Abstract
An aspartate kinase-deficient mutant of Thermus thermophilus, AK001, was constructed. The mutant strain did not grow in a minimal medium, suggesting that T. thermophilus contains a single aspartate kinase. Growth of the mutant strain was restored by addition of both threonine and methionine, while addition of lysine had no detectable effect on growth. To further elucidate the lysine biosynthetic pathway in T. thermophilus, lysine auxotrophic mutants of T. thermophilus were obtained by chemical mutagenesis. For all lysine auxotrophic mutants, growth in a minimal medium was not restored by addition of diaminopimelic acid, whereas growth of two mutants was restored by addition of α-aminoadipic acid, a precursor of lysine in biosynthetic pathways of yeast and fungi. A BamHI fragment of 4.34 kb which complemented the lysine auxotrophy of a mutant was cloned. Determination of the nucleotide sequence suggested the presence of homoaconitate hydratase genes, termed hacA and hacB, which could encode large and small subunits of homoaconitate hydratase, in the cloned fragment. Disruption of the chromosomal copy of hacA yielded mutants showing lysine auxotrophy which was restored by addition of α-aminoadipic acid or α-ketoadipic acid. All of these results indicated that in T. thermophilus, lysine was not synthesized via the diaminopimelic acid pathway, believed to be common to all bacteria, but via a pathway using α-aminoadipic acid as a biosynthetic intermediate.
Amino acid biosynthesis is regulated according to the availability of environmental nutrition. In bacteria, the biosynthetic pathway leading to lysine, methionine, and threonine is controlled at several steps by end products and/or their biosynthetic intermediates in a process called feedback inhibition. In this regard, regulation of aspartate kinase is the most important because the enzyme catalyzes the reaction of the first step in the amino acid biosynthetic pathway and therefore determines the overall flux toward biosynthesis of these amino acids (4). However, control of the flux is not the same among microorganisms producing these amino acids. Corynebacterium glutamicum, for example, is known to contain a single aspartate kinase, whose activity is concertedly inhibited by lysine and threonine (29). Escherichia coli and Bacillus subtilis, on the other hand, have three different aspartate kinases, each regulated by feedback inhibition or repression of gene expression (5, 19, 31). In this biosynthetic pathway, lysine and methionine/threonine biosyntheses are branched at l-aspartate 4-semialdehyde: in the former the compound is converted to 2,3-dihydrodipicolinic acid by dihydrodipicolinic acid synthase, and in the latter the compound is converted to l-homoserine by homoserine dehydrogenase (4). Lysine is then synthesized through several steps from 2,3-dihydrodipicolinic acid via diaminopimelic acid as an intermediate. Since diaminopimelic acid serves as not only a key intermediate in this pathway but also a component of the cell wall in most bacteria, the lysine biosynthetic pathway is usually referred to as the diaminopimelic acid pathway (4, 34).
A previous study showed that an aspartate kinase, product of the askAB genes from the extremely thermophilic bacterium Thermus flavus AT-62, was inhibited only by threonine (17). This could suggest the presence of another lysine-sensitive aspartate kinase in Thermus species. However, the strain did not grow in a minimal medium (unpublished results), indicating that it is auxotrophic for an unknown nutrition source. It was therefore difficult to examine the role of the aspartate kinase in lysine biosynthesis of T. flavus. We then characterized the askAB genes from Thermus thermophilus HB27 (13, 26), which could grow in a minimal medium (in contrast to T. flavus AT-62) and was often used for genetic analysis (13, 26). Phenotypic analysis of a disruptant of the chromosomal askAB genes and a mutant of T. thermophilus generated by a chemical reagent revealed that lysine is synthesized via α-aminoadipic acid as an intermediate but not diaminopimelic acid in T. thermophilus. This finding was further confirmed by direct measurement of homocitrate synthase activity in T. thermophilus cells and cloning of genes encoding homoaconitate hydratase. This study provides the first instance where lysine is not synthesized via diaminopimelic acid in bacteria.
MATERIALS AND METHODS
Enzymes and chemicals.
