Abstract
Background
In December 2020, the Israeli Ministry of Health launched a national vaccination campaign against SARS-CoV-2. Concomitant sporadic reports on anaphylactic responses in other countries raised safety concerns at the outset of this operation.
Objective
To characterize reports on allergic reactions to coronavirus disease 2019 vaccines.
Methods
Allergy events were reported by health care professionals throughout the country to Israeli Ministry of Health Division of Epidemiology via a Web-based computerized national vaccine registry. The study period was from December 19, 2020 to September 13, 2021, during which 14,475,979 injections were administered.
Results
Allergic reactions were reported in 463 subjects, 99.3% of whom received Pfizer-BioNTech BNTT162B2. The reporting rate was 106 per million in December 2020. From January to May 2021, a reduction was observed to 66, 18, 14, eight, and zero per million, and reporting remained low until September. Mean age of subjects was 48.9 ± 16.7 years (range, 15-96 years) with a female preponderance of 78%. Epinephrine was administered in 34 subjects. Validated immediate allergy was observed in only 37 cases (8%), suggesting 2.5 to 3.3 bona fide reactions per million. In subjects with reactions classified as severe (n = 46), plausible allergy was identified in 36% to 41% of cases. A history of allergy was associated with high false reporting of immediate reactions (83%). Allergic events after the first dose did not compromise adherence to subsequent doses.
Conclusions
Excessive reporting of allergy declined over time and did not affect adherence to vaccination. The existence of previous allergy may affect reporting profiles, but not the occurrence of vaccine allergy.
Key words: Vaccine allergy, Anaphylaxis, COVID-19, SARS-CoV-2
Abbreviations used: AE, Adverse events; COVID-19, Coronavirus disease 2019; IMoH, Israeli Ministry of Health; PEG, Polyethylene glycol
What is already known about this topic? mRNA-based vaccines against SARS-CoV-2 were first introduced in December 2020. Cases of allergic reactions at the outset of the vaccination campaign raised safety concerns among the general population.
What does this article add to our knowledge? This communication is based on a national registry and provides a unique account of allergic events during vaccination. It delineates fluctuations in reporting patterns as well as characteristics and reliability of reported allergic reactions.
How does this study impact current management guidelines? The study highlights challenges in monitoring allergic reactions along anti-SARS-CoV-2 vaccination. It may support the design of improved evaluation algorithms in future campaigns.
Introduction
The outbreak of the coronavirus disease 2019 (COVID-19) pandemic created a burden on health care systems throughout the world. This triggered considerable efforts to develop a safe and efficacious vaccine against the causative virus, SAR-CoV-2. Subsequently, on December 2 and 11, 2020, the United Kingdom and the United States, respectively, issued emergency authorizations for Pfizer-BioNTech (New York, NY, USA and Mainz, Germany) BNTT162B2. Allergy had not appeared as a significant adverse event (AE) in the clinical trial that examined this vaccine before its approval for clinical use.1 In this study, a total of 21,720 subjects received the vaccine after excluding patients with a high-risk history of severe reactions to any vaccine or severe allergic reaction to any component of the COVID-19 vaccine.
