Abstract
Background
Patients presenting with early-stage melanoma (AJCC pT1b-pT2a) reportedly have a relatively low risk of a positive SNB (~5–10%). Those patients are usually found to have low-volume metastatic disease after SNB, typically reclassified to AJCC stage IIIA, with an excellent prognosis of ~90% 5-year survival. Currently, adjuvant systemic therapy is not routinely recommended for most patients with AJCC stage IIIA melanoma. The purpose was to assess the SN-positivity rate in early-stage melanoma and to identify primary tumor characteristics associated with high-risk nodal disease eligible for adjuvant systemic therapy
Methods
An international, multicenter retrospective cohort study from 7 large-volume cancer centers identified 3,610 patients with early primary cutaneous melanomas 0.8–2.0 mm in Breslow thickness (pT1b-pT2a; AJCC 8th edition). Patient demographics, primary tumor characteristics, and SNB status/details were analyzed.
Results
The overall SNB-positivity rate was 11.4% (412/3610). Virtually all SNB-positive patients (409/412; 99.3%) were reclassified to AJCC stage IIIA. Multivariate analysis identified age, T-stage, mitotic rate, primary site and subtype, and lymphovascular invasion as independent predictors of sentinel node status. A mitotic rate of >1/mm2 was associated with a significantly increased SN-positivity rate and was the only significant independent predictor of high-risk SNB metastases (>1 mm maximum diameter).
Conclusions
The new treatment paradigm brings into question the role of SNB for patients with early-stage melanoma. The results of this large international cohort study suggest that a reevaluation of the indications for SNB for some patients with early-stage melanoma is required.
Early-stage invasive melanoma can be defined as primary tumors with Breslow thickness 0.8-2.0 mm or T-stage pT1b-pT2a according to the TNM classification system (AJCC 8th edition Staging System).1 The current standard of care for early-invasive melanoma is to stage the patients with a sentinel node biopsy (SNB).2 The incidence of sentinel node metastasis from early-stage invasive primary cutaneous melanoma is low, previously reported at 5-10%.3 In contrast, the risk of nodal metastasis from advanced primary cutaneous melanoma (Breslow thickness >4 mm, pT4b) is 35-50%. The outcomes of the MSLT-1,4 MSLT-2,5 and DeCOG6 studies, in addition to the maturation of data from recent adjuvant systemic therapy trials,7,8 have embedded the role of SNB for accurately staging patients with cutaneous melanoma, whilst simultaneously shifting the treatment paradigm to identifying those eligible for adjuvant systemic therapy, rather than completion lymph node dissection. Nearly all patients with early-stage melanoma who are subsequently found to be SNB-positive (SN+) still have an excellent prognosis, with 5-year survival approaching 90%.1 These patients are mapped to the AJCC Stage IIIA category.1 Adjuvant systemic therapy is usually not routinely recommended for this group, particularly if the maximum diameter of the tumor deposit is 1 mm or less.2 Accordingly, the low incidence of SN-positivity and the very limited role of adjuvant systemic therapy even when SN-positivity is diagnosed brings into question the role of SNB for patients with early-stage melanoma.
The dual purposes of this study were to identify patients with early-stage primary melanomas (AJCC pT1b-pT2a) who were more likely to have micrometastatic disease in a SN and to identify primary tumor characteristics predictive of patients with higher-risk nodal disease, who might potentially benefit from adjuvant systemic therapy.
Methods
Central regulatory approval for this study was granted by the UK NHS Health Research Authority (IRAS ID: 284808). A consortium of seven, high-volume cancer centers from Australia, UK, and North America with prospective, institutionally maintained melanoma databases agreed to collaborate in this study. All centers had similar referral guidelines for SNB based on the current AJCC staging criteria and routinely offered SNB to patients with primary melanomas stage pT1b and above.1,9 All centers routinely undertake internal central pathology review by dedicated dermatopathologists prior to offering SNB. The primary inclusion criteria for this study were adult patients, aged 18 years or older, with pathological stage pT1b and pT2a primary cutaneous melanomas who underwent SNB between 2005 and 2020.
Standard patient demographic data and tumor characteristics were recorded. Mitotic rate (MR), defined as mitoses per square millimeter, was measured as per the AJCC criteria using the “hot-spot” method.1 Details of the sentinel node biopsy report included nodal status and, for the SN+ cases, N-stage, maximum size of tumor deposit.10 Historic completion lymph node dissection (CLND) data were not used in the analysis due to the lack of consistency for the indications for the procedure across the centers during the study period and the irreconcilable bias of several centers participating in the MSLT-2 study at the same time.5
Statistical Analysis
Pseudoanonymised data were analyzed using Jamovi software (Version 1.6, Sydney, Australia https://www.jamovi.org) and R-Studio (version 1.3.1093, Boston, MA), both running R-language (version 3.6, https://cran.r-project.org/). Patients’ characteristics and histopathological parameters were summarized using descriptive statistics stratified by SN status. Differences between groups were tested using Kruskal-Wallis or Student’s t-tests as appropriate for continuous variables and chi-squared tests for categorical variables. Subgroup analyses were performed to assess the incidence of SN+ within pT stage and MR categories.
