Abstract
Purpose
To examine the relations between patient-reported outcomes (PROs) within a conceptual model for adults with sickle cell disease (SCD) ages 18 – 45 years enrolled in the multi-site Sickle Cell Disease Implementation Consortium (SCDIC) registry. We hypothesized that patient and SCD-related factors, particularly pain, and barriers to care would independently contribute to functioning as measured using PRO domains.
Methods
Participants (N = 2054) completed a 48-item survey including socio-demographics and PRO measures, e.g., social functioning, pain impact, emotional distress, and cognitive functioning. Participants reported on lifetime SCD complications, pain episode frequency and severity, and barriers to healthcare.
Results
Higher pain frequency was associated with higher odds of worse outcomes in all PRO domains, controlling for age, gender and site (OR range 1.02–1.10, 95% CI range [1.004–1.12]). Reported history of treatment for depression was associated with 5 of 7 PRO measures (OR range 1.58–3.28 95% CI range [1.18–4.32]). Fewer individual barriers to care and fewer SCD complications were associated with better outcomes in the emotion domain (OR range 0.46–0.64, 95% CI range [0.34–0.86]).
Conclusions
Study results highlight the importance of the biopsychosocial model to enhance understanding of the needs of this complex population, and to design multi-dimensional approaches for providing more effective interventions to improve outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11136-022-03132-z.
Keywords: Patient-reported outcome measures, Sickle cell disease, Implementation science, Models—biopsychosocial
Plain English summary
Sickle cell disease (SCD) is a rare, inherited blood disorder that causes serious and life-threatening complications including pain, stroke and anemia. It is important to understand the burden of the disease, particularly as patients get older, but there are few studies in this area. Patient-reported outcomes (PROs) communicate information about aspects of patients’ lives that can only be provided from their point of view. Our goal was to examine how PROs were inter-related with pain and other sickle cell complications, barriers to care and other social variables for adults with SCD. We gained valuable insights into the impact of pain, depression, employment and income on quality of life for adults with SCD, as measured by the PROs in our study, and we showed how important barriers to care can be. We contributed to the knowledge base about PRO measurement in SCD. PRO measures thus provide meaningful information for providers and patients to improve quality of life, and the most effective interventions to improve health outcomes must be multi-dimensional.
Introduction
The routine assessment of patient-reported outcomes (PROs) in clinical settings lends to creating a patient-centered environment, by enhancing communication and shared-decision making, improving satisfaction, and allowing for monitoring improvement or deterioration of health status [1]. In clinical trials, PROs complement measures of efficacy such as survival and healthcare utilization, allowing translation of results into some benefits that can only be evaluated with patients’ reports.
Sickle cell disease (SCD) is a rare, inherited blood disorder in the U.S., affecting about 100,000 individuals, primarily African Americans [2]. The clinical manifestations of the disease include recurrent, unpredictable and severe acute pain episodes; chronic pain; cerebrovascular disease, including overt stroke; and other serious complications such as renal and cardiopulmonary disease. These manifestations can lead to significant impairment as assessed using PROs [3, 4], and an increased burden of disease as patients age [5].
The National Heart, Lung and Blood Institute (NHLBI), in collaboration with a range of sickle cell stakeholders, developed the Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me®) to provide a means of systematically evaluating disease-specific PRO domains impacted for the growing population of adults with SCD [6]. The ASCQ-Me development used advanced knowledge of psychometrics as it aligned with the development of the National Institutes of Health’s Patient-Reported Outcomes Measurement Information System (PROMIS®).
Previous research has shown that adults and children with SCD who received the disease modifying therapy hydroxyurea, compared to those who did not, reported better PROs [7–10]. Demographic factors associated with worse PRO scores for adults with SCD include age and sex [11, 12]. Other factors associated with worse PRO scores include SCD complications, particularly pain [13, 14].
As lifespans have increased, disparities in quality of life and quality of care are also evident for adults with SCD, including lower socioeconomic status [15, 16], stigma, discriminatory treatment in healthcare settings [17], lack of social support, isolation, and cognitive challenges [6, 18–20]. The prevalence of depression and anxiety is two to three times the national average [21].
A few studies have delineated how this range of challenges, manifested by scores on PROs measures, are associated with healthcare utilization. For example, lower education was found to be independently associated with potentially avoidable emergency department (ED) care [22]. Young adults with worse PRO scores evidenced more frequent SCD-related hospitalizations and ED visits and/or longer hospitalizations [7, 22, 23]. The three-year Comprehensive Sickle Cell Centers (CSCC) Collaborative Data Project that began in 2005 included 1046 participants (median age 28.0 years, 48% male, 73% SS or Sβ0 thalassemia) [5]. Participants reported impaired health related quality of life (HRQoL) on all but the mental health domain on the SF-36, particularly with increasing age [5]. Pain episodes, asthma, or avascular necrosis were associated with worse SF-36 scale scores as was chronic opioid use. Female gender was associated with impaired physical function and vitality scale scores and chronic antidepressant use was associated with worse scores on bodily pain, vitality, social functioning, emotional role, and mental health scales. Few studies have examined the inter-relations between PROs and different patient- and/or SCD-related variables within a conceptual model. Conceptual models may allow us to advance our understanding of how the disease and treatments affect individuals, as more treatment options become available.
The Sickle Cell Disease Implementation Consortium (SCDIC) was established by NHLBI in 2016 to identify and address barriers to quality care in SCD [24]. A key activity was to engage eight sites in diverse regions in the U.S. to create a registry of a minimum of 2400 adolescents and adults with SCD. The goal of the SCDIC registry is to enhance our understanding of SCD acute and chronic complications/comorbidities and treatments, as well as HRQoL and PROs for a modern cohort. We recently described the SCDIC registry methodology [25] and the preliminary evaluation of PROs in this population [26].
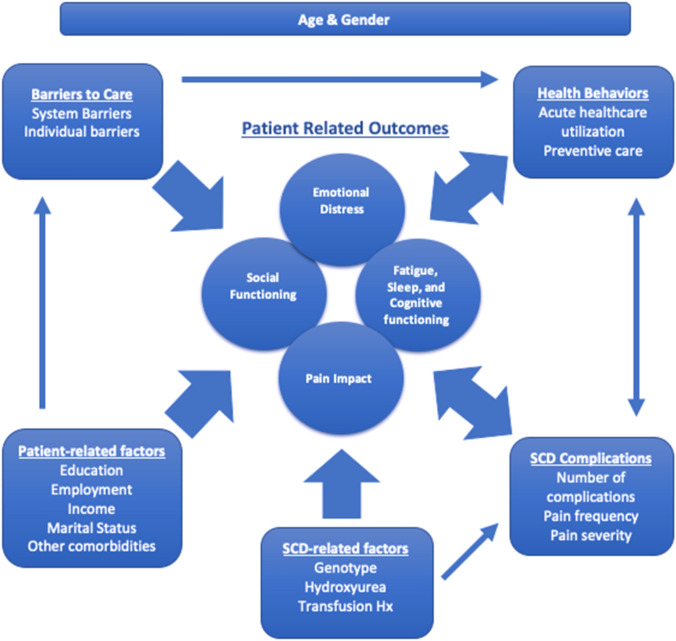
The purpose of the present analysis is to examine the relations between PROs within a conceptual model for adults with SCD ages 18–45 years enrolled in the SCDIC registry. The PRO domains assessed—emotional, pain, fatigue and sleep impacts, and social and cognitive functioning—are influenced by SCD complications, disease modifying therapies, socio-demographics, barriers to care and healthcare utilization. The conceptual model was developed from prior formative research [6], and from the SCDIC's Conceptual Framework for PROs/HRQoL in SCD [27], with input from other national and international experts in the area of PROs in SCD. Figure 1 shows the inter-relations between different variables along with their impact on PROs. Our conceptual model reflects prior research on the inter-relations among variables such as barriers to care [28], utilization patterns [29], morbidity [30], and socio-demographic factors [31, 32] and health outcomes. We hypothesized that patient and SCD-related factors as well as barriers to care would independently contribute to functioning as measured using the PRO domains. We expected that the experience of pain and other SCD-related complications would account for a significant degree of the relation between the variables.