After the introduction of BNTT162B2, safety issues arose regarding its allergenic properties. These concerns were prompted by postmarketing reports of an increased rate of anaphylaxis from the United Kingdom and United States. By the end of December 2020, the United States had recorded 175 cases of possible severe allergic reactions across almost 1.9 million injections.2, 3, 4 Following a review, 21 of these events were confirmed as anaphylaxis, suggesting a rate of 11 reactions in 1 million doses. A report on the Moderna (Cambridge, MA, USA) mRNA-based vaccine suggested that anaphylaxis occurred in 2.5 cases per million doses.5 , 6 A female predominance was noted among allergic episodes in both types of vaccine. All of these cases resolved completely with no fatalities. Another study investigated 64,900 Mass General Brigham Hospital employees during the first 2 months of vaccination.7 That work suggested that severe reactions occurred at a rate of 2.47/10,000 vaccinations. The study was based on self-submitted data, and patients received mRNA vaccines (40% Pfizer-BioNTech and 60% Moderna). In comparison, anaphylaxis is thought to occur in 1 per million doses of vaccines in general.8
The BNTT162B2 vaccine contains a nanoparticle-formulated, nucleoside-modified RNA encoding the SARS-CoV-2 full-length spike, modified by two proline mutations to lock it in the prefusion conformation.8 Its excipient list contains polyethylene glycol (PEG), a component that attracted the attention of investigators as the most likely culprit underlying anaphylaxis.2 Polyethylene glycol is chemically bound to the lipid nanoparticles, increasing their stability. Whereas PEG is a common excipient in numerous drugs, it had not been used in vaccines until that point.2 , 9
The suspicion that PEG induced allergic reactions after vaccination was supported by a previous large case series describing subjects who developed anaphylaxis to medications containing this compound.10 The proposed mechanism involved the recognition of PEG by preexisting specific IgE followed by activation of mast cells and initiation of an anaphylactic response.11
Among the early reports were two cases of subjects from the United Kingdom with a history of severe allergy, who carried epinephrine autoinjectors. These episodes prompted the UK regulator to issue a statement that previous anaphylactic reactions to food, medications, or vaccinations are considered contraindications.8 , 12 This decision, in turn, raised the concern that 3% to 5% of the population would be excluded from vaccination owing to self-report of anaphylaxis to any allergen.12 The contraindication was revoked 3 weeks later and replaced with instructions that only people with a history of allergic reactions to the vaccine components should not receive it.12 Published expert opinions suggested several other risk factors, such as a history of hypersensitivity to other vaccines, mastocytosis, and severe asthma.13
In this communication, we summarize all of the reports on allergic reactions during the first 9 months of the vaccination campaign in Israel. During this time the first, second, and third vaccine doses were administered. The data presented here provide a unique account of allergic reporting on a national level.
Methods
The National Israeli Vaccine Adverse Event Surveillance System
Adverse events were reported to the Israeli Ministry of Health (IMoH) Division of Epidemiology. Reports were submitted via the existing Web-based computerized national vaccine registry (Nachlieli system) operated by the Ministry of Health Public Health Services. Adverse events are submitted using a standardized reporting form based on international vaccine AE forms. For allergic AEs, a form was devised according to Brighton Collaboration recommendations. The registry includes all data pertaining to vaccines, such as demographic details of recipients and vaccine information (components, batch number, date and route of administration, etc). The Nachlieli system provides a unified format of reporting from different sources, with a mechanism for data quality assurance including detection of double reporting, erroneous reporting, and validation of vaccine recipients compared with the national population registry. System users are health care professionals in hospitals, health maintenance organizations, emergency medical services, the IMoH Medical Department and Patient Safety Unit, as well as the Israeli Defense Forces.
For the purposes of this study, serious AEs were defined as those involving one or more of the following: death, life-threatening episode, hospitalization, and persistent or significant disability or incapacity. Included were events requiring intervention to prevent permanent impairment or damage, such as epinephrine injection. For each serious AE involving hospitalization, records and medical backgrounds were requested for further evaluation and investigation.
Study population
This study included all vaccine recipients aged 15 years and older who were registered in the National Computerized Vaccine system, and who received a vaccine dose between December 19, 2020 and September 13, 2021. The surveillance period for AEs was 30 days after vaccine administration (for first, second, and third vaccine doses), as suggested by the Centers for Disease Control and Prevention.
Validation of allergic responses
Reported events were screened and classified into three groups according to their compatibility with systemic allergy (likely, unlikely, and ruled out). Criteria for classification were based on the time from injection to reaction and the involvement of typical organ systems including skin, respiratory, and gastrointestinal systems and hemodynamic findings. Hence, criteria were: (1) likely: a start time of less than 4 hours and a minimum of two systems with at least one objective finding; (2) unlikely: a start time of less than 4 hours with less than two systems or no objective finding; and (3) ruled out: a start time of greater than 4 hours.