Restriction endonucleases, alkaline phosphatase, and T4 DNA ligase were purchased from Takara Shuzo (Kyoto, Japan), Ampli Taq Gold was purchased from Perkin-Elmer Japan (Urayasu, Japan), KOD polymerase was purchased from TOYOBO (Osaka, Japan), and oligonucleotides were purchased from Genset (Tokyo, Japan). A kit for nucleotide sequencing by the M13-dideoxynucleotide method (28) was obtained from Amersham Japan (Tokyo, Japan). ATP and other chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.) and Wako Pure Chemicals Co. (Tokyo, Japan).
Bacterial strains and plasmids.
T. thermophilus HB27 and its derivative TH104 (proC4) (7), as well as plasmid pUC19-39-442Kmr (15), which contains the heat-stable kanamycin nucleotidyltransferase (KNT) gene of Staphylococcus aureus (14), were kindly provided by T. Hoshino (Tsukuba University). E. coli JM105 [Δ(lac-pro) thi strA endA sbcB15 hsdR4 F′ traD36 proAB lacIq lacZΔM15] (27) was used for gene manipulation, and M13 phage propagation was used for determination of nucleotide sequence. E. coli GT3 (thrA1016 metL1005 lysC1004) (10), kindly provided by J.-C. Patte (Laboratoire de Chimie Bacterienne, Centre National de la Recherche Scientifique, Paris, France), was used for production of the aspartate kinase.
Plasmid pAKT101Kmr, which was used to disrupt the chromosomal copy of the askAB genes in T. thermophilus, was constructed as follows. PCR was performed with pUC19-39-442Kmr as the template and two synthetic oligonucleotides, 5′-AGCTTCCGTCGACAACATGAT-3′ and 5′-GGAAACAGCTATGACCATGATTAC-3′, as primers. The former was designed to contain a SalI site just downstream of the KNT-coding region. The PCR conditions were 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min, with a total of 36 cycles. After digestion of the amplified DNA with SalI and XhoI, the resulting restriction fragment of about 850 bp was ligated with the 3.7-kb fragment of pAKT101 digested with XhoI. The resulting plasmid, pAKT101Kmr, contains the KNT gene in the opposite direction from the aspartate kinase gene.
Plasmid pHACA101Kmr, which was used to disrupt the chromosomal copy of the hacA gene (open reading frame [ORF] C; see below) in T. thermophilus, was constructed as follows. PCR was performed with KOD polymerase, using pLYS200 (this study) as the template. The PCR conditions were 98°C for 15 s, 68°C for 2 s, and 74°C for 45 s, with a total of 25 cycles. Two oligonucleotides, 5′-CAGGAAACAGCTATGAC-3′ (M13-RV) and 5′-ACGTCTAGACCCGGATGCCGTGCCGCTTGC-3′, were used to amplify the DNA fragment (nucleotides [nt] 1 to 748) carrying an XbaI site around nt 745. After digestion with BamHI and XbaI, the amplified fragment of about 745 bp was subcloned into pUC18. The plasmid was digested with EcoRI and XbaI, and the resulting restriction fragment (fragment A) was purified by agarose gel electrophoresis. Two other oligonucleotides, 5′-ACCAAGCTTAGGGTGCCCGAGAGCGTCAAG-3′ and 5′-GGGGACGATCCCAAGCTTCACCAGGTTGCG-3′, were designed to amplify the DNA fragment (nt 919 to 2023). The amplified fragment was digested with HindIII and BglII, and the larger fragment (fragment B; nt 923 to 1634) was purified by agarose gel electrophoresis. pLYS200HP was constructed by partial digestion of pLYS200 with HindIII and successive end filling with Klenow fragment of DNA polymerase I to remove the HindIII site in the polylinkers derived from pUC18. pLYS200HP was digested with HindIII and BglII, and the larger fragment was ligated with fragment B. The plasmid was then digested with EcoRI and HindIII, and the larger fragment was ligated with two other DNA fragments, fragment A and the XbaI-HindIII fragment containing the KNT gene. The resulting plasmid was named pHACA101Kmr.