Fig. 1.
Conceptual model for inter-relations of patient-reported outcomes (PROs) in sickle cell disease (SCD). The model includes the inter-relations of four PRO groups (emotional distress, social functioning, pain impact, and fatigue, sleep and cognitive functioning) with health behaviors (acute healthcare utilization and preventive care), SCD complications (number of complications and pain frequency/severity), SCD-related factors (genotype, hydroxyurea, chronic transfusion history), patient related factors (education, employment, income, marital status, diabetes and depression) and barriers to care (systemic and individual). All inter-relations are adjusted for age and gender identity
Method
Participants and procedures
Adolescents and adults with SCD ages 15–45 years were enrolled in the SCDIC registry during an 18-month period between 2017 and 2018. Inclusion criteria was confirmed SCD diagnosis (SS, SC, Sβ-thalassemia, other variants), literate in English, and willing and cognitively able to provide informed consent and complete the Patient Enrollment Survey. SCD diagnosis was confirmed through medical record or confirmatory laboratory test. Individuals with sickle cell trait (e.g., Hb AS), successful bone marrow transplant for SCD, or unwilling/unable to provide consent were excluded. Our sub-sample included 2,054 adults 18—45 years and with completed Patient Enrollment Surveys and Medical Record Abstraction forms.
The study utilized convenience sampling from the eight SCDIC sites, with some outreach into the community. Eligible participants were identified and recruited in-person (e.g., clinic, outreach events), by phone, or via electronic media (e.g., websites, chat rooms). Participants recruited remotely provided verbal informed consent and submitted signatures online. SCDIC research staff were available to answer questions as needed while participants completed the surveys. A member of the local study team completed a medical record abstraction for each participant. Institutional review boards of all SCDIC sites provided approval, with seven of the eight sites providing compensation for participation. All data were collected at one point in time. Details of study methodology were recently published [26].
Measures
Participants completed a 48-item survey including socio-demographics and PRO measures. We did not use complete short forms for some ASCQ-Me® and PROMIS® measures to reduce participant burden. However, the PROs that were selected were developed using item response theory (IRT), that allows for a range of administration and tailoring options [33]. An IRT-calibrated item bank consists of items that correspond with level of symptom severity or function. Any number and combination of items from the same bank can be scored and compared to all other measures derived from the same item bank without loss of precision in measurement of the construct [34]. Individual items were selected for their relevance from domains by the SCDIC investigators. Of note, interpretation of scale scores must take into consideration that the reference populations for ASCQ-Me® and PROMIS® differ, with the former consisting of adults with SCD and the latter adults from the general population. Thus, the “average” score of 50 for the PROMIS measures centers on a sample of individuals that, collectively, matched the U.S. 2000 Census on such demographics as gender, age, race/ethnicity and education, while the “average” score of 50 for the ASCQ-Me measures centers on a sample of adults with SCD 18 years of age and older.
ASCQ-Me® Emotional Impact over the past seven days was assessed using: How often did you … “feel completely hopeless because of your health?” and “were you very worried about needing to go to the hospital?” ASCQ-Me® Social Functioning over the past 30 days was assessed with: How much … “did you rely on others to take care of you because of your health?” and “did your health make it hard for you do things with your friends?” ASCQ-Me® Pain Impact over the past seven days was assessed using: How often … “did you have very severe pain?” and “did you have pain so bad that it was hard to finish what you were doing?” ASCQ-Me® Sleep Impact over the past seven days was assessed using: How often did you … “stay up most of the night because you could not fall asleep?” and “have a lot of trouble falling asleep?” All ASCQ-Me items were scored on a 5-point Likert scale (i.e., Never to Always). Item responses were uploaded to HealthMeasures Scoring Service at assessmentcenter.net, where T-scores and related statistics were generated, using adults with SCD who participated in the ASCQ-Me field test (n = 555) as the reference population [14]. The standardized T-score mean is 50 (standard deviation (SD) = 10), with higher scores indicating better outcomes.
The ASCQ-Me® Pain Episode question set includes five questions regarding the frequency (number of severe pain events in the last 12 months), timing (of most recent event) and severity of the most recent pain event (duration and pain interference). A Pain Episode composite was calculated by creating standard scores for the pain episode frequency and severity composites. A higher score indicates worse frequency, timing and severity of SCD pain.
Cognitive functioning over the past seven days was assessed using the 8-item Neuro-QOL Cognitive Function short form [35] with item responses on a 5-point Likert scale (i.e., Never to Very Often). Item responses were uploaded to the HealthMeasures Scoring Service, where T-scores and related statistics were generated using PROsetta Stone Wave 2 as the reference population, which is representative of the general adult population [36]. A higher T-score indicates better cognitive function.
The 4-item, PROMIS® short form for Emotional Distress-Depression was used to assess depressive symptoms over the past seven days and items were scored on a 5-point Likert scale (i.e., Never to Always). Crosswalk tables have been established using rigorous methodology, to link such “legacy” depression measures as the Patient Health Questionnaire (PHQ)-9, the Beck Depression Inventory-II (BDI-II) and the Center for Epidemiologic Studies Depression Scale (CES-D) with the PROMIS® Emotional Distress-Depression measure [37]. Analyses have shown that PROMIS cutoff scores for depression severity correspond with commonly used legacy measures [38].
A single item (“I felt tired”) from the PROMIS Fatigue item bank was used to measure tiredness in the past seven days, on a 5-point Likert scale from Not at All to Very Much. Item responses were uploaded to the HealthMeasures Scoring Service, where T-scores and related statistics were generated using PROMIS Wave 1 as the reference population, which is representative of the general adult population [39]. Higher T-scores on these PROMIS measures indicate worse outcomes.