Statistical analysis
We performed statistical analysis using the TableOne R package (Kazuki Yoshida, Cambridge, MA, USA). This application automatically recognizes variable types and selects the relevant statistical test. Briefly, in this study, differences between groups were analyzed with the t test or χ2 test when appropriate. P less than .05 was considered statistically significant.
Ethical considerations
Data were collected as part of an ongoing clinical surveillance program for AEs related to the BNT162b2 vaccine as required by national guidelines. Therefore, this study received a waiver for review by the institutional review board. Pfizer–BioNTech had no role in the collection or analysis of the data or in the reporting of the data in this study.
Results
Characteristics of subjects with reported allergic reactions
During the study period, a total of 463 allergic events were reported to the IMoH (Table I ). Mean age was 48.9 years (range, 15-96 years). Most subjects were female (n = 363; 78%) and nearly all subjects, except three, received the Pfizer-BioNTech vaccine. Approximately two-thirds of allergic events were reported after a first-dose injection (n = 314; 68%); the remainder was mostly after the second dose (n = 140; 30%). Only 2% of allergic reactions (n = 9; 2%) were reported after the third dose, which consisted of 20% of all administered injections. The largest group of reactions occurred within 30 minutes of vaccination (n = 163; 35%). In terms of reaction duration, reporting was lacking in a large number of subjects (n = 220; 48%). In 187 events (40%), the site at which the allergic reaction was treated was not mentioned. Among the reported sites, an emergency care unit was the leading choice (n = 129; 28%). Drugs that were most administered to treat allergy included antihistamines (n = 86; 19%) followed by corticosteroids (n = 64; 14%) and epinephrine (n = 34; 7%). Intriguingly, among epinephrine recipients, 91% were female (n = 31).
Table I.
Study population characteristics
| Characteristics | Total (n = 463) | Males (n = 100) | Females (n = 363) | P |
|---|---|---|---|---|
| Age, y (mean ± SD) (range) | 48.9 ± 16.7 (15-96) | 48.4 ± 17.9 (16-90) | 48.9 ± 16.3 (15-96) | .549 |
| Vaccine type, n (%) | ||||
| Pfizer | 460 (99) | 100 (100) | 360 (99) | .838 |
| Moderna | 3 (1) | 0 | 3 (1) | |
| Vaccine dose, n (%) | .725 | |||
| First | 314 (68) | 70 (70) | 244 (67) | |
| Second | 140 (30) | 27 (27) | 113 (31) | |
| Third | 9 (2) | 3 (3) | 6 (2) | |
| Reaction start time, n (%) | .802 | |||
| <30 min | 163 (35) | 34 (34) | 129 (36) | |
| 30 min to 4 h | 72 (16) | 15 (15) | 57 (16) | |
| 4-24 h | 91 (20) | 17 (17) | 74 (20) | |
| 1-7 d | 95 (21) | 24 (24) | 71 (20) | |
| >1 wk | 7 (2) | 2 (2) | 5 (1) | |
| N/A | 35 (8) | 8 (8) | 27 (7) | |
| Duration of reaction, n (%) | .926 | |||
| <30 min | 36 (8) | 8 (8) | 28 (8) | |
| 30 min to 4 h | 47 (10) | 12 (12) | 35 (10) | |
| 4-24 h | 23 (5) | 3 (3) | 20 (6) | |
| 1-7 d | 64 (14) | 15 (15) | 49 (13) | |
| >1 wk | 10 (2) | 3 (3) | 7 (2) | |
| Ongoing | 63 (14) | 15 (15) | 48 (13) | |
| N/A | 220 (48) | 44 (44) | 176 (48) | |
| Site of treatment, n (%) | .566 | |||
| Emergency care unit | 129 (28) | 28 (28) | 101 (28) | |
| Primary care physician | 94 (20) | 22 (22) | 72 (20) | |
| Vaccination center | 21 (5) | 7 (7) | 14 (4) | |
| Hospital admission | 14 (3) | 3 (3) | 11(3) | |
| Phone consultations | 13 (3) | 2 (2) | 11 (3) | |
| Other∗ | 5 (1) | 0 | 5 (1) | |
| N/A | 187 (40) | 38 (38) | 149 (41) | |
| Medical treatment, n (%) | .667 | |||
| Epinephrine | 34 (7) | 3 (3) | 31(9) | |
| Antihistamine | 86 (19) | 17 (17) | 69 (19) | |
| Corticosteroids | 64 (14) | 12 (12) | 52 (14) | |
| Other | 26† (6) | 5 (5) | 21(6) |
N/A, not available.
