The coronavirus disease (COVID)-19 pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected 501 million individuals over the last 3 years and caused more than 6 million deaths worldwide. Gastrointestinal (GI) symptoms have been reported among patients with acute SARS-CoV-2 infection since the early part of the pandemic, with prevalence of up to 30%–60%. The most common symptoms described include diarrhea, nausea, vomiting, and abdominal pain, with up to a fifth of the patients reporting these as the only/predominant symptoms.1
Clinical Problem
An increasingly recognized subset of patients develops post-infection sequelae also described as long COVID or postacute COVID-19 syndrome (PACS). These patients experience a myriad of neurologic, respiratory, cardiac, psychiatric, and/or GI symptoms that persist for 4 weeks or more from the initial diagnosis of SARS-CoV-2.
Epidemiology
In a survey study of 749 survivors, 29% reported at least 1 new chronic GI symptom 6 months after their COVID-19 infection, with heartburn, constipation, diarrhea, and abdominal pain being the most common.2 Of the patients with abdominal pain, 39% met Rome IV criteria for irritable bowel syndrome (IBS). Other studies also reported a 30%–40% prevalence of GI PACS. Additionally, COVID-19 infection was associated with worsening severity of preexisting IBS symptoms. Some risk factors for GI PACS include the presence of GI symptoms during acute infection, psychiatric diagnoses (depression, anxiety) both pre- and post-COVID-19, need for hospitalization during acute illness, and loss of smell and taste. Infectious gastroenteritis is an established risk-factor for development of disorders of gut-brain interaction (DGBI), particularly post-infection IBS (PI-IBS). Many of the risk factors for GI PACS described are also known predisposing factors for PI-IBS, with some exceptions, such as female gender, a risk factor for PI-IBS but not consistently associated with GI PACS. In addition to IBS, other de novo DGBIs, such as functional dyspepsia, heartburn, chest pain, and dysphagia, can be experienced in the spectrum of GI PACS.
Disease Mechanisms
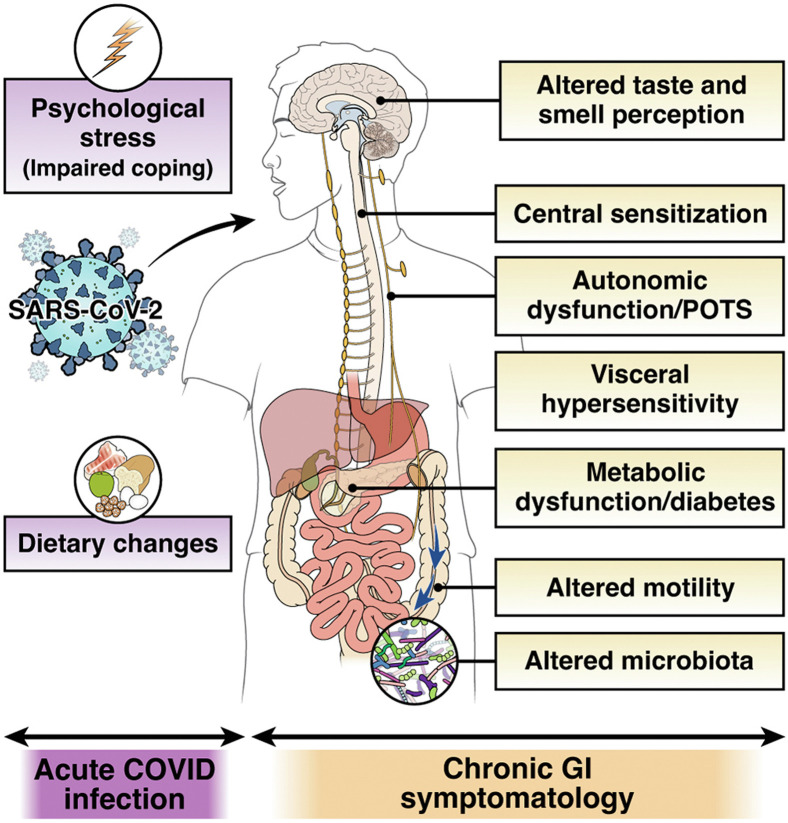
The pathophysiology of PACS including that of the GI manifestations is incompletely understood; however, it is likely multifactorial (Figure 1 ). Epithelial invasion by SARS-CoV-2 is substantiated by the high expression levels of angiotensin-converting enzyme-2 on the enterocytes and colonocytes. The angiotensin-converting enzyme-2 is a negative regulator of the renin-angiotensin system and has a protective cellular role, including in the intestinal tract. Following the entry of SARS-CoV-2 in the cell, angiotensin-converting enzyme-2 protein is downregulated, resulting in an increase in angiotensin-II, the likely molecular mechanism of severe acute respiratory syndrome and systemic inflammatory response development with this coronavirus. Intestinal microbial dysbiosis has also been associated with acute SARS-CoV-2 infection and