A model to predict the likelihood being of SN+ for individual patients was built using multivariable logistic regression and a set of factors clinically selected. Factors predicting high risk of SN tumor burden based on the maximum tumor deposit size among patients with positive SNBs were analyzed using multivariable logistic regression.
Results
Seven centers identified 3610 patients meeting the inclusion criteria. The data was collated from a period ranging from 2005–2020, inclusive.
Sentinel Node Status and Primary Tumor Characteristics
Table 1 provides a full comparison of patients’ and primary tumor characteristics, stratified by the primary outcome variable, SN status. The overall incidence of SN+ was 11.4% (412/3610; 95% confidence interval [CI]: 10.4-12.5%) with a range of 8.3% to 18.3% (X2 = 14.14, p = 0.028) between the treatment centers. SN status was significantly associated with the following variables: age, primary site location, melanoma subtype, pT stage, mitotic rate, and the presence of lymphovascular invasion. Lymphovascular invasion was associated with a 28.6% risk of SN+, but the incidence of this characteristic was 2.0% (70/3,481 cases). The incidence of SN+ was significantly correlated with increasing mitotic rate and increasing Breslow thickness but a significant inverse correlation was seen with age. The median Breslow thickness in the SN+ was 1.3 mm compared with 1.2 mm in the SN− group and the means were 1.38 mm and 1.28 mm, respectively (t-test, df = 2608, p < 0.001; the clinical effect size (Cohen’s d) = 0.33 (95% CIs: 0.23-0.43). For the pT1b (Breslow ≤1 mm) subgroup, the SN+ rate was 7.6% and the pT2a (Breslow 1.01-2 mm) subgroup was 12.8%.
Table 1.
Dataset summary, stratified by sentinel node status
| Dependent: sentinel node status | ||||||
|---|---|---|---|---|---|---|
| Dependent (N)a | Negative N = 3,198 (%) |
Positive N = 410 (%) |
Incidence Positive (%) | Total (%) | Test statistic | |
| Center | Sydney | 1400 (43.8) | 180 (43.7) | 11.4% | 1580 (43.8) | X2(6) = 14.14, p = 0.028b |
| (N = 3610) | Bristol | 227 (7.1) | 36 (8.7) | 13.7% | 263 (7.3) | |
| Leeds | 196 (6.1) | 28 (6.8) | 12.5% | 224 (6.2) | ||
| Liverpool | 134 (4.2) | 30 (7.3) | 18.3% | 164 (4.5) | ||
| Moffitt | 483 (15.1) | 45 (10.9) | 8.5% | 528 (14.6) | ||
| Norwich | 550 (17.2) | 70 (17.0) | 11.3% | 620 (17.2) | ||
| Ottawa | 208 (6.5) | 23 (5.6) | 10.0% | 231 (6.4) | ||
|
Age (N = 3610) |
Median (IQR) | 59.0 (48.0-68.0) | 54.0 (44.0-65.0) | – | 58.0 (47.0-68.0) | F1,3608 = 20.11, p < 0.001c |
| Gender | F | 1490 (46.6) | 177 (43.0) | 10.6% | 1667 (46.2) | X2(1) = 1.94, p = 0.16b |
| (N = 3610) | M | 1708 (53.4) | 235 (57.0) | 12.1% | 1943 (53.8) | |
| Primary site | Torso | 1172 (36.6) | 172 (41.7) | 12.8% | 1344 (37.2) | X2(3) = 22.82, p < 0.001b |
| (N = 3610) | Head/Neck | 448 (14.0) | 57 (13.8) | 11.3% | 505 (14.0) | |
| Upper Limb | 793 (24.8) | 60 (14.6) | 7.0% | 853 (23.6) | ||
| Lower Limb | 785 (24.5) | 123 (29.9) | 13.5% | 908 (25.2) | ||
| Melanoma subtype | SSM | 2291 (71.6) | 311 (75.5) | 12.0% | 2602 (72.1) | X2(4) = 12.94, p = 0.012b |
| (N = 3610) | Nodular | 426 (13.3) | 56 (13.6) | 11.6% | 482 (13.4) | |
| Acral | 38 (1.2) | 9 (2.2) | 19.1% | 47 (1.3) | ||