Cultivation of T. thermophilus and preparation of cell extract.
Thermus strains were cultivated at 70°C in TM medium (13) or MP medium, which is MM medium (32) supplemented with 1 mM proline. Cell extract of T. thermophilus was prepared as follows. T. thermophilus cells were aerobically cultured in MP medium for 12 h, collected by centrifugation at 10,000 × g for 5 min at 4°C, and washed once with 10 mM Tris-HCl (pH 7.5) buffer. The cells were resuspended in the same buffer and disrupted by sonication (Branson sonifier model 250D) in ice water. After centrifugation at 20,000 × g for 20 min at 4°C, supernatant was dialyzed against 10 mM Tris-HCl (pH 7.5) buffer at the same temperature.
DNA manipulation.
Total chromosomal DNAs of T. thermophilus and its derivative were prepared by the method of Saito and Miura (25). The nucleotide sequence was determined by the dideoxy-chain termination method using M13 phages (16, 28). All restriction sites used for cloning on M13 replicative form I DNA were verified by determination as part of an overlapping sequence. Plasmids were purified with Wizard SV Plus minipreps DNA purification system (Promega). Southern (30) and colony (6) hybridizations were performed with a Renaissance chemiluminescence kit (DuPont New England Nuclear).
Cloning of the aspartate kinase gene from T. thermophilus HB27 and production of the enzyme in E. coli cells.
A PstI fragment of approximately 1.6 kb containing the askAB genes from T. thermophilus HB27 was cloned into pUC18 with a portion of the askAB genes from T. flavus AT-62 as the probe for Southern and colony hybridization. Determination of the nucleotide sequence revealed that the amino acid sequences of the two Thermus aspartate kinases differed only at position 126 (Asp in T. flavus aspartate kinase and Glu in the T. thermophilus enzyme). To produce aspartate kinase of T. thermophilus in E. coli, plasmid pAKT202 was constructed by introducing only a small portion from T. thermophilus genes which directed the amino acid replacement into the corresponding region of pAKT102, the plasmid for expression of the askAB genes from T. flavus (17). Aspartate kinase was purified by heat treatment, ammonium sulfate precipitation, and gel filtration to homogeneity to give only two bands corresponding to α and β subunits on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (details will be reported elsewhere). Aspartate kinase from T. thermophilus was inhibited only by threonine, as was the case for the T. flavus enzyme.
Gene disruption of T. thermophilus.
T. thermophilus has a natural transformation system, and its efficiency reaches up to several percent per total viable cells counts for some auxotrophic markers (13). Gene disruption was carried out by the method of Kosuge and Hoshino (12). T. thermophilus TH104 was transformed with pAKT101Kmr by the method of Koyama et al. (13). Kanamycin-resistant colonies were selected as askAB disruptants, because kanamycin resistance was conferred by double-crossover homologous recombination between chromosome and pAK101Kmr, which could not replicate in Thermus cells. Disruption of hacA of T. thermophilus HB27 was performed with pHACA101Kmr in a similar way. Disruptions were confirmed by Southern hybridization.
Enzyme assay.
Specific activity of aspartate kinase was assayed by the method of Black and Wright (2). The reaction mixture contained 180 mM Tris-HCl (pH 7.5), 10 mM MgSO4 · 6H2O, 5 mM l-aspartic acid, 10 mM ATP (adjusted to pH 7.0 with KOH), 160 mM NH2OH · HCl (neutralized with KOH), and an appropriate amount of the enzyme solution. After incubation at 60°C for 15 min, the reaction was terminated by mixing with the same volume of 5% FeCl3 solution. The amount of product was measured by the absorbance at 540 nm with a molecular extinction coefficient of 600 (29). Homocitrate synthase activity was measured by the 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) method (22). The reaction mixture contained 200 mM Tris-HCl (pH 7.5), 5 mM MgSO4, 1 mM α-ketoglutarate, 0.5 mM acetyl coenzyme A (acetyl-CoA), and an appropriate amount of cell extract. After 30 min incubation at 60°C, DTNB was added at the final concentration of 0.1 mM, and the mixture was further incubated for 10 min at 37°C. One unit was defined as the amount of the enzyme that catalyzed the release of 1 μmol of CoA per min.
