disorders, anxiety, depression, behavioral problems and other related terms as listed in Supplementary Table S1. The terms fatigue, nausea or emesis were used as graded as adverse events in the respective clinical trials. The presence of any grade of psychiatric adverse events, fatigue, and a composite item of nausea or emesis was analyzed versus no toxicity as detailed below.
Statistical Analysis
Categorical variables were described using frequencies and percentages. For analysis of association of AED use at the start of concomitant treatment with adverse events during concomitant phase of chemoradiotherapy, univariable and multivariable logistic regression analyses were performed. Given the possibility of repeated adverse events, for assessments during the concomitant treatment, the worst grade for each adverse event was analyzed throughout concomitant treatment until the last day prior to the start of maintenance therapy. For the association of AED use at the start of maintenance treatment with adverse events during maintenance treatment, competing risk models were used, considering treatment discontinuation, disease progression or death before the adverse events as competing events. In these analyses, time to first grade 3 or 4 adverse event were assessed separately for thrombocytopenia, neutropenia, and lymphopenia, and time to first adverse event of any grade separately for anemia, nausea or emesis, fatigue, and psychiatric adverse events. Stratified by trial, the Aalen-Johansen estimator30,31 was used to obtain the cumulative incidence function, and the association of the use of AED with the incidence of adverse events was estimated by the Fine and Gray model.32 Both for the analyses for the concomitant phase and for the maintenance phase, data were adjusted for covariables as indicated. On adjusted analyses, in general, data were used for those patients with all covariables available. However, for patients who did not have information available on the methylation status of the O6-methylguanine-DNA-methyltransferase (MGMT) promotor which was not mandatory in AVAglio and ACT-IV (Supplementary Tables S2 and S3), we decided not to exclude them from adjusted analysis. Interaction of the adjusted variables with AED use was assessed if there was an indication of a possible interaction. In order to account for the assessment of AED use with the variables of interest at two time points, i.e. concomitant phase and maintenance phase, the overall significance level of 5% was split into 2 (2.5% for the concomitant phase analysis and 2.5% for the maintenance phase analysis) and the corresponding 97.5% confidence intervals reported for all the analyses. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
Patient Characteristics at Baseline and at the Start of Maintenance Treatment
Out of 1731 patients that were enrolled in the trials CENTRIC, CORE, and AVAglio, the baseline characteristics of the 1681 patients who started with concomitant treatment are shown in Supplementary Table S2. For the maintenance phase of treatment, we identified 2020 patients from all four trials who started maintenance treatment in the absence of tumor progression (Supplementary Table S3). At the beginning of concomitant treatment, 473 patients (28.1%) were treated with a LEV-containing regimen, 538 patients (32.0%) with other AED alone or in combination, and 670 patients (39.9%) received no AED. At the beginning of maintenance treatment, 714 patients (35.3%) were on a LEV-containing regimen, 475 patients (23.5%) were treated with other AED, and 831 (41.1%) patients received no AED. Patient characteristics stratified by baseline AED use for patients who started concomitant and maintenance treatment are provided in Tables 1 and 2. Overall, patient characteristics among groups were similar. During the baseline period at the start of concomitant and of maintenance treatment, a higher proportion of patients (50.3% and 54.5%) who received a LEV-based regimen used steroids, compared to 37.5% and 48.5% for those who used other AED, and to 38.1% and 41.2% for those who did not use any AED. The incidence of worst grade of adverse events during concomitant and maintenance treatment stratified by AED use is shown in Table 3.
Table 1.
Baseline characteristics of patients who started concomitant temozolomide treatment stratified by antiepileptic drug use
| At the start of concomitant treatment | Total (N = 1681) | |||
|---|---|---|---|---|
| No AED1 (N = 670) | LEV2 with or without other AED (N = 473) | Other AED with or without other AED (N = 538) | ||
| N (%) | N (%) | N (%) | N (%) | |
| Sex | ||||
| Male | 378 (56.4) | 282 (59.6) | 344 (63.9) | 1004 (59.7) |
| Female | 292 (43.6) | 191 (40.4) | 194 (36.1) | 677 (40.3) |
| Age group | ||||
| < 55 years | 239 (35.7) | 202 (42.7) | 259 (48.1) | 700 (41.6) |
| ≥ 55 years | 431 (64.3) | 271 (57.3) | 279 (51.9 | 981 (58.4) |
| WHO performance status | ||||
| 0 | 429 (64.0) | 284 (60.0) | 342 (63.6) | 1055 (62.8) |
| > 0 | 240 (35.8) | 189 (40.0) | 196 (36.4) | 625 (37.2) |
| Missing | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| MGMT promoter status | ||||
| Unmethylated | 267 (39.9) | 221 (46.7) | 221 (41.1) | 709 (42.2) |
| Methylated | 316 (47.2) | 193 (40.8) | 244 (45.4) | 753 (44.8) |
| Unknown | 87 (13.0) | 59 (12.5) | 73 (13.6) | 219 (13.0) |
| Extent of surgery | ||||
| Partial resection or biopsy | 393 (58.7) | 273 (57.7) | 252 (46.8) | 918 (54.6) |
| Gross total resection | 275 (41.0) | 200 (42.3) | 285 (53.0) | 760 (45.2) |
| Missing | 2 (0.3) | 0 (0.0) | 1 (0.2) | 3 (0.2) |
| Steroid use at baseline | ||||
| No | 413 (61.6) | 234 (49.5) | 335 (62.3) | 982 (58.4) |
| Yes | 255 (38.1) | 238 (50.3) | 202 (37.5) | 695 (41.3) |
| Missing | 2 (0.3) | 1 (0.2) | 1 (0.2) | 4 (0.2) |
| Antidepressant use at baseline | ||||
| No | 613 (91.5) | 412 (87.1) | 510 (94.8) | 1535 (91.3) |
| Yes | 57 (8.5) | 61 (12.9) | 28 (5.2) | 146 (8.7) |
1 AED antiepileptic drug.
2 LEV levetiracetam.
Table 2.