| Lentigo Maligna | 122 (3.8) | 5 (1.2) | 3.9% | 127 (3.5) | ||
| Other | 321 (10.0) | 31 (7.5) | 8.8% | 352 (9.8) | ||
|
Breslow (N = 3610) |
Median (IQR) | 1.2 (1.0-1.5) | 1.3 (1.1-1.7) | – | 1.2 (1.0-1.5) | F1,3608 = 39.54, p < 0.001c |
| T stage | T1b | 912 (28.5) | 75 (18.2) | 7.6% | 987 (27.3) | X2(1) = 19.54, p < 0.001b |
| (N = 3610) | T2a | 2286 (71.5) | 337 (81.8) | 12.8% | 2623 (72.7) | |
|
MR (N = 3610) |
Median (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-4.0) | – | 2.0 (1.0-3.0) | F1,3608 = 32.98, p < 0.001c |
| MR category | ||||||
| (N = 3610) | 0 per mm2 | 556 (17.4) | 30 (7.3) | 5.2% | 586 (16.2) | X2(2) = 34.43, p < 0.001b |
| 1 per mm2 | 899 (28.1) | 105 (25.5) | 10.5% | 1004 (27.8) | ||
| >1 per mm2 | 1743 (54.5) | 277 (67.2) | 13.7% | 2020 (56.0) | ||
| MR risk* | Low | 1455 (45.5) | 135 (32.8) | 8.5% | 1590 (44.0) | X2(1) = 24.00, p < 0.001b |
| (N = 3610) | High | 1743 (54.5) | 277 (67.2) | 13.7% | 2020 (56.0) | |
| LVI | Absent | 3027 (98.4) | 384 (95.0) | 11.3% | 3411 (98.0) | X2(1) = 20.04, p < 0.001b |
| (N = 3481) | Present | 50 (1.6) | 20 (5.0) | 28.6% | 70 (2.0) | |
| Perineural invasion | Absent | 1422 (98.8) | 195 (100.0) | 12.1% | 1617 (98.9) | X2(1) = 2.46, p = 0.12b |
| (N = 1635) | Present | 18 (1.2) | 0 (0.0) | 0.0% | 18 (1.1) | |
|
Regression (N = 2942) |
Absent | 1401 (53.8) | 189 (56.1) | 11.9% | 1590 (54.0) | X2(1) = 0.64, p = 0.42b |
| Present | 1204 (46.2) | 148 (43.9) | 10.9% | 1352 (46.0) | ||
| Ulceration* | Absent | 851 (93.3) | 70 (93.3) | 8.2% | 3513 (97.3) | X2(1) = 0.00, p = 0.99b |
| (N = 987) | Present | 61 (6.7) | 5 (6.7) | 8.2% | 97 (2.7) | |
| N stage | N0 | 3198 (100.0) | – | – | 3198 (88.6) | n/a |
| (N = 3610) | N1a | – | 328 (79.6) | – | 328 (9.1) | |
| N2a | – | 81 (19.7) | – | 81 (2.2) | ||
| N3a | – | 3 (0.7) | – | 3 (0.1) | ||
a Number of non-missing values
b Pearson (degrees of freedom)
c Kruskal-Wallis
IQR interquartile range; LVI lymphovascular invasion; MR mitotic rate (per mm2)
MR Risk: High = >1 mitoses per mm2; Low = 0-1 mitoses per mm2
*Ulceration status: pT1 tumors only
Correlation matrix assessment of Breslow thickness and MR demonstrated a significant, positive association between the two variables (Pearson’s r = 0.233, p < 0.001). The subgroup analyses for predictors of SN status are shown in Table 2, focusing on pT stage and MR categories. The rate of SN+ rate ranged from 4.5% to 9.8% across the MR categories in the pT1b group (χ2(2) = 6.19, p = 0.045) and from 5.5% to 14.7% in the pT2a group (χ2(2) = 21.6, p < 0.001). Table 2 shows that 67.2% of all SN+ arose from primary melanomas with a MR >1/mm2, compared with 7.3% of melanomas with MR of 0/mm2 (χ2 = 34.4, df = 2, p < 0.001). Similarly, 81.8% of SN+ were from primary melanomas in the T2 group, compared with 18.2% in the T1 group (χ2 = 19.54, df = 1, p < 0.001; Table 1). Likelihood ratios were calculated for subgroups of patients stratified by T-stage and MR category or MR category alone (Table 2). A MR of 0/mm2 decreased the likelihood of SN+ by a factor of 0.37 (95% CI: 0.20-0.67) in the pT1b group and 0.46 (95% CI: 0.29-0.72) in the pT2a subgroup, and by a factor of 0.42 (0.29-0.60) overall.
Table 2.