Isolation of lysine auxotrophic mutants.
Lysine auxotrophic mutants of T. thermophilus were obtained by random mutation with N-methyl-N′-nitro-N-nitrosoguanidine (NTG) (9). T. thermophilus TH104 cells were grown in 50 ml of TM medium at 70°C. The cells in logarithmic phase after cultivation for approximately 12 h were harvested, suspended in 1 ml of 20 mM sodium phosphate buffer (pH 7.0) containing 40 μg of NTG per ml, and incubated for additional 30 min at 70°C with shaking. The cells were then harvested, washed four times with phosphate-buffered saline containing 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4, and suspended in 50 ml of TM medium. After incubation at 70°C for 3 h, cells were diluted and spread on TM agar plates. After 3 days of incubation at 65°C, colonies were replicated onto MP medium with and without 1 mM lysine. Colonies grown on lysine containing medium but did not on MP medium were picked up.
Cloning of genes involved in lysine biosynthesis in T. thermophilus.
Chromosomal DNA of T. thermophilus HB27 was digested with BamHI, ligated with pUC18 digested with BamHI, and introduced into E. coli JM105. All ampicillin-resistant transformants were replicated onto new agar plates, and the transformants harboring the recombinant plasmids that carried a DNA fragment complementing the lysine auxotrophy of mutant K1004 (see below) were screened by the method of Hoshino et al. (8).
Nucleotide sequence accession number.
The nucleotide sequence reported here has been registered in the EMBL, GenBank, DDBJ databases under accession no. AB013131.
RESULTS
Characterization of aspartate kinase disruptant of T. thermophilus.
We obtained an askAB disruptant of T. thermophilus, termed AK001, as described in Materials and Methods and examined the effect of the askAB disruption on growth in MP medium. T. thermophilus TH104 grew in MP medium but T. thermophilus AK001 did not, which suggested the absence of the functional homologs of the cloned aspartate kinase which could suppress the disruption of the askAB genes in T. thermophilus cells. We next added lysine, methionine, threonine, or methionine plus threonine to MP medium and examined the effects on growth. The growth of AK001 was restored by addition of both methionine and threonine to MP medium (data not shown), suggesting that lysine is synthesized via a pathway independent of the diaminopimelic acid pathway.
Isolation of lysine auxotrophic mutants.
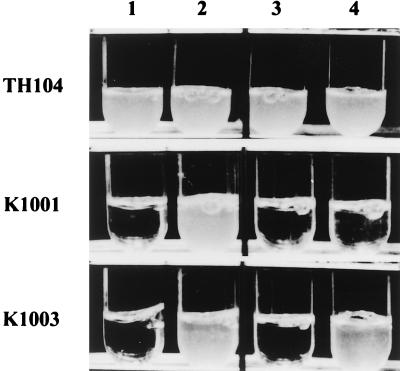
To examine lysine biosynthesis in Thermus species, NTG treatment was carried out to screen for lysine auxotrophic mutants. By this treatment, four lysine auxotrophic mutants (i.e., mutants which could not grow on MP medium without lysine) were isolated. For all the lysine auxotrophic mutants, growth was not restored by addition of diaminopimelic acid, which is known to be a biosynthetic intermediate for lysine in bacteria. On the other hand, two of the mutants, K1003 and K1004, grew on MP medium supplemented with α-aminoadipic acid, a precursor of lysine in biosynthetic pathways of fungi and yeast (Fig. 1).
FIG. 1.
Auxotrophic complementation test of T. thermophilus lysine mutants. Tubes: 1, MP medium; 2, MP medium plus 1 mM lysine; 3, MP medium plus 1 mM diaminopimelic acid; 4, MP medium plus 1 mM α-aminoadipic acid. Mutant K1001, which could not grow in a minimal medium even after addition of 1 mM α-aminoadipic acid, and mutant K1003, which grew after addition of 1 mM α-aminoadipic acid, are shown as examples. Auxotrophy of K1002 and K1004 was the same as that of K1001 and K1003, respectively.