Characteristics of patients who started maintenance treatment in the absence of tumor progression stratified by antiepileptic drug use at the start of maintenance treatment
| At the start of maintenance treatment | Total (N = 2020) | |||
|---|---|---|---|---|
| No AED1 (N = 831) |
LEV2 with or without other AED (N = 714) | Other AED with or without other AED (N = 475) | ||
| N (%) | N (%) | N (%) | N (%) | |
| Sex | ||||
| Male | 521 (62.7) | 449 (62.9) | 300 (63.2) | 1270 (62.9) |
| Female | 310 (37.3) | 265 (37.1) | 175 (36.8) | 750 (37.1) |
| Age group | ||||
| < 55 years | 300 (36.1) | 288 (40.3) | 241 (50.7) | 829 (41.0) |
| ≥ 55 years | 531 (63.9) | 426 (59.7) | 234 (49.3) | 1191 (59.0) |
| WHO performance status | ||||
| 0 | 486 (58.5) | 355 (49.7) | 264 (55.6) | 1105 (54.7) |
| > 0 | 332 (40.0) | 338 (47.3) | 202 (42.5) | 872 (43.2) |
| Missing | 13 (1.6) | 21 (2.9) | 9 (1.9) | 43 (2.1) |
| MGMT promoter status | ||||
| Unmethylated | 392 (47.2) | 380 (53.2) | 200 (42.1) | 972 (48.1) |
| Methylated | 358 (43.1) | 256 (35.9) | 211 (44.4) | 825 (40.8) |
| Unknown | 81 (9.7) | 78 (10.9) | 64 (13.5) | 223 (11.0) |
| Extent of surgery | ||||
| Partial resection or biopsy | 409 (49.2) | 341 (47.8) | 206 (43.4) | 956 (47.3) |
| Gross total resection | 420 (50.5) | 373 (52.2) | 268 (56.4) | 1061 (52.5) |
| Missing | 2 (0.2) | 0 (0.0) | 1 (0.2) | 3 (0.1) |
| Steroid use at the start of maintenance treatment | ||||
| No | 489 (58.8) | 325 (45.5) | 243 (51.2) | 1057 (52.3) |
| Yes | 342 (41.2) | 389 (54.5) | 232 (48.8) | 963 (47.7) |
| Antidepressant use at the start of maintenance treatment | ||||
| No | 772 (92.9) | 617 (86.4) | 434 (91.4) | 1823 (90.2) |
| Yes | 59 (7.1) | 97 (13.6) | 41 (8.6) | 197 (9.8) |
1 AED antiepileptic drug.
2 LEV levetiracetam.
Table 3.
Incidence of adverse events (worst grade) during concomitant and maintenance treatment by use of antiepileptic drugs
| Use of AED1 at the start of concomitant treatment (dataset L, trials CENTRIC, CORE, AVAglio) | Use of AED at the start of maintenance treatment (dataset L, trials CENTRIC, CORE, AVAglio, ACT-IV) | |||||||
|---|---|---|---|---|---|---|---|---|
| No AED (N = 670) |
LEV2 with or without other AED (N = 473) | Other AED with or without other AED (N = 538) | Total dataset L (N = 1681) |
No AED (N = 831) |
LEV with or without other AED (N = 714) | Other AED with or without other AED (N = 475) | Total dataset L (N = 2020) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Anemia | ||||||||
| Grade 0 | 388 (57.9) | 275 (58.1) | 300 (55.8) | 963 (57.3) | 478 (57.5) | 389 (54.5) | 281 (59.2) | 1148 (56.8) |
| Grade 1/2 | 271 (40.4) | 192 (40.6) | 230 (42.8) | 693 (41.2) | 327 (39.4) | 300 (42.0) | 184 (38.7) | 811 (40.1) |
| Grade 3/4 | 5 (0.7) | 1 (0.2) | 4 (0.7) | 10 (0.6) | 2 (0.2) | 1 (0.1) | 3 (0.6) | 6 (0.3) |
| Missing | 6 (0.9) | 5 (1.1) | 4 (0.7) | 15 (0.9) | 24 (2.9) | 24 (3.4) | 7 (1.5) | 55 (2.7) |
| Thrombocytopenia | ||||||||
| Grade 0 | 443 (66.1) | 331 (70.0) | 364 (67.7) | 1138 (67.7) | 390 (46.9) | 343 (48.0) | 220 (46.3) | 953 (47.2) |
| Grade 1/2 | 181 (27.0) | 114 (24.1) | 145 (27.0) | 440 (26.2) | 363 (43.7) | 298 (41.7) | 222 (46.7) | 883 (43.7) |
| Grade 3/4 | 40 (6.0) | 23 (4.9) | 25 (4.6) | 88 (5.2) | 54 (6.5) | 49 (6.9) | 26 (5.5) | 129 (6.4) |
| Missing | 6 (0.9) | 5 (1.1) | 4 (0.7) | 15 (0.9) | 24 (2.9) | 24 (3.4) | 7 (1.5) | 55 (2.7) |
| Neutropenia | ||||||||
| Grade 0 | 549 (81.9) | 381 (80.5) | 400 (74.3) | 1330 (79.1) | 587 (70.6) | 495 (69.3) | 291 (61.3) | 1373 (68.0) |
| Grade 1/2 | 82 (12.2) | 67 (14.2) | 112 (20.8) | 261 (15.5) | 185 (22.3) | 155 (21.7) | 152 (32.0) | 492 (24.4) |
| Grade 3/4 | 31 (4.6) | 20 (4.2) | 20 (3.7) | 71 (4.2) | 33 (4.0) | 39 (5.5) | 23 (4.8) | 95 (4.7) |
| Missing | 8 (1.2) | 5 (1.1) | 6 (1.1) | 19 (1.1) | 26 (3.1) | 25 (3.5) | 9 (1.9) | 60 (3.0) |
| Psychiatric adverse events | ||||||||
| Grade 0 | 619 (92.4) | 406 (85.8) | 500 (92.9) | 1525 (90.7) | 747 (89.9) | 621 (87.0) | 434 (91.4) | 1802 (89.2) |
| Grade 1/2 | 43 (6.4) | 61 (12.9) | 37 (6.9) | 141 (8.4) | 77 (9.3) | 87 (12.2) | 38 (8.0) | 202 (10.0) |
| Grade 3/4 | 8 (1.2) | 6 (1.3) | 1 (0.2) | 15 (0.9) | 7 (0.8) | 6 (0.8) | 3 (0.6) | 16 (0.8) |
| Fatigue | ||||||||
| Grade 0 | 507 (75.7) | 330 (69.8) | 400 (74.3) | 1237 (73.6) | 680 (81.8) | 540 (75.6) | 397 (83.6) | 1617 (80.0) |
| Grade 1/2 | 148 (22.1) | 124 (26.2) | 124 (23.0) | 396 (23.6) | 137 (16.5) | 156 (21.8) | 70 (14.7) | 363 (18.0) |
| Grade 3/4 | 15 (2.2) | 19 (4.0) | 14 (2.6) | 48 (2.9) | 14 (1.7) | 18 (2.5) | 8 (1.7) | 40 (2.0) |
| Nausea or emesis | ||||||||
| Grade 0 | 443 (66.1) | 337 (71.2) | 349 (64.9) | 1129 (67.2) | 546 (65.7) | 504 (70.6) | 323 (68.0) | 1373 (68.0) |
| Grade 1/2 | 219 (32.7) | 131 (27.7) | 181 (33.6) | 531 (31.6) | 276 (33.2) | 203 (28.4) | 144 (30.3) | 623 (30.8) |
| Grade 3/4 | 8 (1.2) | 5 (1.1) | 8 (1.5) | 21 (1.2) | 9 (1.1) | 7 (1.0) | 8 (1.7) | 24 (1.2) |
| Use of AED at the start of concomitant treatment (dataset S, trials CENTRIC, CORE) | AED use at the start of maintenance treatment (dataset S,trials CENTRIC, CORE, ACT-IV) | |||||||
|---|---|---|---|---|---|---|---|---|
| No AED (N = 297) | LEV with or without other AED (N = 233) | Other AED with or without other AED (N = 241) | Total dataset S (N = 771) | No AED (N = 535) | LEV with or without other AED (N = 513) | Other AED with or without other AED (N = 245) | Total dataset S (N = 1293) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Lymphopenia | ||||||||
