Abstract
Objective
The aim of this study is to evaluate the safety and tumor marker level changes of acupuncture plus chemotherapy (FOLFOX4) for advanced gastric cancer.
Methods
One hundred and twenty patients with advanced gastric cancer who were treated at our hospital between May 2019 and April 2021 were recruited for prospective analysis, and all patients were allocated to the control and experimental groups in a 1 : 1 ratio using the random number table method, with 60 patients in each group. They received either chemotherapy using the FOLFOX4 regimen (control group) or the FOLFOX4 chemotherapy plus acupuncture (experimental group). Outcome measures included tumor marker levels, quality of life, and adverse events.
Results
Before treatment, the two groups showed similar tumor markers levels and the MOS 36-item short-form health survey (SF-36) scores (P > 0.05). FOLFOX4 chemotherapy plus acupuncture was associated with significantly lower levels of carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA72-4 versus FOLFOX4 chemotherapy alone (P < 0.05). The patients who were given FOLFOX4 chemotherapy plus acupuncture showed significantly increased SF-36 scores versus monotherapy of the FOLFOX4 regimen (P < 0.05). The joint therapy resulted in a significantly lower incidence of adverse events versus the monotherapy (P < 0.05).
Conclusion
Acupuncture plus chemotherapy using the FOLFOX4 regimen can effectively regulate the serum tumor marker levels of patients with advanced gastric cancer, with a high safety profile, which provides a viable treatment alternative.
1. Introduction
Gastric cancer is a malignant tumour disease with a very high incidence, ranking second in terms of incidence and mortality of malignant tumours in China and posing a serious threat to the health of the population [1–4]. Due to the atypical early symptoms of gastric cancer, the disease mostly progresses to advanced stages by the time of diagnosis [5, 6]. Chemotherapy is the main treatment modality for patients with inoperable advanced gastric cancer [7]. The FOLFOX4 regimen (oxaliplatin + 5-fluorouracil) is a common chemotherapy regimen used to treat patients with advanced gastric cancer and may also involve the use of calcium folinic acid injection (a sensitising agent for fluorouracil injection) [8]. Studies have shown that FOLFOX4 chemotherapy is safe and effective in reducing the pathological stage of gastric cancer, reducing postoperative recurrence and metastasis, and prolonging patient survival [9, 10]. Given the low selectivity in targeting tumour cells and the tendency to harm normal cells, chemotherapy may lead to adverse effects, reduced compliance, poor tolerance, and impaired therapeutic efficacy [11, 12].
Acupuncture provides disease management and health care by treating with acupuncture, whose stimulation of body acupoints regulates the function of breath power, blood, and internal organs [13, 14]. Acupuncture has been reported to reduce adverse events after chemotherapy in patients with gastric cancer, but few studies have combined it with the FOLFOX4 regimen. Therefore, the aim of this study was to evaluate the safety and changes in tumour marker levels of acupuncture combined with chemotherapy (FOLFOX4) in the treatment of advanced gastric cancer.
2. Materials and Methods
2.1. Baseline Data
One hundred and twenty patients with advanced gastric cancer who underwent consultation at our hospital between May 2019 and April 2021 were recruited for prospective analysis, and all patients were allocated to the control and experimental groups in a 1 : 1 ratio using the random number table method, with 60 patients in each group. All patients and their families were informed and asked to sign a consent form, and the study was approved for implementation by the ethics committee of Cangzhou Central Hospital, No. 297901-117.
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
The inclusion criteria were as follows:
those who met the diagnostic criteria of gastric cancer in the Clinical Diagnostic and Treatment Guidelines Oncology Branch [15]
those who met the soft tissue tumor staging criteria for gastric cancer stage IIIB and stage IV by the American Joint Committee on Cancer (AJCC)
those with an expected survival of ≥3 months
2.2.2. Exclusion Criteria
The exclusion criteria were as follows:
those with serious cardiovascular, cerebrovascular, hepatic, renal, and hematological diseases
those with cardiopulmonary dysfunction
those with brain metastases
2.3. Treatment Methods
The patients in the control group were given chemotherapy (FOLFOX4 regimen) [16]. Oxaliplatin injection (Qilu Pharmaceutical Co., Ltd.) 85 mg/m2 was given intravenously for 2 h on the first day, calcium folinate injection (Jiangsu Hengrui Pharmaceutical Co., Ltd.) 200 mg/m2 was given intravenously for 2 h on the first and second days, and 5-fluorouracil (Shanghai Xudong Haipu Pharmaceutical Co., Ltd.) 400 mg/m2 was pushed intravenously, followed by 2 h continuous intravenous of 5-fluorouracil 600 mg/m2. A course of treatment was given every 2 weeks for a total of 4 courses of treatment [17].