Homocitrate synthase activity in T. thermophilus.
The foregoing results suggested the possibility that T. thermophilus synthesized lysine via a pathway similar to the α-aminoadipic acid pathway. We then measured the activity of homocitrate synthase in the cell extract of T. thermophilus, because homocitrate synthase was determined to be involved in lysine biosynthesis in yeast and fungi and was known to catalyze the first reaction in the pathway; the expected specific activity of 0.6 U per mg of protein was detected.
Cloning of genes involved in lysine biosynthesis in T. thermophilus.
We next cloned a DNA fragment which complemented lysine auxotrophy of mutant K1004. A single E. coli transformant was found to contain the recombinant plasmid which complemented the auxotrophy. The plasmid was recovered from the transformant, and the nucleotide sequence of the insert, the BamHI fragment of 4.34 kb, was determined (Fig. 2). The sequence analysis revealed that the cloned DNA fragment contained the DNA sequence (nt 1634 to 4342) previously reported for rimK and argC (12). Compared to the sequence previously reported, the sequence determined in this study had an insertion of G at nt 1974 and a deletion of G at 2727, suggesting typing or experimental errors in the earlier sequence. The nucleotide sequence of the 4.34-kb BamHI fragment contained six (or five) ORFs in addition to those for rimK and argC; two, ORFs A and B, were oriented in the direction opposite the others. ORFs A (nt 187 to 35; 51 amino acids [aa]) and B (nt 452 to 231; 74 aa) had sequences similar to those of ssr1766 and 1765 in Synechocystis sp. strain PCC6803, respectively, though their functions are unknown. An ORF (nt 41 to 445) coding for a protein of 135 amino acid residues whose amino acid sequence showed no homology to those of the proteins registered in the databases was found in the opposite strand of the region possibly encoding ORFs A and B. Considering the high G+C content in the third letter of codons in Thermus genes, it is likely that ORFs A and B encode proteins. However, we do not know which strand is transcribed in this region. The amino acid sequences of ORFs C (nt 475 to 1728; 418 aa) and D (nt 1724 to 2212; 163 aa), which we tentatively designated hacA and hacB, respectively, showed significant identity to those of aconitate/homoaconitate hydratases and α-isopropylmalate isomerase. α-Isopropylmalate isomerase from yeast or fungi is a monomeric enzyme consisting of four structural domains, 1 to 4, whereas its bacterial counterpart is composed of two subunits, large and small, each of which codes for domains 1 to 3 and 4, respectively (20, 23, 24). Since the amino acid sequences of hacA and hacB showed identity to those of domains 1 to 3 and 4 of these enzymes, respectively, we assumed that the products of hacA and hacB associated with each other to exert catalytic activity. The amino acid sequences for ORFs E (nt 2225 to 2536; 104 aa) and F (nt 2532 to 2694; 54 aa) showed no homology to those of the proteins registered in SwissProt and PIR databases. Interestingly, both peptides are composed of repetitive short segments of 20 to 30 aa, all of which contained a C(P/E)×CG motif.
FIG. 2.
Nucleotide and deduced amino acid sequences of the homoaconitate hydratase gene cluster.
Disruption of the hacA gene of T. thermophilus.
The observation that ORFs containing amino acid sequences similar to those of homoaconitate hydratase were included in the DNA fragment complementing the lysine auxotrophy of mutant K1004 suggested that the ORFs encoded homoaconitate hydratase for lysine biosynthesis. To elucidate the function of hacA, hacA disruptants of T. thermophilus were constructed. The disruptants could not grow in MP medium but did grow in MP medium supplemented with lysine (data not shown). In addition, α-aminoadipic acid and α-ketoadipic acid also supported the growth of the disruptants in MP medium (Fig. 3). This result suggested that hacA possibly encoded homoaconitate hydratase.
FIG. 3.