| Grade 0 | 46 (15.5) | 42 (18.0) | 44 (18.3) | 132 (17.1) | 86 (16.1) | 93 (18.1) | 43 (17.6) | 222 (17.2) |
| Grade 1/2 | 167 (56.2) | 128 (54.9) | 143 (59.3) | 438 (56.8) | 296 (55.3) | 270 (52.6) | 144 (58.8) | 710 (54.9) |
| Grade 3/4 | 78 (26.3) | 62 (26.6) | 50 (20.7) | 190 (24.6) | 131 (24.5) | 128 (25.0) | 52 (21.2) | 311 (24.1) |
| Missing | 6 (2.0) | 1 (0.4) | 4 (1.7) | 11 (1.4) | 22 (4.1) | 22 (4.3) | 6 (2.4) | 50 (3.9) |
1 AED antiepileptic drug.
2 LEV levetiracetam.
AED Use is not Associated with Hematologic Adverse Events
There were no significant associations of a LEV-containing regimen or use of other AED compared with no AED with any of the hematologic adverse events of interest, i.e. with any grade of anemia, severe thrombocytopenia, severe neutropenia, and severe lymphopenia during concomitant or maintenance treatment with temozolomide on univariable analysis (Supplementary Table S4). Similarly, no associations of use of LEV or other AED versus no AED with hematologic adverse events during concomitant and maintenance treatment were found on multivariable analysis with adjustments for sex, age, MGMT status, WHO performance status, and steroid use at baseline and at the start of maintenance treatment, respectively (data not shown).
LEV Use is Associated with Reduced Nausea or Emesis During Maintenance Treatment
We observed no significant association of AED use at the start of concomitant treatment with any grade of nausea or emesis during concomitant treatment (LEV-containing regimen: P = .075, other AED: P = .645) (Table 4). In adjusted multivariable analysis, the risk of nausea or emesis during concomitant treatment was higher among females (P < .001), whereas patients aged 55 years or older had a lower risk of nausea or emesis during concomitant treatment (P = .015). At the start of maintenance treatment, on univariable analysis, there was no significant association of nausea or emesis during maintenance treatment with LEV (P = .039) or with other AED (P = .415) compared to no AED. On adjusted multivariable analysis, risk of nausea or emesis during maintenance treatment was lower in patients with use of LEV (P = .017) and in patients aged 55 years or older (P < .001). Steroid use was included in adjusted analysis and did not represent an independent factor associated with nausea or emesis (P = .563). Furthermroe, there was no interaction between the use of AED (including LEV) and steroid use at the start of maintenance treatment with nausea or emesis during maintenance treatment (P = .494). We next explored whether reduced risk of nausea or emesis associated with use of LEV might result from differences in temozolomide exposure. However, we did not observe differences regarding the cumulative dose of temozolomide per cycle in the three groups defined by AED use (Supplementary Table S5).
Table 4.
Risk of nausea or emesis during concomitant treatment and maintenance treatment and use of antiepileptic drug at the start of concomitant and maintenance treatment
| Total N (%) |
Any nausea/emesisN (%) | No nausea/emesis N (%) | Relative risk (97.5% CI) | P-value | Total N (%) |
Any nausea/emesis N (%) | No nausea/emesis N (%) | Hazard ratio (97.5% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concomitant treatment (unadjusted analysis) | Maintenance treatment (unadjusted analysis) | ||||||||||
| AED use | 1681 (100) | 552 (32.8) | 1129 (67.2) | AED use | 2020 (100) | 647 (32.0) | 1373 (68.0) | ||||
| No AED1 | 670 (39.9) | 227 (33.9) | 443 (66.1) | 1 | No AED | 831 (41.1) | 285 (34.3) | 546 (65.7) | 1 | ||
| LEV2 with or without other AED | 473 (28.1) | 136 (28.8) | 337 (71.2) | 0.85 (0.69–1.04) | .075 | LEV with or without other AED | 714 (35.4) | 210 (29.4) | 504 (70.6) | 0.83 (0.68–1.02) | .039 |
| Other AED with or without other AED | 538 (32.0) | 189 (35.1) | 349 (64.9) | 1.04 (0.87–1.24) | .645 | Other AED with or without other AED | 475 (23.5) | 152 (32.0) | 323 (68.0) | 0.92 (0.73–1.16) | .415 |
| Concomitant treatment (adjusted analysis) | Maintenance treatment (adjusted analysis) | ||||||||||
| AED use | 1676 (100) | 549 (32.8) | 1127 (67.2) | AED use | 1977 (100) | 631 (31.9) | 1346 (68.1) | ||||
| No AED | 667 (39.8) | 225 (33.7) | 442 (66.3) | 1 | No AED | 818 (41.4) | 279 (34.1) | 539 (65.9) | 1 | ||
| LEV with or without other AED | 472 (28.2) | 136 (28.8) | 336 (71.2) | 0.88 (0.72–1.07) | .145 | LEV with or without other AED | 693 (35.1) | 203 (29.3) | 490 (70.7) | 0.80 (0.65–0.99) | .017 |
| Other AED with or without other AED | 537 (32.0) | 188 (35.0) | 349 (65.0) | 1.03 (0.87–1.23) | .680 | Other AED with or without other AED | 466 (23.6) | 149 (32.0) | 317 (68.0) | 0.88 (0.70–1.11) | .220 |
| Sex | Sex | ||||||||||
| Male | 1002 (59.8) | 294 (29.3) | 708 (70.7) | 1 | Male | 1243 (62.9) | 372 (29.9) | 871 (70.1) | 1 | ||
| Female | 674 (40.2) | 255 (37.8) | 419 (62.2) | 1.29 (1.11–1.51) | < .001 | Female | 734 (37.1) | 259 (35.3) | 475 (64.7) | 1.19 (0.99–1.43) | .032 |
| Age group | Age group | ||||||||||
| < 55 years | 697 (41.6) | 253 (36.3) | 444 (63.7) | 1 | < 55 years | 811 (41.0) | 303 (37.4) | 508 (62.6) | 1 | ||
| ≥ 55 years | 979 (58.4) | 296 (30.2) | 683 (69.8) | 0.84 (0.72-0.99) | .015 | ≥ 55 years | 1166 (59.0) | 328 (28.1) | 838 (71.9) | 0.69 (0.58-0.83) | < .001 |