Participants reported on lifetime SCD complications using the ASCQ-Me Medical History Checklist (MHC) [14], modified and expanded by the SCDIC investigators from the original list of nine to include 13 treatments and conditions associated with SCD, answered “yes” or “no.” Treatments included daily pain medicine and conditions included lung problems (e.g., acute chest syndrome); kidney, eye, hip or shoulder damage; asthma; pulmonary hypertension; heart failure; blood clots; stroke; leg ulcers; and spleen damage or removal. The score for the checklist is the number of questions with a “yes” response, thus a higher score indicates a greater number of treatments or conditions. Participants reported separately on two comorbidities—diabetes (“yes” or “no”) and current or ever treated for depression. They indicated their current use of disease modifying therapies hydroxyurea and/or regular blood transfusions (“yes” or “no”).
The SCD Barriers to Medical Care consists of 11 reasons for experiencing delays or not receiving needed medical care (“yes” or “no,” grouped into seven Access Barriers (e.g., distance from provider, insurance, challenges obtaining an appointment) and four Individual Barriers (e.g., too busy, previous bad experiences with the healthcare system) [12].
Finally, we tracked healthcare utilization (an aspect of "Health Behaviors" shown in Fig. 1) utilizing (1) outpatient visit with sickle cell specialist or primary care provider within one year of enrollment (“yes” or “no”) and (2) number of acute care visits for pain in the past year, categorized as 0, 1–2 or > = 3 ED visits or hospital admissions, both from medical record abstraction.
Statistical analyses
All analyses were performed on cross-sectional data, with seven PROs (i.e., pain, sleep, emotional and social functioning impacts, emotional distress, tiredness, and cognitive function) included in the analyses. Binary variables were created for all outputs using as cut-points one standard deviation (SD) above the mean for PROs where a higher T-score indicates a worse outcome (PROMIS® Emotional Distress and Fatigue) [41] and one SD below the mean for those where a higher T-score indicates a better outcome as compared to the reference populations (ASCQ-Me® and Neuro-QoL Cognitive Function) [32].
Baseline characteristics and distributions of risk factors are presented as frequencies and percentages for categorical variables, and median and interquartile ranges (IQR) or mean and SD for continuous variables. Categorical variables were analyzed using chi-square, or Fisher’s exact test for sparse tables. Continuous variables were compared using t-test or Mann–Whitney U test, as appropriate.
Univariate analysis was used to evaluate potentially significant variables for inclusion in multivariable models for each PRO to identify factors independently associated with better or worse outcomes. Variables with p < = 0.10 in univariate analysis were included in a multivariable logistic regression with backward elimination. Adherence with hydroxyurea and healthcare utilization (ED and inpatient visits) were not included in the models because not all participants were eligible for or prescribed hydroxyurea and about 27% of records were missing data on utilization. Age, gender and site were included and retained in all models during stepwise reduction regardless of their statistical significance to control for potential confounding effects. To account for multiple testing, a p-value = 0.01 was used as the threshold for statistical significance in the multivariable models. Odds ratios (OR) and corresponding 95% confidence intervals were obtained for variables remaining in the final model. All analyses were conducted in SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Socio-demographics
The median age of the 2054 adults with SCD in this analysis was 28 years (Table 1), and the predominant age group was 24–34 years (43.8%). Over half (56.8%) identified as female, and the majority identified as African American/Black (95.7%), with 4.5% reporting Hispanic ethnicity. The most common educational attainment was some college (35.2%), followed by high school graduate or equivalent (30.3%), with 24.1% attaining a college or advanced degree. Over a third (37.2%) were employed, 25.2% reported being disabled and the remainder were not working, either due to student status (13.5.%), or “other” (24.1%, e.g., maintaining their home or laid off). A significant proportion (74.2%) were never married and over half (54.6%) reported an annual income under $25,000, while the mean household density was 3. Like other SCD populations, almost 60% had Medicaid or other government-sponsored insurance.
Table 1.
Participant socio-demographics
| Characteristic | N = 2054 |
|---|---|
| Age | |
| Mean (SD) years | 29.1 (7.2) |
| Median (IQR) | 28 (23–35) |
| n (%) | |
| 18 to 24 years | 641 (31.2) |
| 25 to 34 years | 900 (43.8) |
| 35 to 45 years | 513 (25.0) |
| Gender | |
| Male | 888 (43.2) |
| Female | 1166 (56.8) |
| Race/Ethnicity | |
| Black/African American | 1918 (95.7) |
| Multi-racial | 67 (3.3) |
| Other Race (American Indian/Alaska Native, Asian, White) | 20 (0.9) |
| Hispanic ethnicity | 91 (4.5) |
| Highest Education | |
| Some high school or less | 209 (10.4) |
| High School (Graduate, GED or equivalent) | 612 (30.3) |
| Some college | 711 (35.2) |
| College graduate or advanced degree | 487 (24.1) |
| Employment | |
| Working now | 748 (37.2) |
| Disabled | 507 (25.2) |
| Student | 272 (13.5) |
| Other (unemployed, retired) | 485 (24.1) |
| Marital status | |
| Married or living together | 313 (16.2) |
| Never married | 1499 (77.6) |
| Not married (divorced/separated, widowed) | 120 (6.2) |
| Insurance | |
| Medicaid, other government-sponsored | 1216 (59.2)a |
| Private | 567 (27.6) |
| Medicare | 468 (22.8) |
| None | 83 (4.0) |
| Other | 16 (0.8) |
| Annual household income | |
| $25,000 or less | 998 (54.6) |
| $25,000—$50,000 | 403 (22.1) |
| $50,001 or more | 426 (23.3) |
|
Household density Mean (SD) |
aPercentages add up to greater than 100% as more than one option could be selected
Clinical characteristics, health behaviors and barriers to care
The majority (72.6%) of participants were diagnosed with sickle cell anemia (SCD genotypes SS or Beta 0 thalassemia—Table 2). Of thirteen potential SCD treatments/complications, participants reported a median of 3 treatments/complications on the ASCQ-Me MHC with 38.7% reporting three or more treatments/complications, 38.7% reporting 2—3 and 22.6% reporting 0—1 SCD-related treatments/complications. Less than three percent reported a diagnosis of diabetes, and 26% reported current or previous treatment for depression. Forty-eight percent were currently using hydroxyurea and 28.8% were currently receiving regular blood transfusions. Over 80% of participants reported no barriers to needed healthcare, with 18.2% reporting 1 or more Access barriers and 18.1% reporting 1 or more Individual barriers. Almost all (92.2%) participants had outpatient visits with their primary care provider or SCD specialist within the past year. More than half (55.2%) had three or more ED or inpatient admissions for acute pain episodes in the past year (27% missing data). For ASCQ-Me® Pain Episode Frequency and Severity T-scores, means and standard deviations were similar to the reference sample, with a pain episode frequency mean (SD) of 49.2 (11) and pain severity mean (SD) of 50.8 (9.7).
Table 2.