Pulmonologist, neurologist, nurse, allergist, infectious disease consultant (n = 1 each).
Antipyretics (n = 18); inhaled bronchodilators (n = 2); intravenous fluid (n = 2); antibiotic (n = 1); metoclopramide (n = 1); acyclovir (n = 1); famotidine (n = 1).
Rates of reporting of allergic reactions to anti-SARS-CoV-2 vaccination
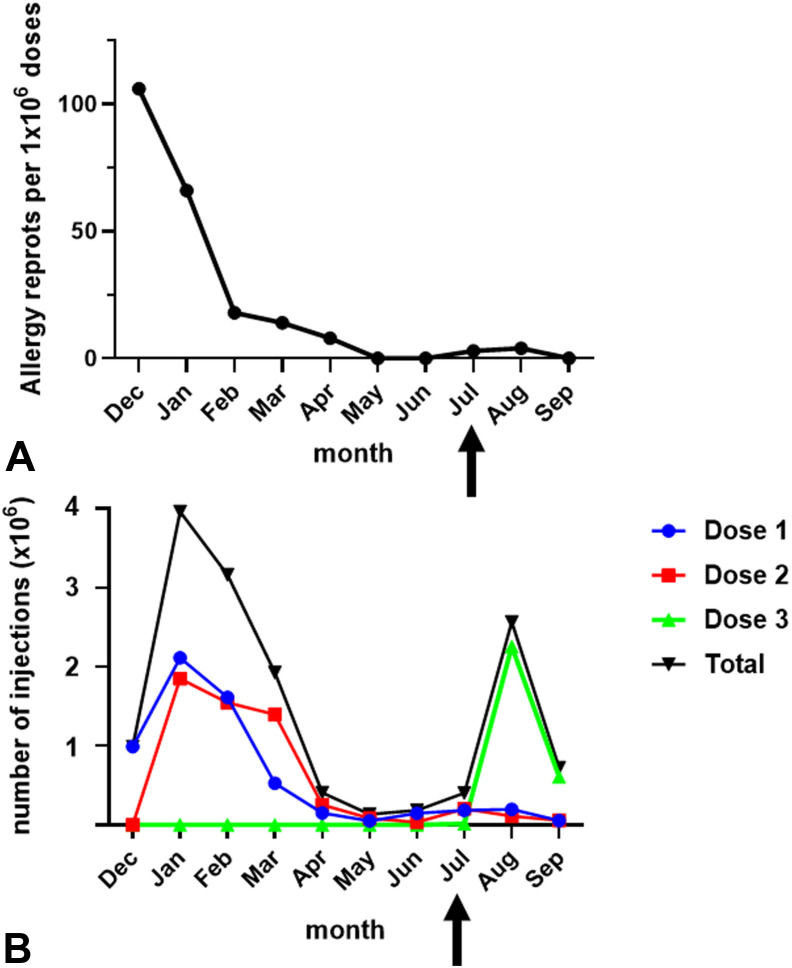
The vaccination campaign in Israel started on December 19, 2020. During the last 13 days of that month, allergic responses were reported at the rate of 106 events per million injections (Figure 1 , A) which was the highest incidence throughout the study period. From January to May 2021, a reduction was observed to 66, 18, 14, eight, and zero allergic episodes per million. No reports were submitted in May and June. In July and August, three and four events per million were documented, respectively, whereas in September no events were noted. The number of vaccine doses administered in December 2020 was 0.99 × 106, consisting exclusively of first-dose injections (Figure 1, B). From January to March 2021, the combination of first and second doses yielded higher numbers of vaccine administration reaching 3.96, 3.16, and 1.93 × 106 injections. This was followed by an interim period of decreased vaccination from April to July (range, 0.14-0.41 × 106/month). A peak of 2.57 × 106 injections was observed in August corresponding to the introduction of a third dose, followed by another drop, to 0.75 × 106 in September.