| WHO performance status | WHO performance status | ||||||||||
| 0 | 1051 (62.7) | 357 (34.0) | 694 (66.0) | 1 | 0 | 1105 (55.9) | 353 (31.9) | 752 (68.1) | 1 | ||
| > 0 | 625 (37.3) | 192 (30.7) | 433 (69.3) | 0.90 (0.77–1.07) | .170 | > 0 | 872 (44.1) | 278 (31.9) | 594 (68.1) | 1.02 (0.84–1.23) | .827 |
| MGMT promoter status | MGMT promoter status | ||||||||||
| Unmethylated | 707 (42.2) | 223 (31.5) | 484 (68.5) | 1 | Unmethylated | 957 (48.4) | 296 (30.9) | 661 (69.1) | 1 | ||
| Methylated | 751 (44.8) | 266 (35.4) | 485 (64.6) | 1.09 (0.92–1.28) | .247 | Methylated | 798 (40.4) | 271 (34.0) | 527 (66.0) | 1.09 (0.89–1.33) | .331 |
| Unknown | 218 (13.0) | 60 (27.5) | 158 (72.5) | 0.87 (0.66–1.15) | .263 | Unknown | 222 (11.2) | 64 (28.8) | 158 (71.2) | 0.88 (0.65–1.21) | .378 |
| Steroid use at baseline | Steroid use at the start of maintenance treatment | ||||||||||
| No | 982 (58.6) | 335 (34.1) | 647 (65.9) | 1 | No | 1035 (52.4) | 329 (31.8) | 706 (68.2) | 1 | ||
| Yes | 694 (41.4) | 214 (30.8) | 480 (69.2) | 0.96 (0.82–1.13) | .571 | Yes | 942 (47.6) | 302 (32.1) | 640 (67.9) | 1.05 (0.87–1.26) | .563 |
| Antidepressants use at baseline | Antidepressant use at the start of maintenance treatment | ||||||||||
| No | 1530 (91.3) | 503 (32.9) | 1027 (67.1) | 1 | No | 1787 (90.4) | 559 (31.3) | 1228 (68.7) | 1 | ||
| Yes | 146 (8.7) | 46 (31.5) | 100 (68.5) | 1.01 (0.76–1.33) | .953 | Yes | 190 (9.6) | 72 (37.9) | 118 (62.1) | 1.28 (0.97–1.69) | .046 |
1 AED antiepileptic drug.
2 LEV levetiracetam.
AED Use is not Associated with Fatigue During Concomitant and Maintenance Treatment with Temozolomide
We next assessed whether any grade of fatigue was associated with AED use (Supplementary Table S6). At the predefined 2.5% significance level, there was a non-significant trend for an association of a LEV-containing regimen compared to no AED with higher risk of fatigue during concomitant and maintenance treatment in unadjusted (P = .025 and .029) and adjusted analyses (P = .101 and P = .041) (Supplementary Table S6). The use of other AED was not associated with fatigue during concomitant and maintenance treatment either (unadjusted: P = .603 and P = .869; adjusted: P = .369 and P = .662).
Psychiatric Adverse Events are Associated with Use of LEV During Concomitant Treatment but not During Maintenance Treatment
The overall incidence of any psychiatric adverse event was 9.3% during concomitant treatment and 10.8% during maintenance treatment. There was an association of psychiatric adverse events during concomitant treatment with use of LEV-containing regimen (P < .001) but not with other AED (P = .744) compared to no AED (Table 5). A higher risk for psychiatric adverse events during concomitant treatment associated with LEV use was confirmed on adjusted analysis (P < .001) with WHO performance status > 0 as a covariable associated with increased risk of psychiatric adverse events (P = .004). However, LEV use at the start of maintenance was not associated with psychiatric adverse events during maintenance treatment on unadjusted and adjusted analysis. There was a higher risk for psychiatric adverse events for patients with WHO performance status > 0 (P = .009) and for patients that used steroids (P = .004), each at the beginning of maintenance treatment. We next explored whether any changes in AED use on the individual patient level or resolvement of adverse events were related to the observation that use of LEV was associated with psychiatric adverse events during concomitant, but not during maintenance treatment. To assess this, we performed subgroup analyses in the patient cohort of CENTRIC, CORE, and AVAglio during maintenance treatment since no data for concomitant treatment were available for ACT-IV. First, we confirmed that LEV was not associated with psychiatric adverse during maintenance treatment in this subcohort, too, both in unadjusted and adjusted analyses (P = .427 and P = .656). To assess the association of psychiatric adverse events on the individual patient level during concomitant and maintenance treatment, data from 1314 patients included in the maintenance phase of CENTRIC, CORE and AVAglio were available. Among them, 110 patients (8.4%) had grade 1 or 2 psychiatric adverse events during concomitant treatment, while 7 patients (0.5%) had grade 3 or 4 severe psychiatric adverse events. Of the 117 patients who had any psychiatric adverse event during concomitant treatment, the adverse event resolved during maintenance treatment in 99 patients (84.6%), one patient (0.1%) had an increase to severe adverse event, and in 17 patients (14.5%) the worst grade psychiatric adverse event remained unchanged. We next asked whether the dose of LEV was reduced or LEV was stopped in patients with adverse events during the concomitant treatment. Since the dose of AED was not recorded in AVAglio, only data from CENTRIC and CORE were assessed. Of 44 patients with psychiatric adverse events during concomitant treatment, LEV was continued at the start of maintenance in 25 patients (56.8%), among them without dose reduction in 23 (92.0%) patients, and 2 (8.0%) patients with dose increase. LEV was stopped and no other AED was used at the start of maintenance treatment in 16 patients (36.4%), and in 3 patients (6.8%) LEV was replaced by another AED.