PACS. Long-term respiratory dysfunction after COVID-19 is associated with altered gut microbiota and persistently elevated lipopolysaccharide-binding protein levels. One study showed that dysbiosis in COVID-19 patients continued throughout their hospitalizations and up to 21 days from disease onset, with a decrease in health-promoting, short-chain fatty acid–forming bacteria.3 Gut microbiome of patients with PACS was characterized by higher levels of Ruminococcus gnavus and Bacteroides vulgatus, and lower levels of Faecalibacterium prausnitzii. Interestingly, presence of butyrate-producing bacteria showed an inverse correlation with development of PACS at 6 months.4 A recent study also suggested that salivary microbiome during acute infection may predict the development of GI PACS.5
Figure 1.
Risk and mechanisms of post-COVID-19 IBS. Hospitalization during COVID-19 infection and high stress during or subsequent to the acute infection might increase the risk of PI-IBS development. Invasion of SARS-CoV-2 in the intestinal epithelium may mediate immune dysregulation, barrier dysfunction, and neuromuscular plasticity. Additionally, changes in luminal milieu caused by altered microbiota or metabolites may mediate changes in sensory-motor function. Patients may develop central sensitization because of the ongoing stress associated with the pandemic. Lastly, established mechanisms of long-COVID, such as autonomic dysfunction and altered taste perception, might influence gut function and eating patterns or diet, thereby leading to chronic gastrointestinal symptomatology. POTS, postural orthostatic tachycardia syndrome.
Other etiologies that may underlie development of GI PACS include the worsening or development of diabetes after SARS-CoV-2 infection.6 Diabetes can predispose to development of DGBI, such as functional dyspepsia, gastroparesis, and diarrhea. Weight gain experienced by a subset of individuals post-COVID-19 can also aggravate gastroesophageal reflux disease or other DGBI symptoms. More recently, a T-cell-based immune dysregulation was reported to play a role in the development of PACS, particularly chronic GI symptoms. In this study, 9% of individuals developed PACS but 18% had anosmia/dysgeusia. GI PACS was associated with multiple positive antibodies during the acute and chronic phases. T-cell receptor and single-cell sequencing also showed that GI PACS is associated with specific T-cell clonal dynamics where there is an expansion of cytotoxic pool. Additionally, SARS-CoV-2-specific CD8 T cells exhibited undifferentiated phenotypes during acute disease and elevated cytotoxic characteristics at chronic stage.7 Bystander activation of cytomegalovirus-specific T cells has also been observed in GI PACS. Increased intestinal permeability, which has been implicated in PI-IBS, has also been reported in patients with severe COVID-19. Lastly, autonomic dysfunction associated with PACS and chronic anosmia/dysgeusia can also play into the pathophysiology of chronic GI symptoms.