Figure 1.
Rates of vaccination and allergy reports. (A) Rates of allergy reporting for each month during the study period from December 19, 2020 until September 13, 2021. (B) Numbers of anti–coronavirus disease 2019 vaccine injections that were administered. Arrows indicate the onset of the third dose administration.
Validation of allergy diagnosis
We screened all allergy reports and evaluated their validity according to simplified criteria specified in the Methods section. According to this assessment, the largest category of events was designated as ruled out (n = 228; 49%) (Table II ). In another 43% of cases (n = 198), the reaction occurred within a time frame compatible with an immediate allergic reaction. However, clinical findings did not support this diagnosis, and they were considered unlikely. In 8% of reports (n = 37), clinical findings were consistent with a likely allergic reaction. Most of these subjects were females (32 of 37; 86%), corresponding to the sex-biased epinephrine injections (Table I). In subjects with likely allergic reactions, the most common findings were related to the skin (33 of 37; 89%) followed by the respiratory system (26 of 37; 70%), hemodynamic changes (n = 10 of 37; 27%), and gastrointestinal tract (4 of 37; 11%). Intriguingly, likely allergic reactions were reported at a rate of eight per million in December 2020 and decreased to 2.5 to 3 per million thereafter (Figure 2 ).
Table II.
Validation of immediate allergic reaction to vaccine
| Validation of all reported cases | Total |
Males |
Females |
P |
|---|---|---|---|---|
| n = 463 | n = 100 | n = 363 | ||
| Level of allergy probability, n (%) | ||||
| Ruled out | 228 (49) | 51 (51) | 177 (49) | .815 |
| Unlikely | 198 (43) | 44 (44) | 154 (42) | .834 |
| Likely |
37 (8) |
5 (5) |
32 (9) |
.305 |
| System involvement of likely allergic reactions | n = 37 | n = 5 | n = 32 | P |
|---|---|---|---|---|
| Involved system, n (%) | ||||
| Skin | 33 (89) | 4 (80) | 29 (91) | .416 |
| Respiratory | 26 (70) | 2 (40) | 24 (75) | .084 |
| Hemodynamic | 10 (27) | 4 (80) | 6 (19) | .822 |
| Gastrointestinal | 4 (11) | 0 | 4 (13) | .274 |
Figure 2.

Rates of likely allergy reporting per month.
Characteristics of severe reactions
We defined severe reactions as those involving epinephrine injections or hospitalization (Table III ). Severe allergic reactions were reported in a total of 46 individuals, including two subjects who were treated with epinephrine and were subsequently hospitalized. Patients who were injected with epinephrine were mostly treated in an emergency care unit (27 of 34; 79%). In both epinephrine-injected and hospitalized subjects, administration of corticosteroids (n = 23 and 4, respectively) exceeded the use of antihistamines (n = 8 and 2, respectively). This observation is in contrast to the general study population in which antihistamines were most common (Table I). Intriguingly, validation showed that the proportion of likely allergic reactions was 41% (14 of 34) and 36% (5 of 14) for epinephrine-injected and hospitalized patients, respectively. The peak incidence of epinephrine injections, hospitalizations, and referrals to the emergency department was observed in January 2021 (Table III and Figure 3 ). The distribution of these findings corresponded to the pattern of total vaccine injections as presented in Figure 1.
Table III.