Table 5.
Risk of psychiatric adverse events during concomitant treatment and maintenance treatment and use of antiepileptic drug at the start of concomitant and maintenance treatment
| Total N (%) |
Any psychiatric adverse events N (%) |
No psychiatric adverse events N (%) |
Relative risk (97.5% CI) | P-value | Total N (%) |
Any psychiatric adverse events N (%) |
No psychiatric adverse events N (%) | Hazard ratio (97.5% CI) | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concomitant treatment (unadjusted analysis) | Maintenance treatment (unadjusted analysis) | ||||||||||
| AED use | 1681 (100) | 156 (9.3) | 1525 (90.7) | AED use | 2020 (100) | 218 (10.8) | 1802 (89.2) | ||||
| No AED1 | 670 (39.9) | 51 (7.6) | 619 (92.4) | 1 | No AED | 831 (41.1) | 84 (10.1) | 747 (89.9) | 1 | ||
| LEV2 with or without other AED | 473 (28.1) | 67 (14.2) | 406 (85.8) | 1.86 (1.26–2.75) | < .001 | LEV with or without other AED | 714 (35.4) | 93 (13.0) | 621 (87.0) | 1.18 (0.84–1.66) | .277 |
| Other AED with or without other AED | 538 (32.0) | 38 (7.1) | 500 (92.9) | 0.93 (0.56–1.55) | .744 | Other AED with or without other AED | 475 (23.5) | 41 (8.6) | 434 (91.4) | 0.99 (0.64–1.54) | .971 |
| Concomitant treatment (adjusted analysis) | Maintenance treatment (adjusted analysis) | ||||||||||
| AED use | 1676 (100) | 156 (9.3) | 1520 (90.7) | AED use | 1977 (100) | 213 (10.8) | 1764 (89.2) | ||||
| No AED | 667 (39.8) | 51 (7.6) | 616 (92.4) | 1 | No AED | 818 (41.4) | 83 (10.1) | 735 (89.9) | 1 | ||
| LEV with or without other AED | 472 (28.2) | 67 (14.2) | 405 (85.8) | 1.88 (1.30–2.73) | <.001 | LEV with or without other AED | 693 (35.1) | 90 (13.0) | 603 (87.0) | 1.09 (0.77–1.54) | .571 |
| Other AED with or without other AED | 537 (32.0) | 38 (7.1) | 499 (92.9) | 0.84 (0.49–1.43) | .469 | Other AED with or without other AED | 466 (23.6) | 40 (8.6) | 426 (91.4) | 0.94 (0.60–1.46) | .741 |
| Sex | Sex | ||||||||||
| Male | 1002 (59.8) | 87 (8.7) | 915 (91.3) | 1 | Male | 1243 (62.9) | 128 (10.3) | 1115 (89.7) | 1 | ||
| Female | 674 (40.2) | 69 (10.2) | 605 (89.8) | 1.19 (0.86–1.64) | .230 | Female | 734 (37.1) | 85 (11.6) | 649 (88.4) | 1.11 (0.81–1.52 | .470 |
| Age group | Age group | ||||||||||
| < 55 years | 697 (41.6) | 62 (8.9) | 635 (91.1) | 1 | < 55 years | 811 (41.0) | 80 (9.9) | 731 (90.1) | 1 | ||
| ≥ 55 years | 979 (58.4) | 94 (9.6) | 885 (90.4) | 0.93 (0.67-1.29) | .627 | ≥ 55 years | 1166 (59.0) | 133 (11.4) | 1033 (88.6) | 1.08 (0.79–1.49) | .568 |
| WHO performance status | WHO performance status | ||||||||||
| 0 | 1051 (62.7) | 85 (8.1) | 966 (91.9) | 1 | 0 | 1105 (55.9) | 90 (8.1) | 1015 (91.9) | 1 | ||
| > 0 | 625 (37.3) | 71 (11.4) | 554 (88.6) | 1.53 (1.10–2.13) | .004 | > 0 | 872 (44.1) | 123 (14.1) | 749 (85.9) | 1.45 (1.05–2.00) | .009 |
| MGMT promoter status | MGMT promoter status | ||||||||||
| Unmethylated | 707 (42.2) | 72 (10.2) | 635 (89.8) | 1 | Unmethylated | 957 (48.4) | 106 (11.1) | 851 (88.9) | 1 | ||
| Methylated | 751 (44.8) | 75 (10.0) | 676 (90.0) | 1.00 (0.72–1.35) | .943 | Methylated | 798 (40.4) | 90 (11.3) | 708 (88.7) | 1.05 (0.75–1.47) | .758 |
| Unknown | 218 (13.0) | 9 (4.1) | 209 (95.9) | 0.38 (0.12–1.16) | .051 | Unknown | 222 (11.2) | 17 (7.7) | 205 (92.3) | 0.86 (0.48–1.55) | .575 |
| Steroid use at baseline | Steroid use at the start of maintenance treatment | ||||||||||
| No | 982 (58.6) | 91 (9.3) | 891 (90.7) | 1 | No | 1035 (52.4) | 90 (8.7) | 945 (91.3) | 1 | ||
| Yes | 694 (41.4) | 65 (9.4) | 629 (90.6) | 0.95 (0.69–1.31) | .739 | Yes | 942 (47.6) | 123 (13.1) | 819 (86.9) | 1.51 (1.10–2.07) | .004 |
| Antidepressants use at baseline | Antidepressant use at the start of maintenance treatment | ||||||||||
| No | 1530 (91.3) | 138 (9.0) | 1392 (91.0) | 1 | No | 1787 (90.4) | 189 (10.6) | 1598 (89.4) | 1 | ||
| Yes | 146 (8.7) | 18 (12.3) | 128 (87.7) | 1.06 (0.64–1.74) | .797 | Yes | 190 (9.6) | 24 (12.6) | 166 (87.4) | 1.02 (0.63–1.66) | .914 |
1 AED antiepileptic drug.
2 LEV levetiracetam.
Discussion
We studied the toxicity profile of a LEV-based regimen or other AED compared with no AED in the context of temozolomide-based chemoradiotherapy in a pooled patient cohort from four prospective clinical trials in newly diagnosed glioblastoma. Use of a LEV-containing regimen at the start of concomitant treatment was associated with a higher risk of psychiatric adverse events during concomitant treatment on univariable and multivariable analyses [Relative risk (RR) 1.68 and 1.88, P < .001], while there was no association of AED use with hematologic toxicity, nausea or emesis, or fatigue (Tables 4, 5 and Supplementary Tables S4, S6). Use of a LEV-containing regimen at the beginning of maintenance temozolomide was associated with reduced risk of nausea/vomiting during maintenance treatment on multivariable analysis [Hazard ratio (HR) 0.8, P = .017] while there were no associations of LEV use with risk of hematologic toxicity, fatigue, or psychiatric adverse events (Tables 4, 5 and Supplementary Tables S4, S6).