The patients in the experimental group were given acupuncture plus chemotherapy (FOLFOX4 regimen). A millineedle of 40 mm or more was used to perform acupuncture at the following acupoints, including Guanyuan, Qihai, Zusanli, Daheng, Neiguan, Xuehai, Diji, Shuidao, and Guilai, followed by the needling techniques of the lifting-thrusting method and the reinforcing-reducing method. A moxa stick about 2 cm long is placed above the needle handle, about 2-3 cm from the skin, lit from the lower end and the skin of the acupuncture point is covered with kraft paper, and the patient feels proper warmth at the point. The acupuncture takes about 20 minutes and the moxa strips are burnt out and the needles are withdrawn. During acupuncture, if the patient feels unbearable heat, a piece of cardboard can be placed over the acupuncture point to reduce the heat. Acupuncture was performed at Zusanli and Hangjian acupoints for patients with liver and stomach disharmony, at Zusanli, Piyu, Geyu, and Sanyinjiao for spleen and kidney yang deficiency. The needles remained at the points for 6 h. Acupuncture once every other day, 20 times as a course of treatment, a total of 28 courses of treatment.
2.4. Outcome Measures
Tumor markers: At the end of the procedure, 2–5 ml of fasting venous blood was collected from the patient, clotted, and centrifuged at 2500 r/min. The serum was separated to determine carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA72-4 using electrochemiluminescence immunoassay (ECLIA) with original matching reagents.
Quality of survival [18]: The MOS 36-item Short Form Health Survey (SF-36) was used to assess quality of life 3 months after the end of treatment in 8 domains: physical functioning, physical role, physical pain, general health, vitality, social functioning, role emotion, and mental health. The total score for each dimension is 100, with higher scores representing a better quality of life for the patient.
Adverse events: Adverse events, including anaemia, nausea and vomiting, malaise, leucopenia, and peripheral neuropathy, occurred during treatment in both groups were recorded and the incidence of adverse events was calculated.
2.5. Statistical Analysis
SPSS 22.0 was used for data analyses, and GraphPad Prism 8 was used for image rendering. The measurement data were expressed as ( ± s) and processed using the t-test. The count data were expressed as the number of cases (rate) and analyzed using the chi-square test. Differences were considered statistically significant at P < 0.05.
3. Results
3.1. Baseline Data
The baseline characteristics of the control group (33 males, 27 females, aged 38–63 (50.45 ± 5.68) years, 42 cases of stage IIIB, and 18 cases of stage IV) were comparable with those of the experimental group (31 males, 29 females, aged 39–66 (50.18 ± 5.94) years, 37 cases of clinical-stage IIIB, and 23 cases of stage IV) (P > 0.05) (Table 1).
Table 1.
Comparison of baseline data ( ± s).
| Groups | n | Gender | Age | Clinical stage | |||
|---|---|---|---|---|---|---|---|
| Male | Female | Range | Mean age | IIIB | IV | ||
| Control group | 60 | 33 | 27 | 42–71 | 50.45 ± 5.68 | 42 | 18 |
| Experimental group | 60 | 31 | 29 | 40–72 | 50.18 ± 5.94 | 37 | 23 |
| t value | — | 0.251 | |||||
| P value | — | 0.802 | |||||
3.2. Tumor Markers Levels
Before treatment, the two groups showed similar tumor markers levels (P > 0.05). FOLFOX4 chemotherapy plus acupuncture was associated with significantly lower levels of CEA, CA19-9, and CA72-4 (13.21 ± 1.31 μg/ml, 158.14 ± 5.14 U/ml, 56.74 ± 5.27 U/ml) versus FOLFOX4 chemotherapy alone (16.25 ± 2.12 μg/ml, 228.52 ± 9.14 U/ml, 70.08 ± 4.13 U/ml) (P < 0.001) (Table 2).
Table 2.