Rates sentinel node metastasis by stage and mitotic rate category
| Stage | Mitotic rate category | N (% stage) |
SN+,N (rate, %)* |
Likelihood ratio (95% CIs) |
High risk SN+, N (rate, %)** | Likelihood ratio (95% CIs) |
Statistica,b |
|---|---|---|---|---|---|---|---|
| T1b | 0/mm2 | 243 (24.6%) | 11 (4.5%) | 0.37 (0.20-0.67) | 1 (0.4%) | 0.11 (0.02-0.78) | |
| 1/mm2 | 336 (34.0%) | 24 (7.1%) | 0.60 (0.40-0.89) | 6 (1.8%) | 0.49 (0.22-1.07) | χ2 = 1.70; p = 0.19a | |
| >1/mm2 | 408 (41.3%) | 40 (9.8%) | 0.84 (0.62-1.15) | 11 (2.7%) | 0.74 (0.42-1.32) | χ2 = 5.87; p = 0.015a | |
| Total | 987 (27.3%) | 75 (7.6%) | 18 (1.8%) | χ2(2) = 6.19; p = 0.045b | |||
| T2a | 0/mm2 | 343 (13.1%) | 19 (5.5%) | 0.46 (0.29-0.72) | 5 (1.5%) | 0.40 (0.17-0.94) | |
| 1/mm2 | 668 (25.5%) | 81 (12.1%) | 1.07 (0.87-1.32) | 19 (2.8%) | 0.78 (0.51-1.19) | χ2 = 11.0; p = 0.001a | |
| >1/mm2 | 1612 (61.5%) | 237 (14.7%) | 1.34 (1.22-1.47) | 88 (5.5%) | 1.55 (1.37-1.75) | χ2 = 20.9; p < 0.001a | |
| Total | 2623 (72.7%) | 337 (12.8%) | 112 (6.9%) | χ2(2) = 21.6, p < 0.001b | |||
| T1b-T2a | 0/mm2 | 586 (16.2%) | 30 (5.1%) | 0.42 (0.29-0.60) | 6 (1.0%) | 0.23 (0.10-0.50) | |
| 1/mm2 | 1004 (27.8%) | 105 (10.5%) | 0.91 (0.76-1.08) | 25 (2.5%) | 0.56 (0.39-0.80) | χ2 = 13.6; p < 0.001a | |
| >1/mm2 | 2020 (56.0%) | 277 (13.7%) | 1.23 (1.15-1.33) | 99 (4.9%) | 1.68 (1.52-1.87) | χ2 = 32.2; p < 0.001a | |
| Total | 3610 (100.0%) | 412 (11.4%) | 130 (3.6%) | χ2(2) = 34.4, p < 0.001b |
aPearson chi-square test with one degrees of freedom
bChi-square test for trend with two degrees of freedom
*SN+ = positive sentinel node; **positive sentinel node with maximum tumor deposit size >1 mm
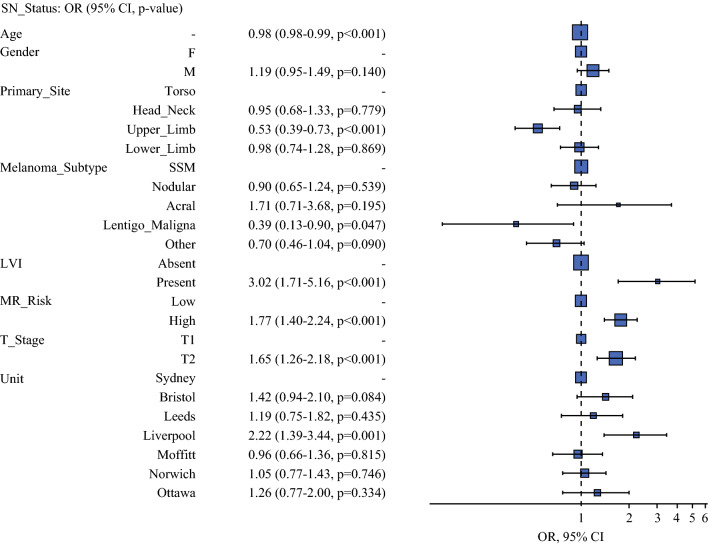
Multivariable logistic regression analysis identified age, primary site, Breslow thickness, MR, and lymphovascular invasion as significant independent predictors of sentinel node status. Figure 1 represents the results of the binomial multivariable analysis as an odds ratio plot. T2 stage, increasing mitotic rate and lymphovascular invasion were significantly associated with increased odds of SN+.
Fig. 1.