Although severe hematologic toxicity associated with LEV, mainly thrombocytopenia, has been reported in several case reports,14–17,33 the combination of LEV with temozolomide-based chemoradiotherapy does not seem to augment the incidence of hematologic adverse events (Supplementary Table S4).
Our data support antiemetic properties of LEV suggested by a case report34 and a retrospective study reporting less chemotherapy-induced nausea and vomiting associated with use of LEV in patients with glioblastoma.19 Limited by the study design, we cannot exclude that imbalances between groups such as steroid use with the potential to exert antiemetic effects (Table 2) may have contributed to the reduced risk of nausea/vomiting during associated with LEV during maintenance treatment. However, this was observed in multivariable analysis adjusted for risk factors relevant in the context of nausea and emesis including age, sex and use of steroids (Table 4). Furthermore, there was no statistical significant interaction between use of AED and use of steroids. However, since the study design did not allow to control for variations of steroid use during the maintenance phase, use of steroids may still represent a confounder regarding the incidence of nausea and emesis. A retrospective series of patients with cycling vomiting syndrome also indicated antiemetic properties of LEV.35 In a placebo-controlled randomized trial in patients with generalized epilepsy, using LEV as adjunctive antiepileptic drug, nausea was rarely reported, but interestingly less frequently in patients treated with LEV, i.e. in 3 of 79 patients (3.8%) versus 7 of 84 patients (8.3%) treated with placebo.36 Given the moderate emetogenic potential of temozolomide,18 chemotherapy-induced nausea and vomiting usually is manageable by serotonin receptor (5-HT-3) antagonists and/or dopamine antagonists. However, in some patients, additional options of antiemetic treatment are necessary. In this context, antiemetic properties of LEV may be beneficial and impact the choice of AED in selected patients. The mechanism of action by which LEV may exert antiemetic effects is unknown. Beyond the antiepileptic properties of LEV mediated by the SV2A protein,5–7 effects on glutamate release upon by targeting presynaptic P/Q-type voltage-dependent calcium channels were suggested as mechanism of action.8 Glutamate receptor signaling in the central nucleus of the amygdala was shown to be involved in cisplatin-induced malaise of rodents.37 We hypothesize that antiemetic properties of LEV in the context of chemotherapy may be interpreted by anti-glutamatergic effects.
We further explored whether fatigue was associated with use of LEV in the context of chemoradiotherapy (Supplementary Table S6) and observed only a non-significant trend towards higher risk of fatigue associated with LEV during concomitant and maintenance treatment in unadjusted and adjusted analyses. Fatigue represents a common side effect of several AED and is a common comorbidity in brain tumor patients especially in the context of radiotherapy. In a meta-analysis of 26 randomized placebo-controlled trials in patients receiving LEV for various indications, somnolence and a composite item of asthenia and fatigue were significantly associated with LEV. Similarly, the SANAD II trial, comparing LEV and zonisamide with lamotrigine as first-line treatment for patients with newly diagnosed focal epilepsy, reported fatigue as part of a composite item of general disorders to be more common in patients receiving LEV or zonisamide versus in patients receiving lamotrigine.11 Extrapolation of these results to patients with brain tumor-associated epilepsy is limited given that the paucity of data derived from comparative studies on AED in brain tumor patients.4,38 In a historical study switching patients with gliomas from phenytoin to LEV for control of postoperative seizures, the percentages of patients with somnolence were comparable.39 A randomized trial reported somnolence as measured by an increase of ≥ 5 points in the Epworth sleepiness scale in 11 of 25 patients (44%) versus 6 of 27 patients (22%) with brain tumors receiving either LEV or pregabalin for epilepsy after switch from phenytoin.40 Despite limited data regarding the choice of the optimal agent, consideration of concomitant medications including AED as potential treatable contributing factor for fatigue is recommended.4,41
Neuropsychiatric side effects represent one of the major concerns in the treatment of epilepsy with LEV.11,42 In our cohort, we observed an association of the use of a LEV-containing regimen with psychiatric adverse events during concomitant but not during maintenance treatment, while the overall incidence of psychiatric adverse events with 9.3% and 10.8% during concomitant and maintenance treatment was comparable (Table 5). Risk-adjusted analysis suggests steroid use as a cofactor associated with psychiatric adverse events during maintenance but not concomitant treatment. Psychiatric disorders represent common side effects of steroids in brain tumor patients.4,41 Importantly, we acknowledge that interpretation of data is limited because variations of use of steroids and use of antidepressants during the period of concomitant and during maintenance therapy may not be excluded as potential confounders regarding the incidence of psychiatric adverse events. At the start of maintenance, LEV had been discontinued in 16 patients (36.4%), and it was replaced by another AED in 3 patients (6.8%). Yet, it remains undetermined whether psychiatric morbidity was the cause for discontinuation of LEV since for most patients AED were stopped altogether and LEV was replaced by other AED only for 6.8% of patients. It may not be excluded that patients prone to psychiatric side effects of LEV have been taken off the drug during the concomitant phase which may explain that LEV was not associated with psychiatric adverse events during maintenance. Data of prospective comparative studies of AED in patients with brain tumors are limited. In a small prospective trial randomizing patients with brain tumor-related epilepsy treated with phenytoin to LEV or pregabalin, adverse events included anxiety and irritability similarly distributed in both groups while depression seemed more prevalent in patients receiving LEV.40 Limited by small patient numbers and lack of statistical comparison, conclusions remain elusive. In cohorts of patients with epilepsy of various origin, replacement of LEV by brivaracetam may be beneficial given an improvement of psychiatric adverse events in a range of 33–83% of patients based 4 retrospective and 1 observational studies, however, spontaneous improvement or placebo effects may not be ruled out by this design.43–48 Lamotrigine may be another alternative to LEV since psychiatric disorders were less frequently reported in a prospective cohort of patients with newly diagnosed focal epilepsy,11 however, whether this finding may be extrapolated to brain tumor patients is debatable.