Comparison of tumor markers levels ( ± s).
| Groups | n | Before treatment | After treatment | ||||
|---|---|---|---|---|---|---|---|
| CEA (μg/ml) | CA19-9 (U/ml) | CA72-4 (U/ml) | CEA (μg/ml) | CA19-9 (U/ml) | CA72-4 (U/ml) | ||
| Control group | 60 | 24.23 ± 2.27 | 255.54 ± 8.17 | 78.25 ± 3.47 | 16.25 ± 2.12 | 228.52 ± 9.14 | 70.08 ± 4.13 |
| Experimental group | 60 | 24.51 ± 2.11 | 256.08 ± 7.94 | 77.94 ± 3.86 | 13.21 ± 1.31 | 158.14 ± 5.14 | 56.74 ± 5.27 |
| t value | — | 0.718 | 0.367 | 0.462 | 9.471 | 51.967 | 15.433 |
| P value | — | 0.474 | 0.714 | 0.645 | <0.001 | <0.001 | <0.001 |
∗ indicates a statistically significant difference (P < 0.05) in the same group between before and after treatment.
3.3. Quality of Life
The patients who were given FOLFOX4 chemotherapy plus acupuncture showed significantly increased SF-36 scores (75.23 ± 6.17) versus monotherapy of the chemotherapy (68.17 ± 6.88) (P < 0.001) (Figure 1).
Figure 1.
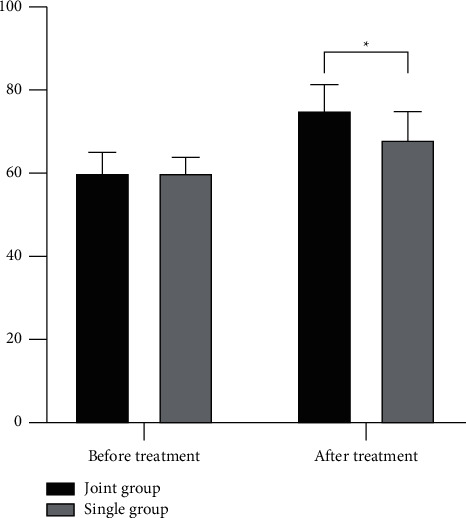
Comparison of SF-36 scores. ∗ indicates a statistically significant difference (P < 0.05) between the two groups.
3.4. Incidence of Adverse Events
The joint therapy resulted in a significantly lower incidence of adverse events (3.34%, including 1 (1.67%) case of nausea and vomiting and 1 (1.67%) case of fatigue) versus the monotherapy (41.67%, including 8 (13.34%) cases of anaemia, 11 (18.34%) cases of nausea and vomiting, 4 (6.67%) cases of fatigue, 1 (1.67%) case of leukopenia, and 1 (1.67%) case of peripheral neuropathy) (P < 0.05) (Table 3).
Table 3.
Comparison of incidence of adverse events (%).
| Groups | n | Anemia | Nausea and vomiting | Fatigue | Leukopenia | Peripheral neuropathy | Total incidence |
|---|---|---|---|---|---|---|---|
| Control group | 60 | 8 (13.34) | 11 (18.34) | 4 (6.67) | 1 (1.67) | 1 (1.67) | 25 (41.67) |
| Experimental group | 60 | 0 (0.00) | 1 (1.67) | 1 (1.67) | 0 (0.00) | 0 (0.00) | 2 (3.34) |
| χx 2 | — | 25.281 | |||||
| P value | — | <0.001 | |||||
4. Discussion
Advanced gastric cancer refers to the invasion of tumour tissue into the stroma or stromal layer of the stomach or the occurrence of extrastromal metastasis. The main clinical manifestations are emaciation, epigastric pain, anaemia, loss of appetite, and corresponding clinical manifestations in distant sites. Currently, the FOLFOX4 regimen is a common chemotherapy regimen in clinical practice [19, 20]. Acupuncture features extensive indications and significant efficacy [21]. The results of this study showed a significant reduction in CEA, CA19-9 and CA72-4 levels after acupuncture combined with FOLFOX4 regimen chemotherapy, indicating the effectiveness of FOLFOX4 regimen chemotherapy in reducing tumour marker levels. It has been shown that CEA is highly sensitive to gastric cancer and its changes correlate with the sensitivity of gastric cancer to chemotherapy, showing great benefit in prognostic assessment and efficacy observation. CA19-9 is a highly specific gastrointestinal tumour-associated antigen whose expression correlates positively with the degree of tumour progression and has a sensitivity of approximately 40% for gastric cancer. CA72-4 is a tumour marker for gastrointestinal tract tumours and ovarian cancer and plays a role in detecting residual tumours and early gastric cancer recurrence. It has been confirmed in numerous studies that serum CEA, CA19-9, and CA72-4 are all common clinical serum tumor markers for gastric cancer, with considerable clinical significance in the diagnosis and treatment of gastric cancer. In traditional Chinese medicine, the process of disease development and regression is essentially the process of the struggle between the positive and evil breath power. Acupuncture plus chemotherapy has a wide range of indications and significant efficacy to better exploit its anticancer effects. Here, the patients given FOLFOX4 chemotherapy plus acupuncture showed significantly increased SF-36 scores and a lower incidence of adverse events versus monotherapy of the chemotherapy, indicating the benefits of quality of life after the intervention of acupuncture, as it stimulates the acupoints to unblock breath power and blood of the stomach, harmonize the Yin and Yang, and the dispel evil breath power, which resulted in enhanced quality of life and fewer adverse events.