Clinical characteristics, barriers to care and health behaviors
| Characteristic | N = 2054 |
|---|---|
| From patient survey | |
| ASCQ-Me Medical History Checklist | |
| Median (IQR) | 3 (2) |
| Range | 0–12 |
| Sickle cell disease diagnosis | n (%) |
| Hb SS or Sβ0 thalassemia | 1490 (72.6) |
| Hb SC | 432 (21.1) |
| Hb Sβ + thalassemia and other variants | 130 (6.3) |
| Diabetes | |
| Yes | 53 (2.6) |
| No | 1953 (97.4) |
| Ever treated for depression | |
| Yes, current | 181 (9.2) |
| Yes, previous | 330 (16.8) |
| No | 1455 (74.0) |
| Hydroxyurea use and adherence | |
| Yes, adherent (6–7 of 7 days) | 628 (31.3) |
| Yes, partially adherent (2–5 of 7 days) | 250 (12.5) |
| Yes, not adherent (0–1 of 7 days) | 91 (4.5) |
| No, not currently using | 1035 (51.7) |
| Regular blood transfusions for SCD | |
| Yes | 587 (28.8) |
| No | 1449 (71.2) |
| Barriers to Care | |
| Access/Accommodations/Insurance | |
| No barriers | 1681 (81.8) |
| 1–2 barriers | 302 (14.7) |
| 3 or more barriers | 71 (3.3) |
| Individual barriers | |
| No barriers | 1683 (81.9) |
| 1–2 barriers | 323 (15.7) |
| 3 or more barriers | 48 (2.3) |
| From Medical Record Abstractions: | |
| Outpatient visit to hematologist or primary care provider within past 12 months | |
| Yes | 1893 (92.2) |
| No | 92 (4.5) |
| Unknown | 69 (3.4) |
| Emergency department (ED) and inpatient admissions for pain within past 12 months | |
| No ED or inpatient admissions | 279 (18.7) |
| 1–2 ED or inpatient admissions | 388 (26.0) |
| 3 or more ED or inpatient admissions | 823 (55.2) |
aPatients with missing data are not included in calculations of percentages unless otherwise specified
Patient-reported outcomes: multivariable models
On the ASCQ-Me® measures, means and standard deviations were similar to the reference sample. Using the Emotional and Social Functioning Impact measures, participants reported mean (SD) scores of 50.5 (8.8) and 51.2 (9.7) respectively and only a few reported T-scores less than 40, with 12.7% reporting worse emotional impact and 14.8% reporting worse social functioning compared to the population norms. Somewhat higher percentages of participants reported T-scores less than 40 for Pain (mean (SD) of 47.1 (9.0)) and Sleep Impact (mean (SD) of 49.2 (9.7)), with 21.5% reporting worse impact of pain and 16.9% reporting worse sleep impact. For Neuro-Qol Cognitive Functioning, the mean (SD) was 50.3 (9.1) with 12.9% of the sample reporting impaired cognitive functioning (T-score < 40). For PROMIS Emotional distress, the mean (SD) was 50.9 (9.6), with 20.1% reporting worse emotional distress (T-score > 60). Finally, for PROMIS® Fatigue (tiredness), the mean (SD) was 55.4 (9.5) with 22% reporting worse tiredness (T-score > 60).
Results for univariate models can be found in supplemental materials (Table S1). Based on these results, age, gender, income, employment status, marital status, ever treated for depression, access and individual barriers to care, pain frequency and severity, and number of reported complications were entered in the multivariable models according to our selection criteria for each outcome.
In the multivariable model for Emotional Impact (Table 3) ever treated for depression, and pain frequency and severity were associated with higher odds for worse outcomes, while fewer individual barriers to care and fewer than three complications on the MHC were associated with better outcomes on Emotional Impact. Employment (disabled or “other” status), ever treated for depression, and pain frequency and severity were associated with higher odds for worse social functioning in the multivariable model for Social Functioning Impact, while fewer individual barriers to care were associated with lower odds of worse social functioning impact. In the model for Pain Impact, disabled and “other” employment status and higher pain frequency/severity was associated with higher odds of poor outcomes. In the model for Sleep Impact, ever treated for depression, income less than $50,000, and increased pain frequency and severity were associated with worse outcomes while fewer than three complications on the MHC were associated with lower odds for poor outcomes. For Neuro-QoL® Cognitive Function, ever treated for depression and income of $25,000 and less were associated with higher odds for worse cognitive functioning, while fewer access barriers to care were associated with lower odds for poor cognitive functioning.
Table 3.
Significant multivariable relations between patient-reported outcomes and demographic and clinical characteristics
| Model | Predictor | OR (95% CI) |
|---|---|---|
| ASCQ-Me® Emotional Impact | ||
| Ever treated for depression | 2.30 (1.67–3.17)** | |
| # Individual barriers to care | ||
| 0 versus 1 or more | 0.50 (0.35–0.70)** | |
| ASCQ-Me® Pain Frequency | 1.05 (1.03–1.07)** | |
| ASCQ-Me® Pain Severity | 1.07 (1.04–1.09)** | |
| ASCQ-Me® SCD-MHC | ||
| Low (0–1) | 0.54 (0.33–0.86)** | |
| Medium (2–3) | 0.49 (0.34–0.70)** | |
| High (> 3) | Ref. | |
| ASCQ-Me® Social Functioning Impact | ||
| Employment | ||
| Disabled | 3.65 (2.50–5.39)** | |
| Student | 1.49 (0.87–2.51) | |
| Other | 2.40 (1.62–3.58)** | |
| Working | Ref. | |
| Ever treated for depression | 1.58 (1.18–2.13)* | |
| # Individual barriers to care | ||
| 0 versus 1 or more | 0.54 (0.39–0.75)** | |
| ASCQ-Me® Pain Frequency | 1.04 (1.03–1.06)** | |
| ASCQ-Me® Pain Severity | 1.08 (1.06–1.11)** | |
| ASCQ-Me® Pain Impact | ||
| Employment | ||
| Disabled | 2.31 (1.68–3.2)** | |
| Student | 1.05 (0.66–1.66) | |
| Other | 2.05 (1.48–2.84)** | |
| Working | Ref. | |
| ASCQ-Me® Pain Frequency | 1.10 (1.08–1.12)** | |
| ASCQ-Me® Pain Severity | 1.10 (1.08–1.12)** | |
| ASCQ-Me® Sleep Impact | ||
| Income | ||
| $25,000 and under | 1.92 (1.3–2.89)** | |
| $25,001—$50,000 | 1.95 (1.24–3.1)** | |
| $50,001 + | Ref. | |
| Ever treated for depression | 2.10 (1.56–2.81)** | |
| ASCQ-Me® Pain Frequency | 1.03 (1.01–1.04)** | |
| ASCQ-Me® Pain Severity | 1.03 (1.01–1.05)** | |
| ASCQ-Me® SCD-MHC | ||
| Low (0–1) | 0.43 (0.27–0.67)** | |
| Medium (2–3) | 0.65 (0.48–0.89)* | |
| High (> 3) | Ref. | |
| Neuro-QoL™ Cognitive Functioning | ||
| Income | ||
| $25,000 and under | 2.03 (1.36- 3.21)** | |
| $25,001—$50,000 | 1.57 (0.97- 2.57) | |
| $50,001 + | Ref. | |
| Ever treated for depression | 2.18 (1.61–2.93)** | |
| # Accessl barriers to care | ||
| 0- versus 1 or more | 0.57 (0.41–0.79)** | |
| PROMIS® Emotional Distress | ||
| Income | ||
| $25,000 and under | 1.97 (1.39–2.85)** | |
| $25,001—$50,000 | 1.36 (0.88–2.09) | |
| $50,001 + | Ref. | |
| Ever treated for depression | 3.28 (2.50–4.32)** | |
| # Individual barriers to care | ||
| 0- versus 1 or more | 0.46 (0.34–0.63)** | |
| ASCQ-Me® Pain Frequency | 1.02 (1.01–1.04)** | |
| ASCQ-Me® SCD-MHC | ||
| Low (0–1) | 0.62 (0.41–0.91)** | |
| Medium (2–3) | 0.64 (0.47–0.86)** | |
| High (> 3) | Ref. | |
| PROMIS® Fatigue/Tiredness | ||
| Gender Identity Male | 0.38 (0.29–0.49)** | |
| Ever treated for depression | 1.87 (1.47–2.39)** | |
| # Access barriers to care | ||
| 0 versus 1 or more | 0.47 (0.36–0.62)** | |
| ASCQ-Me® Pain Severity | 1.02 (1.004–1.03)* | |