Binomial multivariable analysis of predictors for sentinel node status as an odds ratio plot. Increased odds of sentinel node metastasis to the right of the dotted line of null effect
Nodal Risk
The incidences of N1, N2, and N3 disease were 79.6%, 19.7%, and 0.7%, respectively, meaning that 99.3% of the SN+ patients were reclassified to AJCC stage IIIA following their SNB. The current NCCN guidelines2 suggest that SN+ classified with AJCC stage IIIA disease and a maximum deposit size greater than 1.0 mm may be considered for adjuvant systemic therapy. Accordingly, this definition was taken as the cut-point for classifying SN+ disease as low-risk (≤1 mm) or high-risk (>1 mm). Table 2 shows that the overall incidence of high-risk SN+ disease was 3.6% (130/3610). The high-risk SN+ rate in the pT1b group was 1.8% (18/987) and 6.9% (112/2623) in the pT2a group. Binomial logistic regression analysis was performed for 404 complete record sets, which identified MR >1/mm2 as the only primary tumor characteristic that was a significant independent predictor of high-risk SN+ disease (odds ratio [OR] = 1.95; 95% CI: 1.20-3.23, p = 0.008). Of the cohort with SN+ melanomas, 35.8% (98/274) of patients had primaries with MR >1/mm2 and high-risk SN+ disease compared with 21.5% (28/130) of patients with MR 0-1/mm2 and high-risk SN+ disease (X2(1) = 8.32, p = 0.004) (Table 3).
Table 3.
Rates of high-risk sentinel node metastasis (where tumor deposit size >1 mm in maximum diameter) by patient and primary tumor characteristics
| Low N = 278 (%) |
High N = 126 (%) |
Total N = 404 (%) |
Test statistic | Odds ratio multivariable* (95% CIs, statistic) |
||
|---|---|---|---|---|---|---|
| Age | Median (IQR) | 56.0 (44.2-66.0) | 52.0 (43.2-61.8) | 54.0 (44.0-65.0) | F1,402 = 2.81, p = 0.09c | 0.99 (0.97-1.00, p = 0.062) |
| Gender | F | 124 (44.6) | 49 (38.9) | 173 (42.8) | X2(1) = 1.16, p = 0.28b | Referent |
| M | 154 (55.4) | 77 (61.1) | 231 (57.2) | 1.31 (0.84-2.04, p = 0.234) | ||
| Breslow | Median (IQR) | 1.3 (1.1-1.7) | 1.4 (1.2-1.8) | 1.3 (1.1-1.7) | F1,402 = 1.22, p = 0.27c | – |
| T Stage | T1 | 57 (20.5) | 18 (14.3) | 75 (18.6) | χ2(1) = 2.22, p = 0.14b | Referent |
| T2 | 221 (79.5) | 108 (85.7) | 329 (81.4) | 1.38 (0.77-2.55, p = 0.292) | ||
| MR | Median (IQR) | 2.0 (1.0-4.0) | 3.0 (2.0-5.0) | 2.0 (1.0-4.0) | F1,402 = 11.87, p < 0.001c | – |
| MR Risk | Low | 102 (36.7) | 28 (22.2) | 130 (32.2) | χ2(1) = 8.32, p = 0.004b | Referent |
| High | 176 (63.3) | 98 (77.8) | 274 (67.8) | 1.95 (1.20-3.23, p = 0.008) | ||
| LVI | Present | 11 (4.0) | 9 (7.1) | 20 (5.0) | χ2(1) = 1.87, p = 0.17b | Referent |
| Absent | 267 (96.0) | 117 (92.9) | 384 (95.0) | 1.90 (0.73-4.82, p = 0.177) |
a Number of non-missing values
b Pearson (degrees of freedom)
c Kruskal-Wallis
IQR interquartile range; LVI lymphovascular invasion; MR mitotic rate (per mm2)
MR Risk: High = >1 mitoses per mm2; Low = 0-1 mitoses per mm2
*Binomial multivariable analysis stratified by age, sex, primary site, T-stage, MR risk, and LVI
Discussion
Since the publication of the MSLT-1 data, the therapeutic utility of adjuvant systemic therapy for stage III melanoma has been increasingly accepted internationally.2 Furthermore, the MSLT-2 and DeCOG studies have shown that CLND does not confer a significant survival advantage for SN+ patients and it is no longer routinely recommended.5,6 Before this, the main focus of the debate regarding sentinel node biopsy was the threshold (i.e., primary pT-stage, Breslow thickness, ulceration status, mitotic rate) at which the procedure should be offered, given an arbitrary predicted positive test rate of 5%.1,9,11 Some academic centers published algorithms to provide a further, nuanced selection criterion,12–16 and others debated whether SNB should be offered at all.17,18 Ultimately, the role of SNB for accurately staging patients with cutaneous melanoma remains, but the primary rationale for offering the procedure has altered to identifying those most likely to benefit from adjuvant systemic therapy. The focus of the current study is to reappraise those who are least likely to benefit from being staged by the procedure, particularly if the outcome does not alter subsequent management of the patient. The focus of the current analysis has been to assess the early-stage invasive melanoma group, where nearly all of the SN+ patients are subsequently reclassified to the AJCC IIIA group and the prognosis remains excellent, regardless.1
Risk of Sentinel Node Micrometastasis in the pT1b/pT2a Cohort
An initial analysis of our data demonstrated that lymphovascular invasion (LVI; syn. angiolymphatic invasion) had approximately 2.5 times the rate of SN+ compared with the rest of the cohort (p < 0.001), which is consistent with previous findings.3,19–21 This is a clear indication for SNB, yet the incidence of LVI was very low (2.0%), which makes this a poor stratifying variable for the early-stage melanoma cohort generally. Further analysis of the cohort revealed that SN+ was significantly associated with younger age, increasing Breslow thickness (or T-stage), increasing mitotic rate, primary location and tumor subtype. These results are consistent with the literature and are not necessarily specific to the early-stage group.1,20–22
Most current national guidelines use an internationally accepted threshold risk of 5% SN+ rate as an indication for performing SNB.2,23 In the current study, the overall SN+ rate was 7.6% in the pT1b cohort, 12.8% in the pT2a cohort, and 11.4% in the early-stage melanoma group as a whole. These data would seem to suggest that all patients with early-stage invasive melanoma should be offered SNB, yet the 5% threshold is arbitrary, derived from an era where systemic therapy was ineffective and the only successful treatment available at the time was surgery.4 In the modern paradigm, this threshold may need to be reconsidered, particularly when the outcome for ~90% of the cohort is a negative SNB. For two-thirds (278/404; 68.8%) of the SN+ cohort, the outcome is observation, according to current NCCN melanoma treatment guidelines.