The study of Xu demonstrated that acupuncture can reduce adverse events in gastric cancer patients after chemotherapy, for example, a large number of studies have shown that acupuncture at certain specific acupoints, such as the Neiguan point, not only has an effect on the release of wuqiang acid in the vomiting centre but also has a regulatory effect on the release of wuqiang acid in the local tissues of the gastrointestinal tract, providing a good antiemetic effect from effectively reducing damage to the gastric mucosa from chemotherapy drugs and restoring gastrointestinal motility [21]. It has also been shown that acupuncture can have a prestimulatory effect, i.e., some acupuncture can reduce the degree of damage when the body is injured only before the body is injured [22]. A related study by Zhou et al. also showed that acupuncture used early in chemotherapy was more effective in improving gastric motility than later in the course of chemotherapy, suggesting that if acupuncture is used early and at the right time in chemotherapy, it may prevent the onset of vomiting and nausea, or reduce its symptoms when it occurs [23].
However, there are obvious limitations to our study. First, our experimental sample was small, which may lead to some error in the results. Second, we need to improve the monitoring indicators in subsequent trials by adding indicators such as survival after treatment and the clinical remission rate of the efficacy evaluation criteria for solid tumours (RECIST) [24].
5. Conclusion
To sum up, acupuncture plus chemotherapy using the FOLFOX4 regimen can effectively regulate the serum tumor marker levels of patients with advanced gastric cancer, with a high safety profile, which provides a viable treatment alternative.
Acknowledgments
This study was supported by Cangzhou Science and Technology Support Program Project, No. 213106044.
Data Availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Song Z., Wu Y., Yang J., Yang D., Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biology . 2017;39(7) doi: 10.1177/1010428317714626.101042831771462 [DOI] [PubMed] [Google Scholar]
- 2.Digklia A., Wagner A. D. Advanced gastric cancer: current treatment landscape and future perspectives. World Journal of Gastroenterology . 2016;22(8):2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasechnikov V., Chukov S., Fedorov E., Kikuste I., Leja M. Gastric cancer: prevention, screening and early diagnosis. World Journal of Gastroenterology . 2014;20(38):13842–13862. doi: 10.3748/wjg.v20.i38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venerito M., Link A., Rokkas T., Malfertheiner P. Gastric cancer—clinical and epidemiological aspects. Helicobacter . 2016;21:39–44. doi: 10.1111/hel.12339. [DOI] [PubMed] [Google Scholar]
- 5.Li Q., Xu X., Su D., Zhou T., Wang G., Li Z. Long-term survival of an elderly patient with advanced gastric cancer after combination therapy: a case report and literature review. BMC Cancer . 2019;19(1):p. 459. doi: 10.1186/s12885-019-5683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebenhüner A. R., De Dosso S., Helbling D., et al. Advanced gastric cancer: current treatment landscape and a future outlook for sequential and personalized guide: swiss expert statement article. Oncology Research and Treatment . 2021;44(9):485–494. doi: 10.1159/000518107. [DOI] [PubMed] [Google Scholar]
- 7.Liu H., Song J., Yang Z., Zhang X. Effects of cytokine-induced killer cell treatment combined with FOLFOX4 on the recurrence and survival rates for gastric cancer following surgery. Experimental and Therapeutic Medicine . 2013;6(4):953–956. doi: 10.3892/etm.2013.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haghighi S., Kasbkar H., Esmaeilpour K., Yasaei M. Oxaliplatin, 5fluorouracil and leucovorin (FOLFOX4) as first line chemotherapy in elderly patients with advanced gastric cancer. Asian Pacific Journal of Cancer Prevention . 2016;17(7):3277–3280. [PubMed] [Google Scholar]