Auxotrophic complementation test of hacA disruptants of T. thermophilus. Tubes: 1, MP medium; 2, MP medium plus 1 mM lysine; 3, MP medium plus 1 mM α-aminoadipic acid; 4, MP medium plus 1 mM α-ketoadipic acid.
DISCUSSION
Aspartate kinase catalyzes the first reaction in the pathway for lysine, methionine, and threonine biosynthesis in all bacteria studied to date, and the overall enzyme activity present in the cells is controlled principally by both lysine and methionine/threonine (4). The present study revealed that aspartate kinase cloned from T. thermophilus was regulated only by threonine. This could suggest the presence of an unidentified lysine-sensitive aspartate kinase in T. thermophilus. The finding that the askAB disruptant is auxotrophic only for threonine/methionine, however, excluded this possibility but clearly indicated that the strain contained no other aspartate kinase which could suppress the disruption and that lysine was synthesized independently of aspartate kinase. In several microorganisms, expression of the aspartate kinase gene was repressed in the presence of several end products or their intermediates (5, 19, 31). However, preliminary analysis with polyclonal antibodies against T. flavus aspartate kinase showed that addition of lysine or diaminopimelic acid to the culture medium of T. thermophilus did not decrease the production of aspartate kinase (data not shown). This preliminary analysis also supported the independence of lysine in aspartate kinase regulation.
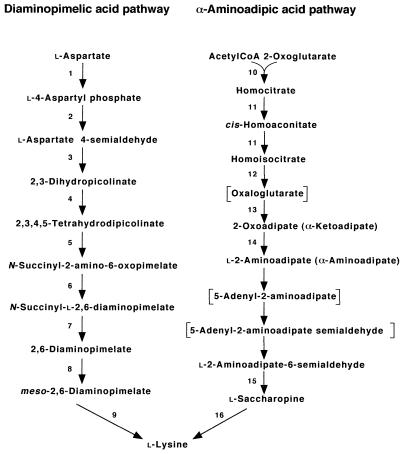
α-Aminoadipic acid is known as an intermediate in lysine biosynthetic pathways of fungi and yeast (1, 3, 35). The pathway called the α-aminoadipic acid pathway initiates from the synthesis of homocitrate from α-ketoglutarate (2-oxoglutarate) and acetyl-CoA by homocitrate synthase and proceeds via α-aminoadipic acid and saccharopine to lysine (Fig. 4). Although actinomycetes possess the enzymes for the synthesis of α-aminoadipic acid (33), as a direct precursor of cephalosporins, from lysine, the organisms synthesize lysine by the diaminopimelic acid pathway (11). In this study, however, T. thermophilus was shown to possess homocitrate synthase, which catalyzes the first step of the reactions in the α-aminoadipic acid pathway. In addition, cloning and nucleotide sequencing of the genes which complemented the growth defect of mutant K1004 in a minimal medium indicated that the cloned DNA fragment contained the genes possibly encoding homoaconitate hydratase. The hypothesis was further supported by the growth defect of hacA disruptants in a minimal medium. Until now no bacterial species utilizing α-aminoadipic acid as an intermediate in lysine biosynthesis have been reported. This therefore becomes the first instance demonstrating the presence of a lysine biosynthetic pathway different from the diaminopimelic acid pathway in bacteria. The observation that aspartate kinase from T. flavus is also threonine sensitive (17) suggests that the diaminopimelic acid-independent lysine biosynthetic pathway is common to all Thermus species.
FIG. 4.
Lysine biosynthetic pathways. Enzymes: 1, aspartate kinase; 2, aspartate semialdehyde dehydrogenase; 3, dihydrodipicolinate synthase; 4, dihydrodipicolinate reductase; 5, tetrahydropicolinate succinylase; 6, succinyldiaminopimelate transaminase; 7, succinyldiaminopimelate desuccinylase; 8, diaminopimarate epimerase; 9, diaminopimelate decarboxylase; 10, homocitrate synthase; 11, homoaconitate hydratase; 12, homoisocitrate dehydrogenase; 13, 2-aminoadipate transaminase; 14, l-aminoadipate semialdehyde dehydrogenase; 15, saccharopine dehydrogenase (NADP+, l-glutamate forming); 16, saccharopine dehydrogenase (NAD+, l-lysine forming).