Mitotic rate has been employed as a stratifying variable in the pT1b subgroup with successive iterations of the AJCC classification for melanoma,1,9,11 and by extension, as an indication for performing SNB. Other retrospective cohorts have investigated mitotic rate as a predictor of SNB status and outcome, and whilst there is no consistent value cutpoint, the previous literature consistently reports that the risks of both survival and SN status are directly proportional to mitotic rate.1,22,24 Data from this study demonstrated that a higher mitotic rate was significantly correlated with increasing Breslow thickness, and high mitotic rate tumors (MR >1/mm2) predominated in the T2 group. In our cohort, the median difference in Breslow thickness between the SN+ and SN− patients was statistically significant but not clinically relevant (1.2 vs. 1.3 mm; δ = 0.1 mm; p < 0.001). Accordingly, when considering the low-risk melanoma cohort (pT1b-pT2a) as a whole, it is reasonable to suggest that mitotic rate should be used as the leading primary tumor characteristic for predicting SN status.
Nodal Disease Burden
Recently, several, prospective, randomized, controlled trials have shown clinical benefit of adjuvant systemic therapy, in terms of recurrence-free survival, for patients with metastatic melanoma.7,25,26 As a result, most modern clinical guidelines recommend it for high-risk resected metastatic melanoma.2,27,28 Whilst the main factor for offering adjuvant systemic therapy is based on the risk of recurrence and/or death from melanoma, the risk of toxicity from the systemic therapy may outweigh the potential benefits of the treatment, and the patient requires careful counseling accordingly. Our data confirm that the N-stage is not helpful as a determining factor for deciding whether to offer adjuvant systemic therapy in the early-stage melanoma patients with SN+ disease. The ongoing phase III adjuvant systemic therapy clinical trials for SN+ patients have, arbitrarily, only included those with a deposit greater than 1 mm, as these were judged to be a higher risk subgroup at trial design.
Data from this study has shown that the proportion of high-risk SNB metastases (>1 mm in maximum diameter) arising from low-risk primary melanomas was 31.3% (132/422). Multivariate analysis indicated that the only variable independently predictive of high-risk SN+ disease was mitotic rate. The odds of pT1b-pT2a primary melanomas with MR >1 mm2 being staged with a high-risk SN+ were almost twice those of melanomas with MR = 0-1/mm2. Furthermore, 77.8% (98/126) of all high-risk SN+ metastases arose from primaries with MR >1/mm2.
Likelihood ratios are useful to aid decision making when determining whether to undertake a test. Rather than predicting the chances of having a disease, using Bayes’ theorem, the ratio updates the clinician as to chances their patient has the disease in question by the magnitude of the likelihood ratio value. Assessment of the likelihood ratios in Table 2 for the mitotic rate categories indicates that, for the MR = 0/mm2 group, the ratio is 0.42 (95% CI: 0.29-0.60), indicating a small to moderate decrease in the likelihood of SN+. In addition, when assessing for likelihood of high-risk nodal disease, MR = 0/mm2 decreases that likelihood by a moderate to large amount (LR = 0.23; 95% CI: 0.1-0.5). Conversely, the likelihood SN+ and high-risk SN+ are both increased with a MR>1/mm2, although this change would be considered minimal from the underlying pretest probabilities of 11.4% and 3.6%, respectively. The likelihood ratio of the MR = 1/mm2 group does not change the odds of SN+ in a clinically relevant manner.