Characterization of events reported as severe allergy
| Epinephrine-injected subjects | Total |
Males |
Females |
P |
|---|---|---|---|---|
| n = 34 | n = 3 | n = 31 | ||
| Site of treatment, n (%) | .653 | |||
| Vaccination center | 5 (15) | 0 | 5 (16) | |
| Emergency care unit | 27 (79) | 3 (100) | 24 (77) | |
| Primary care physician | 0 | 0 | 0 | |
| Hospital admission | 2 (6) | 0 | 2 (7) | |
| Medications, n, (%) | .913 | |||
| Epinephrine only | 14 (41) | 1 (33) | 13 (42) | |
| Antihistamine | 8 (24) | 1(33) | 7 (23) | |
| Corticosteroids | 23 (68) | 2 (67) | 21 (68) | |
| Level of allergy probability, n (%) | .634 | |||
| Ruled out | 5 (17) | 0 | 5 (16) | |
| Unlikely | 15 (44) | 2 (67) | 13 (42) | |
| Likely |
14 (41) |
1(33) |
13 (42) |
| Hospitalization | n = 14 | n = 3 | n = 11 | P |
|---|---|---|---|---|
| Medications, n (%) | .513 | |||
| Epinephrine | 2 (14) | 0 | 2 (18) | |
| Antihistamine | 2 (14) | 1 (33) | 1 (9) | |
| Corticosteroids | 4 (29) | 1 (33) | 3 (27) | |
| Level of allergy probability, n (%) | .683 | |||
| Ruled out | 7 (50) | 2 (67) | 5 (46) | |
| Unlikely | 2 (14) | 0 | 2 (18) | |
| Likely | 5 (36) | 1 (33) | 4 (36) |
Figure 3.
Distribution of (A) epinephrine injections and (B) emergency room and hospitalizations.
Reporting in subjects with a history of allergy
A total of 35 subjects reported a history of allergic disorders with drug hypersensitivity (n = 21 of 35; 60%) and respiratory allergy (n = 6 of 35; 17%) as the leading diagnoses (Table IV ). Three previous reactions to vaccines were noted, with no details on severity. No reports of responses to PEG were documented. Intriguingly, 20% of events were reported as severe (n = 7 of 35) compared with 10% within the entire study population (Table III). Accordingly, in patients with a previous allergy, 83% of the documented reactions (29 of 35) were immediate-unlikely allergic whereas the rate of this category was 43% in the total study group (Table II). Only one subject with a history of allergy (3%) was judged to have had a likely immediate reaction.
Table IV.
Subjects with previous allergy
| Characteristics | Total (n = 35) | Males (n = 5) | Females (n = 30) | P |
|---|---|---|---|---|
| Reported allergy history, n (%) | .847 | |||
| Drugs∗ | 21 (60) | 3 (60) | 18 (60) | |
| Respiratory | 6 (17) | 0 | 6 (20) | |
| Insect venom | 4 (13) | 1 (20) | 3 (10) | |
| Food | 2 (6) | 0 | 2 (7) | |
| Unknown | 6 (17) | 1 (20) | 5 (17) | |
| Others | 1 | 0 | 1 | |
| Reaction reported as severe, n (%) | .876 | |||
| Epinephrine injection | 4 (11) | 0 | 4 (13) | |
| Hospitalization | 3 (9) | 1 (20) | 2 (7) | |
| Level of allergy probability, n (%) | .198 | |||
| Ruled out | 5 (14) | 2 (40) | 3 (10) | |
| Unlikely | 29 (83) | 3 (60) | 26 (87) | |
| Likely | 1 (3) | 0 | 1(3) |
Including three previous reactions to vaccines; no reported allergy to polyethylene glycol.
First-dose allergic responses and adherence to subsequent doses
These data provided evidence about the course of vaccination in 279 subjects who reported allergic responses to the first dose (Figure 4 ). In this group, the rates of adherence to the second and third doses were 88% and 64%, respectively, showing no decrease compared with the general population (92% and 48%, respectively) (Figure 4, A). Among subjects with likely allergic responses to the first dose, we could evaluate adherence in 25 cases. In this subgroup, second and third doses were decreased with rates of 68% and 36%, respectively. Repeated allergic reactions to the first and second doses were found in only six subjects (Figure 4, B). Four of them proceeded to a third dose and only one was compatible with likely allergy. The remaining five subjects experienced events that were judged unlikely allergic.