In established DGBI patients recovering from COVID-19 infection, an overall increase in symptoms, medication use, and health care use has been observed. Multiple studies have highlighted the profound psychological impact of the pandemic on patients with DGBI, with worsening of GI symptoms significantly correlating with increased depression and anxiety.8 Moreover, alterations in dietary habits and behaviors with likely effects on GI symptoms have been reported during the pandemic and confinement, such as increased simple sugar or carbohydrates consumptions, decreased water intake, and reduced physical activity. Psychological stress can also exacerbate underlying eating disorders that may be associated with symptoms of DGBI. These observations may foreshadow a continued rise in patients presenting to gastroenterologist offices with IBS or DGBI symptoms, both from de novo diagnosis and from increase/flare-up of existing symptoms.
Treatment
Currently, there are no diagnostic biomarkers or clinical trials testing therapies for GI PACS. Much of the therapeutic armamentarium is, therefore, based on treatment strategies for non-COVID-19-associated DGBI (Table 1 ). A handful of studies have tested potential targeted therapies for GI PACS. A Lactiplantibacillus plantarum and Pediococcus acidilactici–based probiotic improved nasopharyngeal viral load, lung infiltrates, and duration of GI and non-GI symptoms, even though the fecal microbiota composition did not change.9 In another study, 2-month expanded access to a high-fiber formula improved nausea and appetite loss in PACS, putatively because of increase in short-chain fatty acid–producing bacteria.10
Table 1.
Management Considerations for Post-COVID-19 Irritable Bowel Syndrome
“Positive” diagnosis
Lifestyle modifications
|
NOTE. In the absence of targeted clinical trials, these recommendations are based on expert opinion and on extrapolation of treatment for irritable bowel syndrome. Individual patient factors and side effect profiles should be considered.
Take-Home Message
Gastroenterologists should be aware that SARS-CoV-2 infection can result in the development of chronic DGBI, such as IBS, or GI symptoms that may be part of a systemic PACS. In the absence of alarm symptoms, the diagnostic work-up should be limited and follow paradigms for routine PI-IBS or DGBI, as warranted by the presenting symptoms. In addition to treating GI symptoms using currently available strategies for IBS/DGBI, addressing comorbid (psychosocial) or contributing (autonomic dysfunction, dysgeusia) etiologies may augment response. Future studies will likely provide more insight into mechanisms that can be systematically targeted for management of GI PACS.
Footnotes
Conflicts of interest The authors disclose the following: Walter W. Chan served on the scientific advisory board for Ironwood, Takeda, and Phathom Pharmaceuticals. Madhusudan Grover has received research support from Takeda, Donga, and Alexza Pharmaceuticals; and served as advisor for Alfasigma pharmaceuticals.
Funding Madhusudan Grover is supported by the National Institutes of Health (DK 127998).
References
- 1.Redd W.D., Zhou J.C., Hathorn K.E., et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159:765–767. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackett J.W., Wainberg M., Elkind M.S.V., et al. Potential long coronavirus disease 2019 gastrointestinal symptoms 6 months after coronavirus infection are associated with mental health symptoms. Gastroenterology. 2022;162:648–650. doi: 10.1053/j.gastro.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizutani T., Ishizaka A., Koga M., et al. Correlation analysis between gut microbiota alterations and the cytokine response in patients with coronavirus disease during hospitalization. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.01689-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q., Mak J.W.Y., Su Q., et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 5.Haran J.P., Bradley E., Zeamer A.L., et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight. 2021;6 doi: 10.1172/jci.insight.152346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montefusco L., Ben Nasr M., D'Addio F., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Y., Yuan D., Chen D.G., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamp K.J., Levy R.L., Munson S.A., et al. Impact of COVID-19 on individuals with irritable bowel syndrome and comorbid anxiety and/or depression. J Clin Gastroenterol. 2022;56:e149–e152. doi: 10.1097/MCG.0000000000001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez-Castrellon P., Gandara-Marti T., Abreu Y.A.A.T., et al. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes. 2022;14 doi: 10.1080/19490976.2021.2018899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Wu G., Zhao L., et al. Nutritional modulation of gut microbiota alleviates severe gastrointestinal symptoms in a patient with post-acute COVID-19 syndrome. mBio. 2022;13 doi: 10.1128/mbio.03801-21. [DOI] [PMC free article] [PubMed] [Google Scholar]