In this study, we cloned the DNA fragment that complemented the growth defect of a mutant in a minimal medium and showed that hacA and possibly hacB genes may be involved in lysine biosynthesis in T. thermophilus. In addition to the two genes, six structural genes were contained in the cloned fragment. It is of interest to examine whether these additional genes are involved in lysine biosynthesis of T. thermophilus.
Although Thermus species can grow at temperatures exceeding 70°C and are known to be members of extremophiles, the microorganisms are taxonomically classified as gram-negative bacteria. Thermus species possess ornithine in place of diaminopimelic acid as a cell wall component (21), which may confer an advantage for growth at high temperatures and render dispensable the synthesis of diaminopimelic acid for growth. Another demonstration of the uniqueness of Thermus species is all other bacteria are known to possess mitochondrion-type malate dehydrogenase, whereas Thermus has a mammalian cytoplasm-type enzyme (18). Thermus species, which have developed unique enzyme systems, are therefore interesting with respect to evolution.
Footnotes
Present address for Masaru Tanokura: Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan. Phone: 81-3(3812)2111, ext. 5165. Fax: 81-3(5689)7225. E-mail: utanok@hongo.ecc.u-tokyo.ac.jp.
REFERENCES
- 1.Bhattacharjee J K, Strassman M. Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant. J Biol Chem. 1967;242:2542–2546. [PubMed] [Google Scholar]
- 2.Black S, Wright N G. β-Aspartate kinase and β-aspartylphosphate. J Biol Chem. 1954;213:27–38. [Google Scholar]
- 3.Broquist H P. Lysine biosynthesis (yeast) Methods Enzymol. 1971;17:112–129. [Google Scholar]
- 4.Eggeling L. Biology of l-lysine overproduction by Corynebacteriumglutamicum. Amino Acids. 1993;6:261–272. doi: 10.1007/BF00813746. [DOI] [PubMed] [Google Scholar]
- 5.Graves L M, Switzer R L. Aspartokinase III, a new isozyme in Bacillus subtilis 168. J Bacteriol. 1990;172:218–223. doi: 10.1128/jb.172.1.218-223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunstein M, Hogness D S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA. 1975;72:248–254. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidaka Y, Hasegawa M, Nakahara T, Hoshino T. The entire population of Thermus thermophilus cells is always competent at any growth phase. Biosci Biotechnol Biochem. 1994;58:1338–1339. doi: 10.1271/bbb.58.1338. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino T, Kosuge T, Hidaka Y, Tabata K, Nakahara T. Molecular cloning and sequence analysis of the proC gene encoding delta 1-pyrroline-5-carboxylate reductase from an extremely thermophilic eubacterium Thermus thermophilus. Biochem Biophys Res Commun. 1994;199:410–417. doi: 10.1006/bbrc.1994.1244. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino T, Yoshino Y, Elvira D G, Ishida S, Hiruta T, Fujii R, Nakahara T. Isolation and partial characterization of carotenoid underproducing and overproducing mutants from an extremely thermophilic Thermus thermophilus HB27. J Ferment Bioeng. 1994;77:131–136. [Google Scholar]
- 10.Jeffrey D C, Torin R W, Lisa P, Barry R B, William R J J. Isolation and characterization of the aspartate kinase and aspartate semialdehyde dehydrogenase operon from mycobacteria. Mol Microbiol. 1994;11:629–639. doi: 10.1111/j.1365-2958.1994.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatric J R, Doolin L E, Godfrey O W. Lysine biosynthesis in Streptomyces lipmanii: implication in antibiotic biosynthesis. Antimicrob Agents Chemother. 1973;4:542–550. doi: 10.1128/aac.4.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosuge T, Hoshino T. Molecular cloning and sequence analysis of the lysR gene from the extremely thermophilic eubacterium, Thermus thermophilus HB27. FEMS Microbiol Lett. 1997;157:73–79. doi: 10.1111/j.1574-6968.1997.tb12755.x. [DOI] [PubMed] [Google Scholar]