We acknowledge that our study has several limitations. Although all trials were prospective randomized studies, the main limitation of this study is the post hoc analysis of the association of AED use with adverse events, which was not a predefined endpoint of the respective trials. Based on the study design, limitations of our analysis include that information on indications of AED use, on seizure incidence and seizure control were not available as well as a possible overlap of adverse events induced by epileptic seizures and side effects of the AED. Further limitations include that use of AED varied during the study and the group of other AED including different type of drugs is heterogeneous. Another limitation is that isocitrate dehydrogenase (IDH)-1/2 mutation status was not reported in the respective trials and therefore, we cannot exclude that some of the tumors reported as glioblastoma may be astrocytoma, IDH mutant, WHO grade 4 as defined by the current WHO classification 2021.49 In addition, for some readouts patient numbers with adverse events were small.
Here we explored the clinically highly relevant question whether use of LEV affects the tolerability of temozolomide-based chemoradiotherapy in patients with glioblastoma. Our data suggest antiemetic properties of LEV, which may be exploited in patients suffering from chemotherapy-induced nausea and emesis. In summary, LEV does not decrease tolerability of temozolomide-based chemoradiotherapy in patients with glioblastoma except for a higher risk of psychiatric adverse events in the early phase, i.e. during concomitant, treatment. In conclusion, the choice of AED for patients with glioblastoma and seizures should consider comorbidities and clinical context.
Supplementary Material
Contributor Information
Katharina Seystahl, Department of Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Felix Boakye Oppong, EORTC Headquarters, Brussels, Belgium.
Emilie Le Rhun, Department of Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland; Department of Neurosurgery, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Caroline Hertler, Department of Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Roger Stupp, Malnati Brain Tumor Institute of the Lurie Comprehensive Cancer Center and Departments of Neurosurgery and Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Burt Nabors, University of Alabama at Birmingham, Department of Neurology, Division of Neuro-Oncology, Birmingham, AL, USA.
Olivier Chinot, Aix-Marseille University, AP-HM, Service de Neuro-Oncologie, CHU Timone, Marseille, France.
Matthias Preusser, Division of Oncology, Department of Medicine 1, Medical University of Vienna, Vienna, Austria.
Thierry Gorlia, EORTC Headquarters, Brussels, Belgium.
Michael Weller, Department of Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Funding
This publication was supported by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group. We are grateful to Merck KGaA for supporting the independent EORTC Study 26071. Felix B. Oppong’s work as Fellow at EORTC Headquarters was supported by a grant from the EORTC Brain Tumor Group. We are grateful to Merk KGaA and Roche for providing access to the CORE and AVAGLIO studies. KS was supported by a personal grant (“Filling the gap”) of the University of Zurich, Switzerland and “Walter and Gertrud Siegenthaler Foundation” (no grant number is applicable). CH was supported by a personal grant (“Filling the gap”) of the University of Zurich (no grant number is applicable).
Conflict of Interest
ELR has received honoraria for lectures or advisory board from Adastra, Bayer, Leo Pharma, Seattle Genetics. BN received honoraria for advisory board participation from Karyopharm and Chimerix and serves on data safety and monitoring board for the University of Pennsylvania. OC has received honoraria for lectures or advisory board from AbbVie and Bristol-Myers Squibb. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp and Dome, Tocagen, Adastra, Gan and Lee Pharmaceuticals. MW has received research grants from Apogenix, Merck, Sharp and Dohme, Merck (EMD) and Quercis, and honoraria for lectures or advisory board participation or consulting from Adastra, Bristol Meyer Squibb, Medac, Merck, Sharp and Dohme, Merck (EMD), Nerviano Medical Sciences, Novartis, Orbus, Philogen and yMabs. The other authors report no conflicts of interest.
Authorship statement
Study conception and design: KS, MW. Contribution of clinical trial data: RS, BN, OC, JS, MW. Analysis and interpretation of data: KS, ELR, MP, MW. Statistical analysis: FBO, TG. Writing the first draft of the manuscript: KS, MW. Supervision of the project: MW. Review, editing and approval of the manuscript: all authors.
References
- 1. Walbert T, Harrison RA, Schiff D, et al. . SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro-oncology. 2021;23(11):1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maschio M, Beghi E, Casazza MML, et al. . Patterns of care of brain tumor-related epilepsy. A cohort study done in Italian Epilepsy Center. PLoS One. 2017; 12(7):e0180470. doi: 10.1371/journal.pone.0180470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Julie DAR, Ahmed Z, Karceski SC, et al. . An overview of anti-epileptic therapy management of patients with malignant tumors of the brain undergoing radiation therapy. Seizure. 2019; 70:30–37. [DOI] [PubMed] [Google Scholar]
- 4. Roth P, Pace A, Le Rhun E, et al. . Neurological and vascular complications of primary and secondary brain tumours: EANO-ESMO clinical practice guidelines for prophylaxis, diagnosis, treatment and follow-up†. Ann Oncol. 2021; 32(2):171–182. [DOI] [PubMed] [Google Scholar]
- 5. Lynch BA, Lambeng N, Nocka K, et al. . The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004; 101(26):9861–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vogl C, Mochida S, Wolff C, Whalley BJ, Stephens GJ. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol. 2012; 82(2):199–208. [DOI] [PubMed] [Google Scholar]
- 7. Deshpande LS, Delorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol. 2014; 5:11. doi: 10.3389/fneur.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee CY, Chen CC, Liou HH. Levetiracetam inhibits glutamate transmission through presynaptic P/Q-type calcium channels on the granule cells of the dentate gyrus. Br J Pharmacol. 2009; 158(7):1753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagarkatti N, Deshpande LS, DeLorenzo RJ. Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci Lett. 2008; 436(3):289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Armstrong TS, Grant R, Gilbert MR, Lee JW, Norden AD. Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro-oncology. 2016; 18(6):779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marson A, Burnside G, Appleton R, et al. . The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021; 397(10282):1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scaringi C, De Sanctis V, Minniti G, Enrici RM. Temozolomide-related hematologic toxicity. Onkologie. 2013; 36(7-8):444–9. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–96 [DOI] [PubMed] [Google Scholar]