ASCQ-Me®: Adult Sickle Cell Quality of Life Measurement Information System
ASCQ-Me® SCD-MHC: ASCQ-Me®: Sickle Cell Disease Medical History Checklist
Neuro-QoL™: Quality of Life in Neurological Disorders
PROMIS®: Patient-Reported Outcomes Measurement Information System
*p < .01
**p < .001
aAll models were adjusted for gender, age group, and site. ORs for these variables were included in the table only when statistically significant
In the multivariable model for PROMIS Emotional Distress, incomes of $25,000 and less, ever treated for depression and higher pain frequency were associated with higher odds for worse outcomes, while fewer individual barriers to care and fewer than three complications on the MHC were associated with lower odds for poor outcomes. Finally, in the model for PROMIS Fatigue (tiredness), ever treated for depression and higher pain severity were associated with higher odds for worse reports of tiredness, while male gender and fewer access barriers to care were associated with lower odds for tiredness.
Discussion
We hypothesized that patient and SCD-related factors as well as barriers to care would independently contribute to functioning as measured using PRO domains from the ASCQ-Me®, PROMIS® and Neuro-QoL™ measurement systems. We expected that the experience of pain and other SCD-related complications would account for a significant degree of the relation between the variables and the PRO domains. Generally, our findings were consistent with study hypotheses, with higher pain frequency and history of treatment for depression associated with higher odds of worse outcomes in almost all PRO domains studied, with findings remaining when controlling for age, gender and site. Such socio-demographic variables as lower household income and unemployment, particularly due to disability status, were also associated with higher odds of worse outcomes on some of the PRO domains. Our study includes consideration of barriers to care, and we found that reports of fewer individual barriers to care were associated with better outcomes on measures in the emotion domain. We also found that fewer self-reported SCD complications/treatments were associated with better outcomes in the emotion domain.
Our findings are consistent with previous research [5, 42, 43] that highlighted dimensions of pain experiences associated with worse outcomes on PROs for adults with SCD, as well as depression [44, 45]. However, our study includes the first large, multi-site cohort of adults with SCD who completed contemporary PRO measures that have been developed and validated with state-of-the-science psychometric methods. We thus contribute to the accumulation of information on the precision, applicability and interpretation of these next generation measurement systems.
Reports on the PRO measures for our study participants were on average similar to reference samples, although with considerable variability within and across domains. For several domains, about 20% of participants of large-scale PROMIS reference samples have demonstrated moderate to severe symptomatology or functional impairment [46, 47]. About 20% of participants in the current study reported moderate/severe pain, emotional impact or tiredness. However, the proportion of participants with moderate/severe emotional distress (12.7%) and social functioning (14.8%) and sleep impact (16.9%) on ASCQ-Me were less than that seen in the reference population for PROMIS. In the current study, the two measures therefore appear to be assessing different constructs, in contrast with a recent study including 42 adults who demonstrated severe impairments on most domains assessed on both ASCQ-Me and PROMIS Global Health measures [42]. The Esham sample also experienced more severe and more frequent pain episodes compared with the ASCQ-Me reference sample and the timing of administration of the PROs occurred in relation to hospital admissions, while the SCDIC Registry participants completed the measures as outpatients.
Approximately 13% of respondents fell below the moderate/severe threshold on the Neuro-QOL Cognitive Function short form [35] which measures concerns about general cognition and executive function [48, 49]. The multivariable model was consistent with prior studies in that worse reports of cognitive function were associated with depressive symptoms and with lower incomes [50, 51]. Given that multiple cognitive domains have been shown to be increasingly negatively affected across the lifespan for the SCD population, this is an area of particular importance for future research [52]. Further exploration is also needed of validity, reliability, interpretability, and responsiveness of scores from the SCD specific ASCQ-Me measures and the comprehensive measurement systems including PROMIS and Neuro-QOL using large, multi-site samples [40].
Complex relations were also found among measures. Frequency of pain and history of depression were associated with the highest odds for worse emotional and sleep impacts, consistent with other studies [53, 54]. Pain experiences combined with unemployment (particularly related to disabled status) played a significant role in worse outcomes on social functioning. We consistently found that fewer patient reports of SCD-related complications and treatments were associated with better outcomes on the PRO measures. Thus, when considering clinical and research interventions, there is ample evidence that HRQoL in SCD must be viewed as a complex biopsychosocial phenomenon and there is a need for specific focus on pain experience and depression.
While disparities in HRQoL and quality of care are well-recognized in SCD, particularly for adults [55, 56], the impact of barriers to care has not been widely studied. We used a modified version of the first disease-specific measure of barriers to care in SCD and demonstrated that most participants in the SCDIC registry reported no barriers to needed care, and fewer barriers to care were associated with better outcomes on all PRO measures except pain and sleep impact. In a recent study of 303 adults with SCD, and in the SCDIC needs assessment with over 400 adolescents and adults with SCD, the most reported barriers to receiving care were costs, and perceived discrimination by and mistrust in healthcare professionals [32, 57].
Limitations
Despite participation from multiple sites across the U.S., the generalizability of the sample may still represent a limitation, given that we used convenience sampling and the majority were recruited through sickle cell centers and had seen a sickle cell or primary care provider in the previous year. Due to the shortage of adult sickle cell specialists in the U.S., most adults with SCD do not have access to needed preventive care. The impact of disparities in access to care on HRQoL can only be determined when more patients who are “unaffiliated” with SCD care are recruited into research. Barriers to health care access were reported in less than 20% of our study population and this may be an underestimation due to selection bias of patients who are already established in specialized SCD centers.