On one level, these data would suggest that clinicians targeting maximum potential from their SNB services would be well-served by prioritizing patients with MR >1/mm2 and for considering clinical surveillance of those patients with MR = 0/mm2. Unfortunately, the paradox remains that non-invasive techniques, especially ultrasound, are very poor at identifying nodal tumor deposits less than 1 mm in maximum diameter, even when performed contemporaneously with the SN-localization procedure immediately before SNB.29 Furthermore, data from the MSLT-1 study4 was highly suggestive of the phenomenon of disease progression within the metastatic sentinel nodes, and for a small subgroup of patients, excision of the metastatic focus in the sentinel node may be therapeutic in addition to diagnostic. The theoretical concern remains, therefore, that the significantly smaller proportion of patients with low-risk primary melanomas who are not offered SNB may be clinically disadvantaged with ultimately poorer outcomes in the longer term. With these data, clinicians deciding on prioritizing their services may wish to consider the risk of missing a SN metastasis in a melanoma with a very low likelihood of SN+, who may potentially still be salvaged by surgery and/or systemic therapy at a later date, against the value of SNB to the residual, much larger group who are SN−, who cannot benefit clinically from the procedure yet approximately 10% will develop a recurrence within 5 years. Clinical decision-making algorithms for calculating the threshold for offering a diagnostic test, considering the harms of under- and overtreating patients, and the benefits of correctly identifying treatable disease are potentially useful in this scenario,30,31 although ultimately setting the threshold for offering sentinel node biopsy will be the responsibility of national guidelines committees.
Limitations
We acknowledge that the current study has several limitations, which arose from including multiple databases that were not uniformly aligned in data collection, and from the lack of a centralized pathological review of all cases, which was not practical. The classification of the host response to the primary tumor by the presence or absence of tumor infiltrating lymphocytes is well recognized as a predictor of SN status and patient outcome,32 but this data was not collected uniformly across the investigating centers. Similarly, extracapsular spread (syn. extracapsular extension) is recognized as a significant predictor of high-risk sentinel node metastases,33 but these data were not collected consistently across the units to allow analysis in the current dataset.
Conclusions
In this current study, we have undertaken a large retrospective analysis of the outcome of sentinel node biopsy from 3,610 patients with early-stage primary cutaneous melanoma treated in seven, high-volume, cancer centers across three continents. The results would suggest that further research and a reappraisal of the role of SNB is required for a significant proportion of patients with early-stage invasive primary melanomas, because virtually all SN+ patients are restaged to AJCC IIIA. In the early-stage invasive melanoma group, a mitotic rate of >1/mm2 identifies patients with increased likelihood of SN metastasis and the greater proportion of SN+ who may be considered for adjuvant systemic therapy according to NCCN guidelines. The role of SNB for tumors with a mitotic rate of 1/mm2 tumors needs further clarification.
Acknowledgment
RAS is supported by an NHMRC Practitioner Fellowship (APP1141295). RAS and JFT are recipients of an NHMRC Program Grant (APP1093017). Support from The Cameron Family, as well as from colleagues at the authors’ respective institutions is also gratefully acknowledged.
Disclosure
MDM has received honoraria to present from Sanofi. RAS has received fees for professional services from F. Hoffmann-La Roche Ltd, Evaxion, Provectus Biopharmaceuticals Australia, Qbiotics, Novartis, Merck Sharp & Dohme, NeraCare, AMGEN Inc., Bristol-Myers Squibb, Myriad Genetics, GlaxoSmithKline. Other authors have no conflicts to declare. CN has received honoraria to speak and to be on advisory boards for Novartis, Merck, EMD Sorono and Sanofi. JFT has received honoraria for advisory board participation from BMS Australia, MSD Australia, GSK and Provectus Inc, and travel and conference support from GSK, Provectus Inc and Novartis. JSZ has received fees for professional services from Merck, Amgen, Novartis, Philogen, Delcath Systems, Castle Biosciences. JSZ also has research funding through institution for Provectus, Castle Biosciences, Neracare, Philogen, Delcath Systems. None of which are pertinent to the current publication.