Figure 4.
Follow-up of subjects who reported allergic reactions to the first dose. (A) Subjects with allergic reactions and likely allergic reactions were compared with the total population, as specified. The amount of subjects who received the first dose was defined as 100%, and the absolute number for each group is shown below the graph. (B) Details of individuals who reported an allergic reaction to more than one dose of vaccine.
Discussion
In this communication, we provide an account of COVID-19 vaccine allergy events, based on nationwide reporting to IMoH. The data presented in our work were collected from December 19, 2020 to September 13, 2021. During this time, 14.5 million injections were administered and 463 allergy events were documented with no fatalities. Analysis of these data suggests several insights regarding allergy reactions to the COVID-19 vaccine, the reliability of reporting, and the perception of vaccine allergy among medical staff and the general population.
One of the most striking observations was that the peak of allergy events was recorded immediately after the onset of the vaccination campaign and declined rapidly thereafter. This drop did not correlate with the number of administered injections. However, it is expected that allergic reactions would relate directly to the amount of allergen exposure among the population. This discrepancy may be explained in more than one way. Overreporting could have taken place owing to anticipation of AEs after news about anaphylaxis. We hypothesize that pandemic-related stress may have led health care workers to misinterpret clinical findings as allergy. This effect was presumably more prominent at the outset (first 2 months) of the vaccination campaign. Therefore, the increase in total allergic events and likely allergic reactions could be attributed to evaluation bias. We do not have data that would allow a comparison of the reliability of health care workers and self-reporting by patients. Another intriguing possibility is that emotional stress or anxiety enhanced the rate and severity of true allergic reactions. Although this explanation appears less probable, we could not find evidence that would rule it out entirely. Finally, there may have been a decline in the motivation to report bona fide allergic responses. This option seems to be less likely because fluctuations in validated allergic responses were significantly smaller compared with total numbers of documented events. Accumulation of experience and data regarding vaccine AEs was concomitantly reflected in IMoH recommendations. In December 2020, any history of anaphylaxis was initially regarded as a contraindication. Soon afterward, reactions to specific allergens such as food, insect venoms, and oral drugs were excluded. Along the vaccination campaign, additional contraindications were gradually removed. By June 2021, vaccination was allowed in subjects with previous severe allergic reactions to known allergens, including injected drugs as well as idiopathic anaphylaxis and mastocytosis.
The study population is distinctly characterized by a female preponderance (n = 363; 78%). This finding is in line with previous initial observations.3 , 5 A summary issued by the Centers for Disease Control and Prevention found that 90% of anaphylaxis episodes occurred in female recipients. Sex bias was previously described for drug reactions. Interestingly, a comprehensive meta-analysis showed that the frequency of self-reported drug allergy was significantly higher in women than in men.14 The underlying cause for this skewing may hypothetically be explained by sex-related differences in the awareness to drug AEs. An intriguing alternative explanation could be presensitization owing to exposure to PEG in household products, cosmetics, and medicine. A recent case series presented six subjects with acute hypersensitivity to PEG, all of whom were female subjects.15 However, a previous study on 18 subjects with reactions to the first dose of anti-COVID-19 vaccine proposed that skin testing to PEG is noninformative.16 Accordingly, a history of PEG hypersensitivity was not found in our study.