- 9.Xie H., Lu Q., Wang H., Zhu X., Guan Z. Two postoperative chemotherapies for gastric cancer: FOLFOX4 vs. TPF. Oncology Letters . 2019;17(1):933–936. doi: 10.3892/ol.2018.9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi M., Ji J., Wu J., et al. Cetuximab combined with FOLFOX4 as the first-line treatment for advanced gastric cancer: report of 25 cases from a single institution. Hepato-Gastroenterology . 2012;59(116):1054–1058. doi: 10.5754/hge11874. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Qin S., Liu Y., et al. Camrelizumab combined with FOLFOX4 regimen as first-line therapy for advanced hepatocellular carcinomas: a sub-cohort of a multicenter phase ib/II study. Drug Design, Development and Therapy . 2021;15:1873–1882. doi: 10.2147/dddt.s304857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passardi A., Rapposelli I. G., Scarpi E., et al. Neoadjuvant treatment (FOLFOX4 plus hypofractionated tomotherapy) for patients with locally advanced rectal cancer: a multicenter phase II trial. Therapeutic Advances in Medical Oncology . 2020;12 doi: 10.1177/1758835920977139.175883592097713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K. Acupuncture for quality of life in gastric cancer patients: methodological issues. Journal of Pain and Symptom Management . 2022;63(4):e447–e448. doi: 10.1016/j.jpainsymman.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y. J., Min Q., Huang Y., et al. Efficacy of acupuncture and moxibustion as a subsequent treatment after second-line chemotherapy in advanced gastric cancer. Evidence-Based Complementary and Alternative Medicine . 2020;2020:9. doi: 10.1155/2020/8274021.8274021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoyama T., Yoshikawa T. Adjuvant therapy for locally advanced gastric cancer. Surgery Today . 2017;47(11):1295–1302. doi: 10.1007/s00595-017-1493-y. [DOI] [PubMed] [Google Scholar]
- 16.André T., Iveson T., Labianca R., et al. The IDEA (international duration evaluation of adjuvant chemotherapy) collaboration: prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: trial design and current status. Current Colorectal Cancer Reports . 2013;9(3):261–269. doi: 10.1007/s11888-013-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z., Zhu R. J., Yang G. F., Li Y. Neoadjuvant chemotherapy with FOLFOX4 regimen to treat advanced gastric cancer improves survival without increasing adverse events: a retrospective cohort study from a Chinese center. Scientific World Journal . 2014;2014:10. doi: 10.1155/2014/418694.418694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Beurs E., Carlier I., van Hemert A. Psychopathology and health-related quality of life as patient-reported treatment outcomes: evaluation of concordance between the brief symptom inventory (BSI) and the short form-36 (SF-36) in psychiatric outpatients. Quality of Life Research . 2021;31(5):1461–1471. doi: 10.1007/s11136-021-03019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Jiang L., Ouyang J., Du X., Jiang L. Efficacy and safety of traditional Chinese medicine injections joint with FOLFOX4 regimen for gastric cancer: a protocol for systematic review and network meta-analysis. Medicine (Baltimore) . 2021;100(41) doi: 10.1097/md.0000000000027525.e27525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi Y., Yang J., Yang S., Sun Y., Jia B., Shi Y. Phase I dose-finding study of sorafenib with FOLFOX4 as first-line treatment in patients with unresectable locally advanced or metastatic gastric cancer. Chinese Journal of Cancer Research . 2015;27(3):239–246. doi: 10.3978/j.issn.1000-9604.2015.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X. The effect of warm acupuncture on the recovery of gastrointestinal function after gastric cancer surgery. China Medical Device Information . 2016;22:p. 2. [Google Scholar]
- 22.Choi H. Daegu, Republic of Korea: DGIST; 2018. Surface-modified acupuncture needles offering enhanced therapeutic properties. Ph D. dissertation. [Google Scholar]
- 23.Zhou J., Fang L., Wu W. Y., et al. The effect of acupuncture on chemotherapy-associated gastrointestinal symptoms in gastric cancer. Current Oncology . 2017;24(1):e1–e5. doi: 10.3747/co.24.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y. J., Wu Xy, Wang W., et al. Acupuncture for quality of life in gastric cancer patients undergoing adjuvant chemotherapy. Journal of Pain and Symptom Management . 2022;63(2):210–220. doi: 10.1016/j.jpainsymman.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.