Footnotes
Vernon K. Sondak, John F. Thompson and Jonathan S. Zager are joint senior authors with equal merit (alphabetical).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gershenwald JE, Scolyer RA, Hess KR et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual: Melanoma Staging: AJCC 8 th Edition. CA Cancer J Clin, 2017;67(6):472–92 [DOI] [PMC free article] [PubMed]
- 2.Melanoma: Cutaneous. NCCN Clinical Practice Guidelines in Oncology. Published February 19, 2021. Accessed April 10, 2021. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- 3.Sondak VK, Taylor JMG, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11(3):247–258. doi: 10.1245/ASO.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8. [DOI] [PubMed] [Google Scholar]
- 7.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 8.Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2020;383(12):1139–1148. doi: 10.1056/NEJMoa2005493. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Akkooi ACJ, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248(6):949–955. doi: 10.1097/SLA.0b013e31818fefe0. [DOI] [PubMed] [Google Scholar]
- 11.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 12.El Sharouni MA, Ahmed T, Witkamp AJ, et al. Predicting recurrence in patients with sentinel node-negative melanoma: validation of the EORTC nomogram using population-based data. Br J Surg. 2021;108(5):550–553. doi: 10.1002/bjs.11946. [DOI] [PubMed] [Google Scholar]
- 13.Mocellin S, Thompson JF, Pasquali S, et al. Sentinel node status prediction by four statistical models: results from a large bi-institutional series (n = 1132) Ann Surg. 2009;250(6):964–969. doi: 10.1097/SLA.0b013e3181b07ffd. [DOI] [PubMed] [Google Scholar]
- 14.Mocellin S, Ambrosi A, Montesco MC, et al. Support vector machine learning model for the prediction of sentinel node status in patients with cutaneous melanoma. Ann Surg Oncol. 2006;13(8):1113–1122. doi: 10.1245/ASO.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Sabel MS, Rice JD, Griffith KA, et al. Validation of statistical predictive models meant to select melanoma patients for sentinel lymph node biopsy. Ann Surg Oncol. 2012;19(1):287–293. doi: 10.1245/s10434-011-1979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murali R, Desilva C, Thompson JF, Scolyer RA. Non-Sentinel Node Risk Score (N-SNORE): a scoring system for accurately stratifying risk of non-sentinel node positivity in patients with cutaneous melanoma with positive sentinel lymph nodes. J Clin Oncol. 2010;28(29):4441–4449. doi: 10.1200/JCO.2010.30.9567. [DOI] [PubMed] [Google Scholar]
- 17.Zagarella S. Sentinel Lymph Node Biopsy Still Provides No Benefits for Patients With Melanoma. Am J Dermatopathol. 2020;42(7):481–483. doi: 10.1097/DAD.0000000000001656. [DOI] [PubMed] [Google Scholar]
- 18.Faries MB, Ascierto PA, Blank C, et al. Letter Regarding Editorial by Samuel Zagarella. Am J Dermatopathol. 2021;43(7):539–541. doi: 10.1097/DAD.0000000000001857. [DOI] [PubMed] [Google Scholar]
- 19.Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer. 2007;109(1):100–108. doi: 10.1002/cncr.22382. [DOI] [PubMed] [Google Scholar]
- 20.Chang JM, Kosiorek HE, Dueck AC, et al. Stratifying SLN incidence in intermediate thickness melanoma patients. Am J Surg. 2018;215(4):699–706. doi: 10.1016/j.amjsurg.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murali R, Haydu LE, Quinn MJ, et al. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg. 2012;255(1):128–133. doi: 10.1097/SLA.0b013e3182306c72. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29(16):2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peach H, Board R, Cook M, et al. Current role of sentinel lymph node biopsy in the management of cutaneous melanoma: A UK consensus statement. J Plast Reconstr Aesthet Surg. 2020;73(1):36–42. doi: 10.1016/j.bjps.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Mandalà M, Galli F, Cattaneo L, et al. Mitotic rate correlates with sentinel lymph node status and outcome in cutaneous melanoma greater than 1 millimeter in thickness: A multi-institutional study of 1524 cases. J Am Acad Dermatol. 2017;76(2):264–73.e2. doi: 10.1016/j.jaad.2016.08.066. [DOI] [PubMed] [Google Scholar]
- 25.Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-Mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441–3449. doi: 10.1200/JCO.18.01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 27.Clinical practice guidelines for the diagnosis and management of melanoma. Australian Melanoma Guidelines. Published August 11, 2020. Accessed April 10, 2021. https://wiki.cancer.org.au/australia/Guidelines:Melanoma
- 28.Managing Melanoma - NICE Pathways. Published 2021. Accessed April 10, 2021. https://pathways.nice.org.uk/pathways/melanoma
- 29.Sanki A, Uren RF, Moncrieff M, et al. Targeted high-resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. J Clin Oncol. 2009;27(33):5614–5619. doi: 10.1200/JCO.2008.21.4882. [DOI] [PubMed] [Google Scholar]
- 30.Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1980;302(20):1109–1117. doi: 10.1056/NEJM198005153022003. [DOI] [PubMed] [Google Scholar]
- 31.Felder S, Mayrhofer T. Threshold analysis in the presence of both the diagnostic and the therapeutic risk. Eur J Health Econ. 2018;19(7):1019–1026. doi: 10.1007/s10198-017-0951-1. [DOI] [PubMed] [Google Scholar]
- 32.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 33.Lo M, Robinson A, Wade R, et al. Extracapsular Spread in Melanoma Lymphadenopathy: Prognostic Implications, Classification, and Management. Ann Surg Oncol. 2021;28(3):1642–1653. doi: 10.1245/s10434-020-09099-w. [DOI] [PubMed] [Google Scholar]