The inclination to overdiagnose drug allergy is a well-described phenomenon. For example, over 95% of patients with a history of penicillin allergy that is not severe are actually penicillin-tolerant.17 Therefore, self-report of drug allergy, including vaccines, requires objective validation. At the outset of the vaccination campaign, IMoH defined 4 hours from injection as the limit for immediate reactions. The initial working hypothesis was that allergy would be a considerable AE, and therefore a sensitive, rather than specific, threshold was chosen. The IMoH vaccine AE surveillance system is based on Brighton Collaboration recommendations. This system of evaluation provides a detailed algorithm for diagnosing anaphylaxis. However, we suggest that simplification of these guidelines may significantly facilitate the evaluation of allergic events in a large-scale campaign of vaccination. Therefore, we devised a set of criteria to assess the quality of allergy diagnoses, adopting a 4-hour limit beyond which allergic events were judged to be ruled out. Episodes that occurred within this limit were classified as likely allergic if they were reported to involve at least two of the Brighton Case Definition body systems (skin, respiratory, cardiovascular, and gastrointestinal), with at least one relevant objective finding. Using this scoring system, we obtained these findings: (1) only 8% of all events are likely allergic reactions and the rate increases to 36% to 41% among subjects reported as severe allergy; (2) a previous allergy may increase the likelihood of immediate reaction reporting without affecting validated anaphylaxis; and (3) during most of the study period, the rate of likely allergic reactions remained in the range of 2.5 to 3 regardless of the amount of administered injections or total reporting of allergy.
The finding that allergic reactions to the first dose did not impair adherence to subsequent doses provides several insights regarding the nature of allergic events. This observation is in line with previous studies proposing that a reaction to the first dose does not compromise the safety of subsequent vaccine administration.16 , 18 It also confirms that the decline in AEs is not the result of eliminating genuinely allergic subjects; rather, it reflects trends in the general population. Most important, it implies that the patient’s subjective experience was not severe enough to be discouraging.
In conclusion, reporting was considerably excessive and it is likely that allergic reactions were rare in the Israeli population. Concomitantly, the total number of allergic events declined over time. Finally, the challenge of dependable data collection and accurate diagnosis may remain unmet despite the application of validated forms, which suggests that a simplified system is warranted.
Footnotes
Emilia Anis and Sharon Alroy Preis contributed equally to this work.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. Am J Transplant. 2021;21:1332–1337. doi: 10.1111/ajt.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United states, December 21, 2020-January 10, 2021. Am J Transplant. 2021;21:1326–1331. doi: 10.1111/ajt.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabanillas B., Novak N. Allergy to COVID-19 vaccines: a current update. Allergol Int. 2021;70:313–318. doi: 10.1016/j.alit.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., Wickner P. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner P.J., Ansotegui I.J., Campbell D.E., Cardona V., Ebisawa M., El-Gamal Y., et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14:100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vrieze J. Pfizer's vaccine raises allergy concerns. Science. 2021;371:10–11. doi: 10.1126/science.371.6524.10. [DOI] [PubMed] [Google Scholar]
- 10.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Cabanillas B., Akdis C.A., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76:1617–1618. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 12.Rutkowski K., Mirakian R., Till S., Rutkowski R., Wagner A. Adverse reactions to COVID-19 vaccines: a practical approach. Clin Exp Allergy. 2021;51:770–777. doi: 10.1111/cea.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caminati M., Guarnieri G., Senna G. Who is really at risk for anaphylaxis due to covid-19 vaccine? Vaccines. 2021;9:1–3. doi: 10.3390/vaccines9010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sousa-Pinto B., Almeida Fonseca J., Rebelo Gomes E. Frequency of self-reported drug allergy: a systematic review and meta-analysis with meta-regression. Ann Allergy Asthma Immunol. 2017;119:326–373. doi: 10.1016/j.anai.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Cox F., Khalib K., Conlon N. PEG that reaction: a case series of allergy to polyethylene glycol. J Clin Pharmacol. 2021;61:832–835. doi: 10.1002/jcph.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessel A., Bamberger E., Nachshon L., Rosman Y., Confino-Cohen R., Elizur A. Safe administration of the Pfizer-BioNTech COVID-19 vaccine following an immediate reaction to the first dose. Allergy. 2021;76:3538–3540. doi: 10.1111/all.15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenoy E.S., Macy E., Rowe T., Blumenthal K.G. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321:188–199. doi: 10.1001/jama.2018.19283. [DOI] [PubMed] [Google Scholar]
- 18.Krantz M.S., Kwah J.H., Stone C.A., Jr., et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181:1530–1533. doi: 10.1001/jamainternmed.2021.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]