The cross-sectional nature of the study precludes any conclusions about causal relations between study variables and the PROs. To reduce participant burden, we did not include all items for every PRO measure, thereby limiting full comparison with studies using the complete PRO measures. However, these measures have been constructed to maintain precision even when single, or a few items are used. Our registry data collection included both self-report and information extracted from medical records, however for completeness of data, we only used self-reports of SCD complications experienced, and these reports may suffer from recall bias or may not correspond with actual complications. Further, our reliance on self-report data poses the potential risk of subjectivity and interindividual variation.
We did not have data on several potential contributing factors to the PRO measures, such as other mental health symptoms, e.g., anxiety; actual experience of stigma and discrimination; chronic pain; coping and self-efficacy. We acknowledge that our measure of depression is a self-report of “ever received treatment for depression” so the prevalence of “depression” that we found on the order of 26% may be an underestimate. We did not use data on healthcare utilization from the medical record in these analyses given excess missing data. Study limitations notwithstanding, our research contributes to the literature in its examination of inter-relations between modern PRO measures and SCD-related and other variables within a conceptual model and utilizing a large, geographically diverse sample.
Conclusions
Reliable and valid PRO measurement is essential to the design of clinical trials and other research [58, 59]. Authoritative bodies including the Centers for Medicaid and Medicare Services and the Food and Drug Administration have prioritized the use of PRO measures for clinical and research applications [60, 61]. Results from this study can provide a baseline for longitudinal investigations that can establish sensitivity to change of the PRO measures and advance our understanding of how SCD and its treatments impact outcomes. We highlighted how critical it is to view lives, care and treatments for individuals with SCD within a biopsychosocial model given our sample’s high prevalence of history of depression, impact of pain experiences in every PRO domain, yet positive associations with fewer barriers to care and disease complications and inter-relations between these variables and socio-demographics such as income and employment status. This research supports that PRO measures can provide meaningful information for providers and patients to improve HRQoL, as well as inform multi-dimensional approaches for providing more effective interventions to improve outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Ome-Ollin Ruiz.
Author contributions
Conceptualization: Treadwell, Mushiana, Badawy, Gordeuk, Wun; Methodology: Drs. Treadwell, Glassberg, Gordeuk, Shah, Snyder, Wun; Formal analysis and investigation: Drs. Treadwell, Mushiana, Badawy, Preiss, Ms. Chen; Writing—original draft preparation: Drs. Treadwell, Mushiana, Badawy, Ms. Chen; Writing—review and editing: Drs. Treadwell, Mushiana, Badawy, Preiss, King, Kroner, Glassberg, Gordeuk, Shah, Snyder, Wun, Ms. Chen; Funding acquisition: Drs. Treadwell, Glassberg, Gordeuk, Shah; Resources: Drs. Treadwell, Kroner, Glassberg; Supervision: Drs. Treadwell, Kroner.
Funding
The Sickle Cell Disease Implementation Consortium (SCDIC) has been supported by US Federal Government cooperative agreements HL133948, HL133964, HL133990, HL133996, HL133994, HL133997, HL134004, HL134007, and HL134042 from the National Heart Lung and Blood Institute and the National Institute on Minority Health and Health Disparities (Bethesda, MD).
Data availability
All data and materials support our published claims and comply with field standards.
Code availability
Not applicable.
Declarations
Conflict of interest
Drs. Treadwell, Mushiana, Badawy, Preiss, King, Kroner, Glassberg, Gordeuk, Snyder, Wun and Ms. Chen have none to declare. Dr. Shah receives research funding from Global Blood Therapeutics, is a consultant for Novartis, Emmaus Medical and Forma Therapeutics and a speaker for Global Blood Therapeutics and Novartis.
Ethical approval
This study was approved by the institutional review boards of the participating institutions (Approval Numbers: University of California San Francisco 20–31161; Research Triangle Institute 14157; Washington University, St. Louis 201706016; Mount Sinai PPHS 16–01047; University of Illinois, Chicago 17–061401; Duke University Pro00073506; Augusta University 1078191). The authors certify that all procedures in the study were performed in accordance with the ethical standards as laid down in the 1964 declaration of Helsinki and its latter amendments or comparable ethical standards.
Informed consent
Informed consent to participate in the study was obtained from all participants.
Consent for publication
Informed consent included agreement to have de-identified data published and disseminated in accordance with publication guidelines for the Sickle Cell Disease Implementation Consortium.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dobrozsi S, Panepinto J. Patient-reported outcomes in clinical practice. Hematology. 2015;2015(1):501–506. doi: 10.1182/asheducation-2015.1.501. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. (2020, December 16). Data & Statistics on Sickle Cell Disease | CDC. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/sicklecell/data.html
- 3.Panepinto JA. Health-related quality of life in patients with hemoglobinopathies. Hematology. 2012;2012(1):284–289. doi: 10.1182/asheducation.V2012.1.284.3798319. [DOI] [PubMed] [Google Scholar]
- 4.Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: Past, present, and future. Pediatric Blood & Cancer. 2012;59(2):377–385. doi: 10.1002/pbc.24176. [DOI] [PubMed] [Google Scholar]