- 13.Koyama Y, Hoshino T, Tomizuka N, Furukawa K. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J Bacteriol. 1986;166:338–340. doi: 10.1128/jb.166.1.338-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao H, McKenzie T, Hageman R. Isolation of a thermostable enzyme variant by cloning and selection in a thermophile. Proc Natl Acad Sci USA. 1986;83:576–580. doi: 10.1073/pnas.83.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maseda H, Hoshino T. Development of expression vectors for Thermus thermophilus. J Ferment Bioeng. 1998;86:121–124. [Google Scholar]
- 16.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama M, Kukimoto M, Beppu T, Horinouchi S. An operon encoding aspartokinase and purine phosphoribosyltransferase in Thermus flavus. Microbiology. 1995;141:1211–1219. doi: 10.1099/13500872-141-5-1211. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama M, Matsubara N, Yamamoto K, Iijima S, Uozumi T, Beppu T. Nucleotide sequence of the malate dehydrogenase gene of Thermus flavus and its mutation directing an increase in enzyme activity. J Biol Chem. 1986;261:14178–14183. [PubMed] [Google Scholar]
- 19.Patte J C, Le Bras G, Cohen G N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967;136:245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- 20.Prodromou C, Artymiuk P J, Guest J R. The aconitase of Escherichia coli. Nucleotide sequence of the aconitase gene and amino acid sequence similarity with mitochondrial aconitases, the iron-responsive-element-binding protein and isopropylmalate isomerases. Eur J Biochem. 1992;204:599–609. doi: 10.1111/j.1432-1033.1992.tb16673.x. [DOI] [PubMed] [Google Scholar]
- 21.Quintela J C, Pittenauer E, Allmaier G, Aran V, de Pedro M A. Structure of peptidoglycan from Thermus thermophilus HB8. J Bacteriol. 1995;177:4947–4962. doi: 10.1128/jb.177.17.4947-4962.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddles P W, Blakeley R L, Zerner B. Reassessment of Ellman’s reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- 23.Robbins A H, Stout C D. The structure of aconitase. Proteins. 1989;5:289–312. doi: 10.1002/prot.340050406. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal E R, Calvo J M. The nucleotide sequence of leuC from Salmonella typhimurium. Nucleic Acids Res. 1990;18:3072. doi: 10.1093/nar/18.10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito H, Miura K. Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- 26.Sakaki Y, Oshima T. Isolation and characterization of a bacteriophage infectious to an extreme thermophile, Thermus thermophilus HB8. J Virol. 1975;15:1449–1453. doi: 10.1128/jvi.15.6.1449-1453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiio I, Miyajima R. Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem (Tokyo) 1969;65:849–859. doi: 10.1093/oxfordjournals.jbchem.a129089. [DOI] [PubMed] [Google Scholar]
- 30.Southern E M. Detection of specific sequences among DNA fragments separated by agarose gel electrophoresis. J Mol Biol. 1975;98:513–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 31.Stadtman E R, Cohen G N, LeBras G, deRobichon-Szulmajster H. Feedback inhibition and repression of aspartokinase activity in Escherichia coli and Saccharomyces cerevisiae. J Biol Chem. 1961;236:2033–2038. doi: 10.1111/j.1749-6632.1961.tb35587.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T, Kawano N, Oshima T. Cloning of 3-isopropylmalate dehydrogenase gene of an extreme thermophile and partial purification of the gene product. J Biochem (Tokyo) 1981;89:677–682. doi: 10.1093/oxfordjournals.jbchem.a133245. [DOI] [PubMed] [Google Scholar]
- 33.Trown P W, Smith B, Abraham E P. Biosynthesis of cepharosporin C from amino acids. Biochem J. 1963;86:284–291. doi: 10.1042/bj0860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umbarger H E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- 35.Vogel H J. Distribution of lysine pathways among fungi: evolutionary implications. Am Nat. 1964;98:446–455. [Google Scholar]