- 14. Alzahrani T, Kay D, Alqahtani SA, Makke Y, Lesky L, Koubeissi MZ. Levetiracetam-induced pancytopenia. Epilepsy Behav Case Rep. 2015; 4:45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elouni B, Ben Salem C, Biour M. Levetiracetam-induced pancytopenia. Ann Pharmacother. 2009; 43(5):985. [DOI] [PubMed] [Google Scholar]
- 16. Peer Mohamed B, Prabhakar P. Thrombocytopenia as an adverse effect of levetiracetam therapy in a child. Neuropediatrics. 2009; 40(5):e1243–e1. [DOI] [PubMed] [Google Scholar]
- 17. Meschede A, Runge U, Sabolek M. Thrombocytopenia during levetiracetam therapy. Epilepsy Res. 2008; 80(1):91–2. [DOI] [PubMed] [Google Scholar]
- 18. Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014; 722:197–202. [DOI] [PubMed] [Google Scholar]
- 19. Lee JW, Bromfield EB, Kesari S. Antiemetic properties of the antiepileptic drug levetiracetam. N Engl J Med. 2008; 359(17):1853. [DOI] [PubMed] [Google Scholar]
- 20. Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010; 116(11):2707–15 [DOI] [PubMed] [Google Scholar]
- 21. Mula M, von Oertzen TJ, Cock HR, Yogarajah M, Lozsadi DA, Agrawal N. Fatigue during treatment with antiepileptic drugs: a levetiracetam-specific adverse event? Epilepsy Behav. 2017; 72:17–21. [DOI] [PubMed] [Google Scholar]
- 22. Bedetti C, Romoli M, Maschio M, et al. . Neuropsychiatric adverse events of antiepileptic drugs in brain tumour-related epilepsy: an Italian multicentre prospective observational study. Eur J Neurol. 2017; 24(10):1283–1289 [DOI] [PubMed] [Google Scholar]
- 23. Mula M, Trimble MR, Yuen A, Liu RS, Sander JW. Psychiatric adverse events during levetiracetam therapy. Neurology. 2003; 61(5):704–6. [DOI] [PubMed] [Google Scholar]
- 24. Belcastro V, Pisani LR, Bellocchi S, et al. . Brain tumor location influences the onset of acute psychiatric adverse events of levetiracetam therapy: an observational study. J Neurol. 2017; 264(5):921–927. [DOI] [PubMed] [Google Scholar]
- 25. Stupp R, Hegi ME, Gorlia T, et al. . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014; 15(10):1100–8 [DOI] [PubMed] [Google Scholar]
- 26. Nabors LB, Fink KL, Mikkelsen T, et al. . Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro-oncology. 2015; 17(5):708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014; 370(8):709–22. [DOI] [PubMed] [Google Scholar]
- 28. Weller M, Butowski N, Tran DD, et al. . Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017; 18(10):1373–1385 [DOI] [PubMed] [Google Scholar]
- 29. Oken MM, Creech RH, Tormey DC, et al. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5(6):649–55. [PubMed] [Google Scholar]
- 30. Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous markov chains based on censored observations. Scand J Stat. 1978; 5(3):141–150. [Google Scholar]
- 31. Stegherr R, Beyersmann J, Jehl V, et al. . Survival analysis for AdVerse events with VarYing follow-up times (SAVVY): rationale and statistical concept of a meta-analytic study. Biom J. 2021; 63(3):650–670. [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94(446):496–509 [Google Scholar]
- 33. Kim J, Shin JW. Levetiracetam-induced thrombocytopenia in a patient with status epilepticus. Epileptic Disord Int Epilepsy J Videotape. 2017; 19(1):104–108. [DOI] [PubMed] [Google Scholar]
- 34. Lee JW, Bromfield E, Kesari S. Emesis responsive to levetiracetam. J Neurol Neurosurg Psychiatry. 2008; 79(7):847–849. [DOI] [PubMed] [Google Scholar]
- 35. Clouse RE, Sayuk GS, Lustman PJ, Prakash C. Zonisamide or levetiracetam for adults with cyclic vomiting syndrome: a case series. Clin Gastroenterol Hepatol. 2007; 5(1):44–48. [DOI] [PubMed] [Google Scholar]
- 36. Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U, Group ObotLNS. Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology. 2007; 69(18):1751–1760. [DOI] [PubMed] [Google Scholar]
- 37. Alhadeff AL, Holland RA, Nelson A, Grill HJ, De Jonghe BC. Glutamate receptors in the central nucleus of the amygdala mediate cisplatin-induced malaise and energy balance dysregulation through direct hindbrain projections. J Neurosci. 2015; 35(31):11094–11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol. 2012; 13(9):e375–82. [DOI] [PubMed] [Google Scholar]
- 39. Lim DA, Tarapore P, Chang E, et al. . Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: a randomized phase II pilot study. J Neuro-oncol. 2009; 93(3):349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossetti AO, Jeckelmann S, Novy J, Roth P, Weller M, Stupp R. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study. Neuro-oncology. 2013; 16(4):584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro-oncology. 2015; 17(4):488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen B, Choi H, Hirsch LJ, et al. . Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017; 76:24–31. [DOI] [PubMed] [Google Scholar]
- 43. Steinhoff BJ, Klein P, Klitgaard H, et al. . Behavioral adverse events with brivaracetam, levetiracetam, perampanel, and topiramate: a systematic review. Epilepsy Behav. 2021; 118:107939. doi: 10.1016/j.yebeh.2021.107939. [DOI] [PubMed] [Google Scholar]
- 44. Steinhoff BJ, Bacher M, Bucurenciu I, et al. . Real-life experience with brivaracetam in 101 patients with difficult-to-treat epilepsy-A monocenter survey. Seizure. 2017; 48:11–14. [DOI] [PubMed] [Google Scholar]
- 45. Steinig I, von Podewils F, Möddel G, et al. . Postmarketing experience with brivaracetam in the treatment of epilepsies: a multicenter cohort study from Germany. Epilepsia. 2017; 58(7):1208–1216. [DOI] [PubMed] [Google Scholar]
- 46. Villanueva V, López-González FJ, Mauri JA, et al. . BRIVA-LIFE-A multicenter retrospective study of the long-term use of brivaracetam in clinical practice. Acta neurologica Scandinavica. 2019; 139(4):360–368. [DOI] [PubMed] [Google Scholar]
- 47. Theochari E, Cock H, Lozsadi D, Galtrey C, Arevalo J, Mula M. Brivaracetam in adults with drug-resistant epilepsy and psychiatric comorbidities. Epilepsy Behav. 2019; 90:129–131. [DOI] [PubMed] [Google Scholar]
- 48. Hirsch M, Hintz M, Specht A, Schulze-Bonhage A. Tolerability, efficacy and retention rate of Brivaracetam in patients previously treated with Levetiracetam: a monocenter retrospective outcome analysis. Seizure. 2018; 61:98–103. [DOI] [PubMed] [Google Scholar]
- 49. Louis DN, Perry A, Wesseling P, et al. . The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.