- 5.Dampier C, LeBeau P, Rhee S, Lieff S, Kesler K, Ballas S, Rogers Z, Wang W. Health-related quality of life in adults with sickle cell disease (SCD): A report from the comprehensive sickle cell centers clinical trial consortium. American Journal of Hematology. 2011;86(2):203–205. doi: 10.1002/ajh.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treadwell MJ, Hassell K, Levine R, Keller S. Adult sickle cell quality-of-life measurement information system (ASCQ-Me): Conceptual model based on review of the literature and formative research. The Clinical Journal of Pain. 2014;30(10):902–914. doi: 10.1097/AJP.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badawy SM, Barrera L, Cai S, Thompson AA. Association between participants’ characteristics, patient-reported outcomes, and clinical outcomes in youth with sickle cell disease. BioMed Research International. 2018 doi: 10.1155/2018/8296139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badawy SM, Thompson AA, Lai J-S, Penedo FJ, Rychlik K, Liem RI. Health-related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatric Blood & Cancer. 2017;64(6):2636. doi: 10.1002/pbc.26369. [DOI] [PubMed] [Google Scholar]
- 9.Ballas SK, Barton FB, Waclawiw MA, Swerdlow P, Eckman JR, Pegelow CH, Koshy M, Barton BA, Bonds DR. Hydroxyurea and sickle cell anemia: Effect on quality of life. Health and Quality of Life Outcomes. 2006;4:59. doi: 10.1186/1477-7525-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornburg CD, Calatroni A, Panepinto JA. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. Journal of Pediatric Hematology/Oncology. 2011;33(4):251–254. doi: 10.1097/MPH.0b013e3182114c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JL, Lemanek KL, Clough-Paabo E, Rhodes M. Predictors of health-related quality of life over time among adolescents and young adults with sickle cell disease. Journal of Clinical Psychology in Medical Settings. 2014;21(4):313–319. doi: 10.1007/s10880-014-9406-3. [DOI] [PubMed] [Google Scholar]
- 12.Treadwell, M. J., Barreda, F., Kaur, K., & Gildengorin, G. (2015) Emotional Distress, Barriers to Care, and Health-Related Quality of Life in Sickle Cell Disease. Journal of Clinical Outcomes Management, 22(1)
- 13.Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood C, Lanzkron S, Hand M, Edwards RR, Haythornthwaite JA. An evaluation of central sensitization in patients with sickle cell disease. The Journal of Pain. 2016;17(5):617–627. doi: 10.1016/j.jpain.2016.01.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller SD, Yang M, Treadwell MJ, Werner EM, Hassell KL. Patient reports of health outcome for adults living with sickle cell disease: Development and testing of the ASCQ-Me item banks. Health and Quality of Life Outcomes. 2014;12:125. doi: 10.1186/s12955-014-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky MA, Rodeghier M, Sanger M, Byrd J, McClain B, Covert B, Roberts DO, Wilkerson K, DeBaun MR, Kassim AA. Risk factors for 30-day readmission in adults with sickle cell disease. The American Journal of Medicine. 2017;130(5):601.e9–601.e15. doi: 10.1016/j.amjmed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Shah N, Bhor M, Xie L, Halloway R, Arcona S, Paulose J, Yuce H. Treatment patterns and economic burden of sickle-cell disease patients prescribed hydroxyurea: A retrospective claims-based study. Health and Quality of Life Outcomes. 2019 doi: 10.1186/s12955-019-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues in Mental Health Nursing. 2018;39(8):675–686. doi: 10.1080/01612840.2018.1443530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asnani MR, Fraser R, Lewis NA, Reid ME. Depression and loneliness in Jamaicans with sickle cell disease. BMC Psychiatry. 2010;10:40. doi: 10.1186/1471-244X-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor LEV, Stotts NA, Humphreys J, Treadwell MJ, Miaskowski C. A Biopsychosocial-spiritual model of chronic pain in adults with sickle cell disease. Pain Management Nursing: Official Journal of the American Society of Pain Management Nurses. 2013;14(4):287–301. doi: 10.1016/j.pmn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, Kasten J, Eggleston B, Kesler K, McMahon L, Orringer EP, Harrington T, Kalinyak K, De Castro LM, Kutlar A, Rutherford CJ, Johnson C, Bessman JD, Jordan LB, Armstrong FD. Neuropsychological Dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA : The Journal of the American Medical Association. 2010;303(18):1823–1831. doi: 10.1001/jama.2010.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonassaint CR, Jones VL, Leong S, Frierson GM. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. British Journal of Haematology. 2016;174(1):136–147. doi: 10.1111/bjh.14023. [DOI] [PubMed] [Google Scholar]
- 22.Jonassaint CR, Beach MC, Haythornthwaite JA, Bediako SM, Diener-West M, Strouse JJ, Lanzkron S, Onojobi G, Carroll CP, Haywood C. The Association between educational attainment and patterns of emergency department utilization among adults with sickle cell disease. International Journal of Behavioral Medicine. 2016;23(3):300–309. doi: 10.1007/s12529-016-9538-y. [DOI] [PubMed] [Google Scholar]
- 23.Badawy SM, Thompson AA, Holl JL, Penedo FJ, Liem RI. Healthcare utilization and hydroxyurea adherence in youth with sickle cell disease. Pediatric Hematology and Oncology. 2018;35(5–6):297–308. doi: 10.1080/08880018.2018.1505988. [DOI] [PubMed] [Google Scholar]
- 24.DiMartino LD, Baumann AA, Hsu LL, Kanter J, Gordeuk VR, Glassberg J, Treadwell MJ, Melvin CL, Telfair J, Klesges LM, King A, Wun T, Shah N, Gibson RW, Hankins JS. The sickle cell disease implementation consortium: translating evidence-based guidelines into practice for sickle cell disease. American Journal of Hematology. 2018;93(12):E391–E395. doi: 10.1002/ajh.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glassberg JA, Linton EA, Burson K, Hendershot T, Telfair J, Kanter J, Gordeuk VR, King AA, Melvin CL, Shah N, Hankins JS, Epié AY, Richardson LD. Publication of data collection forms from NHLBI funded sickle cell disease implementation consortium (SCDIC) registry. Orphanet Journal of Rare Diseases. 2020;15(1):178. doi: 10.1186/s13023-020-01457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knisely, M. R., Pugh, N., Kroner, B., Masese, R., Gordeuk, V., King, A. A., Smith, S. M., Gurney, J. G., Adams, R., Wun, T., Snyder, A., Glassberg, J., Shah, N., Treadwell, M., & Sickle Cell Disease Implementation Consortium Patient-reported outcomes in sickle cell disease and association with clinical and psychosocial factors: Report from the sickle cell disease implementation consortium. American Journal of Hematology. 2020;95(9):1066–1074. doi: 10.1002/ajh.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King AA, Badawy SM, Panepinto J, Anie K, Jonassaint C, Treadwell M. Psychosocial Burden in Sickle Cell Disease. In: Gladwin M, Novelli E, Kato G. eds. Sickle Cell Disease. 1ed. San Francisco, CA: McGraw Hill; 2021. ISBN: 978–1–260–45859–6.
- 28.Brennan-Cook J, Bonnabeau E, Aponte R, Augustin C, Tanabe P. Barriers to care for persons with sickle cell disease: the case manager's opportunity to improve patient outcomes. Professional Case Management. 2018;23(4):213–219. doi: 10.1097/NCM.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benenson I, Jadotte Y, Echevarria M. Factors influencing utilization of hospital services by adult sickle cell disease patients A systematic review. JBI Database of Systematic Reviews and Implementation Reports. 2017;15(3):765–808. doi: 10.11124/JBISRIR-2016-002983. [DOI] [PubMed] [Google Scholar]
- 30.Ataga KI, Gordeuk VR, Agodoa I, Colby JA, Gittings K, Allen IE. Low hemoglobin increases risk for cerebrovascular disease, kidney disease, pulmonary vasculopathy, and mortality in sickle cell disease: A systematic literature review and meta-analysis. PLoS ONE. 2020;15(4):e0229959. doi: 10.1371/journal.pone.0229959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asnani MR, Knight Madden J, Reid M, Greene LG, Lyew-Ayee P. Socio-environmental exposures and health outcomes among persons with sickle cell disease. PLoS ONE. 2017;12(4):e0175260. doi: 10.1371/journal.pone.0175260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizio AA, Bhor M, Lin X, McCausland KL, White MK, Paulose J, Nandal S, Halloway RI, Bronté-Hall L. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Quality of Life Research. 2020 doi: 10.1007/s11136-019-02412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stover AM, McLeod LD, Langer MM, Chen W-H, Reeve BB. State of the psychometric methods: Patient-reported outcome measure development and refinement using item response theory. Journal of Patient-Reported Outcomes. 2019;3(1):50. doi: 10.1186/s41687-019-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fries JF, Bruce B, Cella D. The promise of PROMIS: Using item response theory to improve assessment of patient-reported outcomes. Clinical and Experimental Rheumatology. 2005;23(5 Suppl 39):S53–57. [PubMed] [Google Scholar]
- 35.Cella D, Lai J-S, Nowinski CJ, Victorson D, Peterman A, Miller D, Bethoux F, Heinemann A, Rubin S, Cavazos JE, Reder AT, Sufit R, Simuni T, Holmes GL, Siderowf A, Wojna V, Bode R, McKinney N, Podrabsky T, Moy C. Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The patient-reported outcomes measurement information system (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Medical Care. 2007;45(Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychological Assessment. 2014;26(2):513–527. doi: 10.1037/a0035768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clover K, Lambert SD, Oldmeadow C, Britton B, King MT, Mitchell AJ, Carter G. PROMIS depression measures perform similarly to legacy measures relative to a structured diagnostic interview for depression in cancer patients. Quality of Life Research. 2018;27(5):1357–1367. doi: 10.1007/s11136-018-1803-x. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, Hays RD. Representativeness of the patient-reported outcomes measurement information system internet panel. Journal of Clinical Epidemiology. 2010;63(11):1169–1178. doi: 10.1016/j.jclinepi.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller S, Yang M, Treadwell MJ, Hassell KL. Sensitivity of alternative measures of functioning and wellbeing for adults with sickle cell disease: Comparison of PROMIS® to ASCQ-MeSM. Health and Quality of Life Outcomes. 2017;15(1):117. doi: 10.1186/s12955-017-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaraja V, Mara C, Khanna PP, et al. Establishing clinical severity for PROMIS® measures in adult patients with rheumatic diseases. Quality of Life Research. 2018;27:755–764. doi: 10.1007/s11136-017-1709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esham KS, Rodday AM, Smith HP, Noubary F, Weidner RA, Buchsbaum RJ, Parsons SK. Assessment of health-related quality of life among adults hospitalized with sickle cell disease vaso-occlusive crisis. Blood Advances. 2020;4(1):19–27. doi: 10.1182/bloodadvances.2019000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClish DK, Smith WR, Levenson JL, Aisiku IP, Roberts JD, Roseff SD, Bovbjerg VE. Comorbidity, pain, utilization, and psychosocial outcomes in older versus younger sickle cell adults: The PiSCES project. BioMed Research International. 2017 doi: 10.1155/2017/4070547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adam SS, Flahiff CM, Kamble S, Telen MJ, Reed SD, De Castro LM. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Advances. 2017;1(23):1983–1992. doi: 10.1182/bloodadvances.2017006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Master S, Arnold C, Davis T, Shi R, Mansour RP. Anxiety, depression, pain intensity and interference in adult patients with sickle cell disease. Blood. 2016;128(22):1312–1312. doi: 10.1182/blood.V128.22.1312.1312. [DOI] [Google Scholar]
- 46.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Hays R. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the patient-reported outcomes measurement information system (PROMIS) Journal of Clinical Epidemiology. 2010;63(11):1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prussien KV, DeBaun MR, Yarboi J, Bemis H, McNally C, Williams E, Compas BE. Cognitive function, coping, and depressive symptoms in children and adolescents with sickle cell disease. Journal of Pediatric Psychology. 2018;43(5):543–551. doi: 10.1093/jpepsy/jsx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, Uemura K, Lee S, Park H, Suzuki T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Frontiers in Aging Neuroscience. 2014 doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanger M, Jordan L, Pruthi S, Day M, Covert B, Merriweather B, Rodeghier M, Debaun M, Kassim A. Cognitive deficits are associated with unemployment in adults with sickle cell anemia. Journal of Clinical & Experimental Neuropsychology. 2016;38(6):661–671. doi: 10.1080/13803395.2016.1149153. [DOI] [PubMed] [Google Scholar]
- 51.Saunders B, Milyavskaya M, Etz A, Randles D, Inzlicht M. Reported self-control is not meaningfully associated with inhibition-related executive function: A bayesian analysis. Collabra Psychology. 2018;4(1):39. doi: 10.1525/collabra.134. [DOI] [Google Scholar]
- 52.Prussien KV, Jordan LC, DeBaun MR, Compas BE. Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. Journal of Pediatric Psychology. 2019;44(8):948–958. doi: 10.1093/jpepsy/jsz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moscou-Jackson G, Allen J, Kozachik S, Smith MT, Budhathoki C, Haywood C., Jr Acute pain and depressive symptoms: Independent predictors of insomnia symptoms among adults with sickle cell disease. Pain Management Nursing. 2016;17(1):38–46. doi: 10.1016/j.pmn.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallen GR, Minniti CP, Krumlauf M, Eckes E, Allen D, Oguhebe A, Seamon C, Darbari DS, Hildesheim M, Yang L, Schulden JD, Kato GJ, Taylor VI, J. G. Sleep disturbance, depression and pain in adults with sickle cell disease. BMC Psychiatry. 2014;14:207. doi: 10.1186/1471-244X-14-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee L, Smith-Whitley K, Banks S, Puckrein G. Reducing health care disparities in sickle cell disease: A Review. Public Health Reports. 2019;134(6):599–607. doi: 10.1177/0033354919881438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evensen CT, Treadwell MJ, Keller S, Levine R, Hassell KL, Werner EM, Smith WR. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine. 2016;95(35):e4528. doi: 10.1097/MD.0000000000004528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanter J, Gibson R, Lawrence RH, Smeltzer MP, Pugh NL, Glassberg J, Masese RV, King AA, Calhoun C, Hankins JS, Treadwell M. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Network Open. 2020;3(5):206016. doi: 10.1001/jamanetworkopen.2020.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarri G, Bhor M, Abogunrin S, Farmer C, Nandal S, Halloway R, Revicki DA. Systematic literature review and assessment of patient-reported outcome instruments in sickle cell disease. Health and Quality of Life Outcomes. 2018 doi: 10.1186/s12955-018-0930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yount SE, Cella D, Blozis S. PROMIS®: Standardizing the patient voice in health psychology research and practice. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2019;38(5):343–346. doi: 10.1037/hea0000741. [DOI] [PubMed] [Google Scholar]
- 60.CMS Measures Management System Blueprint | CMS. (n.d.). Retrieved January 26, 2022, from https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/mms-blueprint
- 61.Farrell AT, Panepinto J, Carroll CP, Darbari DS, Desai AA, King AA, Adams RJ, Barber TD, Brandow AM, DeBaun MR, Donahue MJ, Gupta K, Hankins JS, Kameka M, Kirkham FJ, Luksenburg H, Miller S, Oneal PA, Rees DC, Zempsky WT. End points for sickle cell disease clinical trials: Patient-reported outcomes, pain, and the brain. Blood Advances. 2019;3(23):3982–4001. doi: 10.1182/bloodadvances.2019000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials support our published claims and comply with field standards.
Not applicable.